Abstract
Purpose of Review
Adoption of poor lifestyles (inactivity and energy-dense diets) has driven the worldwide increase in the metabolic syndrome, type 2 diabetes mellitus and non-alcoholic steatohepatitis (NASH). Of the defining features of the metabolic syndrome, an atherogenic dyslipidaemia characterised by elevated triglycerides (TG) and low plasma concentration of high-density lipoprotein cholesterol is a major driver of risk for atherosclerotic cardiovascular disease. Beyond lifestyle intervention and statins, targeting the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα) is a therapeutic option. However, current PPARα agonists (fibrates) have limitations, including safety issues and the lack of definitive evidence for cardiovascular benefit. Modulating the ligand structure to enhance binding at the PPARα receptor, with the aim of maximising beneficial effects and minimising adverse effects, underlies the SPPARMα concept.
Recent Findings
This review discusses the history of SPPARM development, latterly focusing on evidence for the first licensed SPPARMα, pemafibrate. Evidence from animal models of hypertriglyceridaemia or NASH, as well as clinical trials in patients with atherogenic dyslipidaemia, are overviewed.
Summary
The available data set the scene for therapeutic application of SPPARMα in the metabolic syndrome, and possibly, NASH. The outstanding question, which has so far eluded fibrates in the setting of current evidence-based therapy including statins, is whether treatment with pemafibrate significantly reduces cardiovascular events in patients with atherogenic dyslipidaemia. The PROMINENT study in patients with type 2 diabetes mellitus and this dyslipidaemia is critical to evaluating this.
Keywords: Pemafibrate, Selective peroxisome proliferator-activated receptor alpha modulator, SPPARM, Triglycerides, Metabolic syndrome, Non-alcoholic fatty liver disease
Introduction
The metabolic syndrome poses a global challenge as societies become increasingly urbanised, sedentary and obese. A key requirement for identification is the combination of three or more of the following: increased waist circumference, elevated triglycerides (TG), low plasma concentration of high-density lipoprotein cholesterol (HDL-C), elevated blood pressure and raised fasting blood glucose (Table 1) [1]. Worldwide, 20–30% of adults are affected, although this varies with age, ethnicity and gender [2]. The metabolic syndrome is no longer a disease of affluence, with escalating prevalence in emerging regions coincident with rising rates of obesity [3, 4], or a disease of adults, as globally more than 5% of children and adolescents are affected [4, 5]. The extensive comorbidity of the metabolic syndrome confers a substantial burden, from the increased risk for atherosclerotic cardiovascular disease (ASCVD), affecting multiple vascular territories [6], and type 2 diabetes mellitus [7, 8]. In fact, ASCVD and diabetes are the two leading causes of death.
Table 1.
Harmonised definition of the metabolic syndrome. Derived from Alberti et al [1]
| Waist >94 cm (men) or > 80 cm (women)* together with the presence of two or more of the following: | |
| Fasting blood glucose greater than 5.6 mmol/L (100 mg/dL) or diagnosed diabetes | |
| HDL cholesterol < 1.0 mmol/L (40 mg/dL) in men, < 1.3 mmol/L (50 mg/dL) in women or drug treatment for low HDL-C | |
| Fasting blood triglycerides > 1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides | |
| Blood pressure > 130/85 mmHg or drug treatment for hypertension |
*Based on the International Diabetes Federation thresholds for Europid population, with subsequent regional-specific definitions in men and women
Beyond the increased risk for ASCVD, metabolic derangements that characterise the metabolic syndrome predispose to the development of non-alcoholic fatty liver disease (NAFLD), the most common chronic liver disease worldwide. NAFLD encompasses the spectrum of disease ranging from asymptomatic fatty infiltration of hepatocytes in the absence of inflammation to progression to non-alcoholic steatohepatitis (NASH), liver fibrosis and liver failure. Among NAFLD patients, about half exhibit the metabolic syndrome with dyslipidaemia the most prevalent characteristic (in ~ 70% of patients) [9]. The pathogenesis of NAFLD is multifactorial, involving genetic, environmental and metabolic factors. Of the latter, TG accumulation in the liver, reflecting the imbalance between free fatty acid influx and efflux/catabolism, is a hallmark feature which also drives inflammation [10, 11]. Globally, it is estimated that NAFLD affects about one billion people, with an overall prevalence of ~ 25% in adults, with the highest rates in South America, the Middle East and Asia [9]. The clinical, economic and health-related quality-of-life burden of NAFLD is already substantial and growing [9].
Thus, given the changing landscape of cardiovascular risk associated with escalating obesity, the metabolic syndrome poses a global socioeconomic challenge. Renewed thinking about therapeutic options is imperative.
Pathogenesis
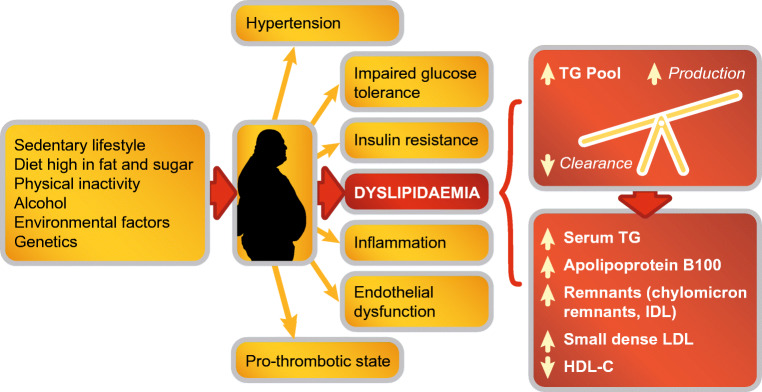
A key driver of the metabolic syndrome is visceral obesity, a marker of ectopic fat deposition [12•, 13•] (Fig. 1). Expansion of visceral adipose tissue to a greater extent than that of subcutaneous adipose tissue is associated with metabolic alterations which promote inflammation. Key amongst these metabolic derangements is atherogenic dyslipidaemia, in particular increases in TG-rich lipoproteins, their remnants and apolipoprotein (apo) C-III [14, 15]. These effects are mediated via crosstalk between a multitude of pathways that promote impaired adipogenesis, adipokine dysregulation and inflammation, and increase free fatty acids, oxidative stress, adipose tissue hypoxia and lipotoxicity (both local and systemic) [16••]. Recently, attention has focused on angiopoietin-like protein 2 (ANGPTL2), a glycoprotein which is expressed abundantly in adipose tissue. Under normal conditions, ANGPTL2-mediated expression contributes to angiogenesis and tissue damage repair, whereas overexpression promotes chronic inflammation [17–19]. In obese women with insulin resistance, ANGPTL2 production by adipocytes was shown to upregulate proinflammatory cytokine production in macrophages, in turn increasing adipose tissue inflammation, systemic insulin resistance and hyperinsulinaemia [20]. Thus, ANGPTL2 provides a link between the metabolic syndrome, NAFLD and ASCVD.
Fig. 1.
Dyslipidaemia is an important feature of the metabolic syndrome. Overproduction of large very low-density lipoprotein particles is a fundamental defect contributing to the increase in the triglyceride pool. This initiates a sequence of lipoprotein changes, leading to higher levels of remnant particles, an increase in small, dense low-density lipoprotein particles and lower plasma concentration of high-density lipoprotein cholesterol. IDL, intermediate-density lipoprotein; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein; TG, triglyceride
Hypertriglyceridaemia is a key component of the dyslipidaemia associated with the metabolic syndrome and NAFLD, its hepatic manifestation. Moderately elevated TG levels (a surrogate for elevated TG-rich lipoproteins and their remnants) result from increased dietary-derived apo B48-containing intestinal chylomicrons, overproduction of hepatic very low-density lipoproteins (VLDL) and reduction in catabolism of TG-rich lipoproteins. Evidence from epidemiologic, mechanistic and genetic studies supports a causal association between TG-rich lipoproteins and their TG-hydrolysed remnants and ASCVD [21•, 22]. Specifically, it is the cholesterol contained within these lipoproteins (i.e. remnant cholesterol) that promotes the development of atherosclerosis and ischaemic heart disease, in part mediated via low-grade inflammation [23, 24]. Postprandial hypertriglyceridaemia is also an emerging contributing factor in residual cardiovascular risk [25•]. Clinically, the combination of elevated TG and increased waist circumference (hypertriglyceridaemic waist) represents a marker of high-risk carotid atherosclerosis features, and highlighting this dual anomaly can improve the identification of individuals with metabolic syndrome and preclinical atherosclerosis beyond traditional risk factors [26•].
PPAR: a Key Therapeutic Target
Lifestyle intervention, encompassing both dietary changes and increased physical activity, is an important first step in the management of mild to moderate hypertriglyceridaemia, but long-term adherence is usually problematic [27]. Beyond lifestyle, agents that target the nuclear receptor peroxisome proliferator-activated receptor (PPAR) are obvious therapeutic candidates given their role in regulating the expression of key genes involved in adipogenesis, lipoprotein metabolism, inflammation and metabolic homeostasis [28••, 29, 30]. Three isoforms are identified to date, PPARα, PPARγ and PPARβ/δ, each encoded by separate genes [31]. PPARα is abundant in energy-demanding tissues, such as the liver, kidney, heart and skeletal muscle; PPARγ is predominantly found in adipose tissue, macrophages and the large intestine, whereas PPARβ/δ is more ubiquitous in distribution [32, 33]. These PPARs are controlled through their interaction with fatty acids and their derivatives and are the pharmacological targets for the lipid-lowering fibrates (PPARα) or the insulin sensitizer thiazolidinediones (PPARγ).
PPARα
Activation of PPARα by binding of endogenous ligands (e.g. fatty acids or eicosanoids), or drugs (fibrates) to the ligand binding domain and subsequent heterodimerisation with the ligand-activated retinoid X-receptor (RXR) trigger a conformational change which influences cofactor recruitment, either promoting (transactivation) or inhibiting (transrepression) expression of target genes. This process is mediated by the interaction between the activated PPARα, the PPAR response element (PPRE) of the target gene and relevant cofactors which render the complex transcriptionally active (or inactive in the case of transrepression) [32]. PPARα activation targets key genes involved in TG metabolism, specifically increasing the production of lipoprotein lipase and apo A-V and decreasing plasma levels of apo C-III; increasing HDL synthesis by targeting genes encoding apo A-I and A-II, scavenger receptor BI, and the ATP binding cassette transporters A1 and G1; and enhancing beta-oxidation by increasing expression of hepatic acyl CoA synthase [34–38]. The net effects are reduction in serum TG, an increase in HDL-C concentration, attenuation of very-low-density lipoprotein (VLDL) particles, as well as a shift in the low-density lipoprotein (LDL) profile to fewer small, dense LDL particles and a proportional increase in larger, less dense LDL particles. There is also transrepression of proinflammatory genes, leading to lower levels of inflammatory mediators such as C-reactive protein, interleukin-6 and prostaglandins [39]. Emerging evidence also suggests that PPARα favourably influences glucose homeostasis and insulin sensitivity, possibly mediated via effects on acetyl-CoA [40], and inhibits thrombogenesis and improves vascular function, although the underlying mechanisms are not fully defined.
Other PPAR Isoforms
PPARγ appears to be important in cell differentiation and energy metabolism, binding to the PPRE of almost all adipogenic genes, including those implicated in glucose and fatty acid metabolism. Although less well characterised, PPARβ/δ appears to regulate lipid metabolism, glucose homeostasis and inflammation, suggesting a role in the maintenance of energy homeostasis [29, 41].
Current Therapeutic Options
PPARα
Given their pharmacological profile, PPARα ligands (fibrates) are appropriate treatments for correcting atherogenic dyslipidaemia that is characteristic of the metabolic syndrome. Current fibrates are either specific for PPARα (fenofibrate and gemfibrozil) or activate all three PPAR subtypes (pan-agonist, bezafibrate). While the TG-lowering efficacy of fibrates is well established, their clinical benefit in terms of reduction in cardiovascular events is less convincing. Two prospective trials with gemfibrozil, the Helsinki Heart Study, a primary prevention trial in men with elevated non-HDL-C [42], and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), a secondary prevention trial in men with low HDL-C [43], showed significant reduction in cardiovascular events. It must be borne in mind, however, that both were essentially monotherapy lipid-lowering trials as these were conducted before widespread statin use. Of the remaining trials, two with fenofibrate, Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) [44] and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid trial [45], both in patients with type 2 diabetes, and one with bezafibrate (Bezafibrate Infarction Prevention [BIP] study) in patients with established coronary disease [46] were inconclusive. None was positive in terms of reduction in their primary endpoint for the total study population, and in the case of the FIELD study, was further complicated by discrepancies in the uptake of statin use between the two groups. The ACCORD Lipid study was the only trial conducted against background statin treatment; the lack of benefit may largely relate to inappropriate patient selection in terms of baseline TG levels and prevalence of atherogenic dyslipidaemia at inclusion [45].
There are, however, important insights from subgroup analyse of the fibrate trials. Analyses of the Helsinki Heart Study and the VA-HIT showed greater reduction in cardiovascular events in patients with the combination of both elevated TG and low HDL-C [47], or with insulin resistance [48], respectively. In the FIELD study, post hoc analysis showed that patients who satisfied the metabolic syndrome criteria (about 80% of the total study population), derived greater clinical benefit, with maximum reduction in cardiovascular events in those with the combination of elevated TG and low HDL-C [49]. Subgroup analysis of the ACCORD Lipid study also showed benefit in type 2 diabetes patients with this atherogenic dyslipidaemia [45]. When data from the major fibrate trials were combined, individuals with atherogenic dyslipidaemia gained significant clinical benefit for reduction in risk of cardiovascular events, whereas those without this lipid profile did not [50].
Longer-term follow-up of the BIP study showed that patients with the combination of three of the five criteria of the metabolic syndrome derived significant benefit in terms of reduction in myocardial infarction [51]; added to this, 22-year follow-up also showed that elevated TG was a significant predictor of all-cause mortality [52]. There is also evidence to suggest a legacy cardiovascular benefit from fenofibrate treatment in the ACCORDION study, an observational follow-up of the ACCORD Lipid study [53].
There are, however, well-recognised safety concerns with the current fibrates. A major issue is an elevation in serum creatinine with fenofibrate [54]; although this is reversible, there are practical disadvantages in stopping and restarting treatment, as well as limitations to its use in patients with renal dysfunction. The potential for drug-drug interactions is another issue, most notably the risk of myopathy with statin coadministration, clearly demonstrated with gemfibrozil [55, 56].
Other PPAR Agonists
PPARγ agonists are currently limited to pioglitazone, indicated as a glucose-lowering agent for the management of type 2 diabetes mellitus [57]. In addition to beneficial effects on atherogenic dyslipidaemia, pioglitazone has been shown to regress atherosclerosis and reduce cardiovascular events in this patient group [58–60]. The IRIS (Insulin Resistance Intervention after Stroke) trial demonstrated cardiovascular benefit in patients with insulin resistance but without diabetes [61], and significantly reduced the development of diabetes [62]. Safety issues in the trial included weight gain and increases in fracture risk and oedema [63].
Despite encouraging experimental findings, the clinical development of PPARβ/δ agonists in metabolic disorders has been disappointing. Seladelpar (MBX-8025) favourably impacted metabolic parameters, reducing apo B100, TG, non-HDL-C and C-reactive protein and increasing HDL-C, in a short-term trial in overweight men and women with mixed dyslipidaemia, with and without atorvastatin treatment [64]. There was, however, no benefit in NASH, leading to termination of clinical development of this agent [65].
Improved understanding of interactions at the PPAR receptor has invigorated the search for selective and potent peroxisome proliferator-activated modulators (SPPARMs), which aim to maximise beneficial effects and minimise the adverse effects of current PPAR agonists [28••].
SPPARMs for the Metabolic Syndrome?
The underlying aim of a SPPARM is to improve specificity and potency (i.e. efficacy) and minimise safety issues with established PPAR agonists, such as fibrates. This rationale borrows from that used in the development of selective oestrogen receptor modulators [66]. Understanding binding interactions at the PPAR has been key to the development of SPPARMs, as previously discussed [28••]. In brief, binding of the ligand (drug) at the receptor induces specific conformational changes and selective recruitment of coactivators which then selectively activate or repress key target genes, with downstream therapeutic effects. This is the paradigm on which the search for a SPPARM is based.
In the history of SPPARM development, some have shown dual activity, such as aleglitazar, a PPARα/γ agonist (terminated) and elafibranor (previously known as GFT505), a PPARα/δ agonist, which had been targeted to the management of NASH. The latest interim analysis of the RESOLVE-IT Phase 3 trial with elafibranor, however, failed to show a significant benefit for the predefined primary endpoint of NASH resolution without worsening of fibrosis, or secondary endpoints related to metabolic parameters versus placebo [67]. Most recently, lanifibranor, a pan-PPAR agonist, met primary and secondary endpoints in a phase 2b study in NASH [68, 69].
The very few pure SPPARMα agonists that have been developed to date include LY518674, GW7647 and most recently, pemafibrate (previously referred to as K-877), now licensed in Japan for the management of dyslipidaemia [70]. LY518674 potently upregulated apo A-I production and catabolism in human subjects with the metabolic syndrome [71] but was not superior to fenofibrate in lowering TG and raising HDL-C in patients with atherogenic dyslipidaemia. Additionally, elevation in serum creatinine (similar to that observed with fenofibrate) was also reported with this agent [72].
Pemafibrate is the culmination of the systematic synthesis of over 1500 compounds which were rigorously screened for SPPARMα activity. As for traditional PPARα agonists, the pemafibrate molecule has an acidic region, but the addition of unique benzoxazole and phenoxyalkyl sidechains gives it a Y-shaped structure. As a result, pemafibrate has an enhanced fit within the ligand-binding domain of the PPARα (Fig. 2) [73, 74, 75•]. This structural differentiation confers an increase in PPARα activation potency compared with other fibrates and a high degree of PPARα subtype selectivity [28, 76]. Transcriptome analysis showed that gene expression profiles also differed between these two agents, particularly in terms of magnitude of effect. For example, pemafibrate induced key target genes such as VLDLR and ABCA1 at a concentration 10-fold lower than fenofibrate [77].
Fig. 2.
Pemafibrate is the realisation of the SPPARMα concept. Understanding binding interactions at the PPAR have been critical in driving the development of SPPARMα. Systematic structural modifications based on a precision medicine approach led to the creation of pemafibrate. This agent demonstrated an enhanced fit completely within the ligand binding domain of PPARα, in contrast to the linear structure of conventional fibrates such as fenofibrate. PPARα, peroxisome proliferator-activated receptor alpha
In preclinical studies, there was more robust reduction in TG and elevation in HDL-C with pemafibrate compared with fenofibrate, as well as enhanced cholesterol efflux from macrophages, upregulation of fibroblast growth factor 21 (FGF-21), reduced inflammation and attenuation of atherosclerosis [78]. Additionally, pemafibrate attenuated postprandial hypertriglyceridaemia in a mouse model [79], by suppressing the postprandial increase in chylomicrons and the accumulation of chylomicron remnants. This response was achieved with a pemafibrate dose 100-fold lower than with fenofibrate [79], implying that pemafibrate is more efficient in decreasing TG and apo B48-containing chylomicron remnants, which are highly atherogenic lipoproteins, more so than those containing apoB100 [80, 81].
Insights from rodent models of NASH have suggested that pemafibrate may have potential in NASH. In a diet-induced amylin NASH mouse model, pemafibrate improved dyslipidaemia, liver dysfunction and NASH features. These effects were attributed in part to stimulation of lipid turnover [82•]. A subsequent study using a STAM NASH mouse model which demonstrates NASH progression resembling the clinical disease showed that pemafibrate improved the histological severity of NASH, as well as inflammatory and fibrosis marker gene expression, without influencing hepatic TG content [83•]. The findings from this study are therefore consistent with current thinking that combination therapy targeting multiple components is needed to manage NASH [84].
Pemafibrate in Metabolic Syndrome: Clinical Profiling
Preclinical data support the SPPARMα concept and suggest potential therapeutic application in managing dyslipidaemia associated with the metabolic syndrome. Additionally, because pemafibrate is metabolised in the liver and excreted into the bile [85], it can be used safely in patients with renal impairment, as borne out in clinical trials [86, 87•].
An early study in Japanese subjects with atherogenic dyslipidaemia (TG > 200 mg/dL and low HDL-C, < 50 mg/dL in men and < 55 mg/dL in women) showed robust TG-lowering (by ~ 45%) with pemafibrate (0.2 or 0.4 mg daily) that was superior to fenofibrate 100 mg daily [88]. Pemafibrate treatment was also effective against other components of the dyslipidaemia associated with the metabolic syndrome, including lowering VLDL-C (43 to 48%), remnant cholesterol (48 to 50%) and apo B and C-III, raising HDL-C (21 to 14%) and promoting a shift to a more favourable lipoprotein profile, with fewer small and very small LDL. Importantly, pemafibrate treatment was well tolerated with no increase in serum creatinine and decreased liver enzymes [88]. Pemafibrate also attenuated postprandial hyperlipidaemia [89], consistent with preclinical findings [90] and reduced inflammatory markers such as serum amyloid A and high-sensitivity C-reactive protein [89].
Furthermore, pemafibrate (0.4 mg daily for 24 weeks) was similarly effective in Japanese patients with type 2 diabetes mellitus and hypertriglyceridaemia (≥ 150 mg/dL or 1.7 mmol/L), as assessed by reduction in TG and other markers of TG-rich lipoproteins. Pemafibrate treatment also lowered fasting glucose, insulin and homeostasis model assessment of insulin resistance (HOMA-IR) levels [91]. In a hyperinsulinaemic-euglycemic clamp study in subjects with hypertriglyceridaemia and insulin resistance, pemafibrate treatment improved hepatic glucose uptake and insulin sensitivity [92]. This effect may be attributed to the stimulation of fatty acid beta-oxidation and amelioration of liver dysfunction [92] and/or mediated by the effect of increases in FGF21, as shown by this and other studies [91, 92], on insulin-dependent hepatic glucose disposal [93]. These findings imply benefit with pemafibrate beyond lipid-lowering, consistent with the pharmacology of PPARα activation.
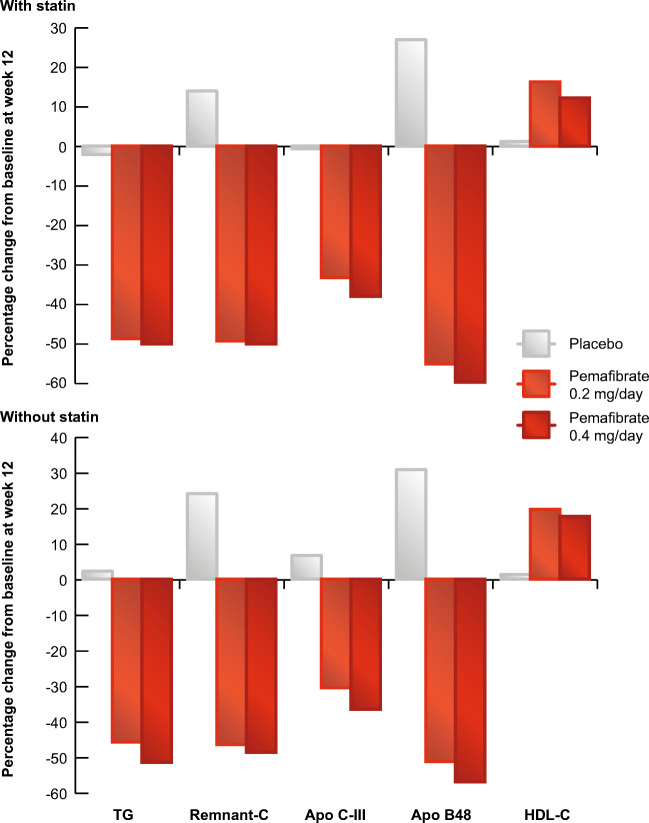
Pooled clinical trial data confirmed the favourable benefit-risk profile for pemafibrate. In one analysis including 1253 patients (677 also treated with a statin) with atherogenic dyslipidaemia in six phase II-III clinical trials [94•], pemafibrate 0.4 mg daily lowered TG by ~ 50%, irrespective of statin treatment, with almost all (98.6% on statin and 97.7% on pemafibrate monotherapy) patients showing an appropriate response. Efficacy against other components of this dyslipidaemia was robust, notably lowering remnant cholesterol by ~ 50% (Fig. 3). The safety of pemafibrate was also reassuring. Regardless of statin use, pemafibrate was well tolerated, with a favourable renal and hepatic safety profile, even among patients with mild to moderate renal impairment [86]. There was no evidence of interaction with concomitant statin therapy [94•].
Fig. 3.
Pemafibrate favourably impacts the atherogenic dyslipidaemia of the metabolic syndrome. Pooled analysis of more than 1200 patients with atherogenic dyslipidaemia showed that pemafibrate treatment for 12 weeks lowered triglycerides (TG) and associated atherogenic lipoproteins and raised HDL-C, irrespective of statin therapy. Data from Yamashita et al [94•]. apo, apolipoprotein; C, cholesterol; HDL-C, high-density lipoprotein cholesterol
Finally, the PROVIDE study provided insights regarding the long-term efficacy and safety of pemafibrate (0.2 or 0.4 mg daily for 52 weeks) in patients with type 2 diabetes mellitus and elevated TG [95•]. Robust lowering of TG and remnant cholesterol with pemafibrate (~ 50%) was sustained over this period, together with improvement in fasting insulin and HOMA-IR. Additionally, pemafibrate treatment improved liver function tests (such as alanine aminotransferase and gamma-glutamyl transferase), and was not associated with clinically meaningful increases in creatine kinase or serum creatinine, supporting the favourable safety profile of this SPPARMα.
Conclusions
SPPARMα poses an attractive approach to managing atherogenic dyslipidaemia associated with the metabolic syndrome. Despite early disappointment with the first compounds tested, the latest candidate, pemafibrate, has shown a promising benefit-risk profile in patients with atherogenic dyslipidaemia or hypertriglyceridaemia, key features of the metabolic syndrome. In addition to robust lowering of TG and remnant lipoproteins and elevation in HDL-C, pemafibrate treatment improved insulin sensitivity and reduced inflammation. Importantly, pemafibrate was well tolerated, with no evidence of clinically meaningful elevation in serum creatinine, a concern with conventional fibrate therapy.
The outstanding question, which has so far eluded fibrates in the setting of current evidence-based treatment including statins, is whether treatment with pemafibrate significantly reduces cardiovascular events in patients with atherogenic dyslipidaemia. This is being tested in the PROMINENT study (Pemafibrate to Reduce cardiovascular OutcoMes by reducing triglycerides IN diabetic patiENTs), a cardiovascular outcomes study in 10,000 patients with type 2 diabetes mellitus and atherogenic dyslipidaemia (TG ≥ 2·3 mmol/L and < 5·6 mmol/L, and low HDL-C), in both primary and secondary prevention settings [96••]. Results are eagerly anticipated in the next 2–3 years.
Finally, despite the ongoing obesity pandemic, there are still no approved treatments for NAFLD, the hepatic manifestation of the metabolic syndrome. As the SPPARMα pemafibrate favourably impacts lipoprotein metabolism and inflammation, it may offer therapeutic potential. Promising results in experimental NASH models and evidence of benefit in lowering liver enzymes in clinical trials are encouraging [97]. On this basis, pemafibrate is being tested in an ongoing trial in NAFLD (ClinicalTrials.gov identifier NCT03350165) [98]. It should, however, be borne in mind that the multifactorial pathogenesis of NAFLD and lack of robust surrogate trial endpoints have presented obstacles to drug development in this area.
The coming 2–3 years are critical in defining whether SPPARMα will offer new approaches to managing the metabolic syndrome and, as a consequence, reducing the associated morbidity, mortality and disability of ASCVD.
Authors’ Contributions
All authors contributed to the writing and review of this manuscript.
Funding
This manuscript was prepared independently of funding.
Data Availability
Data used during the current review are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Conflict of Interest
J-C Fruchart and J Fruchart-Najib report personal fees from Kowa Company; MP Hermans reports no conflict of interest; T Kodama is the recipient of a research grant from Kowa Company.
Ethics Approval
Not applicable.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects discussed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards and international/national/institutional guidelines).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
This article is part of the Topical Collection on Statin Drugs
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jean-Charles Fruchart, Email: Jean-Charles.fruchart@r3i.org.
Michel P. Hermans, Email: michel.hermans@uclouvain.be
Jamila Fruchart-Najib, Email: jamila.fruchart@yahoo.fr.
Tatsuhiko Kodama, Email: kodama@lsbm.org.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lu,ng, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 3.Azizi F, Hadaegh F, Hosseinpanah F, Mirmiran P, Amouzegar A, Abdi H, Asghari G, Parizadeh D, Montazeri SA, Lotfaliany M, Takyar F, Khalili D. Metabolic health in the Middle East and north Africa. Lancet Diabetes Endocrinol. 2019;7:866–879. doi: 10.1016/S2213-8587(19)30179-2. [DOI] [PubMed] [Google Scholar]
- 4.The GBD 2015 Obesity collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, Dalleck LC. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: a pooled analysis. Prev Med. 2017;7:211–215. doi: 10.1016/j.pmedr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Evans MA, Allison MA, Bertoni AG, Budoff MJ, Criqui MH, Malik S, Ouyang P, Polak JF, Wong ND. Multisite atherosclerosis in subjects with metabolic syndrome and diabetes and relation to cardiovascular events: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2019;282:202–209. doi: 10.1016/j.atherosclerosis.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 8.Malik S, Wong ND, Franklin SS, Kamath TV, Gilbert JL, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 10.Marchisello S, Di Pino A, Scicali R, Urbano F, Piro S, Purrello F, Rabuazzo AM. Pathophysiological, molecular and therapeutic issues of nonalcoholic fatty liver disease: an overview. Int J Mol Sci. 2019;20:1948. doi: 10.3390/ijms20081948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caligiuri A, Gentilini A, Marra F. Molecular pathogenesis of NASH. Int J Mol Sci. 2016;17:1575. doi: 10.3390/ijms17091575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.•.Piché EM, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126:1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 13.•.Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137:1391–1406. doi: 10.1161/CIRCULATIONAHA.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Larochelliere E, Cote J, Gilbert G, Bibeau K, Ross MK, Dion-Roy V, et al. Visceral/epicardial adiposity in nonobese and apparently healthy young adults: association with the cardiometabolic profile. Atherosclerosis. 2014;234:23–29. doi: 10.1016/j.atherosclerosis.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Mortera R, Caccavello R, Garay-Sevilla ME, Gugliucci A. Higher ANGPTL3, apoC-III, and apoB48 dyslipidemia, and lower lipoprotein lipase concentrations are associated with dysfunctional visceral fat in adolescents with obesity. Clin Chim Acta. 2020;508:61–68. doi: 10.1016/j.cca.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 16.••.Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 17.Thorin-Trescases N, Thorin E. Angiopoietin-like-2: a multifaceted protein with physiological and pathophysiological properties. Expert Rev Mol Med. 2014;16:e17. doi: 10.1017/erm.2014.19. [DOI] [PubMed] [Google Scholar]
- 18.Thorin-Trescases N, Thorin E. High circulating levels of ANGPTL2: beyond a clinical marker of systemic inflammation. Oxidative Med Cell Longev 2017;2017:1096385. [DOI] [PMC free article] [PubMed]
- 19.Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M, Tazume H, Tian Z, Takahashi O, Terada K, Takeya M, Hao H, Hirose N, Minami T, Suda T, Kiyohara Y, Ogawa H, Kaikita K, Oike Y. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2014;34:790–800. doi: 10.1161/ATVBAHA.113.303116. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Lee SK, Jang YJ, Park HS, Kim JY, Hong JP, et al. Enhanced ANGPTL2 expression in adipose tissues and its association with insulin resistance in obese women. Sci Rep. 2018;8:13976. doi: 10.1038/s41598-018-32419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.•.Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40:537–557. doi: 10.1210/er.2018-00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 23.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 24.Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 25.•.Kolovou GD, Watts GF, Mikhailidis DP, Pérez-Martínez P, Mora S, Bilianou H, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 Expert Panel Statement. Curr Vasc Pharmacol. 2019;17:498–514. doi: 10.2174/1570161117666190507110519. [DOI] [PubMed] [Google Scholar]
- 26.•.LeBlanc S, Coulombe F, Bertrand OF. Bibeau K, Pibarot P, Marette A, et al. Hypertriglyceridemic waist: a simple marker of high-risk atherosclerosis features associated with excess visceral adiposity/ectopic fat. J Am Heart Assoc. 2018;7:e008139. doi: 10.1161/JAHA.117.008139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrne A, Makadia S, Sutherland A, Miller M. Optimizing non-pharmacologic management of hypertriglyceridemia. Arch Med Res. 2017;48:483–487. doi: 10.1016/j.arcmed.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.••.Fruchart JC, Santos RD, Aguilar-Salinas C, Aikawa M, Al Rasadi K, Amarenco P, et al. The selective peroxisome proliferator-activated receptor alpha modulator (SPPARMα) paradigm: conceptual framework and therapeutic potential : a consensus statement from the International Atherosclerosis Society (IAS) and the Residual risk Reduction Initiative (R3i) Foundation. Cardiovasc Diabetol. 2019;18:71. doi: 10.1186/s12933-019-0864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botta M, Audano M, Sahebkar A, Sirtori CR, Mitro N, Ruscica M. PPAR agonists and metabolic syndrome: an established role? Int J Mol Sci. 2018;19:1197. doi: 10.3390/ijms19041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Investig. 2017;127:1202–1214. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 32.Fruchart JC. Selective peroxisome proliferator-activated receptor α modulators (SPPARMα): the next generation of peroxisome proliferator-activated receptor α-agonists. Cardiovasc Diabetol. 2013;12:82. doi: 10.1186/1475-2840-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, −beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 34.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 35.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prieur X, Coste H, Rodriguez JC. The human apolipoprotein AV gene is regulated by peroxisome proliferator-activated receptoralpha and contains a novel farnesoid X-activated receptor response element. J Biol Chem. 2003;278:25468–25480. doi: 10.1074/jbc.M301302200. [DOI] [PubMed] [Google Scholar]
- 37.Staels B, Vu-Dac N, Kosykh VA, Saladin R, Fruchart JC, Dallongeville J, Auwerx J. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995;95:705–712. doi: 10.1172/JCI117717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, de Bosscher K. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr Rev. 2018;39:760–802. doi: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- 39.Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr Opin Cell Biol. 2015;33:125–131. doi: 10.1016/j.ceb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derosa G, Sahebkar A, Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J Cell Physiol. 2018;233:153–161. doi: 10.1002/jcp.25804. [DOI] [PubMed] [Google Scholar]
- 42.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, Mäenpää H, Mälkönen M, Mänttäri M, Norola S, Pasternack A, Pikkarainen J, Romo M, Sjöblom T, Nikkilä EA. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middleaged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 43.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 44.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M, FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 45.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, Leiter LA, Linz P, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bezafibrate Infarction Prevention (BIP) study Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 47.Tenkanen L, Mänttäri M, Manninen V. Some coronary risk factors related to the insulin resistance syndrome and treatment with gemfibrozil. Experience from the Helsinki Heart Study. Circulation. 1995;92:1779–1785. doi: 10.1161/01.cir.92.7.1779. [DOI] [PubMed] [Google Scholar]
- 48.Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VAHIT) Arch Intern Med. 2002;162:2597–2604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 49.Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363:692–694. doi: 10.1056/NEJMc1006407. [DOI] [PubMed] [Google Scholar]
- 51.Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165:1154–1160. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 52.Klempfner R, Erez A, Sagit B-Z, Goldenberg I, Fisman E, Kopel E, Shlomo N, Israel A, Tenenbaum A. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100–108. doi: 10.1161/CIRCOUTCOMES.115.002104. [DOI] [PubMed] [Google Scholar]
- 53.Elam MB, Ginsberg HN, Lovato LC, Corson M, Largay J, Leiter LA, Lopez C, O’Connor PJ, Sweeney ME, Weiss D, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm R, Ismail-Beigi F, Goff DC, Jr, Fleg JL, Rosenberg Y, Byington RP, for the ACCORDION Study Investigators ACCORDION study investigators: association of fenofibrate therapy with long-term cardiovascular risk in statintreated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370–380. doi: 10.1001/jamacardio.2016.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011;54:280–290. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 55.Corsini A, Bellosta S, Davidson MH. Pharmacokinetic interactions between statins and fibrates. Am J Cardiol. 2005;96:44K–49K. doi: 10.1016/j.amjcard.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Bortolini M, Wright MB, Bopst M, Balas B. Examining the safety of PPAR agonists—current trends and future prospects. Expert Opin Drug Saf. 2013;12:65–79. doi: 10.1517/14740338.2013.741585. [DOI] [PubMed] [Google Scholar]
- 57.Ahsan W. The journey of thiazolidinediones as modulators of PPARs for the management of diabetes: a current perspective. Curr Pharm Des. 2019;25:2540–2554. doi: 10.2174/1381612825666190716094852. [DOI] [PubMed] [Google Scholar]
- 58.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 59.Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, de Larochellière R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM, PERISCOPE Investigators Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 60.Nicholls SJ, Tuzcu EM, Wolski K, Bayturan O, Lavoie A, Uno K, Kupfer S, Perez A, Nesto R, Nissen SE. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J Am Coll Cardiol. 2011;57:153–159. doi: 10.1016/j.jacc.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 61.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O'Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR, IRIS Trial Investigators Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy AM, Dagogo-Jack S, Ismail-Beigi F, Korytkowski MT, Pratley RE, Schwartz GG, Kernan WN, IRIS Trial Investigators Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care. 2016;39:1684–1692. doi: 10.2337/dc16-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, Gorman M, Kelly MA, Lovejoy AM, Kernan WN, IRIS Trial Investigators Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab. 2017;102:914–922. doi: 10.1210/jc.2016-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bays HE, Schwartz S, Littlejohn T, Kerzner B, Krauss RM, Karpf DB, et al. MBX-8025, A novel peroxisome proliferator receptor-δ agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. J Clin Endocrinol Metab. 2011;96:2889–2897. doi: 10.1210/jc.2011-1061. [DOI] [PubMed] [Google Scholar]
- 65.CymaBay Therapeutics. Press release CymaBay Therapeutics Halts Clinical Development of Seladelpar. November 25, 2019. https://www.globenewswire.com/news-release/2019/11/25/1951942/0/en/CymaBay-Therapeutics-Halts-Clinical-Development-of-Seladelpar.html (Accessed 15 July 2020).
- 66.Lewis JS, Jordan VC. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat Res. 2005;591:247–263. doi: 10.1016/j.mrfmmm.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 67.Genfit. Press release. GENFIT: announces results from interim analysis of RESOLVE-IT Phase 3 trial of elafibranor in adults with NASH and fibrosis. May 11, 2020. https://ir.genfit.com/news-releases/news-release-details/genfit-announces-results-interim-analysis-resolve-it-phase-3 (Accessed 15 July 2020).
- 68.Lanifibranor in patients with type 2 diabetes & nonalcoholic fatty liver disease. ClinicalTrials.gov Identifier: NCT03459079 https://clinicaltrials.gov/ct2/show/NCT03459079 (Accessed 15 July 2020).
- 69.Inventiva press release. Inventiva’s lanifibranor meets the primary and key secondary endpoints in the Phase IIb NATIVE clinical trial in non-alcoholic steatohepatitis (NASH). 15 June 2020. https://inventivapharma.com/inventivas-lanifibranor-meets-the-primary-and-key-secondary-endpoints-in-the-phase-iib-native-clinical-trial-in-non-alcoholic-steatohepatitis-nash/ (Accessed 15 July 2020).
- 70.Blair HA. Pemafibrate: first global approval. Drugs. 2017;77:1805–1810. doi: 10.1007/s40265-017-0818-x. [DOI] [PubMed] [Google Scholar]
- 71.Millar JS, Duffy D, Gadi R, Bloedon LT, Dunbar RL, Wolfe ML, et al. Potent and selective PPAR-alpha agonist LY518674 upregulates both ApoA-I production and catabolism in human subjects with the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2009;29:140–146. doi: 10.1161/ATVBAHA.108.171223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nissen SE, Nicholls SJ, Wolski K, Howey DC, McErlean E, Wang MD, Gomez EV, Russo JM. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 2007;297:1362–1373. doi: 10.1001/jama.297.12.1362. [DOI] [PubMed] [Google Scholar]
- 73.Yamazaki Y, Abe K, Toma T, Nishikawa M, Ozawa H, Okuda A, Araki T, Oda S, Inoue K, Shibuya K, Staels B, Fruchart JC. Design and synthesis of highly potent and selective human peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett. 2007;17:4689–4693. doi: 10.1016/j.bmcl.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto Y, Takei K, Arulmozhiraja S, Sladek V, Matsuo N, Han SI, Matsuzaka T, Sekiya M, Tokiwa T, Shoji M, Shigeta Y, Nakagawa Y, Tokiwa H, Shimano H. Molecular association model of PPARα and its new specific and efficient ligand, pemafibrate: structural basis for SPPARMα. Biochem Biophys Res Commun. 2018;499:239–245. doi: 10.1016/j.bbrc.2018.03.135. [DOI] [PubMed] [Google Scholar]
- 75.•.Kawasaki M, Kambe A, Yamamoto Y, Arulmozhiraja S, Ito S, Nakagawa Y, et al. Elucidation of molecular mechanism of a selective PPARα modulator, pemafibrate, through combinational approaches of X-ray crystallography, thermodynamic analysis, and first-principle calculations. Int J Mol Sci. 2020. 10.3390/ijms21010361Critical study that elucidated the molecular mechanism of pemafibrate. [DOI] [PMC free article] [PubMed]
- 76.Fruchart JC. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol. 2017;16:124. doi: 10.1186/s12933-017-0602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raza-Iqbal S, Tanaka T, Anai M, Inagaki T, Matsumura Y, Ikeda K, Taguchi A, Gonzalez FJ, Sakai J, Kodama T. Transcriptome analysis of K-877 (a novel selective PPARalpha modulator (SPPARMalpha))-regulated genes in primary human hepatocytes and the mouse liver. J Atheroscler Thromb. 2015;22:754–772. doi: 10.5551/jat.28720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hennuyer N, Duplan I, Paquet C, Vanhoutte J, Woitrain E, Touche V, Colin S, Vallez E, Lestavel S, Lefebvre P, Staels B. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis. 2016;249:200–208. doi: 10.1016/j.atherosclerosis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Sairyo M, Kobayashi T, Masuda D, Kanno K, Zhu Y, Okada T, Koseki M, Ohama T, Nishida M, Sakata Y, Yamashita S. A novel selective PPAR modulator (SPPARM), K-877 (pemafibrate), attenuates postprandial hypertriglyceridemia in mice. J Atheroscler Thromb. 2018;25:142–152. doi: 10.5551/jat.39693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sandoval JC, Nakagawa-Toyama Y, Masuda D, Tochino Y, Nakaoka H, Kawase R, et al. Fenofibrate reduces postprandial hypertriglyceridemia in CD36 knockout mice. J Atheroscler Thromb. 2010;7:610–618. doi: 10.5551/jat.3988. [DOI] [PubMed] [Google Scholar]
- 81.Proctor SD. Intimal retention of cholesterol derived from apolipoprotein B100- and apolipoprotein B48-containing lipoproteins in carotid arteries of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2003;23:1595–1600. doi: 10.1161/01.ATV.0000084638.14534.0A. [DOI] [PubMed] [Google Scholar]
- 82.•.Honda Y, Kessoku T, Ogawa Y, Tomeno W, Imajo K, Fujita K, et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep. 2017;7:42477. doi: 10.1038/srep42477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.•.Sasaki Y, Asahiyama M, Tanaka T, Yamamoto S, Murakami K, Kamiya W, et al. Pemafibrate, a selective PPARα modulator, prevents non-alcoholic steatohepatitis development without reducing the hepatic triglyceride content. Sci Rep. 2020;10:7818. doi: 10.1038/s41598-020-64902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alkhouri N, Lawitz E, Noureddin M. Looking into the crystal ball: predicting the future challenges of fibrotic NASH treatment. Hepatol Commun. 2019;3:605–613. doi: 10.1002/hep4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hounslow N, Mair S, Suganami H, Nakamura M. Pemafibrate has high bioavailability and is principally excreted via the liver. Atheroscler Suppl. 2018;32:157. [Google Scholar]
- 86.Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S, Of The K-Study Group OB. Long-term efficacy and safety of pemafibrate, a novel Selective Peroxisome Proliferator-Activated Receptor-α Modulator (SPPARMα), in dyslipidemic patients with renal impairment. Int J Mol Sci. 2019;20(3):706. [DOI] [PMC free article] [PubMed]
- 87.•.Fruchart JC, Hermans MP, Fruchart-Najib J. Selective peroxisome proliferator-activated receptor alpha modulators (SPPARMα): new opportunities to reduce residual cardiovascular risk in chronic kidney disease? Curr Atheroscler Rep. 2020;22:43. doi: 10.1007/s11883-020-00860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishibashi S, Yamashita S, Arai H, Araki E, Yokote K, Suganami H, et al. Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: a randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis. 2016;249:36–43. doi: 10.1016/j.atherosclerosis.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 89.Yamashita S, Arai H, Yokote K, Araki E, Suganami H, Ishibashi S, K-877 Study Group Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J Clin Lipidol. 2018;12(5):1267–1279. doi: 10.1016/j.jacl.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 90.Sairyo M, Kobayashi T, Masuda D, Kanno K, Zhu Y, Okada T, Koseki M, Ohama T, Nishida M, Sakata Y, Yamashita S. A novel selective PPARα modulator (SPPARMα), K-877 (pemafibrate), attenuates postprandial hypertriglyceridemia in mice. J Atheroscler Thromb. 2018;25:1086. doi: 10.5551/jat.ER39693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, Nakamura J, Maegawa H, Yoshioka N, Tanizawa Y, Watada H, Suganami H, Ishibashi S. Effects of pemafibrate, a novel selective PPARα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2018;41:538–546. doi: 10.2337/dc17-1589. [DOI] [PubMed] [Google Scholar]
- 92.Matsuba I, Matsuba R, Ishibashi S, Yamashita S, Arai H, Yokote K, Suganami H, Araki E. Effects of a novel selective peroxisome proliferator-activated receptor-α modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J Diabetes Investig. 2018;9:1323–1332. doi: 10.1111/jdi.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.•.Yamashita S, Arai H, Yokote K, Araki E, Matsushita M, Nojima T, et al. Efficacy and safety of pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα): pooled analysis of phase 2 and 3 studies in dyslipidemic patients with or without statin combination. Int J Mol Sci. 2019;20:5537. doi: 10.3390/ijms20225537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.•.Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, et al. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52-week data from the PROVIDE study. Diabetes Obes Metab. 2019;21:1737–1744. doi: 10.1111/dom.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.••.Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, et al. Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J. 2018;206:80–93. doi: 10.1016/j.ahj.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 97.Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S. A pooled analysis of pemafibrate phase II/III clinical trials indicated significant improvement in glycemic and liver function related parameters. Atheroscler Suppl. 2018;32:154–155. [Google Scholar]
- 98.A study of pemafibrate in patients with nonalcoholic fatty liver disease (NAFLD). ClinicalTrials.gov Identifier: NCT03350165. https://clinicaltrials.gov/ct2/show/NCT03350165 (Accessed 15 July 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used during the current review are available from the corresponding author on reasonable request.