Abstract
Purpose
To determine the association between low molecular weight heparin (LMWH) use and mortality in hospitalized COVID-19 patients.
Methods
We conducted a retrospective study of patients consecutively enrolled from two major academic hospitals exclusively for COVID-19 in Wuhan, China, from January 26, 2020, to March 26, 2020. The primary outcome was adjusted in-hospital mortality in the LMWH group compared with the non-LMWH group using the propensity score.
Results
Overall, 525 patients with COVID-19 enrolled with a median age of 64 years (IQR 19), and 49.33% men. Among these, 120 (22.86%) were treated with LMWH. Compared with the non-LMWH group, the LMWH group was more likely to be older and male; had a history of hypertension, diabetes, coronary heart disease (CHD), or stroke; and had more severe COVID-19 parameters such as higher inflammatory cytokines or D-dimer. Compared with non-LMWH group, LMWH group had a higher unadjusted in-hospital mortality rate (21.70% vs. 11.10%; p = 0.004), but a lower adjusted mortality risk (adjusted odds ratio [OR], 0.20; 95% CI, 0.09–0.46). A propensity score-weighting analysis demonstrated similar findings (adjusted OR, 0.18; 95% CI, 0.10–0.30). Subgroup analysis showed a significant survival benefit among those who were severely (adjusted OR, 0.07; 95% CI, 0.02–0.23) and critically ill (adjusted OR, 0.32; 95% CI, 0.15–0.65), as well as among the elderly patients’ age > 65, IL-6 > 10 times upper limit level, and D-dimer > 5 times upper limit level.
Conclusions
Among hospitalized COVID-19 patients, LMWH use was associated with lower all-cause in-hospital mortality than non-LMWH users. The survival benefit was particularly significant among more severely ill patients.
Keywords: COVID-19, LMWH, In-hospital mortality
Introduction
The coronavirus disease of 2019 (COVID-19) is a viral illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), now a pandemic by the World Health Organization [1, 2]. Among patients with hospitalized COVID-19, emerging data suggested that hypercoagulable status commonly existed and may be associated with increased mortality risk in COVID-19 [3]. COVID-19 presented a significant hypercoagulable status with elevated D-dimer, prolonger prothrombin time (PT), and fibrinogen degradation products (FDP) [4]. From two singer-center reports in French, among COVID-19 patients with severe clinical features, the proportion of patients with acute pulmonary embolus was 23–30% [5, 6]. The guideline regarding the diagnosis and treatment of thrombotic or thromboembolic disease and COVID-19, endorsed by the International Society on Thrombosis and Hemostasis, confirmed the prophylactic dose of low molecular weight heparin (LMWH) or unfractionated heparin (UFH) for hospitalized COVID-19, either with or without DIC [3]. A most recent large cohort study also showed that systemic anticoagulant therapy might be associated with improved outcomes among hospitalized COVID-19 patients [7]. Nevertheless, the guideline editorial panel called strongly for more data regarding LMWH [3]. In short, the optimal anticoagulation regimen is currently unknown.
Using data from two largest medical centers exclusive for the management of COVID-19 patients in Wuhan, the epicenter of China, we sought to (1) determine the prevalence of practical LMWH use among COVID-19 patients; (2) assess differences in baseline characteristics and treatment patterns by LMWH use status for COVID-19 patients; and (3) delineate the impact of LMWH use on major in-hospital outcomes, by mild/moderate, severely, critically ill patients, respectively.
Method
Study Design and Participants
This retrospective, multi-center study included 525 patients with COVID-19 admitted to two academic hospitals, the Optical Valley branch and Sino-French New City branch affiliated to Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, which exclusively managed COVID-19 patients in Wuhan, the epicenter of China. The Ethics Commission of Tongji Hospital approved this study. Written informed consent was waived for the retrospective study. Our present medical research was conducted according to the principles expressed in the Declaration of Helsinki.
Inclusion criteria were 18 years of age or older, with COVID-19 admitted from January 26 to March 26, 2020. The diagnosis of COVID-19 was according to the New Coronavirus Pneumonia Prevention and Control Program (7th edition) published by the National Health Commission of China and the WHO interim guidance and confirmed by qualitative reverse transcription polymerase chain reaction (RT-RNA) tests on a nose/throat swab positive for COVID-19. Patients with platelet count < 30 × 109·L−1; high risk of major bleeding such as DIC bleeding status, active peptic ulcers, recent surgery, cerebral hemorrhage severe brain trauma, cerebral aneurysm, arteriovenous malformation, gastrointestinal bleeding, and severe chronic liver disease; hemodialysis or continuous renal replacement therapies (CRRT); recipients of organ transplantation; and incomplete medical records (e.g., transfer to other hospitals) were excluded from the analysis.
Data Collection
Data were collected including patient demographic information, history of comorbidities, clinical characteristics, laboratory data, therapeutic interventions (antiplatelet therapy, antiviral treatment, immunological treatment, ventilation, and extracorporeal membrane oxygenation [ECMO]) during the hospitalization, and in-hospital mortality. The patient demographic information (age and sex) and laboratory data on admission (blood cell count, C-reactive protein [CRP], procalcitonin [PCT], D-dimer, organ function markers, serum electrolyte, and inflammatory marker [IL-6]) were collected from the laboratory information system. Comorbidities (hypertension, diabetes, CHD, chronic liver disease, and chronic pulmonary disease) were extracted from medical history. The in-hospital treatments were collected from medical records. All data were double-checked against source documents by two independent researchers.
Definition
LMWH users were defined as receiving continuous LMWH for 7 days or longer. All the patients in the treatment group received at least standard doses of thromboprophylaxis, defined as enoxaparin for VTE prophylaxis 40 mg subcutaneously (SC) once daily and or enoxaparin 40 mg twice a day.
The severity of COVID-19 was classified according to the diagnosis and treatment scheme for COVID-19 of China (7th edition) [8]. Patients were classified as severe COVID-19 group if they met one of the following criteria: (1) shortness of breath, RR ≥ 30 times/min; (2) oxygen saturation ≤ 93%; (3) alveolar oxygen partial pressure/fraction of inspiration O2 (PO2/FiO2) ≤ 300 mmHg; and (4) patients whose pulmonary imaging showed significant progression of lesion > 50% within 24–48 h. Critically ill patients were defined as respiratory failure requiring mechanical ventilation, septic shock, or combined with other organ failure needed intensive care unit (ICU) monitoring and treatment. Mild/moderate COVID-19 patients were defined as the absence of the previously described characteristics with or without a symptom of pneumonia. The onset of COVID-19 was defined as the time when the symptoms were first noticed.
Major bleeding was defined as (1) fatal bleeding and/or (2) symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome, and/or (3) bleeding causing a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells [9]. All clinically relevant minor bleed was defined as an acute or subacute clinically overt bleed that does not meet the criteria for a major bleed but prompts a clinical response, in that it leads to at least one of the following: (1) a hospital admission for bleeding, or (2) a physician-guided medical or surgical treatment for bleeding, or (3) a change in antithrombotic therapy (including interruption or discontinuation of study drug) [10].
Statistical Analysis
Continuous variables were expressed as median and interquartile range (IQR), and categorical variables were expressed as number and percentage (%). Statistical differences between two groups were analyzed using the Mann-Whitney U test for continuous variables, while categorical variables were compared using Fisher’s exact test or χ2 test. The risk of in-hospital mortality and the corresponding odds ratio (OR) were calculated using the logistic regression model to compare the LMWH group versus the non-LMWH group. Multivariable adjustment including age, sex, comorbidities (hypertension, diabetes, previous lung disease, CHD), and in-hospital antiplatelet treatment (aspirin and clopidogrel) was performed. A two-side α less than 0.05 was considered statistically different. Data were analyzed in R-3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS Statistics (version 26.0, IBM, Armonk, NY, USA).
Propensity Score-Weighting Analysis
Inverse probability of treatment weighting (IPTW) was used to control the baseline difference between the LMWH and non-LMWH groups based on a propensity score. Propensity scores were generated for cohorts by IPTW estimator that included variables which were expected to be potential confounders associated with the use of LMWH, including age, comorbidities (hypertension, diabetes, lung disease, and CHD), and severity classification. In-hospital mortality was analyzed again in our IPTW-adjusted analyses. The result has been repeated after those whose hospital stay less than 7 days have been censored.
Result
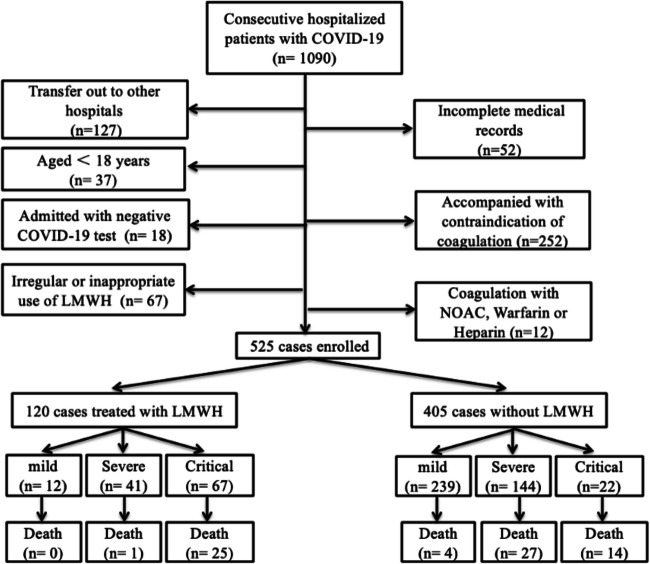
This study cohort began with 1090 patients hospitalized with COVID-19 from two academic hospitals exclusive for managing COVID-19 patients in Wuhan, China. From this cohort, we excluded the following: 252 patients with contraindication to anticoagulant treatment; 127 patients transferred out (because in-hospital treatment and endpoint cannot be fully profiled), with 52 incomplete medical records (without clear medical records for disease severity classification and comorbidities); 37 patients age < 18 years old; 18 patients admitted as the clinical diagnosis of COVID-19 but with repeatedly negative SARS-CoV2 virus PCR test after admission; 67 patients with irregular LMWH use; and 12 patients treated with a novel oral anticoagulant (NOAC), warfarin, or previous heparin use. Our analysis’ final population consisted of 525 patients enrolled from January 26, 2020, to March 26, 2020 (Fig. 1).
Fig. 1.
Patients’ flowchart of enrollment
Our study comprised 251 (47.81%) mild/moderate patients, 185 (35.24%) severe patients, and 89 (16.95%) critically ill patients. Among these 525 COVID-19 patients, 120 (22.86%) were on LMWH use (median age 70 [IQR 17] years; 55.83% men), and 405 (77.14%) did not used any LMWH (median age 63 [IQR 21] years; 47.41% men). The median start day was 1.4 day (1–3.5 days). Among the mild/moderate patients, 12 of 251 (4.78%) patients were on LMWH; among severe patients, 41 of 185 (22.16%) were on LMWH; among critically ill patients, 25 of 89 (28.1%) patients were on LMWH. In short, LMWH was more commonly used for those severer patients compared with milder patients.
Overall and per-group baseline characteristics are presented in Table 1. Baseline characteristics showed an overall median (IQR) age of 64 (IQR 19) and female (50.67%). Hypertension (196 of 525, [37.33%]), diabetes (93 of 525, 17.71%), and CHD (55 of 525, 10.48%) were the most frequent comorbidities. Compared to the non-LMWH group, the LMWH group were older, more likely to be male, and had a higher prevalence of comorbidities such as hypertension, diabetes, CHD, and stroke. LMWH group had a severer manifestation of COVID-19, including worse laboratory profile results such as higher WBC, CRP, IL-6, PCT, and D-dimer level.
Table 1.
Baseline characteristics of the study population according to LMWH use
| Variables | All (N = 525) | On LMWH (N = 120) | Non-LMWH (N = 405) | p |
|---|---|---|---|---|
| Age (years) | 64 (19) | 70 (16.75) | 63 (21) | 0 |
| Sex | – | – | – | 0.105 |
| Male | 259 (49.33) | 67 (55.83) | 192 (47.41) | |
| Female | 266 (50.67) | 53 (44.17) | 213 (52.59) | |
| Hypertension (%) | 196 (37.33) | 64 (53.33) | 132 (32.59) | 0.699 |
| DM (%) | 93 (17.71) | 29 (24.17) | 64 (15.8) | 0.375 |
| COPD (%) | 29 (5.52) | 5 (4.17) | 24 (5.93) | 0.047 |
| CHD (%) | 55 (10.48) | 18 (15) | 37 (9.14) | 0.884 |
| Stroke (%) | 33 (6.29) | 12 (10) | 21 (5.19) | 0.816 |
| Liver disease (%) | 4 (0.76) | 0 (0) | 4 (0.99) | 1 |
| Tumor (%) | 9 (1.71) | 1 (0.83) | 8 (1.98) | 0.248 |
| ALT (u/l) | 24 (26) | 26 (28.75) | 23 (25) | 0.07 |
| AST (u/l) | 27 (21) | 31.5 (29) | 26 (19) | 0.004 |
| Creatinine (μmol/l) | 68 (30.18) | 71 (30.75) | 67 (28.25) | 0.32 |
| Hb (g/l) | 125 (27.75) | 125 (27.5) | 126 (27) | 0.463 |
| WBC (× 109/l) | 6.46 (3.92) | 8 (4.34) | 6.01 (3.49) | 0 |
| PLT(× 109/l) | 225 (126) | 199 (122) | 227.5 (121) | 0.094 |
| hs-CRP (mg/l) | 26.35 (74.55) | 63.5 (100.01) | 18.4 (59.75) | 0 |
| IL-6 (pg/ml) | 12.11 (40.29) | 25.2 (59.27) | 7.89 (34.08) | 0 |
| PCT (ng/ml) | 0.08 (0.17) | 0.14 (0.24) | 0.07 (0.11) | 0 |
| D-dimer (μg/ml) | 1.05 (1.73) | 2.49 (4.29) | 0.83 (1.34) | 0 |
| Invasive ventilation (%) | 57 (10.86) | 40 (33.33) | 17 (4.2) | 0.178 |
| Non-invasive ventilation (%) | 118 (22.48) | 49 (40.83) | 69 (17.04) | 0.624 |
| ECMO (%) | 2 (0.38) | 2 (1.67) | 0 (0%) | 1 |
| Aspirin (%) | 40 (7.62) | 17 (14.17) | 23 (5.68) | 0.002 |
| Clopidogrel (%) | 24 (4.57) | 11 (9.17) | 13 (3.21) | 0.006 |
| Antiviral treatment (%) | 274 (52.19) | 108 (90.00) | 166 (40.99) | 1 |
| Immunological treatment (%) | 171 (32.57) | 76 (63.33) | 95 (23.46) | 0.0001 |
| Severity classification | 0 | |||
| Mild/moderate | 251 (47.81) | 12 (10) | 239 (59.01) | |
| Severe | 185 (35.24) | 41 (34.17) | 144 (35.56) | |
| Critically ill | 89 (16.95) | 67 (55.83) | 22 (5.43) |
DM diabetes mellitus, CHD coronary heart disease, ALT alanine aminotransferase, AST aspartate transaminase, Hb hemoglobin, WBC white blood cell, PLT platelet, CRP C-reactive protein, IL-6 interleukin-6, PCT procalcitonin, BiPAP bilevel positive airway pressure, ECMO extracorporeal membrane oxygenation
Continuous variables are presented as median (IQR), categorical variables are presented as n percentage
In terms of in-hospital treatment, the overall proportion of invasive ventilation was 10.86%, with 33.33% in the LMWH group and 4.2% in the non-LMWH group; noninvasive ventilation was 22.48% overall, with 40.83% in the LMWH group and 17.04% in the non-LMWH group; all patients received antiviral therapy. The LMWH group had a higher percentage of patients receiving immunological treatment and antiplatelet therapy such as aspirin (14.17% vs. 5.68%, p = 0.002) and clopidogrel (9.17% vs. 3.21%, p = 0.006) than patients in the non-LMWH group (Table 1).
For dose difference in the LMWH group, 26.67% of patients had LMWH twice a day, with 8.33% in the mild/moderate group, 12.2% in the severe group, and 38.8% in the critically ill group. The critically ill group had the highest proportion of LMWH twice a day use.
In terms of severity classification, patients in the LMWH group had 55.83% of critically ill patients, 34.17% of severe cases, and 10% mild/moderate cases; the non-LMWH group had 5.43% critically ill patients, 35.56% severe cases, and 59.01% mild/moderate cases. Briefly, the LMWH group was more likely to be severer than the non-LMWH group.
In-hospital mortality rate in our subjects was 71 out of 525 patients (13.52%). Crude fatality rate was 21.67% (26 of 120 patients) in the LMWH group and 11.1% (45 of 405 patients) in the non-LMWH group (unadjusted OR 2.21, 95% CI 1.30–3.77). Among mild/moderate patients, 0 in LMWH vs. 1.7% (4 of 239 patients) in non LMWH (p = 1) died; in severe patients, 2.4% (1 of 41 patients) in LMWH vs. 18.8% (27 of 144 patients) died (unadjusted OR 0.11, 95% CI 0.014–0.82, p = 0.03); and critically ill patients, 37.31% (25 of 67 patients) in LMWH vs. 63.63% (14 of 22 patients) in non LMWH died (unadjusted OR 0.03, 95% CI 0.13–0.92, p = 0.04) (Table 2).
Table 2.
Crude in-hospital outcomes according to LMWH use
| Outcome | Overall | Cumulative incidence | Unadjusted OR* (95% CI) | p | |
|---|---|---|---|---|---|
| LMWH users | Non-LMWH users | ||||
| In-hospital mortality | 71/525 (13.5%) | 26/120 (21.7%) | 45/405 (11.1%) | 2.21 1.30–3.77 | p = 0.004 |
| Mild/moderate | 4/251 (1.59%) | 0/12 (0%) | 4/239 (1.7%) | N/A | p = 1 |
| Severe | 28/185 (15.14%) | 1/41 (2.4%) | 27/144 (18.8%) | 0.11 (0.014–0.82) | p = 0.032 |
| Critically ill | 39/89 (43.82%) | 25/67 (37.3%) | 14/22 (63.6%) | 0.34 (0.13–0.92) | p = 0.035 |
*OR odds ratio of in-hospital mortality between LMWH and non-LMWH (reference)
In the logistic regression model after adjusting for age, sex, comorbidities, in-hospital medications (antiplatelet therapy), and severity classification, the use of LMWH was associated with lower all-cause mortality (adjusted OR, 0.20; 95% CI, 0.09–0.46; p = 0.001) versus non-LMWH group (Table 3). In subgroup analyses, LMWH was significantly associated with decreased mortality in those severely and critically ill patients (adjusted OR 0.08, 95% CI 0.01–0.62; adjusted OR 0.32, 95% CI 0.10–0.996, respectively) (Table 3). Further subgroup analysis demonstrated a significant survival benefit among elderly patients older than 65 years (adjusted OR, 0.16; 95% CI, 0.06–0.44), IL-6 > 10times upper limit level (adjusted OR, 0.06; 95% CI, 0.22–0.30), and D-dimer > 5 times upper limit level (adjusted OR, 0.06; 95% CI, 0.014–0.27) (Fig. 2).
Table 3.
Adjusted in-hospital mortality rate compared by LMWH versus non-LMWH, before and after propensity score weighting, respectively
| Unweighted | Weighted | |||
|---|---|---|---|---|
| Outcomes | Adjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
| In-hospital mortality | 0.20 (0.09–0.46) | p = 0.001 | 0.18 (0.10–0.30) | p = 0 |
| Mild/moderate | N/A | p = 1 | N/A | p = 1 |
| Severe | 0.08 (0.01–0.62) | p = 0.016 | 0.07 (0.02–0.23) | p = 0 |
| Critically ill | 0.32 (0.10–0.996) | p = 0.049 | 0.315 (0.15–0.65) | p = 0.002 |
Fig. 2.
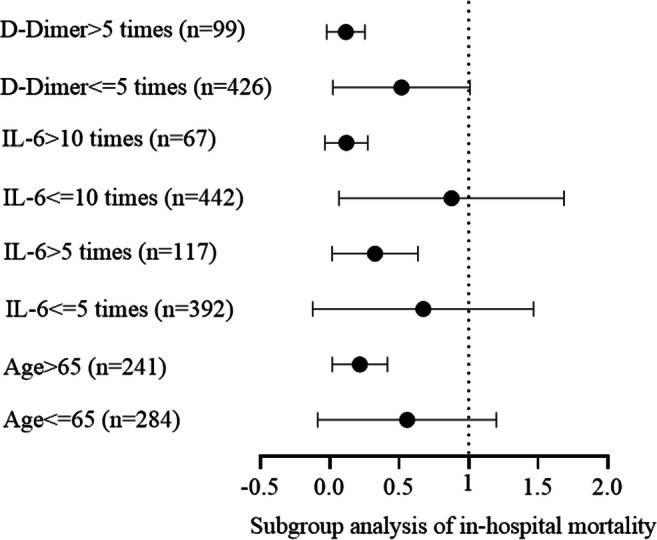
Subgroup analysis for in-hospital mortality. Subgroup analysis demonstrated a significant survival benefit among elderly patients older than 65 years (adjusted OR, 0.16; 95% CI, 0.06–0.44), IL-6 > 10 times upper limit level (adjusted OR, 0.06; 95% CI, 0.22–0.30), and D-dimer > 5 times upper limit level (adjusted OR, 0.06; 95% CI, 0.014–0.27)
These results were validated using a propensity score-weighting analysis. With I.P.W. adjustment, the results remained consistent and statistically significant, demonstrating a lower risk of in-hospital mortality in the LMWH group (adjusted OR, 0.18; 95% CI [0.10–0.30], p = 0). The survival benefits were most prominent among severe cases with adjusted OR 0.07 (95% CI, 0.02–0.23) and critically ill patients with OR 0.32 (95% CI, 0.15–0.65; p = 0.002) (Table 3).
To avoid survival bias, we censored those whose hospital stay was less than 7 days. The results remained consistent and statistically significant, demonstrating a lower risk of in-hospital mortality in the LMWH group (adjusted OR, 0.35; 95% CI [0.20–0.60], p = 0).
Overall, there were three major bleeding events recorded in the electric medical system. Among them, two cases received LMWH treatment, and one did not. All three cases were critically ill on admission and, unfortunately, died of severe gastrointestinal bleeding. It is worth mentioning that these three cases have received glucocorticoids intravenously before their gastrointestinal bleeding. Two cases of the LMWH group were male, 69 and 85 years old, respectively. The 69-year-old case had atrial fibrillation and was treated with LMWH for 10 days, while the 85-year-old case received 7 days of LMWH. The case from the non-LMWH group was a 79 years old male who also died of gastrointestinal bleeding.
Discussion
In this retrospective study, in-hospital use of LMWH was associated with a lower risk of all-cause in-hospital mortality than the non-LMWH group among COVID-19 patients. The benefit was most significant among more severely ill patients, elderly patients, and those with high IL-6 and D-dimer levels. Although unmeasured confounding and indication bias may have contributed to the observed protective association, these data echoes the most recent large cohort study that in-hospital use of anticoagulant therapy was associated with improved outcomes in COVID-19 [7]. These findings provide additional clinical evidence of LWMH benefit in support of recently published guideline statements to initiate prophylactic anticoagulated therapy in patients with COVID-19, particularly among severe patients. Given this study’s retrospective nature, these data need further validation in randomized controlled trials to determine the efficacy of LMWH use in patients and COVID-19.
Hypercoagulable status was found in previous coronavirus pneumonia, such as severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS). Notably, hypercoagulable status contributed to the significant morbidity and mortality in SARS and MERS [4, 11]. The clinical picture about coagulation consisted of vascular endothelial damage in pulmonary vessels, pulmonary thromboembolic, pulmonary infarction, diffuse microthrombosis, and DIC to significant organ failure [12]. During the progression of COVID-19 severity, the D-dimer increased, along with prolonged PT and gradually decreased fibrinogen (FBG) and platelet, consistent with the diagnosis of the hypercoagulable phase of DIC, leading to organ failure [4]. From two singer-center reports in French, among COVID-19 patients with severe clinical features, the proportion of patients with acute pulmonary embolus was 23–30%, and a recent study using the Padua model showed that 40% of hospitalized patients were at high risk of VTE [6, 13, 14]. Another study reporting stroke occurrence in five young COVID-19 patients without risk factors has raised the vast concern of this hypercoagulative status in COVID-19 [15]. The underlying pathogenic mechanism is yet unknown. Whether the thrombotic changes are specific effects of COVID-19, a consequence of cytokine storm caused by systemic inflammatory response syndrome (SIRS) as in SARS [16] or a specific immunological change with elevated antiphospholipid antibodies is still a question [17]. Further studies are needed to define the underlying biological mechanisms involved in the association between hypercoagulative status and adverse outcomes in COVID-19.
In managing thrombotic status in COVID-19 patients, data was minimal. In previous vitro cell studies of SARS and heparin, exogenous heparin inhibited 50% cell infection by inhibiting SARS’s attachment and entry into the cell [18, 19]. Among ARDS and SIRS patients, therapeutic anticoagulation largely reverted DIC and resolute disease progression [20, 21]. LMWH was the most commonly applied anticoagulant, not only for its anticoagulant properties but also for anti-inflammatory activities [20]. Heparin use in smoke-induced acute lung injury or ARDS attenuated pulmonary coagulopathy and reduced mechanical ventilation days without adverse effect [22–24]. For COVID-19, existing data was limited in a subgroup analysis (N = 97) from a single retrospective study, which suggested that the LMWH use was associated with a lower 28-day mortality in COVID-19 [25]. However, the study had limited control for potential confounders. A most recent US cohort study (N = 2773) showed that a longer duration of anticoagulation treatment was associated with a reduced risk of mortality (adjusted HR of 0.86 per day, 95% confidence interval 0.82–0.89, p < 0.001), yet without the information of illness severity classification [7]. Our study collected data at an early stage of this pandemic when the hypercoagulable status of COVID-19 had not been revealed. During the study time, it was not a common practice for clinicians to widely apply anticoagulant therapy in COVID-19 in March 2020. Antithrombotic treatment was decided upon individual clinician’s judgment of hypercoagulable status, such as high D-dimer level, signs of the early stage of DIC, and high risk for DVT. Therefore, our study had 405 (77%) patients not on LMWH, allowing us to compare the LMWH users and nonusers in a natural early stage of the pandemic. The prevalence of LMWH use was similar to other studies conducted at the same time [25]. Our study with adjusted results showed that even though LMWH users were patients with worse clinical manifestations, LMWH use was associated with decreased in-hospital mortality risk. Such survival benefit existed particularly among severity classification as severely and critically ill patients. Compared to mild/moderate patients, severe and critically ill patients were more likely to have higher LMWH doses—twice a day— which may contribute to the survival benefit of LMWH, especially in critically ill patients.
Physicians from the front-line suggested that early anticoagulation may reduce microthrombus, thereby lowering the risk of significant organ damages [3, 4]. Guideline of the diagnosis and treatment of thrombotic or thromboembolic disease and COVID-19 confirmed the prophylactic dose of LMWH or UFH for hospitalized COVID-19 [3]. Major bleeding is uncommon in COVID-19 per se. However, the risk may be enormously increased in the setting of DIC. Early anticoagulation therapy may modify the course of DIC and protect patients from DIC bleeding. Physicians preferred LMWH for its limited contact of patients with medical services for INR monitoring compared with UFH and vitamin K antagonists (VKAs), easy to reserve when bleeding than novel oral anticoagulants (NOAC), and no found drug interaction with antiviral medications compared with NOAC [3]. Regarding major bleeding, our study identified three cases of major bleeding in these major sites for COVID-19, two of them received LMWH. The observation did not show a clinically meaningful difference in bleeding risk by LMWH use. However, more extensive studies are warranted to determine the risk further.
Our study provides evidence supporting LMWH use in COVID-19 patients, especially in subgroups such as elderly patients with a high inflammatory index such as high IL-6 level, and patients with confirmed hypercoagulable status such as high D-dimer level. Older age, high IL-6, and D-Dimer level were risk factors for thrombosis of COVID-19 and contributed to increased mortality [26, 27]. The protective effect of LMWH may result from the anti-inflammatory pathway, which suggested by our result that obvious benefit among high IL-6 patients. Further studies with a larger population for high IL-6 patients are warranted.
Limitations
First, this study was limited to patients who were hospitalized with COVID-19 in Wuhan. The current findings may not be generalizable to all patients with different geographic origins and races [28]. The limited population size of subgroup studies suggested that further more extensive studies are needed for specific groups, such as high IL-6 patients. Second, the study was observational, and treatment with LMWH was nonrandomized. While we adjusted our analyses for baseline differences in disease severity using both logistic regression and propensity score weighting analyses, confirmation via randomized clinical trials are warranted. Third, our study did not have the metrics to examine the dose difference of LMWH use between prophylactic and therapeutic doses. Also, two ECMO cases were included because they have initiated LMWH days before their ECMO use. Notably, both of them were non-survivors. As a result, including the two ECMO cases may have underestimated the benefit of LMWH treatment on COVID-19 patients but have not changed the direction of our results. Finally, we did not examine the regular heparin use in our study. Furthermore, we excluded patients treated with NOAC or heparin, with a limited number of patients (n = 12) in the preliminary population pool. Further studies will be needed to examine the difference between various anticoagulants such as oral anticoagulants, LMWH, and VKA.
Conclusion
Among patients hospitalized with COVID-19, in-hospital treatment with LMWH was associated with a lower risk of all-cause mortality than non-LMWH users. The survival benefit was prominent in more severely ill patients. Prospective randomized clinical trials are warranted to determine the survival benefits of LMWH in COVID-19 patients.
Authors’ Contributions
L. Shen, L. Qiu, D. Liu, and L. Wang contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript; H. Huang, H. Ge, Y. Xiao, Y. Liu, J. Ji, and X. Liu contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript; D.W. Wang, E.D. Peterson, Ben He, and Ning Zhou contributed to the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China to L. Shen (81900308) and N. Zhou (81570261) and Multi-Centered Clinical Research Foundation, Shanghai Jiaotong University, School of Medicine, to B. He (DLY201512).
Data Availability
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
This study was approved by the Ethics Commission of Tongji Hospital. Written informed consent was waived for the retrospective study.
Footnotes
Drs. Ben He and Ning Zhou both provided mentorship and are serving as corresponding authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lan Shen, Lin Qiu, Dong Liu and Li Wang contributed equally to this work.
Contributor Information
Ben He, Email: heben241@126.com.
Ning Zhou, Email: zhouning@tjh.tjmu.edu.cn.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World health organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH, Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186–E1E8. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;201561:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Commission CNH. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition). 2020. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. 2020-03-16.
- 9.Schulman S, Kearon C, Subcommittee on control of anticoagulation of the S, standardization committee of the International Society on T, Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 10.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJY. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 12.Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VE, Meijers JC, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84(10):1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e3e3. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;201544:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, de Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang J, Yang N, Deng J, Liu K, Yang P, Zhang G, Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6(8):e23710. doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicenzi E, Canducci F, Pinna D, Mancini N, Carletti S, Lazzarin A, Bordignon C, Poli G, Clementi M. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg Infect Dis. 2004;10(3):413–418. doi: 10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camprubi-Rimblas M, Tantinya N, Bringue J, Guillamat-Prats R, Artigas A. Anticoagulant therapy in acute respiratory distress syndrome. Ann Transl Med. 2018;6(2):36. doi: 10.21037/atm.2018.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons J. Pittet JF The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28(2):227–236. doi: 10.1097/ACO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon B, Schultz MJ, Hofstra JJ, Campbell DJ, Santamaria JD. Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury. Crit Care. 2010;14(5):445. doi: 10.1186/cc9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon B, Schultz MJ, Smith R, Fink JB, Santamaria JD, Campbell DJ. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care. 2010;14(5):R180. doi: 10.1186/cc9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami K, McGuire R, Cox RA, Jodoin JM, Bjertnaes LJ, Katahira J, Traber LD, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock. 2002;18(3):236–241. doi: 10.1097/00024382-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR, Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(Suppl 4):S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.