Abstract
Spending time in nature is known to benefit human health and well-being, but evidence is mixed as to whether biodiversity or perceptions of biodiversity contribute to these benefits. Perhaps more importantly, little is known about the sensory modalities by which humans perceive biodiversity and obtain benefits from their interactions with nature. Here, we used a ‘phantom birdsong chorus' consisting of hidden speakers to experimentally increase audible birdsong biodiversity during ‘on' and ‘off' (i.e. ambient conditions) blocks on two trails to study the role of audition in biodiversity perception and self-reported well-being among hikers. Hikers exposed to the phantom chorus reported higher levels of restorative effects compared to those that experienced ambient conditions on both trails; however, increased restorative effects were directly linked to the phantom chorus on one trail and indirectly linked to the phantom chorus on the other trail through perceptions of avian biodiversity. Our findings add to a growing body of evidence linking mental health to nature experiences and suggest that audition is an important modality by which natural environments confer restorative effects. Finally, our results suggest that maintaining or improving natural soundscapes within protected areas may be an important component to maximizing human experiences.
Keywords: biodiversity, recreation, soundscapes, healthy parks healthy people, social-ecological systems
1. Background
Humans in developed countries spend much of their time indoors and in urban landscapes that bear little resemblance to the environment in which our species evolved. For example, a large survey based in the USA suggested that a typical citizen spends 87% of their time indoors and an additional 6% of their time in vehicles [1]. Living almost entirely apart from nature can lead to an overall disconnection from nature that has negative consequences for environmental conservation [2–7] and can deprive individuals of the health and well-being benefits that nature provides [8].
Nature provides a variety of beneficial ecosystem services that contribute to human well-being, including psychological [9,10], cognitive [11,12], physical health [13,14] and social health benefits [15,16]. Although many studies have identified various benefits humans obtain from interacting with nature, few studies have explored why humans benefit from nature. For example, Fuller et al. [9] found a positive relationship between greenspace biodiversity (measured by the species richness of plants, butterflies and birds) and self-reported well-being among greenspace visitors. Dallimer et al. [17], using similar methods to Fuller et al. [9] but with an expanded survey, determined that greenspace visitors' self-reported well-being was more strongly associated with their perceptions of biodiversity (i.e. level of biodiversity a visitor thought was present) than actual levels of biodiversity. Instead, tree cover, rather than true biodiversity levels, was more strongly linked with visitors' biodiversity estimates [17]. Another study conducted in public gardens found that regular visitors were unable to detect experimentally increased levels of plant, bird and butterfly biodiversity, yet nevertheless indicated a preference for higher species richness in the gardens [18]. Although these limited studies show mixed outcomes about the role of actual versus perceived biodiversity, it is clear that biodiversity can play a role in human well-being.
In addition to the sights and smells of nature, natural sounds are a key factor of human experiences in nature [19–21] and may contribute to perceptions of biodiversity. Natural sounds enhance the quality of nature-based experiences [22] by adding to overall satisfaction [19,21,22], enhancing perceptions of natural landscapes [23] and improving mood [24]. In particular, birdsong is regarded by most people as enjoyable [20,21,25–27], perhaps owing to its ubiquity throughout human evolution [28,29] or its association with forthcoming or current pleasant weather (i.e. spring and summer, respectively). Hedblom et al. [30] also found that more diverse birdsong was appreciated more than less diverse birdsong, and enhanced participants' perceptions of photos of urban landscapes. Additionally, surveys conducted in unnatural settings and laboratory-based studies suggest birdsong can improve stress recovery [31,32] and cognitive function [33]. However, whether birdsong influences perceptions of biodiversity and well-being among people in real natural settings has not yet been explored.
Here, we investigated how an experimental increase in birdsong influenced self-reported perceptions of biodiversity and concepts of well-being among natural area visitors. To do so, we implemented a ‘phantom chorus' in week-long ‘on' and ‘off' (i.e. ambient conditions) blocks by experimentally increasing bird acoustic biodiversity on hiking trails via playback through speakers. We used intercept surveys at the end of our experimental trail sections to record self-reported well-being by hikers. Based on previous research reporting significant and marginally non-significant positive correlations between actual and perceived plant and bird biodiversity, respectively [9], we predicted that an experimental increase in birdsong would lead to an increase in perceived bird biodiversity by hikers. Other studies conducted in non-natural settings have linked birdsong to psychological benefits [31–33]. If hikers in nature exposed to acoustic stimuli reflective of high bird diversity also experience greater well-being, hikers exposed to the phantom chorus would self-report higher scores for well-being concepts than those that did not experience the phantom chorus. Finally, based on the notion that perceptions of biodiversity are linked to well-being [17], we also expected a positive association between people's self-reported perceptions of bird biodiversity and concepts of well-being.
2. Methods
Our study was conducted with the approval of the California Polytechnic State University Institutional Review Board (protocol number 2017-112). Data collection occurred from 15 July to 4 September 2017 in Boulder Open Space and Mountain Parks (OSMP), Colorado, USA. These dates were selected for several reasons. First, breeding activity among birds nesting in the ponderosa pine (Pinus ponderosa) woodlands of OSMP typically peaks in June (e.g. [34–36]). By timing our playback of the phantom chorus later in the season, we not only minimized potential disruption of breeding birds' behaviours caused by playback (e.g. [37]), but created the necessary contrast in birdsong for our study objectives. Playback of the phantom chorus simulated song activity typical of the early/mid breeding season when song is common for mate attraction and territorial defense, which contrasted strongly with the low song activity associated with the late nesting or fledgling stages of the breeding season [38,39] in mid-July and later in the summer. We applied the phantom chorus treatment (see below) in weekly intervals at each trail, alternating with a ‘quiet' week of no broadcast, allowing each trail to serve as its own control and ensuring surveys of visitors exposed to the playback of the phantom chorus or ambient conditions were not related with unusual weather events, such as high temperatures or rain that could also influence survey responses. Treatment broadcasts also alternated across trails so that when the playback was ‘on' along one trail, it was ‘off' along the other.
(a). Phantom chorus treatment
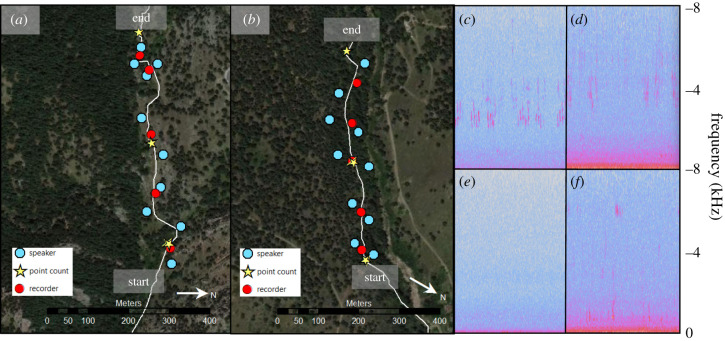
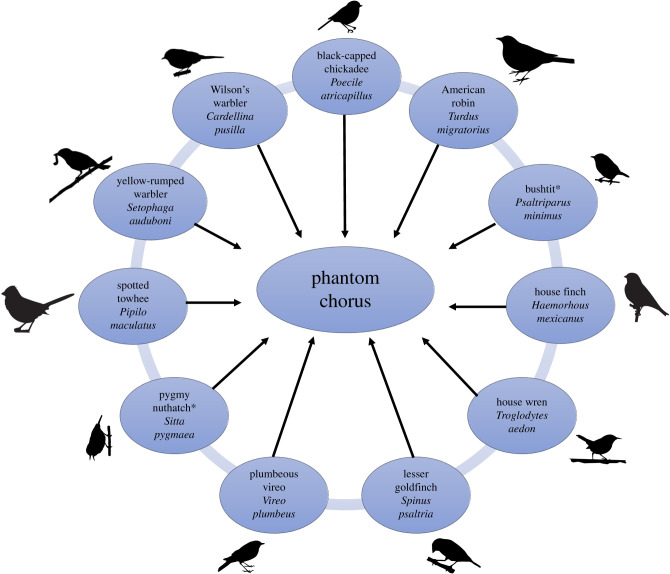
During treatment weeks, 10 hidden, evenly spaced Eco Extreme waterproof speakers (Grace Digital., Inc.) were placed approximately 15–30 m away from the trail in 500 m stretches of the Upper McClintock (McClintock) and Lower Gregory Canyon (Gregory Canyon) trails (figure 1). Each of the 10 speakers broadcast a different looping 5 min file containing songs and calls from one common native species, with the exception of one file that contained two species (figure 2), using Olympus LS-P2 devices (Olympus Corporation). We broadcast recordings from 9:00 to 15:00 5 days a week, including weekends. We set broadcast amplitude at 80 A-weighted decibels (maximum sound level [LAFmax], fast response, re. 20 µPa) using a MicW i436 omnidirectional microphone and the SPLnFFT smartphone application, which serves as a type 2 sound level meter [40]. This amplitude is typical of songbird playback because it falls within the range of natural sounds (reviewed in [41]). Phantom chorus files were created with edited song and call recordings using recordings taken as close to our study trails as possible to account for regional variation in song. We also selected songs with higher signal-to-noise ratios. The files were edited using Audacity 2.1.3 software (audacityteam.org) to remove background noise and vocalizations from other species. We also standardized amplitude among all files using Raven Pro 1.5 (ravensoundsoftware.com). Speakers were placed in realistic microhabitat for each species. For example, the speaker broadcasting spotted towhee (Pipilo maculatus) was placed near the ground in shrubs where this species is often found. The same suite of species was used at both trails, but the order of species differed due to variations in habitat.
Figure 1.
Speaker, recorder and point count locations at (a) Gregory Canyon and (b) McClintock trails. Panels (c) and (d) denote spectrograms of 30 s clips of audio recordings taken during the phantom chorus treatment at Gregory Canyon and McClintock, respectively; panels (e) and (f) were taken on control days at each site (i.e. the phantom chorus treatment was off). Control conditions were not necessarily quiet; for example, Panel (f) illustrates sounds produced by a hummingbird flying and a human talking at McClintock. (Online version in colour.)
Figure 2.
Species composition of the phantom chorus. *Pygmy nuthatch and bushtit sounds were combined into a single file. (Online version in colour.)
(b). Point counts
To quantify actual avian species richness along the study trails, we conducted weekly point counts at three locations at each trail between 3 August and 3 September 2017 (figure 1). We recorded the number of individuals of all species of birds seen or heard within a 5 min period at each location. Bird activity is often highest at dawn [42] and surveys typically take advantage of this increased activity [43]. However, our visitor surveys did not begin until 9:00, thus we timed our point counts to better measure bird activity perceptible to visitors later in the morning by starting them approximately an hour after sunrise and finishing them shortly before our visitor surveys started at 9:00.
(c). Ambient sound levels
To determine whether sound levels systematically varied between trails or between time intervals with and without playback, on each trail, we set up five Olympus LS-P2 recorders (Olympus Corporation), each spaced approximately halfway between successive speakers and no more than 5 m from the trail to record hourly ambient sound levels (see below; figure 1). Recording started just before surveying began at 9:00 each day and stopped after surveying was completed for the day. We positioned each recorder approximately 1.5 m off the ground and surrounded by a custom windscreen, which also served as camouflage.
(d). Survey administration
We intercepted hikers from approximately 09:00 to 15:00, 5 days a week for the duration of the study. Monitoring by park personnel has shown weekday visitation is reflective of local demographics (Boulder OSMP staff 2020, personal communication). To provide a sample with increased generalizability to a more diverse population, we focused our sampling efforts on weekends when there was more non-local visitation. To ensure that each participant experienced the full treatment, only hikers walking uphill on each trail were intercepted. This was possible because the trail sections used for our experiment were not connected to other trails. We administered surveys approximately 50 m beyond the last speaker location. We gave each participant a laminated copy of the survey to follow along with the verbal instructions given by a researcher, who entered responses into the iSURVEY program (harvestyourdata.com) on an iPad. We did not ask hikers who were running or wearing headphones to participate.
We collected responses from visitors using a questionnaire. The instrument contained two main sections relevant to this study: perceived biodiversity and self-reported well-being. To operationalize these concepts, perceived biodiversity was measured as perceived bird species diversity and self-reported well-being was measured as perceived psychological restoration [44–46]. We also collected demographic information for descriptive purposes.
To measure hikers' perceptions of bird species diversity, we asked respondents ‘Based on your experience on the trail today, about how many different types or species of birds would you say are in the last quarter mile or last 7 min of your walk on the trail?' Responses were recorded on a 5-point scale, where 1 = 0–3 different types of birds, 2 = 4–7 different types of birds, 3 = 8–11 different types of birds, 4 = 12–15 different types of birds and 5= more than 15 different types of birds.
There are numerous measures of human well-being (reviewed in [47]). In this study, we drew from Payne's [46] work focused on a soundscape's potential to provide psychological restoration, which was the most appropriate operationalization of environmental restoration theory for our study involving the restorative potential of birdsong. This approach isolates the perceived restorativeness of the acoustic environment as opposed to the audio-visual experience of hikers in a field-based experiment. Payne's [46] operationalization includes five concepts: fascination, being-away-to, being-away-from, compatibility and extent (electronic supplementary material, table S1). ‘Fascination’ refers to the ability of a stimulus to hold an individual's attention in such a way that it does not inhibit their ability to focus on other stimuli or cause attentional fatigue [45]. An example from the survey included: ‘Sounds on the trail today make me wonder about things'. ‘Being-away-to' refers to the ability of the soundscape to contribute to the restorative qualities of the destination not typically found in the one's daily life. An example from our survey was: ‘The trail's acoustic environment is different from what I usually hear in my daily life'. ‘Being-away-from' refers to the soundscape's ability to serve as a refuge from the stress of one's daily life. An example from our survey is: ‘Hearing sounds on the trail today made me feel free from work, routine and responsibilities'. ‘Compatibility' refers to how compatible the trail's soundscape is with one's personal preferences and motivations for visiting. An example from our survey was: ‘The trail's acoustic environment fits with my personal preferences'. ‘Extent' refers to the environment's ability to be ‘rich and coherent enough to constitute another world' and engage the mind with few distractions [45]. An example from our survey was: ‘All the sounds merge to form a coherent acoustic environment.' Hikers were asked, ‘Based on your experience from the last quarter mile of trail or seven minutes of your hike today, how much do you agree with each of these statements?' Responses were recorded on a 7-point scale, where 0 = not at all and 6 = completely (electronic supplementary material, table S1).
(e). Analysis
We first analysed whether the phantom chorus increased the number of potential species detected (i.e. avian species richness) along trails. For each point count week, we calculated the sum of unique species observed during point count surveys from all point count locations per trail plus the number of additional species represented in the phantom chorus that had not been detected on point count surveys (i.e. the sum of unique species from point counts and the phantom chorus). We then compared this combined total of real and simulated bird species to the total species from just the point counts on a trail using linear models with the lm function in program R 3.6.1 [48]. In an initial analysis, we considered the influence of week of observation, trail and count method (i.e. the number of species detected in point counts versus that number plus additional unique species added by the phantom chorus), plus an interaction between trail and measurement, but found the interaction and week of observation did not improve model performance (not shown). Thus, the final model included only the influence of trail and count method.
Analyses of ambient noise recordings were completed using the National Park Service's Acoustic Toolbox software [49]. We used L50 A-weighted decibels (dB) to characterize sound levels along the trails. L50 represents a median of fluctuating sound levels such that sound levels exceed this value 50% of the time. We used linear mixed effect models (LMER) with the lmer function in the lme4 R package [50] to model hourly sound levels. For fixed effects, we included a variable denoting whether the phantom chorus was on or off. Because general sound levels could differ between trails for other reasons and sound levels can also fluctuate seasonally, we also included trail and pseudo-date (where 15 July = 1) as fixed effects and considered an interaction between treatment and trail. Given the hierarchical structure of sound collection, we included hour within recording location as nested random effects.
We used the Statistical Package for the Social Sciences (SPSS) and Amos to perform analyses of the survey data. Three different methods were used: a principal component analysis (PCA), confirmatory factor analysis (CFA) and structural equation modelling (SEM). Maximum-likelihood estimation was used in all CFA and SEM procedures. Data screening showed that some variables had one or two missing data points. Because the maximum-likelihood estimation model we used requires no missing data, we deleted cases with missing data points to be as conservative as possible. This left a final sample size of 665 surveyed hikers. We did not assume multivariate normality, and thus applied bootstrapping to correct for this using bias-corrected confidence intervals (95%) for all CFA and SEM procedures.
We used PCA to examine the underlying structure of the perceived psychological restoration scale in preparation for the CFA. We used this exploratory process first because this scale has achieved mixed results in different settings [46] and we wanted to keep the CFA in the ‘spirit' of a confirmatory process. For the PCA, we used varimax rotation to aid in loading interpretation. We checked assumptions about the appropriateness of the method using the Kaiser–Meyer–Olkin (KMO) statistics (KMO > 0.50) and Bartlett's test of sphericity (p < 0.05). Only components with an eigenvalue greater than 1 were extracted from the data. Using guidance from prior research [46], we interpreted loadings with absolute value greater than 0.40 as belonging to a particular axis [51] and we removed cross-loaded items. The reliability of items measuring a component was examined using Cronbach's alpha, with α > 0.65 being sufficient [52].
We used CFA to test an a priori specified structure that represents the relationships among variables. Based on past research [46], we conceptualized perceived soundscape restoration as a second-order factor composed of several first-order factors. The first-order factors were informed through the PCA. Similar to the PCA, a CFA model should generally have factor loadings greater than 0.40, with values greater than 0.60 considered high [51]. We also used Cronbach's alpha to establish factor reliability in the CFA.
In addition to the criteria above, goodness-of-fit (GOF) statistics allow researchers to evaluate how well the data match the specified model. Here, we provide several commonly used GOF statistics, including both relative and absolute fit statistics. This includes χ2, BSboot (a χ2 that accounts for the bootstrapping procedure), the comparative fit index (CFI), the Tucker–Lewis index (TLI), the root mean square error of approximation (RMSEA) and the standardized root mean square residual (SRMR).
Interpretation of the GOF statistics can vary. Both the χ2 and BSboot are likely to be rejected with larger samples (n ≥ 200), and results of p < 0.05 are largely ignored in CFA and SEM procedures. Instead, other fit statistics are preferred. For CFI and TLI, values should be ≥ 0.90, with ≥ 0.95 indicative of an excellent fit [53]. For SRMR, values ≤ 0.08 are acceptable, with values closer to 0 indicative of a better fit [54]. RMSEA values ≤ 0.10 are considered sufficient, with values ≤ 0.05 considered excellent [55,56]. Collectively, these GOF statistics indicate whether the theoretical model accurately represents the relationships among the data.
The last step in the analysis was building SEMs to represent the relationships among the phantom chorus treatment, perceived bird species diversity, and perceived psychological restoration. Phantom chorus treatment was dummy coded in the models, with 0 = control (phantom chorus off) and 1 = treatment (phantom chorus on). Like the CFA, bootstrapping was applied in the SEMs and GOF statistics are reported. Lastly, standardized path coefficients and their statistical significance are reported using the bias-corrected confidence intervals (95%). trails (i.e. McClintock and Gregory Canyon) were analysed using separate SEMs.
3. Results
(a). Phantom chorus treatment
The phantom chorus increased acoustic bird species richness by approximately six species (t = 6.797, p < 0.001). Gregory Canyon tended to have higher bird species richness than McClintock (t = −2.650, p = 0.017; electronic supplementary material, table S2). Sound levels were significantly higher during phantom chorus playback weeks than during non-playback weeks on McClintock (LMER, t = 5.932, p < 0.001), but were significantly lower during playback weeks than non-playback weeks on Gregory Canyon (LMER, t = −2.048, p < 0.001; electronic supplementary material, figure S1). However, the differences were less than 1 dB in both contrasts. Sound levels also increased across the season (LMER, t = 2.639, p = 0.008; electronic supplementary material, table S3).
(b). Sample characteristics
Overall, the samples at Gregory Canyon and McClintock appeared different (electronic supplementary material, table S4). Respondents at McClintock were older and contained more females when compared to Gregory Canyon respondents. To evaluate for non-response bias, qualitative comparisons were made between respondents in this study and respondents in a recent study conducted in the same area (table 2 of [57]). Differences existed when comparing the overall sample in this study to that conducted from VanderWoude & Kellogg [57]. The sample within this study appeared to be younger, out-of-state residents hiking in larger groups. This indicates that there may be some characteristic differences between the respondents in this current study and the general visitor to Boulder OSMP , so caution should be used in generalizing the results from this study to all visitors at OSMP.
(c). Principal component analysis of perceived restoration measures
KMO (0.916) and Bartlett's test of sphericity (p < 0.001) indicated PCA was an appropriate method to apply. Although the PCA succeeded in extracting four components, several items cross-loaded (perceived restoration, ‘PR' axes 1 and 11), and others had low loadings (less than 0.40; PR15). We removed the cross-loaded and low loading items and reran the PCA analysis. This PCA analysis also met the assumptions for the analysis (KMO = 0.904; Bartlett's test of sphericity, p < 0.001) and extracted four components that explained 69.70% of the variance in the data (see electronic supplementary material, table S1). Reliability for three of the components was sufficient (α > 0.65) and ranged from 0.85 to 0.90. However, the items of one component failed to display acceptable reliability (α = 0.492), and thus the component and items were removed from further analyses. This left three useable components to inform future analyses. We named these components according to Payne's [46] previous research: sound fascination, sound compatibility and sound coherence (electronic supplementary material, table S1).
(d). Second-order confirmatory factor analysis for perceived restoration measures
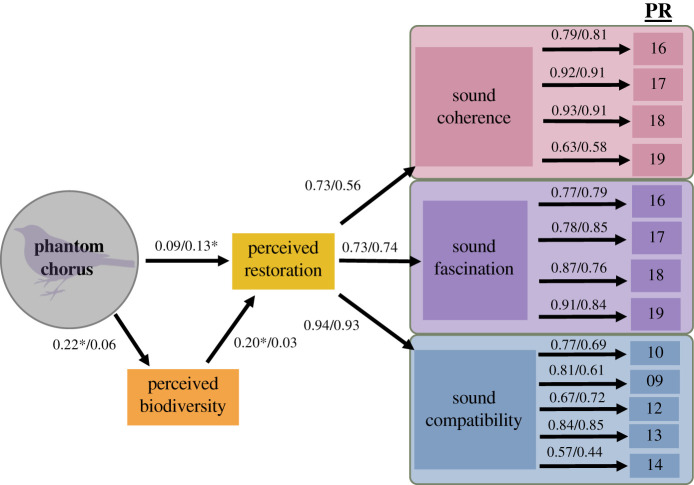
Using the results of the PCA, we constructed a second-order CFA (electronic supplementary material, figure S2). In this CFA, perceived psychological restoration was a second-order factor consisting of three first-order factors: sound fascination, sound compatibility and sound coherence. Although both the χ2 (χ2 = 279.419, d.f. = 62, p < 0.001) and BSboot (p = 0.002) were significant, the rest of the GOF statistics supported the model (RMSEA = 0.073; SRMR = 0.049; CFI = 0.958; TLI = 0.947). Additionally, all factor loadings were ≥ 0.60 and statistically significant. Reliability for first-order factors was already examined in the PCA (electronic supplementary material, table S1), and reliability for the second-order factor of perceived psychological restoration was α = 0.77.
(e). Structural equation model of the phantom chorus, perceived bird species diversity and perceived psychological restoration
(i). McClintock trail
The χ2 (χ2 = 151.262, d.f. = 86, p < 0.001) for the SEM was significant, but the rest of the GOF statistics supported the model (BSboot, p = 0.088; RMSEA = 0.049; SRMR = 0.0427; CFI = 0.975; TLI = 0.970). Overall, the model explained about 6% of the variance in perceived restoration (figure 3). The model identified a significantly positive, but small (p = 0.003) direct effect from the phantom chorus on perceived bird species diversity (‘perceived biodiversity' in figure 3) [58]. There was also a small but significant (p = 0.008) direct effect of perceived bird species diversity on perceived psychological restoration [58]. However, there was not a significant direct effect between the phantom chorus and perceived psychological restoration (p = 0.20). A further look at the indirect effect between the phantom chorus and perceived psychological restoration through perceived bird species diversity revealed a small but significant positive indirect effect (standardized indirect effect = 0.073; p = 0.003). The lack of a direct effect between the phantom chorus treatment and perceived psychological restoration in conjunction with the presence of an indirect effect indicates that perceived bird species biodiversity mediates the relationship between the phantom chorus treatment and perceived psychological restoration.
Figure 3.
Structural equation model for the relationships between the phantom chorus treatment, perceived bird species diversity and perceived psychological restoration at the McClintock and Gregory Canyon trails. Values above structural paths listed before and after the forward slash symbol reflect paths on McClintock and Gregory Canyon trails, respectively. All values marked with an asterisk symbol denote p ≤ 0.05 and all factor loadings were statistically significant (p ≤ 0.05). Numbers below ‘PR' reflect perceived restoration scale variable codes (see electronic supplementary material, table S1). McClintock trail model fit: χ2 = 151.262, d.f. = 86, p < 0.001; BSboot, p = 0.088; RMSEA = 0.049; SRMR = 0.0427; CFI = 0.975; TLI = 0.970. Structural paths marked with * denote p < 0.05. All factor loadings are statistically significant (p < 0.05). Gregory Canyon trail model fit: χ2 = 242.601, d.f. = 86, p < 0.001; BSboot, p = 0.002; RMSEA = 0.072; SRMR = 0.0557; CFI = 0.939; TLI = 0.925. Structural paths marked with an asterisk denote p < 0.05. All factor loadings are statistically significant (p < 0.05). (Online version in colour.)
(ii). Gregory Canyon trail
The χ2 (χ2 = 242.601, d.f. = 86, p < 0.001) and BSboot (p = 0.002) for the SEM were significant, but the rest of the GOF statistics supported the model (RMSEA = 0.072; SRMR = 0.056; CFI = 0.939; TLI = 0.925). Overall, the model explained about 2% of the variance in perceived psychological restoration (figure 3). The model showed the only significant effect (p = 0.028) was a positive but small direct effect from the phantom chorus on perceived psychological restoration (figure 3) [58]. The indirect effect between the phantom chorus and perceived psychological restoration through perceived bird species diversity was not significant (p = 0.478).
4. Discussion
We showed that the experimental addition of a phantom bird chorus increased perceived psychological restoration of hikers on both the McClintock and Gregory Canyon trails. Our results add to a growing body of evidence linking improvements in mental health to nature experiences [47]. Additionally, our study adds a novel field-based experimental approach to understand whether specific sensory modalities are involved in acquiring psychological benefits from nature. In general, our results provide mixed support for the link between perceived biodiversity and human well-being that has been reported previously [9,17,18]. At the McClintock trail, the SEM supported previous research demonstrating people perceive increases in biodiversity and that it positively impacts their sense of well-being [17]. Additionally, the model suggests that the change in perceived psychological restoration in response to the phantom chorus was mediated by visitor perceptions of bird diversity. In other words, increases in visitor perceptions of bird species diversity may contribute to higher levels of perceived psychological restoration and the perceptions of bird species diversity were positively influenced by the phantom chorus. Recent research has connected increased birdsong to human well-being through concepts like attention restoration [32,33]. The evidence from the McClintock trail SEM builds on this connection by indicating that perceived biodiversity may play a key role in the relationship between nature exposure and perceived human health and well-being outcomes, but its role may depend on context (see below). Although some research has explored this area [17], future inquiries should continue to refine this relationship between perceived biodiversity and human health and well-being, and also explore how forms of natural history-based recreation and nature study (e.g. hunting, fishing, rock hounding, botanical sketching, insect collecting, mushroom foraging, etc.) are related to perceived biodiversity and human health and well-being.
In contrast to the results described above, the SEM explaining results from the experiment at Gregory Canyon was in line with the findings that show that people do not perceive increases in biodiversity when it is elevated experimentally [18]. Yet exposure to birdsong still improved hikers' perceived psychological restoration. Although these results are quite different than the results at McClintock, both SEMs demonstrated that the phantom chorus had a positive impact on perceived psychological restoration, either directly (at Gregory Canyon) or indirectly (at McClintock) through perceived bird species diversity. Despite the difference, the end product is clear: hearing more birds improves perceived psychological restoration (electronic supplementary material, figure S3).
Why the results differed between Gregory Canyon and McClintock is unclear. Study attributes probably contributed to the differences between trails and also to the relatively small effects that we documented. Previous studies suggestive of the positive influence of nature on well-being have been based on living near greenspace [59] or have relied on experiences with nature that last 90 min [60]. In our study, visitors were only exposed to conditions on each trail for 7–10 min, thus it may not be surprising that the documented effect was small. Other recent work suggests that increases in self-reported well-being become apparent only for people that spent at least 120 min in a natural area on a single visit during the last seven days [61], although it was unclear how multiple visits to natural areas and the time interval between the natural experience and survey influenced these results. Visitors in our study provided responses immediately after exposure, raising questions about the longevity of any benefits derived from exposure to nature. Thus, in addition to more research focused on how perceptions of biodiversity influence well-being, more work is needed to identify the length of nature experiences necessary to obtain benefits of nature, as are studies that evaluate how long benefits persist and the conditions that prolong or degrade their persistence.
Besides the short duration of exposure, an explanation for differences between trails that seems plausible in hindsight is the different terrain at each trail affected hikers' abilities to perceive bird species diversity. Gregory Canyon is much steeper and rockier than McClintock, and it is possible that these conditions demanded increased focus and attention to footing and movements at the expense of a conscious or subconscious awareness of sounds. Differences in the characteristics of the samples at Gregory Canyon and McClintock could also have affected the results; particularly notable are the differences in ages and proportion of out-of-state hikers at each trail. Besides sample differences between trails, there may also be limited generalizability to a greater population, as this study was conducted in a geographically constrained area with sample characteristics that differ from overall characteristics across all Boulder OSMP locations (electronic supplementary material, table S4).
Another explanation for variation in results between trails could involve the different effects of the phantom chorus on overall sound levels on the two trails. Unlike the increase in sound levels during phantom playback on McClintock trail, there were lower ambient sound levels during playback versus control days at Gregory Canyon. There are no obvious explanations for this difference. However, an intriguing prospect for future research is the possibility that audible birdsong and time spent talking among visitors negatively covary. That is, the slightly higher sound levels on control weeks could reflect more time spent talking among visitors than during weeks with the phantom chorus. Use of wearable, ambulatory recording devices on hikers, similar to on-animal recorders used in wildlife studies [62], could provide a powerful approach for testing these possibilities.
Our results also provide insights into the utility of perceived psychological restoration in psychological ecosystem service research. Our analyses supported perceived psychological restoration as a second-order concept composed of three first-order concepts: sound fascination, sound compatibility and sound coherence. When compared with previous research [46], our model displayed a different structure. For instance, several items failed to display acceptable scale measurement properties, such as reliability and sufficient factor loadings. Future research should focus on continuing to refine valid and reliable tools for measuring the perceived psychological restoration scale, including configural and metric invariance tests in diverse and cross-cultural populations.
The finding that acoustic bird species diversity improves visitors' perceived psychological restoration has implications beyond this study. Taken to its extreme, our findings could be used to justify replacing real experiences in nature with recordings of birdsong, potentially facilitating an even greater disconnect between people and nature and straining already difficult environmental conservation efforts. We should resist temptations to substitute real experiences in nature with copies of it for many reasons. Among them, a recent meta-analysis found that real experiences in natural settings improved mood better than virtual experiences [63]. Thus, even though copies of natural stimuli abound, such as playback of a babbling creek in restaurants, products that make the home smell like various natural environments or wall-sized images of forests, if they provide restorative benefits, they likely fail to provide the same benefit as the rich multi-sensory experience of being in nature. Still, although imitations of aspects of nature may not provide the same benefit as the real thing, their use may still be beneficial for populations that are disconnected to nature for reasons beyond their immediate control. First, however, we should untangle the multi-sensory natural experiences through creative experiments that can identify the singular and combined effects of different sensory modalities involved in improved well-being.
A second implication of our findings involves managing protected areas to promote hearing natural sounds and experiencing wildlife. Observing wildlife is a key motivation to visit parks and protected areas [64], yet human presence can reduce the abundance of wildlife or displace them away from human activity, which makes them more difficult to observe [65]. Recent research shows educational signs instructing visitors to reduce their noise significantly reduced noise levels, increased bird biodiversity, increased visitor perceptions of bird biodiversity and improved visitor experiences at Muir Woods National Monument [21,66]. This simple, cost-effective measure could help mitigate noise pollution and improve visitors' experiences and well-being in protected areas without restricting the number of visitors.
Because an emotional affinity towards nature has been shown to motivate conservation-oriented behaviours [3,67,68], managing noise in parks has the potential to create a feedback loop where increased biodiversity improves visitors' experiences, improves their well-being, and increases their emotional affinity towards nature, thereby motivating actions that will further benefit biodiversity (reviewed in [28]). Further supporting this idea, Larson et al. [69] found that birdwatching is linked to pro-environmental behaviours (recycling, donating to environmental causes, etc.) both directly and indirectly by strengthening participants' attachment to a place. Place attachment, or the positive emotional connection that a person has with a particular environment, is influenced by a variety of factors, which in a natural setting can include things such as scenery, peacefulness or wildlife [70]. Our study demonstrates the importance of natural sounds to having positive experiences in nature, which may further motivate pro-conservation behaviours. As the world's population grows and natural areas become increasingly fragmented and impacted by noise, preserving acoustic resources will be important for both biodiversity and human well-being.
Supplementary Material
Acknowledgements
We thank Crystal Van and Tara Samuels for field assistance and City of Boulder Open Space and Mountain Parks (OSMP) staff, particularly Heather Swanson, for logistical support.
Data accessibility
The data supporting this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.wwpzgmsgx [71].
Authors' contributions
D.M.F., L.AF., B.D.T., J.R.B., P.N. and C.D.F. designed the research. D.M.F. performed the field research. D.M.F. and Z.D.M. analysed the data. D.M.F., Z.D.M. and C.D.F. wrote the manuscript and all authors contributed to drafts and gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
This project was funded by National Science Foundation grant no. 1414171 to J.R.B., C.D.F. and P.N., a City of Boulder OSMP Research Program grant to C.D.F. and funding from the National Park Service Natural Sounds and Night Skies Division and the Cal Poly William and Linda Frost Fund.
References
- 1.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. 2001. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 11, 231–252. ( 10.1038/sj.jea.7500165) [DOI] [PubMed] [Google Scholar]
- 2.Cardoso P, Erwin TL, Borges PAV, New TR. 2011. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 144, 2647–2655. ( 10.1016/j.biocon.2011.07.024) [DOI] [Google Scholar]
- 3.Kals E, Schumacher D, Montada L. 1999. Emotional affinity toward nature as a motivational basis to protect nature. Environ. Behav. 31, 178–202. ( 10.1177/00139169921972056) [DOI] [Google Scholar]
- 4.Miller JR. 2005. Biodiversity conservation and the extinction of experience. Trends Ecol. Evol. 20, 430–434. ( 10.1016/j.tree.2005.05.013) [DOI] [PubMed] [Google Scholar]
- 5.Nisbet EK, Zelenski JM, Murphy SA. 2009. The nature relatedness scale: linking individuals' connection with nature to environmental concern and behavior. Environ. Behav. 41, 715–740. ( 10.1177/0013916508318748) [DOI] [Google Scholar]
- 6.Pauly D. 1995. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430 ( 10.1016/s0169-5347(00)89171-5) [DOI] [PubMed] [Google Scholar]
- 7.Rosa CD, Profice CC, Collado S. 2018. Nature experiences and adults’ self-reported pro-environmental behaviors: the role of connectedness to nature and childhood nature experiences. Front. Psychol. 9, 1055 ( 10.3389/fpsyg.2018.01055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capaldi CA, Dopko RL, Zelenski JM. 2014. The relationship between nature connectedness and happiness: a meta-analysis. Front. Psychol. 5, 976 ( 10.3389/fpsyg.2014.00976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller RA, Irvine KN, Devine-Wright P, Warren PH, Gaston KJ. 2007. Psychological benefits of greenspace increase with biodiversity. Biol. Lett. 3, 390–394. ( 10.1098/rsbl.2007.0149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita E, Fukuda S, Nagano J, Hamajima N, Yamamoto H, Iwai Y, Nakashima T, Ohira H, Shirakawa T. 2007. Psychological effects of forest environments on healthy adults: Shinrin-yoku (forest-air bathing, walking) as a possible method of stress reduction. Public Health 121, 54–63. ( 10.1016/j.puhe.2006.05.024) [DOI] [PubMed] [Google Scholar]
- 11.Berman MG, Jonides J, Kaplan S. 2008. The cognitive benefits of interacting with nature. Psychol. Sci. 19, 1207–1212. ( 10.1111/j.1467-9280.2008.02225.x) [DOI] [PubMed] [Google Scholar]
- 12.Shin WS, Shin CS, Yeoun PS, Kim JJ. 2011. The influence of interaction with forest on cognitive function. Scand. J. For. Res. 26, 595–598. ( 10.1080/02827581.2011.585996) [DOI] [Google Scholar]
- 13.Rook GA. 2013. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc. Natl Acad. Sci. USA 110, 18 360–18 367. ( 10.1073/pnas.1313731110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulrich RS. 1984. View through a window may influence recovery from surgery. Science 224, 420–421. ( 10.1126/science.6143402) [DOI] [PubMed] [Google Scholar]
- 15.Cox DT, Shanahan DF, Hudson HL, Fuller RA, Anderson K, Hancock S, Gaston KJ. 2017. Doses of nearby nature simultaneously associated with multiple health benefits. Int. J. Environ. Res. Public. Health 14, 172 ( 10.3390/ijerph14020172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinew KJ, Glover TD, Parry DC. 2004. Leisure spaces as potential sites for interracial interaction: community gardens in urban areas. J. Leis. Res. 36, 336–355. ( 10.1080/00222216.2004.11950027) [DOI] [Google Scholar]
- 17.Dallimer M, Irvine KN, Skinner AMJ, Davies ZG, Rouquette JR, Maltby LL, Warren PH, Armsworth PR, Gaston KJ. 2012. Biodiversity and the feel-good factor: understanding associations between self-reported human well-being and species richness. BioScience 62, 47–55. ( 10.1525/bio.2012.62.1.9) [DOI] [Google Scholar]
- 18.Shwartz A, Turbé A, Simon L, Julliard R. 2014. Enhancing urban biodiversity and its influence on city-dwellers: an experiment. Biol. Conserv. 171, 82–90. ( 10.1016/j.biocon.2014.01.009) [DOI] [Google Scholar]
- 19.Marin LD, Newman P, Manning R, Vaske JJ, Stack D. 2011. Motivation and acceptability norms of human-caused sound in Muir Woods National Monument. Leis. Sci. 33, 147–161. ( 10.1080/01490400.2011.550224) [DOI] [Google Scholar]
- 20.Miller ZD, Taff BD, Newman P. 2018. Visitor experiences of wilderness soundscapes in Denali National Park and Preserve. Int. J. Wilderness 24, 32–43. [Google Scholar]
- 21.Pilcher E, Newman P, Manning RE. 2009. Understanding and managing experiential aspects of soundscapes at Muir Woods National Monument. Environ. Manage. 43, 425–435. ( 10.1007/s00267-008-9224-1) [DOI] [PubMed] [Google Scholar]
- 22.Newman PB, Taff BD, Weinzimmer D, Lawson S, Trevino K, Fristrup K, McKenna M. 2013. Monitoring and managing anthropogenic noise in national parks: lessons learned from field and laboratory studies. In 42nd International Congress and Exposition on Noise Control Engineering 2013, INTER-NOISE 2013: Noise Control for Quality of Life, pp. 5178–5185. Vienna, Austria: Osterreichischer Arbeitsring fur Larmbekampfung. [Google Scholar]
- 23.Weinzimmer D, Newman P, Taff D, Benfield J, Lynch E, Bell P. 2014. Human responses to simulated motorized noise in national parks. Leis. Sci. 36, 251–267. ( 10.1080/01490400.2014.888022) [DOI] [Google Scholar]
- 24.Benfield JA, Taff BD, Smyth J. 2014. Natural sound facilitates mood recovery. APA PhycNet. 6, 183–188. [Google Scholar]
- 25.Carles JL, Bernáldez F, de Lucio JV.. 1992. Audio-visual interactions and soundscape preferences. Landsc. Res. 17, 52–56. ( 10.1080/01426399208706361) [DOI] [Google Scholar]
- 26.Miller ZD, Hallo JC, Sharp JL, Powell RB, Lanham JD. 2014. Birding by ear: a study of recreational specialization and soundscape preference. Hum. Dimens. Wildl. 19, 498–511. ( 10.1080/10871209.2014.921845) [DOI] [Google Scholar]
- 27.Viollon S, Lavandier C, Drake C. 2002. Influence of visual setting on sound ratings in an urban environment. Appl. Acoust. 63, 493–511. ( 10.1016/S0003-682X(01)00053-6) [DOI] [Google Scholar]
- 28.Francis C, et al. 2017. Acoustic environments matter: synergistic benefits to humans and ecological communities. J. Environ. Manage. 203, 245–254. ( 10.1016/j.jenvman.2017.07.041) [DOI] [PubMed] [Google Scholar]
- 29.Senter P. 2008. Voices of the past: a review of Paleozoic and Mesozoic animal sounds. Hist. Biol. 20, 255–287. ( 10.1080/08912960903033327) [DOI] [Google Scholar]
- 30.Hedblom M, Heyman E, Antonsson H, Gunnarsson B. 2014. Bird song diversity influences young people's appreciation of urban landscapes. Urban For. Urban Green. 13, 469–474. ( 10.1016/j.ufug.2014.04.002) [DOI] [Google Scholar]
- 31.Alvarsson JJ, Wiens S, Nilsson ME. 2010. Stress recovery during exposure to nature sound and environmental noise. Int. J. Environ. Res. Public. Health 7, 1036–1046. ( 10.3390/ijerph7031036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratcliffe E, Gatersleben B, Sowden PT. 2013. Bird sounds and their contributions to perceived attention restoration and stress recovery. J. Environ. Psychol. 36, 221–228. ( 10.1016/j.jenvp.2013.08.004) [DOI] [Google Scholar]
- 33.Abbott LC, Taff D, Newman P, Benfield JA, Mowen AJ. 2016. The influence of natural sounds on attention restoration. J. Park Recreat. Adm. 34, 5–15. [Google Scholar]
- 34.Prather JW, Munger LM, Cruz A. 2002. Breeding biology of the black-backed lesser goldfinch in ponderosa pine forests on the Colorado Front Range. Wilson J. Ornithol. 114, 192–196. ( 10.1676/0043-5643(2002)114[0192:BBOTBB]2.0.CO;2) [DOI] [Google Scholar]
- 35.Swanson HM, Kinney B, Cruz A. 2004. Breeding biology of the chipping sparrow in ponderosa pine forests of the Colorado Front Range. Wilson J. Ornithol. 116, 246–251. ( 10.1676/03-105) [DOI] [Google Scholar]
- 36.Walsh JJ, Tuff TA, Cruz A, Chace JF. 2015. Differential parasitism between two suitable cowbird hosts. Open Ornithol. J. 8, 32–38. ( 10.2174/1874453201508010032) [DOI] [Google Scholar]
- 37.Kleist NJ, Guralnick RP, Cruz A, Francis CD. 2016. Anthropogenic noise weakens territorial response to intruder's songs. Ecosphere 7, e01259 ( 10.1002/ecs2.1259) [DOI] [Google Scholar]
- 38.Hyman J. 2005. Seasonal variation in response to neighbors and strangers by a territorial songbird. Ethology 111, 951–961. ( 10.1111/j.1439-0310.2005.01104.x) [DOI] [Google Scholar]
- 39.Yahner RH, Ross BD. 1995. Seasonal response of wood thrushes to taped-playback songs. Wilson Bull. 107, 4. [Google Scholar]
- 40.Kardous CA, Shaw PB. 2014. Evaluation of smartphone sound measurement applications. J. Acoust. Soc. Am. 135, EL186–EL192. ( 10.1121/1.4865269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luther DA, Danner R, Danner J, Gentry K, Derryberry EP. 2017. The relative response of songbirds to shifts in song amplitude and song minimum frequency. Behav. Ecol. 28, 391–397. ( 10.1093/beheco/arw172) [DOI] [Google Scholar]
- 42.Aschoff J. 1966. Circadian activity pattern with two peaks. Ecology 47, 657–662. ( 10.2307/1933949) [DOI] [Google Scholar]
- 43.Ralph CJ, Geupel GR, Pyle P, Martin TE, DeSante DF. 1993. Handbook of field methods for monitoring landbirds. Albany, CA: US Forest Service, Pacific Southwest Research Station.
- 44.Kaplan R, Kaplan S. 1989. The experience of nature: a psychological perspective. New York, NY: Cambridge University Press. [Google Scholar]
- 45.Kaplan S. 1995. The restorative benefits of nature: toward an integrative framework. J. Environ. Psychol. 15, 169–182. ( 10.1016/0272-4944(95)90001-2) [DOI] [Google Scholar]
- 46.Payne SR. 2013. The production of a perceived restorativeness soundscape scale. Appl. Acoust. 74, 255–263. ( 10.1016/j.apacoust.2011.11.005) [DOI] [Google Scholar]
- 47.Bratman GN, et al. 2019. Nature and mental health: an ecosystem service perspective. Sci. Adv. 5, eaax0903 ( 10.1126/sciadv.aax0903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.RStudio Team. 2016. RStudio: integrated development environment for R. Boston, MA: RStudio, Inc; See http://www.rstudio.com/. [Google Scholar]
- 49.National Park Service. 2008. Acoustic monitoring toolbox. Fort Collins, CO: US Department of the Interior. [Google Scholar]
- 50.Bates D, Maechler M, Bolker B. 2012. lme4: Linear mixed-effects models using S4 classes.
- 51.Kline P. 1994. An easy guide to factor analysis. New York, NY: Routledge. [Google Scholar]
- 52.Vaske JJ. 2008. Survey research and analysis: applications in parks, recreation and human dimensions. State College, PA: Venture Publishing, Inc. [Google Scholar]
- 53.Hu L, Bentler PM. 1998. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol. Methods 3, 424–453. ( 10.1037/1082-989X.3.4.424) [DOI] [Google Scholar]
- 54.Hu L, Bentler PM. 1999. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 6, 1–55. ( 10.1080/10705519909540118) [DOI] [Google Scholar]
- 55.Browne MW, Cudeck R. 1992. Alternative ways of assessing model fit. Sociol. Methods Res. 21, 230–258. ( 10.1177/0049124192021002005) [DOI] [Google Scholar]
- 56.Kline RB. 2011. Principles and practice of structural equation modeling, 3rd edn New York, NY: The Guilford Press. [Google Scholar]
- 57.VanderWoude D, Kellogg A. 2018. 2016-2017 Visitor survey report Boulder, CO: City of Boulder Open Space and Mountain Parks Department. [Google Scholar]
- 58.Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd edn Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- 59.Bratman GN, Hamilton JP, Hahn KS, Daily GC, Gross JJ. 2015. Nature experience reduces rumination and subgenual prefrontal cortex activation. Proc. Natl Acad. Sci. USA 112, 8567–8572. ( 10.1073/pnas.1510459112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White MP, Alcock I, Wheeler BW, Depledge MH. 2013. Would you be happier living in a greener urban area? A fixed-effects analysis of panel data. Psychol. Sci. 24, 920–928. ( 10.1177/0956797612464659) [DOI] [PubMed] [Google Scholar]
- 61.White MP, Alcock I, Grellier J, Wheeler BW, Hartig T, Warber SL, Bone A, Depledge MH, Fleming LE. 2019. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci. Rep. 9, 7730 ( 10.1038/s41598-019-44097-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch E, Angeloni L, Fristrup K, Joyce D, Wittemyer G. 2013. The use of on-animal acoustical recording devices for studying animal behavior. Ecol. Evol. 3, 2030–2037. ( 10.1002/ece3.608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Browning MHEM, Shipley N, Hartig T, Yu C-P, Mcanirlin O, Dzhambov AM. 2020. An actual natural setting improves mood better than its virtual counterpart: a meta-analysis of experimental data. Front. Psychol. 11, 2200 ( 10.3389/fpsyg.2020.02200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manfredo MJ. 2008. Who cares about wildlife? Social science concepts for exploring human–wildlife relationships and conservation issues. New York, NY: Springer. [Google Scholar]
- 65.Karp DS, Guevara R. 2011. Conversational noise reduction as a win–win for ecotourists and rain forest birds in Peru. Biotropica 43, 122–130. ( 10.1111/j.1744-7429.2010.00660.x) [DOI] [Google Scholar]
- 66.Levenhagen MJ, et al. In press. Ecosystem services enhanced through soundscape management link people and wildlife. People Nat. [Google Scholar]
- 67.Halpenny EA. 2010. Pro-environmental behaviours and park visitors: the effect of place attachment. J. Environ. Psychol. 30, 409–421. ( 10.1016/j.jenvp.2010.04.006) [DOI] [Google Scholar]
- 68.Soga M, Gaston KJ. 2016. Extinction of experience: the loss of human–nature interactions. Front. Ecol. Environ. 14, 94–101. ( 10.1002/fee.1225) [DOI] [Google Scholar]
- 69.Larson LR, Cooper CB, Stedman RC, Decker DJ, Gagnon RJ. 2018. Place-based pathways to proenvironmental behavior: empirical evidence for a conservation–recreation model. Soc. Nat. Resour. 31, 871–891. ( 10.1080/08941920.2018.1447714) [DOI] [Google Scholar]
- 70.Stedman RiC. 2003. Is it really just a social construction? The contribution of the physical environment to sense of place. Soc. Nat. Resour. 16, 671–685. ( 10.1080/08941920309189) [DOI] [Google Scholar]
- 71.Ferraro DM, Miller ZD, Ferguson LA, Taff BD, Barber JR, Newman P, Francis CD. 2020. Data from: The phantom chorus: birdsong boosts human well-being in protected areas Dryad Digital Repository. ( 10.5061/dryad.wwpzgmsgx) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ferraro DM, Miller ZD, Ferguson LA, Taff BD, Barber JR, Newman P, Francis CD. 2020. Data from: The phantom chorus: birdsong boosts human well-being in protected areas Dryad Digital Repository. ( 10.5061/dryad.wwpzgmsgx) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.wwpzgmsgx [71].