Abstract
The present study characterized the potential probiotic properties of Pediococcus acidilactici TMAB26 strain isolated from traditional Indian tomato pickle, and evaluated its possible therapeutic applications as an anti-cancer and anti-inflammatory agent in vitro. The 16S rRNA sequencing and primary screening demarcated TMAB26 strain as an ideal probiotic candidate, with distinctive properties of acid tolerance (58.02% at pH 2.5), bile tolerance (55.53% at 0.5%), and efficient adherence to the mucosal surface of the human intestinal cells in vitro, along with antagonistic, anti-inflammatory, and anti-cancer properties. The strain exhibited antagonism against standard intestinal pathogenic strains Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, E. coli, Klebsiella pneumonia, and Salmonella typhi with zones of inhibition in the range of 6–18 mm. The cytotoxicity evaluation of the probiotic isolate TMAB26 culture supernatant (1:1 dilution) showed significant cytotoxicity on HT-29 (94.91% ± 1.27) and Caco-2 (92.63% ± 0.63) cancer cells when compared to that of the peripheral blood mononuclear cells (PBMCs) alone. Furthermore, the strain culture supernatant reduced the mRNA levels of the proinflammatory cytokine tumor necrosis factor-alpha (TNF-α) by threefold, Interleukin-6 (IL-6) by eightfold and increased the mRNA levels of the anti-inflammatory cytokine Interleukin-10 (IL-10) in lipopolysaccharide (LPS) pretreated HT-29 and peripheral blood mononuclear cells (PBMCs), suggesting the potential role of TMAB26 isolate, i.e., Pediococcus acidilactici MTCC 13014 in alleviating gut inflammation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-020-02570-1.
Keywords: Fermented foods, Probiotic bacteria, Pediococcus acidilactici, Anti-cancer activity, Anti-inflammatory, Cytokines
Introduction
Fermentation is one of the efficient ways of preserving foods and improving the nutritional value. Consumption of such fermented foods enriched with many good bacteria obviously helps in improving digestion, reduce cholesterol, pathogens, and so on Prakash (2016). Efforts have been made to find potential bacterial strains with probiotic properties from indigenously processed fermented foods that actually reduce the severity of intestinal cancer and inflammation. The probiotic bacteria are potential therapeutic dietary constituents that act against pathogenic bacteria by inhibiting their colonization of the gut epithelium while also enhancing the growth of beneficial gut microbiota thereby conferring numerous benefits and are therefore well known as “gut warriors” (Nagpal et al. 2012; Yadav and Shukla 2020). Probiotic bacteria have been extensively studied for therapeutic applications like irritable bowel syndrome and antibiotic-associated diarrhea, systemic allergies, and age-related disorders (Verna and Lucak 2010; Yadav and Shukla 2019). Probiotics are also known to have the potential to replenish the beneficial gut microflora destroyed by drug therapy (Plaza-Díaz et al. 2017). These therapeutic properties of probiotics are species-specific and therefore it is essential to investigate native and unexplored strains for their probiotic properties (Delgado et al. 2007). Probiotics are generally taken orally, thereby allowing them to have adequate adherence and a good survival rate while enabling their passage through the stomach and the small intestine. Accordingly, one of the key functional selection criteria for probiotics is resistance to the low pH and the bile salts of stomach and small intestine, respectively (Yadav et al. 2016, 2018). Among the most widely known probiotic microbes, Lactobacillus and Pediococcus have been extensively studied. Various lactic acid bacteria (LAB) have been isolated from a wide range of fermented foods for their potential usage as probiotics. Dowarah et al. (2018) evaluated the probiotic potential of LAB such as L. Plantarum, L. acidophilus, Pediococcus acidilactici, and Bifidobacterium spp. isolated from swine intestine. Studies reported that various LAB were isolated from fermented vegetables, apart from their common sources like dairy foods and animal intestine (Rivera-Espinoza and Gallardo-Navarro 2010). It has been observed that L. brevis DF01 and P. acidilactici K10 strains isolated from traditional Korean fermented pickled vegetable dish ‘Kimchi’ were shown to have more potent probiotic activity than those isolated from animal sources (Kim et al 2019). In another study, Pediococcus strain isolated from traditional Iranian dish ‘Tarkhineh’ or ‘Tarhana’ prepared using fermented and dried vegetables along with fermented mixture of grains was shown to exhibit potential probiotic properties along with anti-microbial and anti-adhesion properties against pathogenic bacterial strains (Vasiee et al 2020). Diet interventions and natural bioactive supplements that advance gut immunity and demonstrate anti-cancer activity have been extensively studied to reduce the risk of colon cancer, multidrug resistance, etc. (Collins and Gibson 1999; Chau and Cunningham 2006). Consumption of probiotics has been shown to improve immunity against gut infections by increasing anti-inflammatory response (Plaza-Díaz et al. 2017; Mousavi et al 2011). Probiotic LAB is known to alleviate intestinal inflammation as different strains are known to induce different stimulatory effects on anti- and pro-inflammatory cytokines (Plaza-Díaz et al. 2017). Menard et al. (2004) demonstrated the effect of conditioned media (CM) obtained from probiotic strains, i.e., lactic acid and commensal Gram-positive bacteria, on LPS-induced secretion of TNF-α by PBMCs or the THP-1 cell line. This study concluded that the metabolites released by probiotic bacteria led to an increased anti-TNF-α activity. The lactic acid bacterial strains and their secretory products have been extensively studied for their role as probiotics and in the regulation of mechanisms involved in gut inflammation (Plaza-Diaz et al. 2019). A recent report used qRT-PCR to evaluate the anti-proliferative and anti-metastatic activity of Lactobacillus crispatus supernatant and Lactobacillus rhamnosus supernatant on human colon and cervical adenocarcinoma cell lines (Nouri et al. 2016). Wang et al. (2019) studied the effect of combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici in weaned pigs and reported a decrease in the concentration of serum proinflammatory cytokines IL-6 and interferon gamma (IFN-γ).
Our study is the first indication of the inherent probiotic potential of the indigenously prepared tomato pickle which has strains like Pediococcus acidilactici. This emphasizes the role and importance of traditional fermented vegetables as an essential source of probiotics. These probiotics display good adherence ability to the intestinal mucosal surface and cytotoxicity against the intestinal cancer cell lines HT-29 and CaCo2, improves gut immunity by alleviating gut inflammation.
Materials and methods
Isolation, screening, and characterization of probiotic lactic acid bacteria
Isolation
Lactic acid bacteria were isolated from different fermented foods like fermented batter, curd, mango and tomato pickles obtained from various places of Telangana state, India. The samples were serially diluted, inoculated on MRS agar plates (De Man Rogosa and Sharpe, HiMedia, India), and incubated at 37 °C for 24–48 h. The colonies obtained were characterized by cell morphology, Gram staining, and biochemical characterization. The carbohydrate fermentation pattern of the isolates was determined by their acid and gas production ability using different sugars.
Primary screening
The primary screening of the selected bacterial isolates evaluated their probiotic properties. Based on the probiotic properties like acid, bile, and temperature tolerance, the efficiency of auto-aggregation and cell-surface hydrophobicity, one isolate was characterized and selected for further studies. The detailed study of in vitro screening for probiotic properties (hemolytic activity, acid, bile, auto-aggregation, and cell-surface hydrophobicity) is presented in the supplementary data.
Molecular characterization
Molecular characterization was carried out based on 16S rRNA sequence analysis (Macrogen, South Korea). The partial sequencing of 16S rRNA gene was carried out using primer sets 27F (5′-AGAGTTTGAYCCTGGCTCAG-3′) and 1492R (5′-GGCTACCTTGTTACGACTT-3′). The online program BLASTn was used to find the related sequences from the taxonomic information available at the NCBI database (http://www.ncbi.nlm.nih.gov/ BLAST). Nucleotide sequence data of the isolate were deposited in the GenBank sequence database. A phylogenetic tree was constructed with MEGA (X) 11 version software (Kumar et al. 2018).
Antagonistic activity against pathogens
The actively growing selected probiotic bacterial culture was centrifuged at 10,000 rpm for 5 min and the supernatant was filtered using 0.22-μm membrane filters. The anti-microbial activity was determined by agar well diffusion method as described by Srisesharam et al. (2018). Pathogenic cultures were inoculated on nutrient agar plates by spread plate technique and 6 mm diameter wells were prepared in each plate. The cell-free supernatant (100 μL) of the probiotic isolate was loaded in each well and plates were incubated for 24 h at 37 °C. After incubation, the length of inhibition zones was measured and documented.
In vitro studies to determine the effect of probiotic isolate on different cell lines
Propagation and maintenance of cell lines
The Caco-2 and HT-29 human intestinal cell lines were procured from NCCS (National Centre for Cellular Sciences), Pune, India. The Minimum Essential Medium (MEM), Dulbecco's Modified Eagle's medium (DMEM), RPMI-1640, Fetal Bovine Serum (FBS) were obtained from GIBCO, Invitrogen, USA. The cell lines were cultured in MEM with 20% FBS and DMEM with 10% FBS, respectively, and incubated at 37 °C with 5% CO2. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of human volunteers using Histoplaque (Sigma, USA). Whole blood was carefully layered on the top of Histoplaque at a dilution of 1:1 with Dulbecco’s phosphate buffer saline (DPBS) and centrifuged at 1000 rpm for 30 min. The cells were then washed with PBS, followed by suspending in RPMI-1640 medium and cell viability was determined by trypan blue method.
The intestinal cell adhesion ability
The in vitro adhesion assay was carried out to assess the adhesion ability of the probiotic bacterial isolate to HT-29 intestinal cells. Cell suspension comprising 105 cells/mL in 4 mL of DMEM supplemented with 10% (v/v) heat-inactivated FBS was prepared and transferred into six-well tissue culture plates. The medium was changed every alternate day until the cells attain 80% confluence. Before the 24-h cell adhesion assay, cells were serum-starved in medium without antibiotics. Cells were subsequently washed with DPBS and fresh medium without serum and antibiotics was added to the culture plates, followed by incubation at 37 °C for 30 min. At the end of incubation, about106 cells/mL of probiotic bacteria suspended in plain DMEM without antibiotics were added to the cells and incubated for 2 h at 37 °C with 5% CO2, for allowing adherence to the intestinal cells. Later, the cells were washed with sterile DPBS and fixed by addition of 3 mL methanol, followed by subsequent incubation at room temperature for 10 min. The fixed cells were then stained with Giemsa stain (Himedia, India) and incubated for 20 min. Later, the cells were washed with ethanol and air-dried at room temperature. The number of bacterial cells was counted in 20 random microscopic fields and grouped into the non-adhesive, adhesive, and strong adhesive categories (Duary et al. 2011) by observing under an inverted microscope at 40 × magnification.
Cytotoxic effect of probiotic isolate on intestinal cancer cell lines and PBMCs
The MTT assay was carried out to evaluate the cytotoxic effect of probiotic bacterial isolate on intestinal cancer cell lines and PBMCs. The Caco-2 and HT-29 cells (1 × 105 /mL) were cultured in a 96-well plate until they attain full confluency, following which the cells were serum-starved for 24 h. PBMC (105 cells/mL) were cultured in a separate 96-well plate and serum-starved for 4 h. The culture medium was removed from 96-well plates and probiotic bacterial cell-free supernatant with fresh cell culture medium was added in a ratio of 1:1, 1:2, 1:3, 1:4, 1:5, and 1:6 dilutions. Cells treated without bacterial cell-free supernatant but with cell culture medium were taken as the negative control. All the plates with Caco-2 and HT-29 cells were incubated for 24 h and PBMCs were incubated for 8 h. After incubation, the cells were washed with DPBS and added with 10 μL/well of MTT solution (5 mg/mL) as per the manufacturer’s instructions. After incubation for 2 h at 37 °C and 5% CO2, 100 µL of dimethyl sulfoxide was added, and absorbance was measured at 570 nm using a microplate ELISA reader (Agilent technologies, USA). The cell viability was calculated using the following formula:
where, ABS is the absorbance, ABS sample is the absorbance of cell-free supernatant of P. acidilactici TMAB26-treated cells, ABS blank is the absorbance of dimethyl sulfoxide, ABS control is the absorbance of untreated cells.
The cytotoxic effect of the probiotic bacteria was measured in terms of cell growth inhibition percentage and expressed as IC50, which is the concentration of the bacterial cell-free supernatant at which the absorbance of the treated cells decreases by 50% when compared to the control (untreated cells).
Anti-inflammatory effect of probiotic bacteria
Treatment of HT-29 and PBMCs with probiotic bacterial conditioned medium
Conditioned media were prepared by taking DMEM and RPMI-1640 media for HT-29 and PBMCs, respectively, with 10% FBS without antibiotic, inoculated with probiotic bacterial isolate, and incubated for 24 h at 37 °C. Subsequently, the bacterial cell-free supernatant conditioned media were collected and filtered through a 0.2-μm membrane filter. The HT-29 cells (106/mL) were seeded in T25 flasks and grown until they attain 80% confluence. At the same time, PBMCs (3 × 106 cells/mL) were cultured separately in T25 suspension flasks. Both the cell lines were serum-starved for 6 h at 37 °C in a CO2 incubator with 5% CO2. The cells were then inoculated with the bacterial cell-free supernatant conditioned media and incubated for 6, 12, and 24 h with and without the addition of inflammatory inducer LPS of Klebsiella pneumoniae (Sigma, USA).
RNA isolation from HT-29 and PBMCs
The total RNA from the cells was isolated by Trizol method. The monolayer cell lines were rinsed with PBS, added with 1 mL Trizol (Sigma-Aldrich) and homogenized. Subsequently, 200 μL of chloroform was added, followed by vortexing and centrifuged at 13,000 rpm for 15 min. The upper aqueous layer was carefully collected into a sterile 1.5 mL Eppendorf tube and mixed with 500 μL isopropanol, followed by incubation for 10 min. The precipitate was centrifuged at 13,000 rpm for 15 min and subsequently washed with 70% (v/v) ethanol. The resultant pellet was air-dried and re-suspended in diethyl pyrocarbonate-treated water and preserved for further analysis.
Effect of probiotic bacteria on inflammatory cytokine levels in HT-29 cell lines and PBMCs
The total RNA isolated from HT-29 cells and PBMCs was estimated spectrometrically at 260/280 nm. Synthesis of cDNA was carried out using 5 µg RNA, reverse transcriptase, and oligodT primers according to the manufacturer’s protocol (Thermo Scientific, USA). The primer sequences for RT-PCR were obtained from qprimer depot (Cui et al. 2007) and were synthesized by Eurofins (Germany) (Table 1). The qRT-PCR was carried out using Fast Start Universal SYBR Green Master (Invitrogen, USA). Thermocycling conditions comprised initial 5 min denaturation at 95 °C followed by 40 cycles of PCR (15-s annealing at 50–61 °C with 15-s extension at 72 °C) and a final extension. Gene expression was calculated using the relative quantification method (Livak and Schmittgen 2001), where the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as the reference for normalization.
Table 1.
Percent tolerance of isolates at acidic pH, high bile and higher ranges of temperature along with their percent auto-aggregation, hydrophobicity and ± values are standard deviation of mean
| S. No | Isolate | % Acid tolerance (pH 2.5) after 4 h | % Bile tolerance (0.5%) After 4 h |
Range of temperature tolerance (°C) | % Auto-aggregation after 5 h | % Hydrophobicity (hexadecane, xylene) |
|---|---|---|---|---|---|---|
| 1 | TMAB | 58.02 ± 0.19 | 55.53 ± 0.3 | 37–46 | 68.30 ± 1 | 78 ± 0.5, 74 ± 0.51 |
| 2 | LCK | 56.24 ± 0.2 | 52.24 ± 0.32 | 37–40 | 58.26 ± 0.5 | 65 ± 1, 68 ± 0.5 |
| 3 | LTD1 | 54.26 ± 0.18 | 52.86 ± 0.24 | 37–42 | 65.62 ± 0.5 | 72 ± 1, 68 ± 1 |
| 4 | LC1 | 56.24 ± 0.19 | 53.24 ± 0.18 | 37–40 | 66.26 ± 1 | 68 ± 1.5, 66 ± 0.5 |
| 5 | LBM | 54.00 ± 0.21 | 58.12 ± 0.19 | 37–38 | 62.20 ± 2 | 75 ± 0.5, 74 ± 1 |
| 6 | LBS | 52.46 ± 0.2 | 48.94 ± 0.28 | 37–38 | 56.26 ± 1 | 64 ± 0.8, 68 ± 2 |
| 7 | LHB | 48.00 ± 0.24 | 53.16 ± 0.24 | 37–40 | 66.52 ± 0.5 | 72 ± 1, 69 ± 1.5 |
| 8 | LFH | 53.14 ± 0.16 | 56.16 ± 0.19 | 37–40 | 58.28 ± 1 | 76 ± 0.75, 74 ± 1 |
| 9 | LID1 | 56.84 ± 0.19 | 52.60 ± 0.24 | 37–40 | 65.82 ± 1.6 | 66 ± 2, 72 ± 3 |
| 10 | LTD2 | 48.86 ± 0.14 | 52.46 ± 0.28 | 37–40 | 66.28 ± 1.5 | 68 ± 1, 66 ± 0.5 |
| 11 | LCY | 54.24 ± 0.18 | 53.46 ± 0.19 | 37–40 | 64.28 ± 1 | 72 ± 3.5, 76 ± 2 |
| 12 | LYT | 56.24 ± 0.22 | 56.46 ± 0.26 | 37–40 | 64.52 ± 0.5 | 66 ± 1, 70 ± 1.5 |
| 13 | LPK | 56.46 ± 0.20 | 53.26 ± 0.19 | 37–45 | 58.62 ± 1 | 72 ± 1, 75 ± 2 |
| 14 | LCM | 56.86 ± 0.16 | 48.46 ± 0.28 | 37–40 | 65.42 ± 1 | 75 ± 2, 68 ± 3 |
| 15 | LBO1 | 52.24 ± 0.19 | 53.46 ± 0.18 | 37–40 | 66.42 ± 0.5 | 68 ± 2, 72 ± 1.5 |
| 16 | LPA | 50.26 ± 0.2 | 52.56 ± 0.26 | 37–40 | 64.20 ± 0.75 | 75 ± 1, 68 ± 2 |
| 17 | LGI | 56.00 ± 0.19 | 52.24 ± 0.32 | 37–38 | 58.62 ± 2 | 70 ± 1.5, 68 ± 3 |
| 18 | LBO2 | 50.84 ± 0.16 | 52.36 ± 0.05 | 37–40 | 65.60 ± 1.5 | 75 ± 2, 66 ± 1 |
| 19 | LBO3 | 52.24 ± 0.18 | 52.26 ± 0.26 | 37–40 | 64.20 ± 1 | 68 ± 0.5, 72 ± 3 |
| 20 | LBO4 | 52.86 ± 0.19 | 52.16 ± 0.18 | 37–40 | 58.20 ± 0.5 | 66 ± 1, 68 ± 2 |
| 21 | LBO5 | 51.94 ± 0.20 | 50.26 ± 0.18 | 37–40 | 66.56 ± 1.5 | 70 ± 2, 72 ± 3 |
| 22 | LLP | 53.45 ± 0.19 | 52.20 ± 0.24 | 37–45 | 58.62 ± 2 | 74 ± 2, 68 ± 3 |
| 23 | LFN | 46.86 ± 0.2 | 58.86 ± 0.24 | 37–40 | 62.20 ± 0.5 | 70 ± 2, 65 ± 1.5 |
| 24 | LCS | 46.12 ± 0.16 | 53.14 ± 0.16 | 37–40 | 64.42 ± 1 | 65 ± 1, 70 ± 2 |
| 25 | LDW | 48.50 ± 0.18 | 52.16 ± 0.18 | 37–40 | 48.72 ± 1.5 | 68 ± 1, 72 ± 3 |
| 26 | LID2 | 55.24 ± 0.2 | 52.60 ± 0.16 | 37–40 | 65.24 ± 2 | 66 ± 2, 70 ± 1.5 |
| 27 | S.thermophilus NCIM 2412 | 56.48 ± 0.14 | 54.36 ± 0.24 | 37–45 | 68.20 ± 1 | 78 ± 1, 76 ± 0.5 |
| 28 | L.acidophilus NCIM 2285 | 54.68 ± 0.18 | 51.40 ± 0.28 | 37–40 | 66.40 ± 1.8 | 75 ± 2, 72 ± 1 |
Statistical analysis
The statistical analysis was carried out by calculating the mean of the triplicates and the respective standard deviations. The results were statistically evaluated by analysis of variance (ANOVA) using Tukey’s post hoc test, with p < 0.05 being considered as significant (Ribeiro et al. 2014).
Results
Based on differential colony morphology, microscopic and cultural characteristics, 26 lactic acid bacterial strains were isolated. Morphological, biochemical characterization, and probiotic properties of total 26 lactic acid bacterial strains are represented in supplementary data.
Molecular characterization of lactic acid bacteria by 16S rRNA analysis
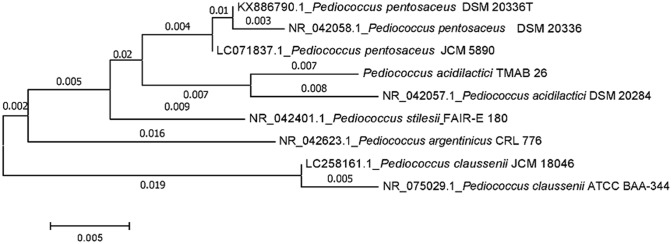
The bacterial strain TMAB26 was identified as Pediococcus acidilactici TMAB26. The sequence search (BLAST) in the NCBI database showed that the 16S rRNA sequence of TMAB26 strain (GenBank accession number: MH 211386) had 99% similarity with P. acidilactici type strain DMS 20284. The species-level comparison studies with different Pediococcus species showed a similarity of less than 98.5%, which was the cut-off value for identification (Choi et al. 2018). Phylogenetic analysis of the 16S rRNA gene sequence of P. acidilactici TMAB26 validated its close relatedness to P. acidilactici DMS 20284 (Fig. 1).
Fig. 1.
Phylogenetic tree showing similarity of bacterial isolate TMAB26 with eight Pediococcus strains of NCBI database
Assessment of probiotic properties
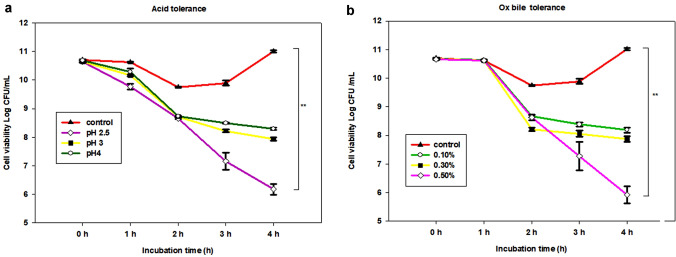
The isolate P. acidilactici TMAB26 showed γ-hemolytic activity which is an essential factor for non-pathogenicity as indicated by the absence of zone around the colonies. Acid tolerance by P. acidilactici TMAB26 showed significant survival rates of 58.02%, 74.53%, and 77.62% after a 4-h incubation period at low pH values of 2.5, 3, and 4, respectively (Fig. 2). The tolerance of probiotic bacteria to Ox bile after an incubation of 1, 2, 3, and 4 h, displayed that there was a significant difference (p < 0.05) in the viability of the strain at different Ox bile concentrations. The probiotic bacterial isolate showed a survival efficiency of 76.75%, 73.85%, and 55.53% at 0.1%, 0.3%, and 0.5% Ox bile concentrations, respectively, after continuous incubation of 4 h (Fig. 2).
Fig. 2.
Cell viability of Pediococcus acidilactici TMAB26 at a different pH indicating acid tolerance and b different Ox bile concentrations indicating bile tolerance. Error bars represent standard deviation of the mean values obtained from statistical analysis by Tukey’s post-test, **P < 0.01 (n = 3)
The probiotic isolate P. acidilactici TMAB26 exhibited strong autoaggregation ability, ranging between 26.3 and 68.3% at 1–5 h incubation with the highest percentage achieved after 5 h of incubation (Fig. 3). In the present study, the probiotic isolate showed 78% and 74% hydrophobicity toward hexadecane and xylene, respectively.
Fig. 3.
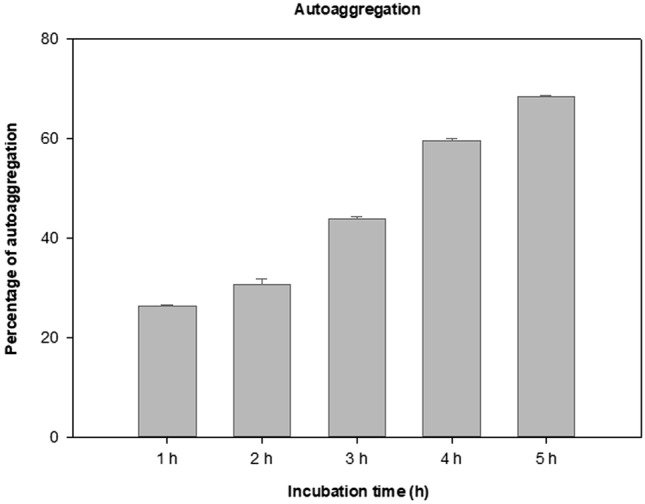
Autoaggregation ability of the isolate Pediococcus acidilactici TMAB26. Error bars represent standard deviation of the mean values obtained from statistical analysis by Tukey’s post-test
Adhesion efficiency
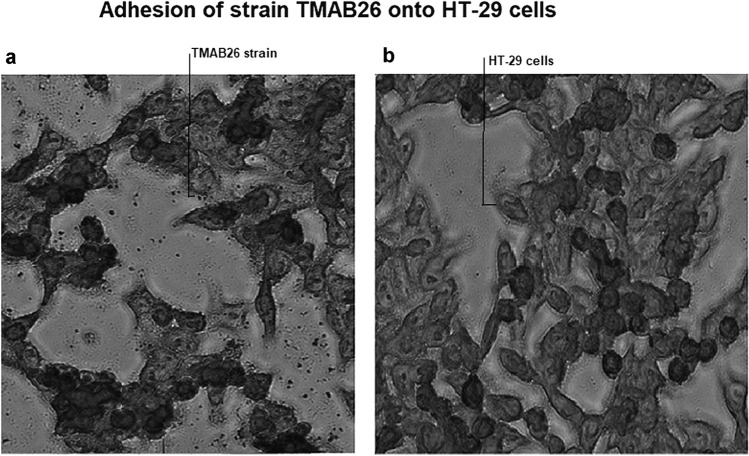
The adhesion efficiency of P. acidilactici TMAB26 strain was examined on HT-29 cells by Giemsa staining under an inverted light microscope. The high adhesion efficiency of the strain was proved by a significant count of 197 ± 17 which showed the presence of more than a hundred bacteria in the intestinal cells in the 20 microscopic fields that had been observed (Fig. 4).
Fig. 4.
Adhesion ability of the isolate Pediococcus acidilactici TMAB26 to HT-29 cells a HT-29 cells treated with bacterial isolate b HT-29 cells without bacterial treatment
Therapeutic applications of P. acidilactici TMAB26
Antagonistic activity against pathogenic bacteria
Antagonistic activity of P. acidilactici TMAB26 against standard pathogenic strains including, Staphylococcus aureus (ATCC 29213), Pseudomonas aeruginosa (ATCC 27853), Bacillus subtilis (ATCC 23857), Escherichia coli (ATCC 8739), Klebsiella pneumonia (ATCC 33499), and Salmonella typhi (ATCC CVD 909 (202117)) was evaluated. The results were shown as zone of inhibition in mm for S. aureus (18 mm), P. aeruginosa (14 mm), B. subtilis (12 mm), E. coli (10 mm), K. pneumonia (8 mm), and S. typhi (6 mm). Among the six pathogenic strains, the maximum and minimum zones of inhibition were observed with S. aureus and S. typhi, respectively (Fig. 5).
Fig. 5.
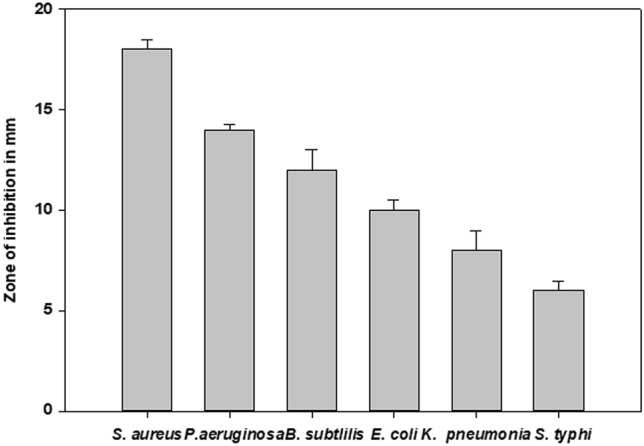
Antagonistic effect of Pediococcus acidilacti TMAB26 on different intestinal pathogens Error bars represent standard deviation of the mean values obtained from statistical analysis by Tukey’s post-test
Anti-cancer activity
The anti-cancer activity of P. acidilactici TMAB26 was tested in HT-29 and Caco-2 cells by incubating the cells with the bacterial cell-free supernatant. The cell viability for different dilutions of bacterial cell-free supernatant was analyzed by MTT assay, in which the results of lower dilutions were associated with increased cell death whereas higher dilutions were associated with decreased cell death.
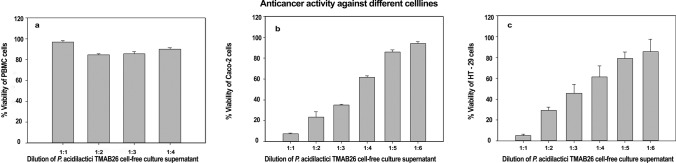
Cell cytotoxicity of the probiotic bacterial isolate after 24 h incubation was observed using PBMCs and intestinal cancer cells and was compared with untreated control cells wherein MRS broth was used for the treatment of cells. Among all the dilutions of cell-free filtrate for the anti-proliferative activity of P. acidilactici TMAB26 on HT-29 and Caco-2 cells, 1:1 showed the highest cytotoxicity levels of 5.1% and 7.37% in HT-29 and Caco-2 cells, respectively, while 1:6 showed the lowest cytotoxicity levels of 85.45% and 94.15% in HT-29 and Caco-2 cells, respectively (Fig. 6). The cell-free filtrate of P. acidilactici TMAB26 showed IC50 values at 1:3.43 and 1:3.42 dilutions in HT-29 and Caco-2 cell lines, respectively, after 24-h incubation. The results indicated an increased cytotoxic effect and cell death in cancer cells as compared to PBMCs. The isolate P. acidilactici TMAB26 showed specific cytotoxic activity and anti-cancer properties against only the intestinal cancer cells (Fig. 6).
Fig. 6.
Cytotoxic effect of Pediococcus acidilactici TMAB26 on a HT-29, b CaCo-2 and c PBMCs. Error bars represent standard deviation of the mean values obtained from statistical analysis by Tukey’s post-test.
Anti-inflammatory role in HT-29 and PBMCs
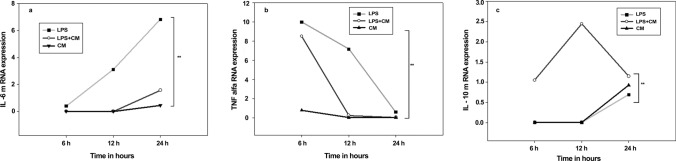
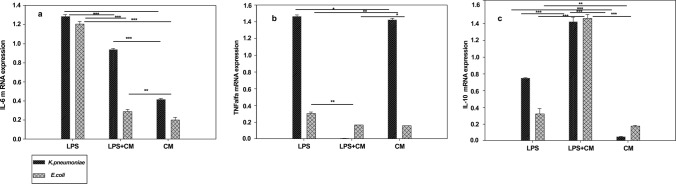
The mRNA expression analysis of the inflammatory cytokines revealed that the Pediococcus TMAB26 strain conditioned media (CM) upregulate the mRNA expression of IL-10 anti-inflammatory cytokine while downregulating the mRNA expression of the proinflammatory cytokines (TNF-α and IL-6). The HT-29 cell lines, when treated with LPS (6 h) followed by incubation with TMAB26, conditioned media (12 h) showed a twofold increase in mRNA levels of IL-10 from 6 to 12 h. On the other hand, TNF-α (eightfold) and IL-6 (threefold) showed decreased expression of mRNA levels when LPS-stimulated cells were treated with conditioned media (Fig. 7). All cytokine levels for the conditioned medium-treated cells were observed to be below those of the control cells after 24 h. Similar results were observed in PBMCs, where proinflammatory cytokines (IL-6 and TNF-α) showed decreased expression when compared to anti-inflammatory cytokine (IL-10) after 8 h of treatment (Fig. 8). The results from both experiments indicate that the probiotic bacteria P. acidilactici TMAB26 which is available as P. Pediococcus acidilactici MTCC 13,014 has an anti-inflammatory response toward LPS, the bacterial endotoxin.
Fig. 7.
Effect of Pediococcus acidilactici TMAB26 on inflammatory cytokine levels in HT-29 intestinal cells a IL-6, b TNF-α, and c IL-10 in presence of LPS (100 ng). HT-29 cells were treated with cell-free supernatant of Pediococcus acidilactici TMAB26 in presence or absence of LPS for 6, 12, and 24 h. mRNA levels were analyzed by qRT-PCR, and gene expression was normalized with GAPDH reference gene. ***P < 0.001, **P < 0.01, *P < 0.05 (n = 4)
Fig. 8.
Effect of Pediococcus acidilactici TMAB26 on inflammatory cytokine levels in PBMCs a IL-6, b TNF-α, and c IL-10 in presence of LPS (100 ng). PBMCs were pretreated with LPS for 4 h in presence or absence of Pediococcus acidilactici TMAB26 cell-free supernatant for 12 h. mRNA levels were analyzed by qRT-PCR, and gene expression was normalized with GAPDH reference gene. ***P < 0.001, **P < 0.01, *P < 0.05 (n = 4)
Discussion
In recent years, probiotics have received increasing attention and have been characterized for their potential therapeutic benefits against gastrointestinal tract inflammatory diseases and infections in humans (Borges et al. 2013). Previous studies on the regulation of gut inflammation have been focused on employing probiotics to alleviate inflammation, resulting in the identification of several different bacterial strains with good probiotic properties; these strains have been shown to enhance immunity without disturbing the normal gut microflora (Menard et al. 2004; Duary et al. 2014; Wang et al. 2020).
In the present study, we have explored the probiotic potential of few indigenously processed fermented foods and thereby identified and investigated P. acidilactici TMAB26 as a probiotic strain and its potential therapeutic effect in human-derived HT-29 intestinal cancer cells. This study focuses on the ability of TMAB26 strain to reduce cancer cell growth in vitro and its impact on anti-inflammatory cytokine levels in the presence of bacterial endotoxin. A previous study on probiotics focused on different probiotic genera of lactic acid bacteria such as Lactobacillus, Bifidobacterium, Streptococcus, and their bioavailability on intestinal health (Nagpal et al. 2012). In the present study, the acid and bile tolerance of P. acidilactici TMAB26 was found to be 58.07% at 2.5 pH and 55.53% at 0.5% bile concentration, respectively, after 4-h incubation. The acid and bile tolerance reported in this study was higher as compared to a previous study with Pediococcus spp. where the survival rate of Pediococcus was 15.7% in 0.5% bile concentration, and decreased to 6.4% in 1.0% bile (Attri et al. 2015).
Further characterization of probiotic properties of P. acidilactici TMAB26 revealed good autoaggregation and hydrophobicity, which would enable biofilm formation, thereby ensuring good adherence to the intestinal epithelial cells. In the present study, the isolate exhibited strong adhesion toward intestinal cancer cell lines (HT-29). The probiotic P. acidilactici TMAB26 showed 68.3% autoaggregation which is in agreement with Gómez et al. (2016) who reported 67% auto-aggregation by lactic acid bacteria Weissella viridescens 113 strain after 24 h of incubation.
The probiotic isolate P. acidilactici TMAB26 showed antagonistic activity against various pathogenic bacterial strains. The restoration of normal gut mucosal immunity is achieved by adhesion of lactic acid bacteria to the intestinal epithelium, this process inhibits the colonization of pathogens (Pringsulaka et al. 2015). In this study, P. acidilactici TMAB26 with a count of 197 cells adhered to HT-29 epithelial cell lines is supported by the studies of Duary et al. (2011) where the adhesion score of probiotic lactobacillus strain to Caco-2 and HT-29 epithelial cell lines was shown to be in a range between 131.0 (Lp75) to 342.7 (Lp91) and 44.7 (CH4) to 315.7 (Lp91), respectively. Gómez et al. (2016) reported a connection between the hydrophobicity of cell surface, colonization, and bacterial attachment.
The anti-microbial activity analysis revealed that P. acidilactici TMAB26 strain exhibited a decent zone of inhibition with all the pathogenic strains used. Anti-microbial activity is attributed to the secretion of several metabolites such as acid, H2O2, and bacteriocin (Ladha and Jeevaratnam 2020). Colorectal cancer (CRC) is the fourth-most leading cause of death worldwide. Probiotics are known to have an ability to modulate the gut microbiota and increase protective immune response by reducing the bacterial translocation via enhancing gut barrier function. They have also shown to increase pro-apoptotic or anti-proliferative activities (Pino et al 2020). The P. acidilactici TMAB26 strain shows cytotoxic specificity toward intestinal colon cancer cell lines (Caco-2 and HT-29) causing them to die plausibly either through apoptosis or necrosis but not for PBMCs. The anti-cancer activity of P. acidilactici TMAB26 against the HT-29 cell line was indicated as a percentage of cell death which was 94.9% and 14.0% for the lowest and highest dilutions of cell-free filtrate (1:1 and 1:6), respectively. Similarly, the percentage of cell death for the Caco-2 colorectal cancer cell line ranged from 92.6% to 5.8%. Significant cytotoxicity (IC50 values) was observed for 1:3.43 and 1:3.42 dilutions in HT-29 and Caco-2 cell lines, respectively. Our findings with P. acidilactici TMAB26 strain cell-free filtrate are in agreement with Dallal et al. (2015), who reported that the cytotoxicity inhibition rate of L. acidophilus and L.casei cell-free supernatant suppressed cell proliferation in a dose-dependent manner on Caco-2 cells.
Several studies have shown that probiotics enhance immunity by regulating cytokine response. Different lactic acid bacteria like L. plantarum, L. acidophilus, P. acidilactici, and Bifidobacterium spp. have been proved to have efficient probiotic properties. However, very few Pediococcus strains have been reported to show probiotic properties, therefore, further studies on the probiotic efficiency of Pediococcus strain are required. In the present study, we have evaluated the response of inflammatory mediators in the presence of P. acidilactici TMAB26 and studied the anti-inflammatory property of the strain using LPS-pretreated HT-29 and PBMCs. We observed that LPS-stimulated HT-29 cells in the presence of P. acidilactici TMAB26 cell-free conditioned media reduced TNF-α mRNA expression levels, indicating that the strain has an ability to regulate and reduce the pro-inflammatory cytokine levels, which might be beneficial in reducing inflammation caused by bacterial infection. Chon et al. (2009) reported that L. plantarum KFCC11389P showed immune-modulating activity by up- and/or downregulation of cytokines in the presence of bacterial endotoxin (LPS)-induced inflammation. Al Obeed et al. (2014) suggested that high levels of TNF-α expression was found to be an independent diagnostic indicator of CRC, indicating that TNF-α might be a promising prognostic tool for the assessment of the clinical stages of CRC. Furthermore, Mendes et al. (2018) showed that supplementation of probiotics like L. rhamnosus, L. acidophilus, and B. bifidum, reduced colitis-associated colon cancer (CAC) in C57BL/6 mice, wherein CAC is prevented as a result of decreased colitis which is made possible by alteration of the microbiota arrangement and controlled cytokine caused inflammatory responses. Besides, P. acidilactici TMAB26 showed decreased levels of IL-6 mRNA along with increased levels of IL-10. IL-6 expression is known to be strictly controlled by transcriptional and post-transcriptional mechanisms. Any pathological infection can lead to an increase in IL-6 levels as seen in infections accompanied by inflammation in a condition such as inflammatory bowel disease (Tanaka et al. 2014).
Previous in vitro studies on the probiotic lactic acid bacteria Lp9 and Lp91 have shown that they have the ability to enhance the anti-inflammatory reaction in LPS-stimulated HT-29 cells, indicating downregulation of pro-inflammatory cytokines such as Interleukin-8, TNF-α, Interleukin-12p35, IFN-γ, and upregulation of anti-inflammatory cytokines such as Interferons alfa, and transforming growth factor beta (Carol et al. 2006). In the present study, P. acidilactici TMAB26 was found to enhance anti-inflammatory responses and also reduce the pro-inflammatory cytokine levels in response to LPS stimulation. This was indicated by increased mRNA expression of anti-inflammatory genes such as IL-10 and decreased mRNA expression of pro-inflammatory genes such as TNF-α and IL-6 in cell-free conditioned medium-treated HT-29 cells and PBMCs. However, the response was much higher in HT-29 intestinal cancer cells than in PBMCs, indicating its marked effect on intestinal cancer cells.
It can be concluded from the findings of the present study that P. acidilactici TMAB26 strain, isolated from tomato pickle, a south Indian fermented food recipe prepared from tomatoes with tamarind and spices like chilly, fenugreek seeds, mustard seeds, and turmeric is a potential probiotic bacterium that possesses good acid and bile tolerance, is non-hemolytic, and displays anti-bacterial, anti-proliferative and anti-cancer activity on the cancer cells. Further, it has shown the ability to regulate the cytokine levels during bacterial endotoxin-induced inflammation, upregulation of IL-10 (anti-inflammatory), and downregulation of IL-6 and TNF-α (pro-inflammatory cytokines) in HT-29 cells and PBMCs. Our findings demonstrate the promising potential role of TMAB26 isolate available as Pediococcus acidilactici MTCC 13014 in cancer cells and in alleviating inflammation in the digestive system thereby providing better insight into the plausible future usage of this organism in colorectal cancer treatment studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Authors are grateful to RUSA 2.0, MHRD, Govt. of India; Science and Engineering Research Board (SERB), Ministry of Science and Technology, Govt. of India (YSS/2015/000173, SB/EMEQ-258/2013) for their financial support and University Grants Commission (UGC), Govt. of India for providing fellowship to carry out this research work.
Author contributions
AB designed all the experiments and carried out. BB designed the concept, monitored the experiments, and edited the manuscript prepared by AB. Both the authors read and approved the final manuscript.
Funding
This study was funded by RUSA 2.0, MHRD, Govt. of India; Science and Engineering Research Board (SERB), Ministry of Science and Technology, Govt. of India (YSS/2015/000173, SB/EMEQ-258/2013).
Compliance with ethical standards
Conflict of interest
Anuradha Barigela declares that she has no conflict of interest. Bhima Bhukya declares that he has no conflict of interest.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
References
- Al Obeed OA, Alkhayal KA, Al Sheikh A, Zubaidi AM, Vaali-Mohammed M-A, Boushey R, Mckerrow JH, Abdulla M-H. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J Gastroenterol WJG. 2014;20:18390. doi: 10.3748/wjg.v20.i48.18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attri P, Jodha D, Gandhi D, Chanalia P, Dhanda S. In vitro evaluation of Pediococcus acidilactici NCDC 252 for its probiotic attributes. Int J dairy Technol. 2015;68:533–542. doi: 10.1111/1471-0307.12194. [DOI] [Google Scholar]
- Borges S, Barbosa J, Silva J, Teixeira P. Evaluation of characteristics of Pediococcus spp. to be used as a vaginal probiotic. J Appl Microbiol. 2013;115:527–538. doi: 10.1111/jam.12232. [DOI] [PubMed] [Google Scholar]
- Carol M, Borruel N, Antolin M, Llopis M, Casellas F, Guarner F, Malagelada JR. Modulation of apoptosis in intestinal lymphocytes by a probiotic bacteria in Crohn’s disease. J Leukoc Biol. 2006;79:917–922. doi: 10.1189/jlb.0405188. [DOI] [PubMed] [Google Scholar]
- Chau I, Cunningham D. Adjuvant therapy in colon cancer—what, when and how? Ann Oncol. 2006;17:1347–1359. doi: 10.1093/annonc/mdl029. [DOI] [PubMed] [Google Scholar]
- Choi A-R, Patra JK, Kim WJ, Kang S-S. Antagonistic activities and probiotic potential of lactic acid bacteria derived from a plant-based fermented food. Front Microbiol. 2018;9:1963. doi: 10.3389/fmicb.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon H, Choi B, Lee E, Lee S, Jeong G. Immunomodulatory effects of specific bacterial components of Lactobacillus plantarum KFCC11389P on the murine macrophage cell line RAW 264· 7. J Appl Microbiol. 2009;107:1588–1597. doi: 10.1111/j.1365-2672.2009.04343.x. [DOI] [PubMed] [Google Scholar]
- Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052s–1057s. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- Cui W, Taub DD, Gardner K. qPrimerDepot: a primer database for quantitative real time PCR. Nucleic Acids Res. 2007;35:D805–D809. doi: 10.1093/nar/gkl767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallal MMS, Mojarrad M, Baghbani F, Raoofian R, Mardaneh J, Salehipour Z (2015) Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2). Arch Iran Med 18:167–172 [PubMed]
- de Ribeiro MC, O, Vandenberghe LP de S, Spier MR, Paludo KS, Soccol CR, Soccol VT, Evaluation of probiotic properties of Pediococcus acidilactici B14 in association with Lactobacillus acidophilus ATCC 4356 for application in a soy based aerated symbiotic dessert. Brazil Arch Biol Technol. 2014;57:755–765. doi: 10.1590/S1516-8913201402258. [DOI] [Google Scholar]
- Delgado S, O’sullivanFitzgeraldMayo EGB. Subtractive screening for probiotic properties of Lactobacillus species from the human gastrointestinal tract in the search for new probiotics. J Food Sci. 2007;72:M310–M315. doi: 10.1111/j.1750-3841.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Dowarah R, Verma AK, Agarwal N, Singh P, Singh BR. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE. 2018;13(3):e0192978. doi: 10.1371/journal.pone.0192978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duary RK, Rajput YS, Batish VK, Grover S. Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Indian J Med Res. 2011;134:664. doi: 10.4103/0971-5916.90992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duary RK, Batish VK, Grover S. Immunomodulatory activity of two potential probiotic strains in LPS-stimulated HT-29 cells. Genes Nutr. 2014;9:398. doi: 10.1007/s12263-014-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez NC, Ramiro JMP, Quecan BXV, de Melo Franco BDG. Use of potential probiotic lactic acid bacteria (LB) biofilms for the control of Listeria monocytogenes, Salmonella typhimurium, and Escherichia coli O157: H7 biofilms formation. Front Microbiol. 2016;7:863. doi: 10.3389/fmicb.2016.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim WJ, Kang SS. Inhibitory effect of bacteriocin-producing Lactobacillus brevis DF01 and Pediococcus acidilactici K10 isolated from kimchi on enteropathogenic bacterial adhesion. Food Bioscience. 2019;30:100425. doi: 10.1016/j.fbio.2019.100425. [DOI] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladha G, Jeevaratnam K. Characterization of purified antimicrobial peptide produced by Pediococcus pentosaceus LJR1, and its application in preservation of white leg shrimp. World J Microbiol Biotechnol. 2020;36:1–12. doi: 10.1007/s11274-020-02847-w. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes MCS, Paulino DSM, Brambilla SR, Camargo JA, Persinoti GF, Carvalheira JBC. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J Gastroenterol. 2018;24:1995. doi: 10.3748/wjg.v24.i18.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi ZE, Mousavi SM, Razavi SH, Emam-Djomeh Z, Kiani H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J Microbiol Biotechnol. 2011;27:123–128. doi: 10.1007/s11274-010-0436-1. [DOI] [Google Scholar]
- Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- Nouri Z, Karami F, Neyazi N, Modarressi MH, Karimi R, Khorramizadeh MR, Taheri B, Motevaseli E. Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J. 2016;18:127. doi: 10.22074/cellj.2016.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino A, De Angelis M, Chieppa M, Caggia C, Randazzo CL. Gut microbiota, probiotics and colorectal cancer: a tight relation. WCRJ. 2020;7:1456. [Google Scholar]
- Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9:555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10:S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash J (2016) Probiotic effect on gastrointestinal flora. In: Prakash V, Martín-Belloso O, Keener L, Astley S, Braun S, Mcmahon H, Lelieveld H (eds) Regulating safety of traditional and ethnic foods. Academic Press, Elsevier Inc., p 536
- Pringsulaka O, Rueangyotchanthana K, Suwannasai N, Watanapokasin R, Amnueysit P, Sunthornthummas S, Sukkhum S, Sarawaneeyaruk S, Rangsiruji A. In vitro screening of lactic acid bacteria for multi-strain probiotics. Livest Sci. 2015;174:66–73. doi: 10.1016/j.livsci.2015.01.016. [DOI] [Google Scholar]
- Rivera-Espinoza Y, Gallardo-Navarro Y. Non-dairy probiotic products. Food Microbiol. 2010;27:1–11. doi: 10.1016/j.fm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Srisesharam S, Park HS, Soundharrajan I, Kuppusamy P, Kim DH, Jayraaj IA, Choi KC. Evaluation of probiotic Lactobacillus plantarum against foodborne pathogens and its fermentation potential in improving Lolium multiflorum silage quality. 3 Biotech. 2018;10:8–443. doi: 10.1007/s13205-018-1449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M. Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiee A, Falah F, Behbahani BA, Tabatabaee-Yazdi F (2020) Probiotic characterization of Pediococcus strains isolated from Iranian cereal-dairy fermented product: interaction with pathogenic bacteria and the enteric cell line Caco-2. J Biosci Bioeng 130:471–479 [DOI] [PubMed]
- Verna EC, Lucak S. Use of probiotics in gastrointestinal disorders: what to recommend? Therap Adv Gastroenterol. 2010;3:307–319. doi: 10.1177/1756283X10373814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yao B, Gao H, Zang J, Tao S, Zhang S, Huang S, He B, Wang J. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC Vet Res. 2019;15:239. doi: 10.1186/s12917-019-1991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xie Q, Zhang Y, Ma W, Ning K, Xiang J-Y, Cui J, Xiang H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl Microbiol Biotechnol. 2020;104:335–349. doi: 10.1007/s00253-019-10259-6. [DOI] [PubMed] [Google Scholar]
- Yadav M, Shukla P. Recent systems biology approaches for probiotics use in health aspects: a review. 3 Biotech. 2019;12:9–448. doi: 10.1007/s13205-019-1980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Shukla P. Efficient engineered probiotics using synthetic biology approaches: a review. Biotechnol Appl Biochem. 2020;67:22–29. doi: 10.1002/bab.1822. [DOI] [PubMed] [Google Scholar]
- Yadav R, Puniya AK, Shukla P. Probiotic properties of Lactobacillus plantarum RYPR1 from an indigenous fermented beverage Raabadi. Front Microbiol. 2016;7:1683. doi: 10.3389/fmicb.2016.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Kumar V, Baweja M, Shukla P. Gene editing and genetic engineering approaches for advanced probiotics: a review. Crit Rev Food Sci Nutr. 2018;58:1735–1746. doi: 10.1080/10408398.2016.1274877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.