Abstract
P-glycoprotein (P-gp), which was first identified in cancer cells, is an ATP-dependent efflux transporter that expels a wide variety of cytotoxic compounds out of cells. This transporter can decrease the bioavailability of therapeutic drugs by preventing their sufficient intracellular accumulation. Over expression of P-gp in cancer cells lead to multidrug resistance (MDR) phenotype that is one of the main reasons for the failure of chemotherapy. Hence, P-gp inhibition is a favorable method to reverse MDR. In this study, the lignanamides from Cannabis sativa were docked against P-gp to recognize potential binding affinities of these phytochemicals. Tariquidar and zosuquidar, two well-known P-gp inhibitors, were selected as the control ligands. It was observed that cannabisin M and cannabisin N exhibited higher binding affinities (− 10.2 kcal/mol) to drug-binding pocket of P-gp when compared with tariquidar and zosuquidar that showed binding affinities of − 10.1 and − 9.6 kcal/mol, respectively. Based on these findings, cannabisin M and cannabisin N could be good drug candidates against P-gp.
Keywords: P-glycoprotein, ABC transporter, Cannabis, Lignanamide, Molecular docking
Introduction
P-glycoprotein (P-gp) is located at the cell surface where it mediates the ATP-dependent efflux of a wide range of hydrophobic compounds including anticancer drugs, hydrophobic drugs, steroids, peptides, detergents and lipids (Liu et al. 2000; Sharom 2006; Ambudkar et al. 1999). P-gp is composed of about 1280 amino acids arranged as a single chain with two homologous halves having 43% amino acid identity. A linker region of ~ 60 amino acids connects the two halves of the protein (Chen et al. 1986). Each half has six transmembrane domains (TM) and a hydrophilic domain containing an ATP-binding site, known as nucleotide binding domain (NBD) (Loo and Clarke 1994, 1995, 1996, 1999; Kast et al. 1996).
This transporter is clinically important because it can reduce the bioavailability of therapeutic drugs (Kim 2006). For example, its expression at the blood–brain barrier can reduce the efficacy of agents used to treat epilepsy, infections and brain tumors (Kim et al. 1998; Miller et al. 2008). P-gp, which was first identified in cancer cells, is encoded by MDR1/ABCB1 gene in humans. The gene shows an exclusive over expression in cancer cells. The correlation between the up regulation of MDR1 mRNA transcription and the over expression of the P-gp transport system, led to multidrug resistance (MDR) phenotype, has been well established during cancer chemotherapy and several microbial infections (Srivalli and Lakshmi 2012). P-gp inhibition as a method to reverse MDR in cancer patients has been studied extensively, but the results have generally been disappointing (Gadhe and Cho 2011).
Phytocompounds of Cannabis sativa L. are among the natural compounds influencing Pgp activity. In multidrug-resistant mouse lymphoma cells, cannabinol, cannabispirol and cannabidiol increased cytotoxic drug accumulation, whereas cannabidiolic acid, tetrahydrocannabidiolic acid and ∆9-tetrahydrocannabinol (THC) reduced it (Molnar et al. 2000).
According to the literature reports, Cannabis sativa L. has various medicinal usages such as the treatment of enteric infections, inflammatory conditions, disorders of motility, emesis and abdominal pain etc. (Russo 2007). A growing set of the secondary metabolites such as cannabinoids, flavonoids, stilbenoids, terpenoids, alkaloids, and lignanamides have been isolated in Cannabis sativa L. (Flores-Sanchez and Verpoorte 2008), among which the lignanamide family exhibited interesting and diverse biological activities including feeding deterrent activity, insecticidal effects, antitumour-promoting effect, and antiinflammatory activity (Li et al. 2012). Here, we studied binding modes and potential binding affinities of lignanamides with P-gp via molecular docking.
Materials and methods
Molecular docking simulation
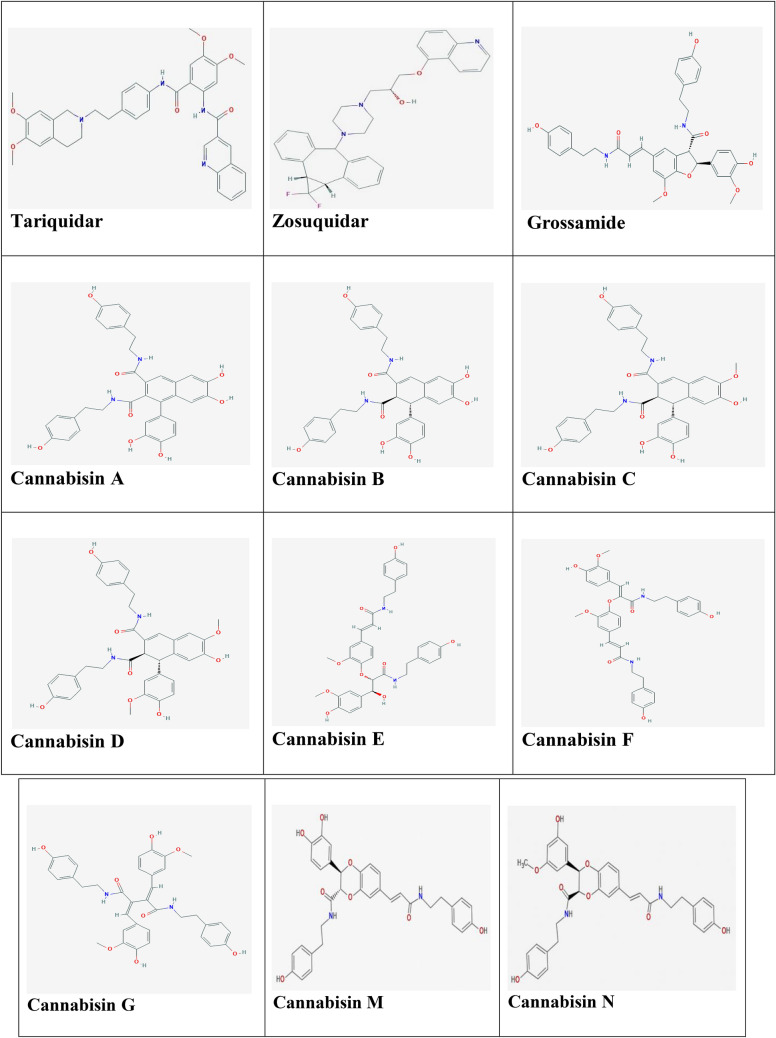
To study the binding modes of ligands in drug-binding pocket of P-gp (Aller et al. 2009), the crystal structure of mouse P-gp (PDB ID: 4Q9H; 87% identical amino acid sequence to human P-gp; Dolghih et al. 2011) was retrieved from Protein Data Bank (PDB; http://www.RCSB.org; Berman et al. 2002). The chemical structures of the control ligands including tariquidar and zosuquidar (Nobili et al. 2006) and the test ligands including cannabisin A, B, C, D, E, F, G and grossamide (Brenneisen and ElSohly 2007; Yan et al. 2015; Zhou et al. 2018) were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) database in SDF format and 3D structures of cannabisin N and M (Yan et al. 2015) that are not available on the PubChem, were generated by Chemdraw software (Fig. 1). To get ready the structures, adding hydrogens and energy minimizing were performed by Molegro Virtual Docker (Thomsen and Christensen 2006) and Chimera 1.13 (http://www.rbvi.ucsf.edu/chimera). Thereafter docking calculations were done by using Autodock vina in PyRx 0.8 (Trott and Olson 2010) to determine the best pose of each of the ligands (the most negative binding affinity). Finally, LigPlot+ was used to analyze protein–ligand complexes based on the type of interactions (bonding and nonbonding) (Wallace et al. 1995).
Fig. 1.
Structures of the ligands (The control ligands; tariquidar and zosuquidar and test ligands; the lignanamides from Cannabis sativa
ADMET assay
Over the past decade, the computation of absorption, distribution, metabolism, excretion and toxicity (ADMET) properties has become one of the most important issues in the process of drug discovery and development. Since in vivo and in vitro evaluations are costly and laborious, in silico techniques had been widely used to estimate ADMET properties of chemical compounds (Shen et al. 2010). In this study, the canonical SMIlES of the ligands were submitted to SwissADME to analyze ADME properties including molecular weight, number of H-bond acceptors, number of H-bond donors, MlogP, gastrointestinal (GI) absorption and blood–brain barrier (BBB) permeability (Daina et al. 2017).
Results and discussion
P-gp efflux drastically affects the bioavailability of its substrates by decreasing their effective therapeutic plasma levels. Co-administration of the P-gp substrate-therapeutics with the P-gp inhibitors can prevent/overcome the substrate expulsion by P-gp and present the purposeful therapeutic benefits of the substrate drugs (Srivalli and Lakshmi 2012). Third-generation inhibitors have high potency and specificity for P-gp. Furthermore, according to the pharmacokinetic studies, significant impact on drug metabolism and clinically drug interactions with common chemotherapy agents, not seen yet. The continued development of these agents may establish the true therapeutic potential of P-gp-mediated MDR reversal (Gadhe and Cho 2011).
In present study, two selective inhibitors of P-gp (tariquidar and zosuquidar) that are belong to third generation inhibitors (Nobili et al. 2006) were designated as control ligands and molecular docking was performed for the ligands (lignanamides and controls) against P-gp using Autodock vina. Binding affinity values have been demonstrated in Table 1. Tariquidar is one of the most potent inhibitors of the P-gp drug pump. It has shown the most promise in clinical trials to improve chemotherapy and increase brain penetration of drugs (Fox and Bates 2007). The mechanism of P-gp inhibition by tariquidar is unclear yet. Due to its promise in a clinical setting, understanding the mechanism of tariquidar is needed to develop better inhibitors. Better inhibitors are required because P-gp inhibition by tariquidar in the blood brain barrier appeared to be far from ideal (Wagner et al. 2009). Zosuquidar also is one of the most potent Pgp inhibitors described to date. In fact, it inhibits P-gp at nanomolar concentrations in vitro and in vivo (Dantzig et al. 2001; Green et al. 2001) and there is evidence that it is not an inhibitor of MRP or BCRP (Dantzig et al. 2001). The mechanism of action of zosuquidar is still unclear but a noncompetitive inhibitory mechanism has been suggested since it is not a substrate for P-gp and cannot be transported by the ABC transporter (Dantzig et al. 2001). Binding affinities of tariquidar and zosuquidar were found to be − 10.1 and − 9.6 kcal/mol against P-gp, respectively (Table 1).
Table 1.
Docking results of the ligands with P-gp
| Compound | Binding affinity (kcal/mol) | RMSD/up | RMSD/low |
|---|---|---|---|
| Tariquidar (control) | − 10.1 | 12.785 | 6.095 |
| Zosuquidar (control) | − 9.6 | 8.196 | 3.376 |
| Grossamide | − 10.0 | 2.871 | 2.256 |
| Cannabisin A | − 10.1 | 1.357 | 0.712 |
| Cannabisin B | − 10.1 | 8.769 | 1.275 |
| Cannabisin C | − 10.1 | 3.422 | 2.63 |
| Cannabisin D | − 10.1 | 2.704 | 2.058 |
| Cannabisin E | − 8.9 | 13.803 | 8.097 |
| Cannabisin F | − 9.4 | 2.444 | 1.732 |
| Cannabisin G | − 9.2 | 1.913 | 1.422 |
| Cannabisin M | − 10.2 | 16.497 | 10.559 |
| Cannabisin N | − 10.2 | 9.245 | 5.246 |
RMSD root mean-square deviation is the measure of the average distance between the atoms
Lignanamides are natural plant secondary metabolites derived from oxidative coupling mechanism with hydroxycinnamic acid amides as intermediates. These compounds display powerful anti-inflammatory, antioxidant, anti-cancer and anti-hyperlipidemic capacities in vitro, cell culture and in vivo studies (Leonard et al. 2020).
Grossamide, a representative lignanamide in hemp seed, has been reported to possess potential antiinflammatory effects (Luo et al. 2017). Its binding affinity was found to be − 10.0 kcal/mol against P-gp (Table 1). This compound interacted with drug-binding pocket of P-gp via hydrophobic interactions (Tyr303, Ile302, Phe339, Ile336, Leu335, Tyr306, Phe724) and hydrogen bonds (Phe332, Gln721; Fig. 2). Cannabisin A is an arylnaphthalene lignanamide from fruits of Cannabis sativa L. (Sakakibara et al. 1991). Similar to tariquidar, cannabisin A binding affinity was found to be − 10.1 kcal/mol against P-gp (Table 1). This compound has been docked with drug-binding pocket of P-gp via hydrophobic interactions (Gln721, Ile302, Phe724, Leu335, Tyr306, Phe339, Tyr303) and hydrogen bonds (Gln986; Fig. 2). Cannabisin B, a lignanamide from hemp seed, was first identified in 1992 (Sakakibara et al. 1992). Its binding affinity was found to be − 10.1 kcal/mol against P-gp (Table 1). Cannabisin B interacted with drug-binding pocket of P-gp via hydrophobic interactions with Tyr306, Phe724, Gln721 and Met982 and through hydrogen bonds with Gln986 and Tyr303 (Fig. 2). Cannabisin C binding affinity also was found to be -10.1 kcal/mol against P-gp (Table 1). This compound interacted with drug-binding pocket of P-gp via hydrophobic interactions (Met982, Phe724, Tyr303, Ile302, Leu335, Phe339, Tyr306, Gln721) and hydrogen bond (Gln986; Fig. 2). Cannabisin D binding affinity was found to be − 10.1 kcal/mol against P-gp (Table 1). As shown in Fig. 2, involved residues in binding of cannabisin D to drug-binding pocket of P-gp are Gln721, Gln986, Met982, Ser975, Phe724, Phe332, Tyr306 and Tyr303. Cannabisin-E binding affinity was found to be − 8.9 kcal/mol against P-gp (Table 1). This compound interacted with drugbinding pocket of P-gp via hydrophobic interactions (Phe974, Ser975, Tyr949, Ile864, Phe339, Val978, Leu335, Phe332, Ile336, Leu64) and hydrogen bond (Tyr306; Fig. 2). Cannabisin F belongs to the lignanamides, and it was first isolated from cannabis fruits and roots in 1995 (Sakakibara et al. 1995). Its binding affinity was found to be − 9.4 kcal/mol against P-gp (Table 1). This compound interacted with drug-binding pocket of P-gp hydrophobically (Gln721, Phe724, Ile336, Phe339, Leu335, Phe332, Tyr306, Tyr303, Ile302; Fig. 2). Cannabisin G was first isolated from the fruits of Cannabis sativa L. in 1995 (Xia et al. 2010). Its binding affinity was found to be − 9.2 kcal/mol against P-gp (Table 1). Cannabisin G also interacted with drugbinding pocket of P-gp via hydrophobic interactions with Gln986, Tyr306, Phe724, Phe332 and Tyr303. In addition, this compound cantacted with Met982 and Gln721 through hydrogen bonds (Fig. 2). Cannabisin M and cannabisin N were top-ranked ligands that binding affinities of both were found to be − 10.2 kcal/mol against P-gp (Table 1). Binding modes of these two compounds in drug-binding pocket of P-gp have been shown in Fig. 3. Cannabisin M interacted with drugbinding pocket of P-gp via hydrophobic interactions (Ile864, Tyr949, Phe339, Phe332, Phe728, Leu335, Leu64, Val978, Phe974) and hydrogen bonds (Tyr306, Ile336; Fig. 2). Cannabisin N also interacted with this area via hydrophobic interactions (Phe974, Ser975, Phe724, Gln721, Tye303, Tyr306, Phe332) and hydrogen bond (Tyr949; Fig. 2).
Fig. 2.
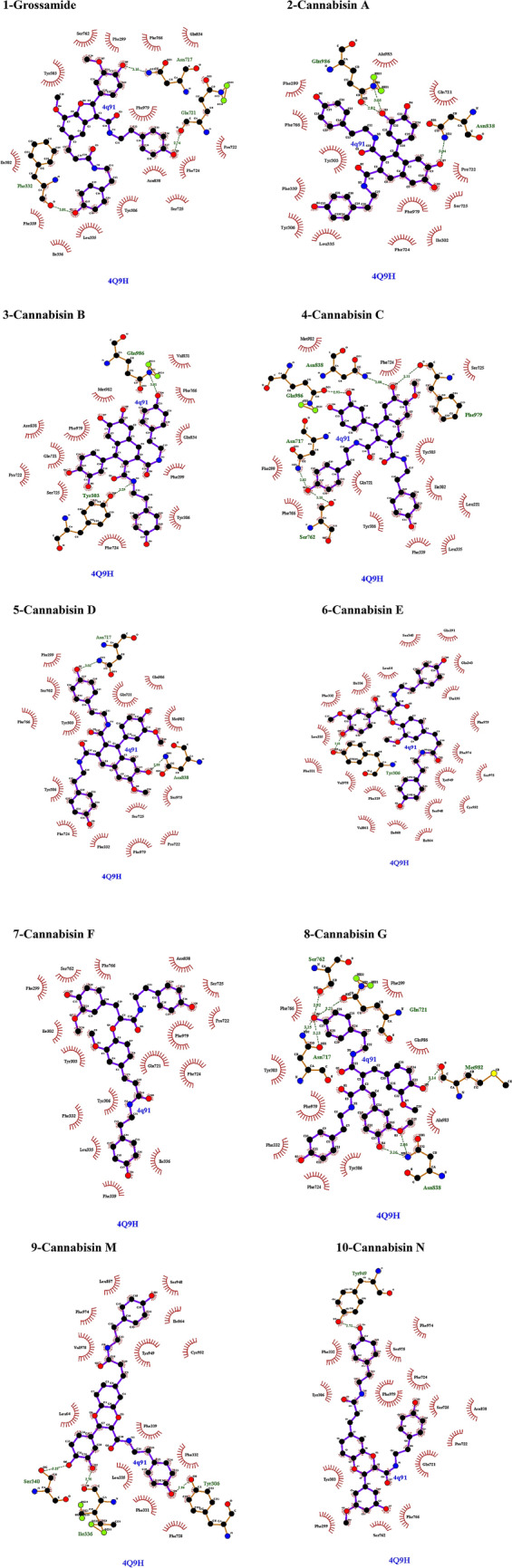
Bonding and nonbonding interactions of the lignanamides with P-gp (PDB code: 4Q9H). Green dashed lines are hydrogen bonds and dashed half-moons present hydrophobic interactions with the corresponding amino acid residues of the protein
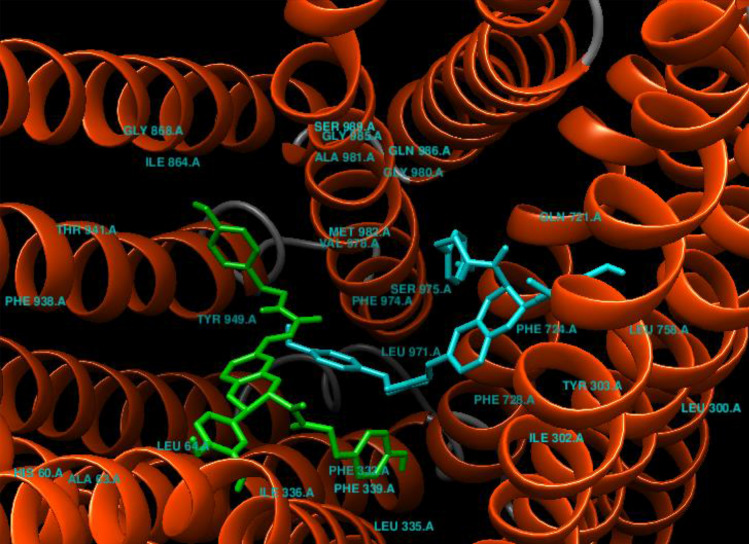
Fig. 3.
Binding modes of top-ranked ligands in drug-binding pocket of P-gp: Cannabisin N (blue), Cannabisin M (green)
ADME property values have been shown in Table 2 The Lipinski’s rule of five (ROF) enlist some criteria that is needed for a compound to be considered to be drug like in nature, this criterion includes a molecular weight that is less than 500 Da, hydrogen bond donors that is less or equal to 5 (≤ 5), hydrogen bond acceptors that is less or equal to (≤ 10) and octanolwater partition coefficient (ClogP) that is less than 5 (or MIogP < 4.15). Therefore, compounds that are coherent with this rule are considered to be drug-like in nature (David et al. 2018; Kharkar et al. 2009). Tariquidar, zosuquidar, grossamide, cannabisin-F and cannabisin-N obeyed ‘Rule of 5′ with 1 violation (MW > 500). Whereas others violated two properties by having MW > 500 and NHorOH > 5. Among these compounds only zosuquidar has high GI absorption and can be crossed from BBB.
Table 2.
ADMET properties of the ligands by SwissADME
| Compound | MW | MlogP | HBA | HBD | Lipinski violations | GI absorption | BBB permeant |
|---|---|---|---|---|---|---|---|
| Tariquidar (control) | 646.73 g/mol | 2.68 | 8 | 2 | 1 violation: MW > 500 | Low | No |
| Zosuquidar (control) | 527.60 g/mol | 3.66 | 7 | 1 | 1 violation: MW > 500 | High | Yes |
| Grossamide | 624.68 g/mol | 1.90 | 8 | 5 | 1 violation: MW > 500 | Low | No |
| Cannabisin A | 594.61 g/mol | 2.36 | 8 | 8 | 2 violations: MW > 500, NHorOH > 5 | Low | No |
| Cannabisin B | 596.63 g/mol | 1.53 | 8 | 8 | 2 violations: MW > 500, NHorOH > 5 | Low | No |
| Cannabisin C | 610.65 g/mol | 1.72 | 8 | 7 | 2 violations: MW > 500, NHorOH > 5 | Low | No |
| Cannabisin D | 624.68 g/mol | 1.90 | 8 | 6 | 2 violations: MW > 500, NHorOH > 5 | Low | No |
| Cannabisin E | 642.69 g/mol | 1.53 | 9 | 6 | 2 violations: MW > 500, NHorOH > 5 | Low | No |
| Cannabisin F | 624.68 g/mol | 2.22 | 8 | 5 | 1 violation: MW > 500 | Low | No |
| Cannabisin G | 624.68 g/mol | 2.22 | 8 | 6 | 2 violations: MW > 500, NHorOH > 5 | Low | No |
| Cannabisin M | 596.63 g/mol | 1.53 | 8 | 6 | 2 violations: MW > 500, NHorOH > 5 | Low | No |
| Cannabisin N | 610.65 g/mol | 1.72 | 8 | 5 | 1 violation: MW > 500 | Low | No |
MW Molecular weight, HBA Num. H-bond acceptors, HBD Num. H-bond donors, BBB Blood brain barrier, GI Absorption Gastro intestinal absorption
Conclusion
Our study demonstrates that the lignanamids interacted with important residues in drugbinding pocket of P-gp with acceptable binding affinity ranging from − 8.9 to − 10.2 kcal/mol. Among the ligands best binding affinities were related to cannabisin N and cannabisin M that both have been docked with P-gp with most negative binding affinity (− 10.2 kcal/mol) compared to tariquidar and zosuquidar (binding affinity: − 10.1 and − 9.6 kcal/mol respectively). Overall, these data suggest that lignanamids might be potential therapeutic candidates against P-gp. More studies are needed in order to prove these results.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for polyspecific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev PharmacolToxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, Fagan P. The protein data bank. ActaCrystallogr D BiolCrystallogr. 2002;58:899–907. doi: 10.1107/S0907444902003451. [DOI] [PubMed] [Google Scholar]
- Brenneisen R. Chemistry and analysis of phytocannabinoids and other cannabis constituents. In: ElSohly MA, editor. Marijuana and the Cannabinoids (Forensic Science and Medicine) Totowa, New Jersey: Humana Press Inc; 2007. pp. 17–49. [Google Scholar]
- Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Daina A, Olivier M, Vincent Z. "SwissADME": a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig A, Law KL, Cao J, Starling JJ. Reversal of multidrug resistance by the Pglycoprotein modulator, LY335979, from the bench to the clinic. Curr Med Chem. 2001;8(1):39–50. doi: 10.2174/0929867013373903. [DOI] [PubMed] [Google Scholar]
- David TI, Adelakun NS, Omotuyi OI, Metibemu DS, Ekun OE. Molecular docking analysis of phyto-constituents from Cannabis sativa with pfDHFR. Bioinformation. 2018;14(9):574–579. doi: 10.6026/97320630014574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolghih E, Bryant C, Renslo AR, Jacobson MP. Predicting binding to P-glycoprotein by flexible receptor docking. PLoSComputBiol. 2011;7(6):e1002083. doi: 10.1371/journal.pcbi.1002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sanchez IJ, Verpoorte R. Secondary metabolism in cannabis. Phytochem Rev. 2008;7:615–639. doi: 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7:447–459. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- Gadhe CG, Cho SJ. Modulation of multidrug resistance in cancer by P-glycoprotein. J Chosun Natural Sci. 2011;4(1):23–30. [Google Scholar]
- Green LJ, Marder P, Slapak CA. Modulation by LY335979 of P-glycoprotein function in multidrug-resistant cell lines and human natural killer cells. BiochemPharmacol. 2001;61(11):1393–1399. doi: 10.1016/s0006-2952(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Kast C, Canfield V, Levenson V, Gross P. Transmembrane organization of mouse pglycoprotein determined by epitope insertion and immunofluorescence. J BiolChem. 1996;271:9240–9248. doi: 10.1074/jbc.271.16.9240. [DOI] [PubMed] [Google Scholar]
- Kharkar PS, Deodhar MN, Kulkarni VM. Design, synthesis, antifungal activity, and ADME prediction of functional analogues of terbinafine. Med Chem Res. 2009;18(6):421–432. doi: 10.1007/s00044-008-9138-8. [DOI] [Google Scholar]
- Kim RB. Transporters and drug discovery: why, when, and how. Mol Pharm. 2006;3:26–32. doi: 10.1021/mp050084o. [DOI] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W, Zhang P, Ying D, Fang Z (2020) Lignanamides: sources, biosynthesis and potential health benefits–a minireview. Critical Reviews in Food Science and Nutrition 1–11. [DOI] [PubMed]
- Li YZ, Tong AP, Huang J. Two new norlignans and a new lignanamide from Peperomiatetraphylla. ChemBiodivers. 2012;9:769–776. doi: 10.1002/cbdv.201100138. [DOI] [PubMed] [Google Scholar]
- Liu R, Siemiarczuk A, Sharom FJ. Intrinsic fluorescence of the P-glycoprotein multidrug transporter: sensitivity of tryptophan residues to binding of drugs and nucleotides. Biochemistry. 2000;39:14927–14938. doi: 10.1021/bi0018786. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Reconstitution of drug-stimulated ATPase activity following coexpression of each half of human P-glycoprotein as separate polypeptides. J BiolChem. 1994;269:7750–7755. [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Membrane topology of a cysteine-less mutant of Human Pglycoprotein. J BiolChem. 1995;270:843–848. doi: 10.1074/jbc.270.2.843. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. The minimum functional unit of human P-glycoprotein appears to be a monomer. J BiolChem. 1996;271:27488–27492. doi: 10.1074/jbc.271.44.27488. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. The transmembrane domains of the human multidrug resistance Pglycoprotein are sufficient to mediate drug binding and trafficking to the cell surface. J BiolChem. 1999;274:24759–24765. doi: 10.1074/jbc.274.35.24759. [DOI] [PubMed] [Google Scholar]
- Luo Q, Yan X, Bobrovskaya L, Ji M, Yuan H, Lou H, Fan P. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol Cell Biochem. 2017;428(12):129–137. doi: 10.1007/s11010-016-2923-7. [DOI] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood–brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar J, Szabo D, Pusztai R, Mucsi I, Berek L, Ocsovszki I, Kawata E, Shoyama Y. Membrane associated antitumor effects of crocine, ginsenoside and cannabinoid derivates. Anticancer Res. 2000;20:861–867. [PubMed] [Google Scholar]
- Nobili S, Landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7:861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. ChemBiodivers. 2007;4:1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- Sakakibara I, Katsuhara T, Ikeya Y, Hayashi K, Mitsuhashi H. Cannabisin A, an arylnaphthalenelignanamide from fruits of Cannabis sativa. Phytochemistry. 1991;30(9):3013–3016. doi: 10.1016/S0031-9422(00)98242-6. [DOI] [Google Scholar]
- Sakakibara I, Ikeya Y, Hayashi K, Mitsuhashi H. Three phenyldihydronaphthalenelignanamides from fruits of Cannabis sativa. Phytochemistry. 1992;31(9):3219–3223. doi: 10.1016/0031-9422(92)83479-I. [DOI] [Google Scholar]
- Sakakibara I, Ikeya Y, Hayashi K, Okada M, Maruno M. Three acyclic bis-phenylpropanelignanamides from fruits of Cannabis sativa. Phytochemistry. 1995;38:1003–1007. doi: 10.1016/0031-9422(94)00773-M. [DOI] [PubMed] [Google Scholar]
- Sharom FJ. Shedding light on drug transport: structure and function of the P-glycoprotein multidrug transporter (ABCB1) Biochem Cell Biol. 2006;84:979–992. doi: 10.1139/o06-199. [DOI] [PubMed] [Google Scholar]
- Shen J, Cheng F, Xu Y, Li W, Tang Y. Estimation of ADME properties with substructure pattern recognition. J ChemInf Model. 2010;50:1034–1041. doi: 10.1021/ci100104j. [DOI] [PubMed] [Google Scholar]
- Srivalli KMR, Lakshmi PK. Overview of P-glycoprotein inhibitors: a rational outlook. Braz J Pharm Sci. 2012;48(3):353–367. doi: 10.1590/S1984-82502012000300002. [DOI] [Google Scholar]
- Thomsen R, Christensen MH. Moldock: a new technique for high–accuracy molecular docking. J Med Chem. 2006;49(11):3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J ComputChem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CC, Bauer M, Karch R, Feurstein T, Kopp S, Chiba P, Kletter K, Löscher W, Müller M, Zeitlinger M, Langer O. A pilot study to assess the efficacy of tariquidar to inhibit Pglycoprotein at the human blood–brain barrier with (R)-11C-verapamil and PET. J Nucl Med. 2009;50:1954–1961. doi: 10.2967/jnumed.109.063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. ProtEng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- Xia Y, Guo Y, Wen Y. The total synthesis of cannabisin G. J Serb ChemSoc. 2010;75(12):1617–1623. doi: 10.2298/JSC091016128X. [DOI] [Google Scholar]
- Yan X, Tang J, Dos Santos PC, Nurisso A, Simões-Pires CA, Ji M, Lou H, Fan P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholine sterase inhibitory activities. J Agric Food Chem. 2015;63(49):10611–10619. doi: 10.1021/acs.jafc.5b05282. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang S, Lou H, Fan P. Chemical constituents of hemp (Cannabis sativa L.) seed with potential anti-neuroinflammatory activity. PhytochemLett. 2018;23:57–61. [Google Scholar]