Abstract
This study aimed to explore the effect of Dendrobium officinale (DO) on the diversity of intestinal mucosal flora in high-fat diet mice and provided an experimental basis for the development and research of DO and its series products. Twenty-four mice were randomly assigned to four equal groups of six mice, namely the control (bcm) group, model (bmm) group, Dendrobium officinale (bdm) group, and positive control (bjm) group. Mice in the bdm group were administrated at the dose of 2.37 g·kg−1·days−1, and those in bjm group were given the Lipid-lowering decoction at the concentration of 1.19 g·kg−1·days−1, and sterile water was used as a placebo control twice a day for 40 consecutive days. We measured the dynamic weight changes and intestinal mucosal flora changes in mice. The analysis showed that DO had a regulatory effect on weight change induced by a high-fat diet in mice. DO could also regulate the changes in the diversity of the intestinal mucosa of mice, which was specifically reflected in the changes of Chao 1, ACE, Shannon and Simpson index. The sample information of the bdm group was relatively concentrated, but the distance from the bmm group was relatively scattered. The relative abundance results showed dominant bacteria phylum (such as Bacteroidetes, Actinobacteria, Verrucomicrobia) and bacterial genus (such as Bifidobacterium, Ruminococcus, Ochrobactrum) in the intestinal mucosa of the four groups. And significant differences in the major microbiota between the bdm and bjm groups. In addition, DO changed the carbohydrate, energy, and amino acid metabolism of intestinal mucosal flora. To sum up, DO has a regulatory effect on weight change induced by high-fat diet in mice and can improve the diversity of intestinal mucosal flora, promote the abundance of Ochrobactrum, inhibit the abundance of Bifidobacterium and Ruminococcus, and influence the intestinal flora to positively affect high-fat diet-induced negative effects in mice.
Keywords: Dendrobium officinale, High-fat diet, Intestinal mucosal flora, Intestinal microenvironment
Introduction
With the acceleration of the pace of life, great changes have taken place in people's eating habits. People consumed more diets with high fat and high protein but took fewer exercises, thus resulting in severe fat accumulation, and rapid rise in the incidence of hyperlipidemia (Chen et al. 2019). As shown in a large number of epidemiological and clinical observations, high-fat diet is easy to cause the disorder of lipid metabolism in the human body, causing atherosclerosis, acute myocardial infarction, and other diseases (Bruce-Keller et al. 2010; Boardman et al. 2020). While some progress has been made in developing drugs to treat hyperlipidemia, the side effects of traditional medicines are also becoming apparent over time (Liu et al. 2019; Lei 2020; Yuan et al. 2019). Therefore, healthy and safe diet interventions have attracted widespread attention.
There are a large number of symbiotic microorganisms in the human intestinal tract, collectively referred to as "intestinal microbiota", which are essential for maintaining the integrity of the intestinal mucosal barrier function, absorbing nutrients, and balancing energy (Ley et al. 2006). Studies have found that intestinal microbiota plays an important role in the development of hyperlipidemia and its complications (Jiang et al. 2020). High-fat diet may induce changes or even disorders in the structure of intestinal flora. For example, the number of Bacteroides in the intestines of mice of high-fat diet decreased, and Firmicutes and Proteobacteria increased, which could cause hyperlipidemia by improving food energy intake, accelerating fatty acid synthesis, and promoting triglyceride and cholesterol deposition (Nicole et al. 2012; Rong et al. 2017; Stephens et al. 2018). However, some by-products are produced during lipid metabolisms, such as secondary cholic acid and hydrogen sulfide. It could damage intestinal mucosa, cause mucosal inflammation, and destroy the microenvironment on which the bacteria live (Ren et al. 2010). Therefore, intestinal flora plays an important role in the occurrence and development of hyperlipidemia. Regulating the composition and metabolic function of intestinal flora becomes a key factor to inhibit the development of metabolic disorders related to hyperlipidemia.
Dendrobium officinale (DO) is a perennial herb of the genus dendrobium of Orchidaceae. Its main components are alkaloids and polysaccharides (Ma et al. 1997). It is rich in amino acids, including 7 essential amino acids (except tryptophan) and 3 semi-essential amino acids (Huang et al. 1997). Modern pharmacological studies have shown that DO has the functions of reducing blood fat and blood glucose, changing the structure of gastrointestinal mucosa, improving digestive enzyme activity, and enhancing gastrointestinal motility (Yan et al. 2016; Yao et al. 2019; Sun et al. 2019). It can adjust the intestinal microecological balance to treat spleen deficiency constipation (Cao et al. 2014). Dendrobium polysaccharides can not only promote beneficial bacteria such as Lactobacillus and Bifidobacteria, but also effectively inhibit the growth of Escherichia coli, Staphylococcus aureus (Li et al. 2011; Zhang et al. 2012). In 2018, the National Health Commission planned to include DO into the list of "both food and medicine" items, which means that the health care value of DO has been focused (Xiao et al. 2020). As shown in the existing literature, DO has a certain preventive effect of hyperlipidaemia, but its mechanism of reducing serum lipid is still unclear (Jia 2016). Based on these, this study aimed to investigate the effect of DO on the diversity of intestinal mucosal flora in mice with high-fat diet, expecting to provide theoretical support for the health efficacy of DO from the perspective of intestinal microecology, and contribute to the experimental basis for the development of DO and its series of products.
Materials and methods
Animals
Twenty-four 6-week-old specific pathogen-free Kunming mice (half male and half female), weighing 20 ± 2 g, were purchased from Hunan Slaccas Jingda Laboratory Animal Co., Ltd (SCXK(Xiang) 2016–0002). The mice were raised under stable conditions (temperature 23–25 ℃, relative humidity 50–70%, 12 h light/dark cycles) in the laboratory animal center of Hunan University of Chinese Medicine. The process of animal experiments was conducted under animal protocols approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine.
Feed
The normal feed is 100% basal feed; and high-fat diet refers to the mixture of 88% basal feed with 2% cholesterol and 10% lard (Zhu et al. 2018).
Medicine
After repeated rinsing with distilled water, DO (from Hunan Longshishan Dendrobium officinale Base Co., Ltd.) was dried with absorbent paper and then cut into pieces. Next, 10 times the amount of water was added, which was boiled twice (30 min each time), and mixed together.
Lipid-lowering decoction (Wang et al. 2012): the semen cassia (30 g), rhizoma alismatis (10 g), curcuma aromatic (10 g), salvia miltiorrhiza (10 g), lotus leaf (10 g), and seaweed (30 g) were weighed. Then add water and boil to concentrate to 0.92 g·mL−1. Finally, the solution was diluted to 0.17 g·mL−1 at a ratio of 1:5.4 (V/V).
Animal groups
Twenty-four Kunming mice were randomly divided into four groups: control (bcm) group, model (bmm) group, Dendrobium officinale (bdm) group, and positive control (bjm) group. The bcm group was fed with basal feed, and other groups were fed with high-fat feed. Six mice in each group, half male and half female, were raised in cages.
Medication and dosage
Equivalent intragastric administration was performed for mice in the above groups according to the clinical dose. Mice in the bdm group were administrated at the dose of 2.37 g·kg−1·days−1, and those in bjm group were given the Lipid-lowering decoction at the concentration of 1.19 g·kg−1·days−1, and sterile water was used as a placebo control twice a day for 40 consecutive days.
Detection of mice weight
The bodyweight of the mice was measured on day 0 (initial) and day 40 respectively, and the rate of weight change was calculated.
Rate of weight change (%) = (weight gain / initial weight) × 100.
Extraction of mice intestinal mucosa
After 40 days of feeding, mice in each group were sacrificed rapidly by cervical dislocation, placed on a super clean workbench, and intestinal mucosa of mice was collected in a sterile manner for use (Feng et al. 2015; Zeng et al. 2012). An appropriate amount of liquid nitrogen was used to fully grind the intestinal mucosa of mice and 0.5 mL Tris was added to each 10–20 mg of the mucosa. After mixing the solution, placed it at room temperature for 5–10 min, and completely separate the nucleoprotein and nucleic acid. Then, 0.2 mL chloroform was added and centrifuged at 4 ℃ (12,000 rpm) for 10 min to collect the supernatant, which was then added with 1/2 times of absolute ethanol, and evenly mixed. After placing for 2 min, the solution was centrifuged (12,000 rpm) at 4 ℃ for 3 min, and the waste solution was discarded. 500 μL RPE solution was added and centrifuged at 4 ℃ (10,000 rpm) for 30 s. Finally, the waste solution was discarded. The process was repeated once. The adsorption column was placed in the collecting pipe and centrifuged (10,000 rpm) at 4 ℃ for 2 min. 30 μL DEPC-Treated ddH2O was added and centrifuged at 4 ℃ for 2 min (12,000 rpm) for 5 min. After detection and analysis with 1.5% agarose gel electrophoresis, the obtained DNA solution was stored at -70 ℃ for future use.
Intestinal flora was analyzed by 16S rRNA high-throughput sequencing
PCR amplification was realized by Q5 high-fidelity DNA polymerase (NEB Company); the extracted DNA was taken as a template, to strictly control and minimize the number of amplification cycles, but maintain the same amplification conditions. 16S rRNA V4 variable region was used for amplification, and the amplification products were measured by electrophoresis detection; for further fluorescence quantification, the samples should be mixed in a corresponding proportion. Using the fluorescent quantitation method of Promega, the recycled products were quantitatively expanded based on the preliminary electrophoretic results; the samples were mixed in a corresponding proportion based on the fluorescent quantitation results. The sequencing library was prepared by Illumina in the following operational approach: First, sequence end repair was performed, the bulged base of DNA sequence 5′ end was removed by End Repair Mix2 in the kit, and a phosphate group was added to supplement the missing base of 3′ end, to ensure that the target sequence was connected to the sequencing joint, and fix the DNA molecules on the Flow Cell. The self-connected fragment was removed, and the DNA fragment was amplified by PCR, and the library system after adding the joint was further selected and purified. Sequencing was completed by Wuhan Frasergen Genetic Information Company (Caporaso et al. 2010).
Bioinformatics and statistical analysis
Bacterial diversity index (including Chao1, ACE, Simpson, and Shannon) in the intestinal mucosa were measured by MOTHUR (version v.1.30.1, https://www.mothur.org/) based on the Operational Taxonomic Units (OTUs). Principle component analysis (PCA) and nonmetric multidimensional scaling (NMDS), and LDA effect size analysis (LefSe) were conducted with the R package (https://www.R-project.org/) to analyze the main distribution characteristics and the similarity of community samples. Functional analysis is to compare the 16S rRNA gene sequence data obtained by sequencing with the Green genes database. "Map" the flora composition data to the known gene function profile database to realize the prediction of the metabolic function of the bacterial flora.
All the data were expressed as means and standard deviation (n = 6). The data were subjected to one-way analysis of variance using the SPSS 24.0 software (IBM Corp, Armonk, NY, USA). The differences among groups were examined by independent samples t-tests. p < 0.05 was considered to be significant.
Results
Effect of DO on weight change of mice on high-fat diet
Compared with male bcm group mice, the bodyweight of bmm group mice decreased. Compared with bmm group mice, the bodyweight of the bdm group mice increased and the bjm group mice decreased. It might be related to the activity and food intake of male mice in bdm group. Compared with female bcm group mice, bmm group mice had increased body weight. Compared with bmm group mice, both bdm and bjm group mice lost weight. It suggested that DO may have better lipid-lowering effect on female mice (Fig. 1).
Fig. 1.

Effect of DO on weight change of mice on high-fat diet (n = 6) Blue was for male mice and pink was for female mice. Data were no statistical difference between the above (p > 0.05). bcm: control group, bmm: model group, bdm: Dendrobium officinale group and bjm: positive control group
Effects of DO on the number and distribution curve of intestinal mucosal flora OTU in mice with high-fat diet
OTU Venn diagram analyzed the unique or common OTUs among different sample groups, which intuitively showed the similarity and uniqueness of samples at the OTU level. Using the Qiime software platform, OTU clustering was performed according to 97% similarity (Fig. 2a). There were 1559 OTUs in the bcm group, 1750 in the bmm group, 1795 in the bjm group and 1981 in the bdm group. There were 704 overlapping OTUs in the four groups. As for the shared OTU, there were 1090 ones in the bcm and bmm group, 985 ones in the bjm group, and 1019 ones in the bdm group. There were 1056 OTUs in the bmm and bjm group, and 1173 OTUs in the bdm group. There were 1263 OTUs in the bjm and bdm group. The results showed that after intervention with DO, the number of OTU in the intestinal mucosa of mice was increased.
Fig. 2.
Four groups of intestinal mucosa microbial composition (n = 6) (a) OTUs distribution Venn diagram of mice in the four groups. b OTU rank abundance distribution curve of mice in the four groups. The abscissa represented the OTU arranged by abundance, and the ordinate represented the abundance of each OTU in this sample. bcm: control group, bmm: model group, bdm: Dendrobium officinale group and bjm: positive control group
Then, we plotted the rank abundance distribution curve according to the abundance log2 value to analyze the community structure of intestinal mucosal flora in each group (Fig. 2b). The rank abundance distribution curve could provide information on resource allocation and species richness among species. The abscissa showed the level of OTU, and the ordinate showed the abundance of OTUs of each sample. They represented the difference of relative abundance of four groups of OTUs. The longer the broken line, the greater the number of OTUs in the sample. The smoother the slope, the better the uniformity of the community, and the steeper the broken line, the lower the evenness of the community. As shown in the Fig. 2b, the number of OTU in the bdm group was the largest, which was consistent with the above-mentioned reports on OTU.
Effects of DO on alpha diversity of intestinal mucosal flora in high-fat mice
Alpha diversity analysis reflected the richness and uniformity in a specific area or ecosystem, including Chao 1, Ace, Simpson, and Shannon index. In the experiment, we used Chao1 and ACE indexes to evaluate the abundance of intestinal mucosal flora (Fig. 3a, b). Shannon and Simpson indexes were used to reflect the community diversity (Fig. 3c, d).
Fig. 3.
Effects of DO on Alpha diversity of intestinal mucosal Flora in high-fat mice (n = 6) (a) Chao1 index. b ACE index. Chao1 and ACE indexes represented the richness of intestinal mucosal flora. c Simpson index. d Shannon index. Shannon and Simpson indexes were used to reflect community diversity. Data were no statistical difference between the above (p > 0.05). bcm: control group, bmm: model group, bdm: Dendrobium officinale group and bjm: positive control group
The rank of Chao1 index from high to low was the bdm, bjm, bcm and bmm group. As for Ace, the ranking was as follows: bdm, bcm, bjm and bmm group, and there was no statistical difference between the above. Simpson index from high to low ranked as: bmm, bjm, bdm, bcm group. As for Shannon, the ranking was as follows: bdm, bmm, bjm, bcm group, all of which had no statistical difference. The results showed that DO could affect the diversity of the intestinal microbial community in mice.
Effects of DO on beta diversity of intestinal mucosal flora in high-fat mice
PCA was a visualization method that can be used to study the similarity or difference of data, and observe the differences between individuals or communities. In the figure, each point represented a sample, and samples of the same color came from the same group. The larger the distance between every two points, the greater the difference between the two samples would be. As shown in Fig. 4a, samples of the bcm and bmm group were in the first and third quadrants and relatively concentrated. Samples of the bdm group were in the second quadrant. The samples of bjm group were concentrated in the second and third quadrants, suggesting that the high-fat diet changed the homogeneity of intestinal mucosal flora. The samples of the bdm and bjm group were close to each other, and the bdm group was relatively concentrated. The above two groups had a certain distance from the bmm group, indicating that the bdm and the bjm group could promote the recovery of intestinal flora in high-fat diet mice, and the treatment effect of the bdm group was better than the bjm group.
Fig. 4.
Effects of DO on Beta diversity of intestinal mucosal Flora in high-fat mice (n = 6) (a) PCA principal coordinate analysis figure. b NMDS analysis diagram. Points of different colors or shapes represent sample groups under different conditions. The more similar the sample composition, the closer the distance reflected. Data were no statistical difference (p > 0.05). bcm: control group, bmm: model group, bdm: Dendrobium officinale group and bjm: positive control group
NMDS was used to analyze the distance matrix information between samples. In Fig. 4b, each point represented a sample, and the points in different colors belonged to different samples. The closer the two points were, the higher the genetic similarity would be, and the difference would be smaller. Samples from the bcm, bdm and bjm group were distributed in a concentrated manner, but the distribution space of samples from the bmm group was relatively large, indicating that diet and high-fat diet had different effects on intestinal microbial composition, and diet was a key factor affecting intestinal microbial community. After the intervention of DO, the diversity of intestinal mucosal flora of high-fat diet mice was restored.
Effect of DO on relative abundance of intestinal mucosal flora in mice with high-fat diet
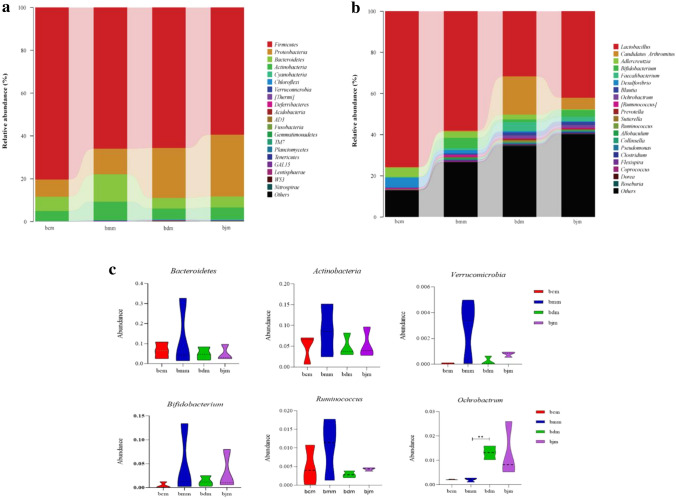
The relative abundance of the intestinal mucosal flora in mice was shown in the phylum horizontal bar chart: the dominant phylum ranked in the top 20 in phylum horizontal content. The histogram of relative abundance of genera showed that the dominant genera with significant flora content were in the top 20, and the genera with extremely low content or not annotated in the database were listed as "Other". The abscissa of the bar chart indicated the group, the ordinate indicated the relative abundance of the horizontal intestinal mucosa bacteria of species phylum (or genus), the squares of different colors represented the names of different species, and the length of the squares indicated the relative abundance of the species.
Figure 5a showed the relative abundance of bacteria in the intestinal mucosa of mice at the phylum level, in which Firmicutes、Proteobacteria、Bacteroidetes、Actinobacteria and Cyanobacteria in the four groups accounted for a large proportion of the dominant phylum. Compared with the bcm group, the relative abundance of Bacteroidetes and Actinobacteria in the bmm group increased (p > 0.05; p > 0.05), while Verrucomicrobia decreased (p > 0.05). Compared with the bmm group, the relative abundance of Bacteroidetes and Actinobacteria in the bdm and bjm group was decreased (p > 0.05; p > 0.05), and Verrucomicrobia was increased (p > 0.05) (Fig. 5c).
Fig. 5.
Effect of DO on relative abundance of intestinal mucosal flora in mice with high-fat diet (n = 6) (a) Relative abundance of phylum level of intestinal mucosal microflora in mice. b Relative abundance of genus level of intestinal mucosal flora in mice. c Level of phylum and genus level of dominant bacteria in intestinal mucosal flora of mice. bcm: control group, bmm: model group, bdm: Dendrobium officinale group and bjm: positive control group
Figure 5b showed the relative abundance of intestinal mucosal bacteria in mice at the genus level. Lactobacillus、Candidatus-Arthromitus、Adlercreutzia、Bifidobacterium and Faecalibacterium were the dominant bacteria that accounted for a large proportion in the four groups. Compared with the bcm group, the relative abundance of Bifidobacterium and Ruminococcus in the bmm group was increased (p > 0.05; p > 0.05), while Ochrobactrum decreased (p > 0.05) Compared with the bmm group, the relative abundance of Bifidobacterium and Ruminococcus in the bdm and bjm group decreased (p > 0.05; p > 0.05), while Ochrobactrum increased (p < 0.05) (Fig. 5C).
Effect of DO on intestinal mucosal characteristic flora of high-fat diet mice
In order to identify the characteristic flora of intestinal mucosa in mice after intervention with DO, LEfSe analysis was carried out in bdm and bjm group. Generally, the abscissa in the LDA score graph represented the LDA score, and the ordinate was composed of the taxonomic units with obvious differences among the groups. The longer the bar, the greater the difference. The different colors of the bar chart could be used to indicate that a specific taxon corresponds to a higher sample group. The cladogram diagram showed the species or species structure of different microbial communities from phylum to species (from inner ring to outer ring). The size of nodes in the graph corresponded to the average relative abundance of taxon. Yellow nodes represented taxons with no significant differences between groups, while other colors (such as red and green) indicated taxons with significant differences between groups.
The letters indicated the names of taxons with obvious differences among different categories. As shown in Fig. 6a, 16 and 17 dominant groups were found in bdm and bjm group respectively. Alphaproteobacteria、Rhizobiales、Ochrobactrum and Brucellaceae were the main microflora in the bdm group. The main microbiota in the bjm group were Clostridiales、Clostridia、Clostridiaceae and Betaproteobacteria. The results showed that there were significant differences in intestinal mucosal flora between bdm and bjm group, and there were significant differences in the characteristic flora between the two groups. Figure 6b showed a taxonomy that represented the structure of intestinal microbes and their main bacteria, and showed the largest taxa difference between the two groups. It further proved the difference in intestinal microbial structure between the bdm and the bjm group.
Fig. 6.
Effect of DO on intestinal mucosal characteristic flora of high-fat diet mice (n = 6) (a) LDA scores derived from LEfSe analysis. The abscissa in the LDA score graph represented the LDA score, and the ordinate was composed of the taxonomic units with obvious differences among the groups. b Cladogram generated based on LEfSe analysis showing the relationship between taxa. The size of nodes in the graph corresponded to the average relative abundance of taxon. bcm: control group, bmm: model group, bdm: Dendrobium officinale group and bjm: positive control group
Functional analysis of DO on intestinal mucosal flora of mice on high-fat diet
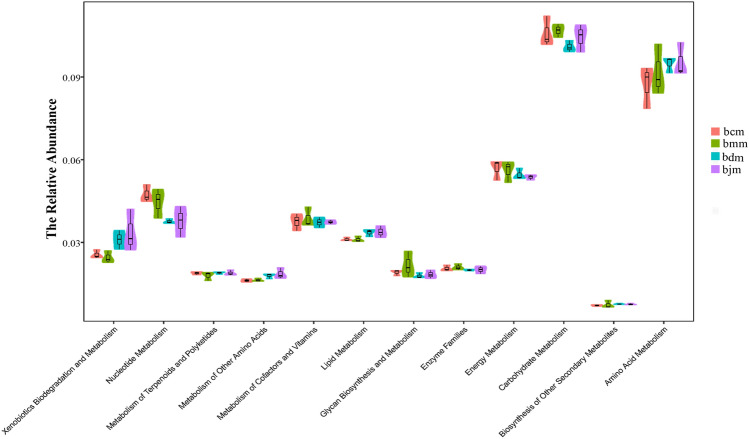
Combined with the data of intestinal microflora in mice, we performed a functional analysis of its metabolic pathway. It can be seen from Fig. 7 that the intestinal mucosal flora of mice was mainly affected by carbohydrate metabolism, amino acid metabolism, and energy metabolism. Compared with the bcm group, the metabolic pathways of mice after the high-fat diet changed significantly, including the increase in carbohydrate metabolism, the decrease in amino acid metabolism, and energy metabolism. After the intervention of DO, the carbohydrate metabolism and energy metabolism were decreased, while amino acid metabolism was increased. It was suggested that DO might treat hyperlipidemia by intervening the above metabolic pathways.
Fig. 7.
Functional analysis of DO on intestinal mucosal flora of mice on high-fat diet (n = 6) The abscissa represented specific metabolic function information, and the ordinate represented its relative abundance value. Different color squares in the figure represented different groups. bcm: control group, bmm: model group, bdm: Dendrobium officinale group and bjm: positive control group
Discussion
Intestinal flora is composed of trillions of symbiotic microorganisms, which plays an important role in maintaining normal metabolism and physiological function of the body (Van et al. 2017; Ames et al. 2017). In recent years, the research on intestinal microbial regulation of lipid metabolism has become a basic and clinical focus, and changes and dysfunctions of intestinal microbial component have gradually become potential new targets for the prevention and treatment of hyperlipidemia (Jia et al. 2017). Studies have shown that intestinal microorganisms can not only participate in the synthesis and metabolism of bile acid, regulate bile acid metabolism, but also affect lipid metabolism through carbohydrate fermentation, that is, the steady-state of intestinal microbes can help maintain lipid metabolism (Ryan et al. 2014; Yue et al. 2020). Chang et al. found that the intestinal microflora of hyperlipidemia animal model could change specifically, and the ratio of Firmicutes/Bacteroidetes increased (Chang et al. 2015). High-fat diet is likely to cause changes in the intestinal flora of mice, reducing the abundance of dominant flora and increasing non-dominant flora. At the same time, some metabolites produced by high-fat diet may damage intestinal mucosa, increase intestinal permeability and endotoxin level, and destroy the flora ecology (Resta et al. 2009). Combined with the results of this experiment, the proportion and abundance of intestinal flora in mice after high-fat diet changed. Compared with the bcm group, the number of OTU in the intestinal mucosa of the bmm group increased, and the diversity and richness of its species changed. The relative abundance of dominant bacteria in the bmm group such as Bifidobacterium and Ruminococcus increased, while the relative abundance of Ochrobactrum decreased. The above results suggested that the intestinal microbial community of mice has changed after high-fat feeding. This was consistent with the previous report that there were differences in the composition and structure of intestinal flora in the occurrence of hyperlipidemia (Hildebrandt et al. 2009).
Studies have found that the advantages of Traditional Chinese medicine (TCM) in the treatment of hyperlipidemia have become increasingly prominent, which can affect the composition and metabolic function of intestinal flora through interaction with intestinal microorganisms, so as to achieve the purpose of treatment or influence the curative effect (Nishida et al. 2016; Lu et al. 2018; Liu et al. 2019). DO, as a characteristic medicinal material for both food and food use of TCM, is rich in dendrobium polysaccharides, amino acids, trace elements, and other chemical components, and has certain clinical efficacy and pharmacological effects on metabolic diseases such as hyperlipidemia (Yan et al. 2019). According to reports (Li et al. 2020), DO can affect the occurrence and development of diseases by regulating the balance of intestinal microorganisms. The active ingredients in DO can not only promote the proliferation of beneficial bacteria and inhibit the excessive reproduction of harmful bacteria through competition, but also regulate the diversity of intestinal flora and affect the metabolic pathways of microorganisms, thereby regulating the balance of intestinal microorganisms. At the same time, it can also provide biochemical functions and enzymes that the host does not have, and plays a promoting role in its efficacy (Xie et al. 2019). Based on the results of this experiment, the number of OTU in the intestinal mucosa of mice in the bdm group was higher than that of the bmm group. Our results showed that DO had a profound effect on the diversity and microbial community of intestinal mucosal flora in mice. On the analysis of Alpha diversity results, Chao 1, ACE, and Shannon index were increased (p > 0.05; p > 0.05; p > 0.05). This higher species diversity was supported by more unique OTUs and total OTUs in the bdm group. However, the Simpson index of the intestinal mucosal flora in mice showed a decreasing trend (p > 0.05), which might be due to individual differences in mice. According to the results of Beta diversity analysis, PCA principal coordinate analysis and NMDS analysis showed that the sample information of bdm group was relatively concentrated, and there was a certain distance from the bmm group, indicating that the bdm group could promote the recovery of intestinal flora in mice fed with high-fat diet. These results seem to be consistent with previous studies, that was, after Yao et al. intervened with the aqueous extract of DO, the intestinal microbial biodiversity of mice increased, and the intestinal microbial community similarity coefficient showed significant differences, which might be influenced by DO (Yao et al. 2019). Based on the above analysis, we speculated that DO has positive effects on regulating the richness, diversity and microbial community of intestinal mucosal flora in mice induced by high-fat feeding.
By comparing the rich changes of the intestinal mucosal flora of mice in the bdm and bmm group, we could further understand how DO changed the intestinal microbial environment. Compared with the bmm group, the bdm group increased the abundance of Verrucomicrobia, but decreased the abundance of Bacteroidetes and Actinobacteria. This supported the role of DO in rebuilding the normal microbial environment (Mirjana et al. 2007). At the genus level, compared with the bmm group, the abundance of Bifidobacterium and Ruminococcus in the intestinal mucosa of mice in the bdm group decreased, while Ochrobactrum increased. Many studies have reported the role of these bacteria in intestinal health. Among them, Bifidobacterium has been shown to reduce endotoxin levels in the gastrointestinal tract of rodents, thereby enhancing the intestinal mucosal barrier function (Wang et al. 2006). It can produce carbohydrate polymers and extracellular polysaccharides can reduce lipids (Brodmann et al. 2017). In this experiment, the abundance of Bifidobacterium in the bmm group increased and decreased in the bdm group, which might be related to the components and ratios of the ingredients in the high-fat diet and the complex chemical components in DO. Ruminococcus is often closely related to the adverse effects of high-fat diets (Huang et al. 2020). Peters et al. found that obesity was positively associated with Ruminococcus (Peters et al. 2018). A longitudinal study of healthy women Study found that Ruminococcus was protective of long-term weight gain (Menni et al. 2017). The above research findings indicated that diet could induce changes in the composition of gut microflora, thereby affecting the host's metabolism to lose weight. Ruminococcus played an important role in the process of lipid-lowering. We could reduce the content of Ruminococcus to improve the adverse effects of a high-fat diet and DO did this. In the LEfSe analysis, there were significant differences in the characteristic flora between the bdm and bjm group, which further proved the difference in intestinal microbial structure between the bdm and bjm group. Later, we analyzed the functional analysis of the metabolic pathways of the intestinal mucosal flora of mice and discovered that DO might mainly interfere with the treatment process through multiple pathways affecting carbohydrate metabolism, amino acid metabolism, and energy metabolism in mice.
In addition, we also found that DO could regulate the changes in body weight in high-fat diet mice, but it might be influenced by gender. Zhang pointed out that there was a gender difference in fat metabolism by comparing the difference in energy metabolism between males and females, which might be related to the difference in lipid content in muscle tissue (Zhang 2009). The oxidative metabolism of lipids in muscle tissue requires the involvement of related hormones. However, intestinal flora can regulate the enterohepatic circulation of estrogen and androgen, and affect local and systemic sex hormone levels (Cross et al. 2018). Based on this, we speculated that the reason why DO affects the weight change of mice with high-fat diet of different gender may be related to the difference of intestinal flora in female and male mice, but its specific mechanism still needs to be further explored.
Conclusions
To sum up, DO has a regulatory effect on weight change induced by high-fat diet in mice, and can improve the diversity of intestinal mucosal flora, promote the abundance of Ochrobactrum, inhibit the abundance of Bifidobacterium and Ruminococcus, and influence the intestinal flora to positively affect HFD-induced negative effects in mice.
Acknowledgements
We thank all the scholars who provided relevant guidance for the study.
Author contributions
XL analyzed the data and drafted the manuscript; XP performed the experiments; KG checked the manuscript; ZT designed the study. All authors read and approved the final manuscript. The decision to submit the manuscript for publication was made by all the authors.
Funding
None.
Data availability
This study was approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Institutional animal care and use committee statement
This study was approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine.
Contributor Information
Kangxiao Guo, Email: 49824329@qq.com.
Zhoujin Tan, Email: tanzhjin@sohu.com.
References
- Ames NJ, Ranucci A, Moriyama B, Wallen GR. The human microbiome and understanding the 16S rRNA gene in translational nursing science. Nurs Res. 2017;66(2):184–197. doi: 10.1097/NNR.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman NT, Pedersen TM, Rossvoll L, Hafstad AD, Aasum E. Diet-induced obese mouse hearts tolerate an acute high fatty acid exposure that also increases ischemic tolerance. AJP-Heart circ physiol. 2020;319(3):H682–H693. doi: 10.1152/ajpheart.00284.2020. [DOI] [PubMed] [Google Scholar]
- Brodmann T, Endo A, Gueimonde M, Vinderola G, Kneifel W, de Vos WM, Salminen S, Gomez-Gallego C. Safety of novel microbes for human consumption: practical examples of assessment in the European Union. Front Microbiol. 2017;8:1725. doi: 10.3389/fmicb.2017.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Richard AJ, Fernandez-Kim SO, Ribnicky DM, Salbaum M, Newman S, Carmouche R, Stephens JM. Fenugreek counters the effects of high fat diet on gut microbiota in mice: links to metabolic benefit. Sci Rep. 2010;10(10):2284–2289. doi: 10.1038/s41598-020-58005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang H, Wu WJ, Tan ZJ, Xiao XY, Wu LF, Zhang HL, Zhao XB. Effect of ultra-micro Dendrobium officinale powder on the in mice with spleen-deficiency constipation. Chin J Microecol. 2014;26(09):1011–1015. doi: 10.13381/j.cnki.cjm.201409006. [DOI] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YYM, Young JD, Lai HC. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Conmun. 2015;6:7489. doi: 10.1038/ncomms16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HP, Zeng F, Li SM, Liu YL, Gong SY, Lv XC, Zhang JC, Liu B. Spirulina active substance mediated gut microbes improve lipid metabolism in high-fat diet fed rats. J Funct Foods. 2019;59:215–222. doi: 10.1016/j.jff.2019.04.049. [DOI] [Google Scholar]
- Cross TL, Kasahara K, Rey FE. Sexual dimorphism of cardiometabolic dysfunction: Gut microbiome in the play? Mol Meta. 2018;15:70–81. doi: 10.1016/j.molmet.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng MJ, Xie JN, Zhang YX, Tan ZJ, Tang B. Influence of lipid-lowering decoction on hepatic morphology and pathological changes in rats with nonalcoholic fatty liver disease. World Chin J Dig. 2015;23(6):2532–2538. doi: 10.11569/wcjd.v23.i16.2532. [DOI] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterol. 2009;137(5):1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Hai J, Huang H, Wu H, Zhang G, Zhang K, Chen Y. Supercritical-CO2fluid extraction in extracting volatile constituents from Juniperus formosana. J Chin Med Materials. 1997;20(1):32–33. [PubMed] [Google Scholar]
- Huang CY, Ming DX, Wang WH, Wang ZJ, Hu YF, Ma X, Wang FL. Pyrroloquinoline quinone alleviates jejunal mucosal barrier function damage and regulates colonic microbiota in piglets challenged with enterotoxigenic Escherichia coli. Front Microbiol. 2020;11:1754. doi: 10.3389/fmicb.2020.01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia GZ (2016) Influence of aqueous extract od Dendrobium on the changes of intestinal flora of fat diet mice. Dissertation, Guangdong Pharmaceutical University.
- Jia LQ, Song N, Zhang N, Ma YX, Lv MJ, Lv XM. Exploration of the relationship between intestinal microbial homeostasis and grease transfer: based on the theory of “Spleen governing transportation and transformation”. J Tradit Chin Med. 2017;58(18):1554–1557. doi: 10.13288/j.11-2166/r.2017.18.007. [DOI] [Google Scholar]
- Jiang YN, Zeng ZJ, Fu LY, Sheng YX, Zeng GW, Yao LL, Wang WW, Zhou ZY, Xu GL, Liu HN. Study on the mechanism of gegen qinlian decoction for lowering blood lipids and preventing blood glucose increase based on intestinal flora. China Pharm. 2020;31(15):1823–1829. doi: 10.6039/j.issn.1001-0408.2020.15.06. [DOI] [Google Scholar]
- Lei YJ. Effects of high doses of simvastatin in treating elderly patients with hypertension and hyperlipidemia. J Clin Med Pract. 2020;24(18):64–67. [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Li L, Ding CC, Li FH. Study on the antibacterial effects of two Dendrobium polysaccharides. J Anhui Agrice Sci. 2011;39(10):5753–5754. doi: 10.13989/j.cnki.0517-6611.2011.10.174. [DOI] [Google Scholar]
- Li M, Yue H, Wang YQ, Guo CL, Du ZY, Jin C, Ding K. Intestinal microbes derived butyrate is related to the immunomodulatory activities of Dendrobium officinale polysaccharide. Int J Biolo Macromol. 2020;149:717–723. doi: 10.1016/j.ijbiomac.2020.01.305. [DOI] [PubMed] [Google Scholar]
- Liu SZ. Clinical efficacy and adverse reactions of atorvastatin in the treatment of elderly patients with hyperlipidemia. Guide China Med. 2020;18(27):57–58. doi: 10.15912/j.cnki.gocm.2020.27.028. [DOI] [Google Scholar]
- Liu L, Jiang HN, Xu HR, Han YB, Sui YB, Zhou GL. The relationship between metabolic syndrome and intestinal flora and the current status of prevention and treatment with TCM. Chin J Microecol. 2019;31(05):605–609. doi: 10.13381/j.cnki.cjm.201905025. [DOI] [Google Scholar]
- Lu YL, Du YM, Qin L, Wu D, Wang W, Ling L, Ma FF, Ling H, Yang L, Wang CH, Wang ZT, Zhou YQ, Ohta Y. Gypenosides altered hepatic bile acids homeostasis in mice treated with high fat diet. Evid Based Complement Alternat Med. 2018;2018:8098059. doi: 10.1155/2018/8098059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Zhang P, Yu SP, Li MF. Analysis of the total alkaloids and polysaccharides in yunnashixiantao. Tradit Chin Herb Drugs. 1997;28(09):561–563. [Google Scholar]
- Menni C, Jackson MA, Pallister T, Steves CJ, Spector TD, Valdes AM. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int J Obes Lond. 2017;41(7):1099–1105. doi: 10.1038/ijo.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirjana RS, Hauke S, Willem MDV. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9(9):2125–2136. doi: 10.1111/j.1462-2920.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- Nicole DW, Muriel D, Hanneke BV, Els O, Shohreh K, Caroline D, Michiel K, Michael M, Roelof VDM. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointrst Liver Physiol. 2012;303(5):G589–G599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- Nishida M, Kondo M, Shimizu T, Saito T, Sato SJ, Hirayama M, Konishi T, Nishida H. Antihyperlipidemic effect of Acanthopanax senticosus (Rupr. et Maxim) Harms leaves in high-fat-diet fed mice. J Sci Food Agric. 2016;96(11):3717–3722. doi: 10.1002/jsfa.7557. [DOI] [PubMed] [Google Scholar]
- Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, Friedlander C, Hayes RB, Ahn J. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8(1):9749. doi: 10.1038/s41598-018-28126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren TT, Lu FG, Zhang YL, Cheng ZM, Xu M. Intake of a high-fat diet alters intestinal flora in rats. World Chin J Dig. 2010;18(25):2694–2697. doi: 10.11569/wcjd.v18.i25.2694. [DOI] [Google Scholar]
- Resta SC. Effects of probiotics and commensals on intestinal epithelial physiology: implication for nutrient handling. J Physiol. 2009;587(17):4169–4174. doi: 10.1113/jphysiol.2009.176370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong ZH, Liang SC, Lu JQ, He Y, Luo YM, You C, Xia GH, Prabhakar M, Li P, Zhou HW. Effect of intermittent fasting on physiology and gut microbiota in presenium rats. J South Med Univ. 2017;37(4):423–430. doi: 10.3969/j.issn.1673-4254.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nat. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RW, Arhire L, Covasa M. Gut microbiota:from microorganisms to metabolic organ influencing obesity. Obes. 2018;26(5):801–809. doi: 10.1002/oby.22179. [DOI] [PubMed] [Google Scholar]
- Sun L, Chen XM, Wu CM, Guo SX. Effects of aqueous extract of dendrobium candidum on intestinal microorganism and lipid metabolism in DM mice. Inf Tradit Chin Med. 2019;36(2):44–49. doi: 10.19656/j.cnki.1002-2406.190043. [DOI] [Google Scholar]
- Van Denelsen LW, Poyntz HC, Weyrich LS, Young W, Forbes-Blom EE. Embracing the gut microbiota:the new frontier for inflammatory and infectious diseases. Clin Transl Immunol. 2017;6(1):e125. doi: 10.1038/cti.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006;61:650–657. doi: 10.1097/01.ta.0000196574.70614.27. [DOI] [PubMed] [Google Scholar]
- Wang XK, Shi YH, Wang CZ, Chen ML, Yuan DD. Comparative study on hyperlipidemia rat models with different high-fat diets. Jiangsu Agric Sci. 2012;40:182–184. doi: 10.15889/j.issn.1002-1302.2012.01.039. [DOI] [Google Scholar]
- Xiao KX, Zhu YJ, Chen R, Sheng M, Tian WY. Research progress in pharmacological action of Tiepi Shihu. Henan Tradit Chi Med. 2020;40(05):788–792. doi: 10.16367/j.issn.1003-5028.2020.05.0199. [DOI] [Google Scholar]
- Xie GZ, Hui HY, Yu ZZ, Peng MJ, Tan ZJ. Effects of Dendrobium officinale polysaccharides on intestinal microecology in mice. Lishizhen Med Mater Medica Res. 2019;30(11):2603–2605. doi: 10.3969/j.issn.1008-0805.2019.11.015. [DOI] [Google Scholar]
- Yan MQ, Chen SH, Lv GY. Advances in pharmacological research on Dendrobii Caulis based on strengthening enterogastric function. Tradit Chin Herb Drugs. 2016;47(21):3918–3924. doi: 10.7501/j.issn.0253-2670.2016.21.028. [DOI] [Google Scholar]
- Yan MJ, Yang ZY, Shi QQ, Wang T, Chen SH, Lv GY. Research progress on protective effects and mechanism of Dendrobii Caulis on metabolic disturbances. Chin Tradit Herb Drugs. 2019;50(10):2491–2497. doi: 10.7501/j.issn.0253-2670.2019.10.033. [DOI] [Google Scholar]
- Yao Y, Zhao L, Li JK. Effects of aqueous extract of Dendrobium candidum on intestinal microorganism and lipid metabolism in DM mice. Inf Tradit Chin Med. 2019;36(02):44–49. doi: 10.19656/j.cnki.1002-2406.190043. [DOI] [Google Scholar]
- Yuan YQ, Liu QB, Zhao FQ, Cao J, Shen XR, Li C. Holothuria leucospilota polysaccharides ameliorate hyperlipidemia in high-fat diet-induced rats via short-chain fatty acids production and lipid metabolism regulation. Int J Mol Ences. 2019;20(19):4738. doi: 10.3390/ijms20194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue CH, Li M, Li J, Han X, Zhu HW, Yu GP, Cheng JJ. Medium-, long- and medium-chain-type structured lipids ameliorate high-fat diet-induced atherosclerosis by regulating inflammation, adipogenesis, and gut microbiota in ApoE-/- mice. Food Func. 2020;11(6):5142–5155. doi: 10.1039/d0fo01006e. [DOI] [PubMed] [Google Scholar]
- Zeng A, Zhang HL, Tan ZJ, Cai Y, Cai GX, Zhou SN. The construction of mice diarrhea model due todysbacteriosis and curative effect of ultra-micro Qiweibaizhusan. J Microbiol. 2012;39(09):1341–1348. doi: 10.13344/j.microbiol.china.2012.09.012. [DOI] [Google Scholar]
- Zhang Y. The gender differences in energy expenditure and substrate metabolism in college students during cycling. Chin J Sports Med. 2009;28(5):491–494. [Google Scholar]
- Zhang ZY, Yang CM, Lan Z, Huang Q, Liang LY. Study on the value of polysaccharide in Dendrobium in antibacterial action. Guide China Med. 2012;10(33):439–440. doi: 10.15912/j.cnki.gocm.2012.33.050. [DOI] [Google Scholar]
- Zhu XL, Zhang W, Gao Y, Zhang GQ. The preventive effect of the saponins from Asparagus officinalis L. by-products on experimental hyperlipidemiain mice. J Yunnan Univ (Nat Sci) 2018;40(05):1017–1023. doi: 10.7540/j.ynu.20170631. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study was approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine.