Abstract
B lymphocytes play a central role in host immunity. They orchestrate humoral immune responses that modulate activities of other immune cells and produce neutralizing antibodies that confer lasting immunity to infectious diseases including smallpox, measles and poliomyelitis. In addition to these traditional functions is the recent recognition that B cells also play critical role in maintaining peripheral tolerance and suppressing the development or severity of autoimmune diseases. Their immune suppressive function is attributed to relatively rare populations of regulatory B cells (Bregs) that produce anti-inflammatory cytokines including interleukin 10 (IL-10), IL-35 and transforming growth factor-β. The IL-35-producing B cell (i35-Breg) is the newest Breg subset described. i35-Bregs suppress central nervous system autoimmune diseases by inducing infectious tolerance whereby conventional B cells acquire regulatory functions that suppress pathogenic Th17 responses. In this review, we discuss immunobiology of i35-Breg cell, i35-Breg therapies for autoimmune diseases and potential therapeutic strategies for depleting i35-Bregs that suppress immune responses against pathogens and tumor cells.
Keywords: regulatory B cell (Breg), Breg immunotherapy, interleukin 35, interleukin 10, interleukin-35-producing Breg (i35-Breg)
Introduction
The immune system has developed multiple layers of defense against existing and emerging pathogens and these proinflammatory strategies are counterbalanced by an equally elaborate network of regulatory mechanisms that restrain exuberant effector immune responses that cause pathogenic autoimmunity. The proinflammatory effector branch of the immune system is orchestrated by T-helper cells (Th1, Th2, Th9, Th17, TFH) and myeloid cells (macrophages, monocytes, neutrophils, dendritic cells, eosinophils) that secrete inflammatory cytokines (IFN-γ, TNF-α, IL-2, IL-4, IL-17, IL-12, IL-23), chemokines, histamine, bradykinin, prostaglandins or leukotrienes. On the other hand, countervailing suppressive responses that check effector responses are mediated in part by myeloid-derived suppressor cells, immune-suppressing dendritic cells and macrophages. However, specialized regulatory lymphocytes are most critical for suppressing the expansion and functions of pathogenic T cells that cause autoimmune diseases. Thus, susceptibility or resistance to systemic or organ-specific autoimmune diseases requires a healthy balance between responses induced by effector and regulatory arms of the immune system.
Research by Gershon, Kondo, Nishizuka and Sakakura half a century ago laid the foundation to our understanding of the role lymphocytes play in immune suppression and led to the widely accepted notion that immune tolerance to a pathogen is transferable to another mouse or human [1–4]. A quarter century later, Sakaguchi et al. described the CD4+ CD25+ Foxp3+ naturally occurring thymic-derived regulatory T cell (nTreg) population that mediates suppression through production of IL-10 [5]. The development of a simple, rapid and reliable assay for the in vitro measurement of suppressor activity by Thornton and Shevach eventually led to the confirmation that CD4+ CD25+ Tregs do indeed exist in humans [6–9]. Other Treg subsets, generically referred to as peripherally induced Tregs (iTregs), were soon described and these include the Tr1 subset (CD4+ CD25− Foxp3−) that inhibits T cell responses through IL-10 and inhibitory receptors (CTLA-4, PD-1) and Th3 cells (CD4+ CD25+ Foxp3+) that produce transforming growth factor-β (TGF-β) [10,11]. The newest iTreg member described in 2010 suppresses inflammation via production of IL-35 (iTR35), an immune-suppressive cytokine of the IL-12 family [12].
Evidence that B cells may also suppress inflammation was provided as early as 1968 in a report showing that adoptive transfer of spleen cells from hyperimmunized mice inhibited antigen-specific immune responses but at that time the suppression of inflammation was proposed to be mediated through production of inhibitory antibodies [13]. Subsequent studies revealed that the B cells induce differentiation of Treg cells that mediated tolerance to the antigen in the recipient mice [14–16]. Existence of regulatory B cells (Bregs) that produce immune-suppressive cytokines including IL-10, IL-35 and TGF-β in mice is now widely accepted [17,18]. Although studies that established the critical roles of Bregs in regulating inflammation and autoimmune diseases came from mouse models of autoimmune diseases, studies of several human autoimmune diseases have revealed numerical and functional Breg defects in SLE, RA, MS, and psoriasis indicating importance of Breg cells in the context of human immunity [19–21]. An aberrant increase in human Bregs has also been implicated in other immune-related pathologies, such as, cancers, and chronic infections, underscoring the role in health and disease. However, because of the limited number of human i35-Breg studies, our focus will be on mouse Breg studies [22–24]. Although our focus will be on i35-Breg cells, we begin by providing a brief overview of the various Breg subtypes and their development before highlighting unique features that make i35-Bregs an attractive Breg subtype for immunotherapy against autoimmune and neurodegenerative diseases.
Breg Subsets
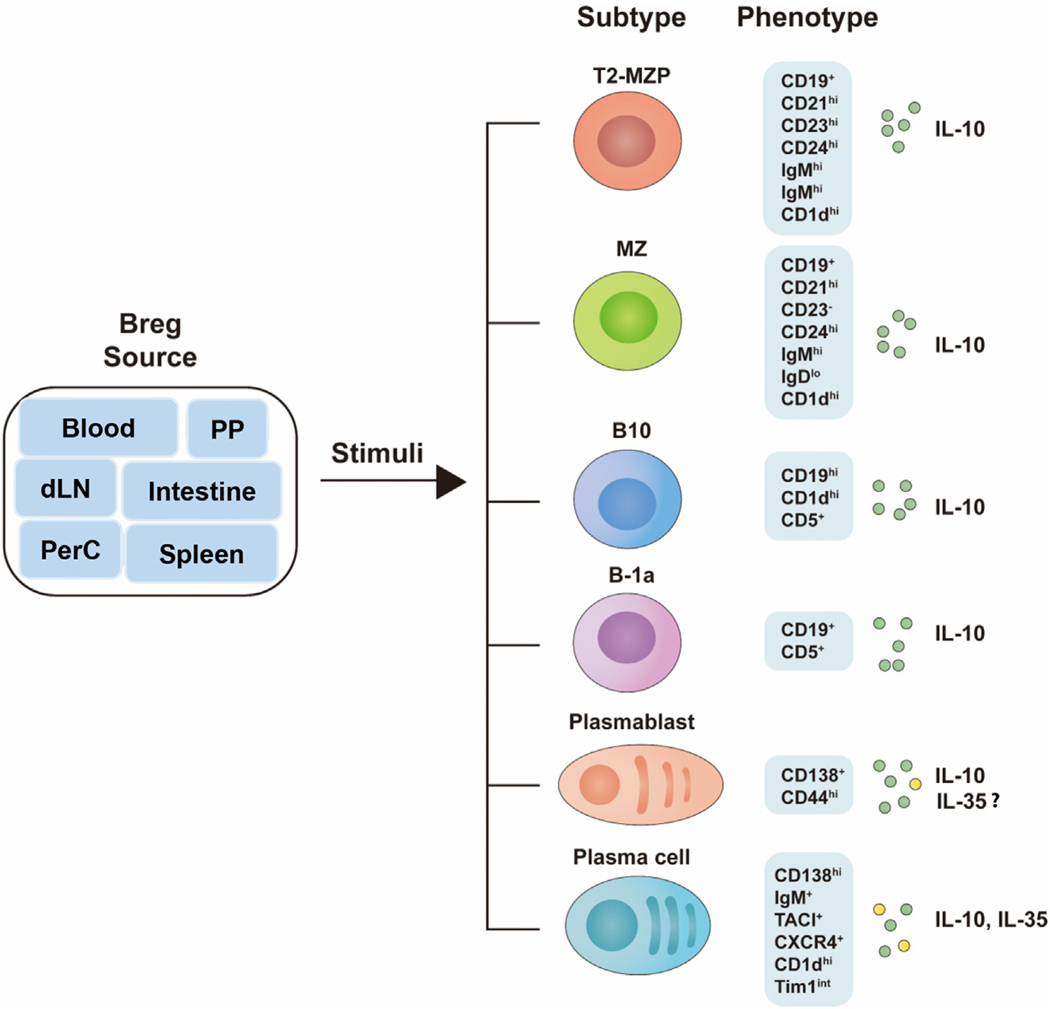
Breg cells comprise 1%–3% of B cells in the mouse spleen with smaller numbers in the blood, lymph nodes, Peyer’s patches, intestinal tissues and peritoneal cavity and their numbers increase significantly during autoimmune and infectious diseases in humans and mice. They derive from cells at all B-cell developmental stages including marginal zone (MZ) (CD19+ CD21hiCD23−CD24hiIgMhiIgDloCD1dhi), transitional 2-MZ precursor (T2-MZP) (CD19+ CD21-hiCD23hiCD24hi IgMhiIgDhiCD1dhi, B10 (CD19hiCD1dhiCD5+) cells, B-1a (CD19+ CD5+), plasmablasts (CD138+ CD44hi) and plasma cells (CD138-hiIgM+TACI+CXCR4+CD1dhiTim1int). However, the developmental origin of Breg cells is unknown and it is also not known whether Bregs are a dedicated B-cell lineage with a master transcription factor that specifies its gene expression program, akin to the nTreg lineage. However, it is of note that activated follicular, MZP and MZ B cells migrate to regional draining lymph nodes (dLN) where they acquire regulatory functions through interactions with myeloid cells that secrete immune-regulatory cytokines, suggesting that Bregs might resemble iTreg cells that acquire regulatory functions during inflammation in response to anti-inflammatory cytokines in the dLN [25,26]. It is, however, of note that for ex vivo development of Breg cells, pre-activation of the B cells by Toll-like receptor (TLR) agonist, CD40 ligand (CD40L) or B-cell receptor (BCR) signals is not sufficient. Efficient generation of significant numbers of Bregs requires the addition of inflammatory cytokines and phenotypic diversity of the various Breg subsets is thought to derive in part from nature of the stimuli in the culture medium (Figure 1).
Figure 1.
Phenotypes of mouse Breg subsets. PP, Peyer’s patch; dLN, draining lymph nodes; Intestine, intestinal tissues; PerC, peritoneal cavity; T2-MZP, transitional 2-marginal zone precursor; MZ, marginal zone.
Immunoregulatory Functions of Bregs
Regulatory role of B cells in autoimmune diseases was first suggested by reports of the exacerbation of inflammatory disorders in B cell-deficient mice and role of IL-10-producing B cells (i10-Bregs) was subsequently established by the development of severe experimental allergic encephalomyelitis (EAE) in mice with targeted deletion of il10 in B cells [27–29]. Adoptive transfer of MZP i10-Breg cells suppressed arthritis or SLE-like disease in mice and human MZP i10-Breg cells were found to be functionally impaired in SLE patients, providing additional evidence of immune-suppressive function of i10-Breg cells [20,30,31]. Suppression of inflammation by the i10-Breg cells was latter shown to correlate with increase of Treg cells and inhibition of the expansion of proinflammatory Th1 and Th17 cells [32,33]. Besides MZP and MZ i10-Breg cells, other B-cell types also suppress inflammation through IL-10-dependent and independent mechanisms [34–36]. To distinguish i10-Breg that utilize IL-10-dependent from IL-10-independent mechanisms, B cells that exclusively mediate immune regulatory functions though IL-10-dependent mechanisms are denoted as B10 cells; they are characterized by CD1d+ CD5+ immunophenotype and considered to be the most abundant Breg cells in the spleen and [34–37].
Other Breg Subsets
Increasing number of B cells that exhibit regulatory functions continue to be identified and characterized by the cell surface markers they express or mechanisms by which they suppress inflammation. Among these are: human and mouse Breg cells that express the T-cell Ig mucin domain-1 (TIM-1) transmembrane glycoprotein [38]; Bregs that suppress inflammation by IL-10 independent mechanisms include PD-L1hi Bregs [39]; plasma cells signaling through TGF-β receptor [40] and CD73+ adenosine producing Breg cells [41]. Although splenic B10 cells, MZP and MZ Breg cells suppress autoimmune diseases, it was not known whether B cells at later stages of development could also acquire the capacity to produce IL-10 and suppress inflammation in vivo. That B cells could differentiate into IL-10-producing CD138+ plasmablasts provided evidence that B cells at most stages of development can acquire Breg functions [25,42]. In 2014, a novel Breg subset corresponding to B220loCD19+ CD5+ or CD138hiTACI+ CXCR4+ CD1dintTim1int plasma cells was described and shown to suppress CNS inflammation in mice through production of IL-35 and IL-10 [43,44].
Discovery of IL-35-Producing Breg Cell (i35-Breg)
Collison et al. showed that IL-35-producing Treg cells (iTR35) suppressed colitis in the mouse, spurring interest in whether Breg cells can also produce IL-35 [45]. Two groups using two complementary approaches simultaneously provided evidence that IL-35-producing cells play critical role in immunity during autoimmune diseases [43,44]. Wang et al. induced experimental autoimmune uveitis (EAU), treated the mice with mouse recombinant IL-35 (rIL-35) and suppression of uveitis was found to correlate with the expansion of B cells secreting IL-10 and IL-35 (i35-Bregs) [43]. In addition, transfer of ex vivo generated i35-Breg suppressed EAU but not in mice that do not respond to IL-35 signal (IL12Rβ2−/−) [43]. On the other hand, Shen et al. showed that mice with B-cell-restricted deficiency in IL-p35 (p35−/−) or EBI3 (Ebi3−/−) developed exacerbated experimental autoimmune encephalomyelitis (EAE) [44]. Together these studies indicate that B cells limit pathogenesis of EAU or EAE through provision of IL-35.
Immunobiology of IL-35 and IL-12 Family Cytokines
IL-35 is a member of the IL-12 family and IL-12 cytokines have emerged as important regulators of host immunity [46]. During Ag-priming, lymphocytes differentiate into pro-inflammatory or immune-suppressive subsets and IL-12 family cytokines play critical roles in lymphocyte developmental decisions that determine the relative abundance of lymphocyte subsets that participate in the ensuing immune response. The family comprises IL-12 (IL12p35/IL12p40), IL-23 (IL23p19/IL12p40), IL-27 (IL27p28/Ebi3), IL-35 (IL12p35/Ebi3) and IL-39 (IL23p19/Ebi3) [46–48]. Each member is composed of an α-subunit with a helical structure similar to type 1 cytokine, IL-6, and a β-subunit structurally related to the soluble IL-6 receptor (IL-6Rα). Thus, each IL-12 family cytokine is in essence a heterodimer that comprises a cytokine and a cytokine receptor. IL-12 cytokines signal by activating the Janus kinase/signal transducer and activator of transcription (JAK–STAT) pathway, and each IL-12 member binds to its cognate heterodimeric receptors, with IL-12 signaling through IL12Rβ1/ IL12Rβ2, IL-23 via IL12Rβ1/IL23R and IL-27 through gp130/IL27Rα (WSX-1) [46]. In T cells, IL-35 is thought to signal through IL12Rβ2/lL12Rβ2, IL12Rβ2/gp130, and gp130/gp130, whereas in B cells, IL-35 signals via IL-12Rβ2/IL27Rα [43,49]. Upon binding to cognate receptors (IL12Rβ1, IL12Rβ2, IL23R, IL27Rα, or gp130), receptor-associated Janus kinases (Jak1, Jak2, Tyk2) are activated, providing phosphortyrosine-docking sites that recruit specific members of the STAT transcription factors. IL-12 mediates signaling via pSTAT4, IL-23 via pSTAT3 and pSTAT4, IL-27 through pSTAT1 and pSTAT3. IL-35 utilizes pSTAT1/pSTAT4 in T cells and pSTAT1/ pSTAT3 in B cells [43,49]. The pro-inflammatory members are IL-12, IL-23 and IL-39, while IL-27 and IL-35 are considered the immune suppressive members. Published reports suggest that during Ag-priming, microenvironment of lymph nodes containing elevated levels of dendritic cells producing IL-12 and/or IL-23 skew naïve T-cell differentiation toward proinflammatory Th1 or Th17 developmental pathway, respectively [50,51]. On the other hand, intense inflammation elicits recruitment of dendritic cells and other antigen presenting cells that produce IL-35 and/or IL-27, thereby promoting the differentiation of regulatory lymphocytes that curtail exuberant immune responses that cause autoimmune disease [47]. In contrast to IL-12 and IL-23, the α and β chains of IL-27 or IL-35 are not covalently linked and it is assumed that the IL12p35, IL27p28 and Ebi3 subunits are independently secreted and form IL12p35:Ebi3 and IL27p28:Ebi3 heterodimers in vivo during the inflammatory response. Understanding of the mechanisms that regulate stability of these heterodimeric cytokines in vivo has remained elusive.
IL-35 Signaling Pathways in B Cells
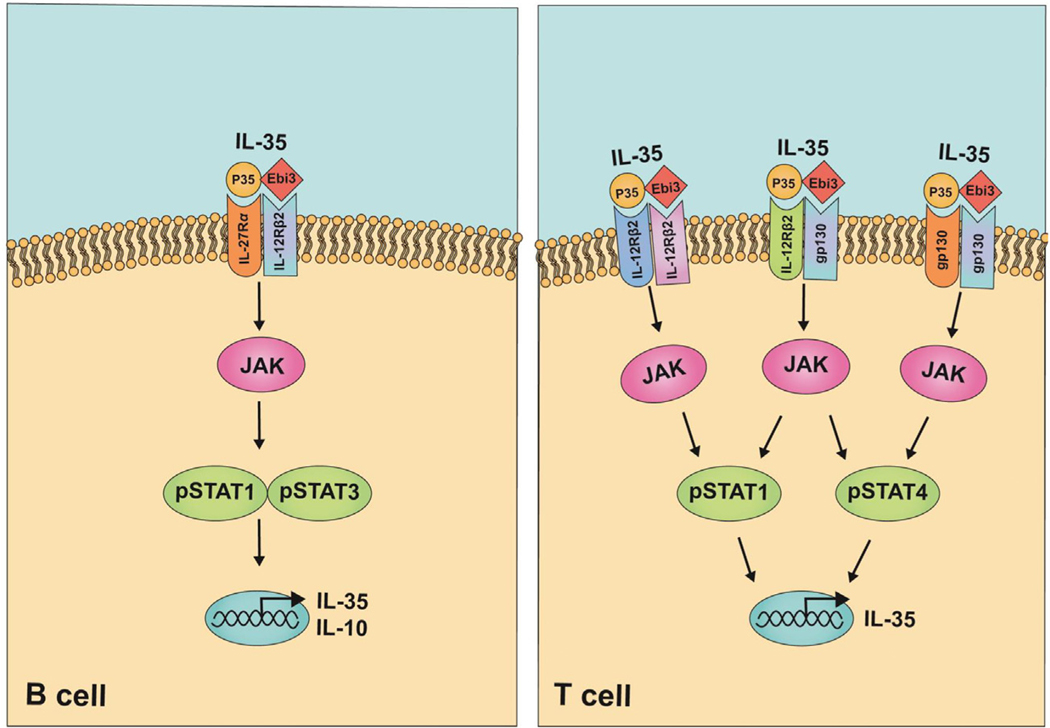
How IL-35 interacts with its cognate receptor and orchestrates downstream signaling pathways that mediate its biological effects is poorly understood. In the absence of X-ray crystallographic data for any of the IL-12 family cytokines, predictions of IL-35 extracellular signaling complex have mostly been inferred from the “site 1–2–3” architectural paradigm proposed for IL-6 and this model is based on hexameric structure and assembly of the IL-6/IL-6Rα/gp130 complex [52]. Structural models of cytokine-receptor complexes formed by IL-12 cytokines predict that “site 1” might mediate interaction between the four-helix bundle of the α-chain (p19, p28, p35) and the β-chain (Ebi3, p40). The nature of the interactions of various combinations of α/β subunits with sites 2 and 3 regions is thought to activate divergent signaling pathways, which underlie unique physiological effects of each IL-12 family cytokine [46]. However, applying the site 1–2–3 paradigm to the understanding of signaling upstream or downstream of the IL-35 receptor is confounded by the fact that IL-6 is a monomer, while IL-35 is a heterodimeric cytokine. In addition, while IL-6 signaling is mediated by classic IL-6 activation via membrane-bound IL-6 receptors (IL-6R) and gp130, IL-6 also utilizes an alternative signaling pathway (IL-6 trans-signaling) involving soluble IL-6R (sIL-6R) with the association of the IL6/sIL-6R complex with gp130 bypassing the requirement of IL-6Rα [53,54]. It is therefore notable that a recent report identified a membrane-bound IL-12p35 (mIL-12p35) on tumor cells and another revealing an Ebi3-mediated IL-6like trans-signaling pathway, raising the possibility that IL-12p35 might utilize mIL-12p35/Ebi3/gp130 trans-signaling mechanisms independent of heterodimeric IL-35 [55,56]. As noted above, IL-35 activates STAT1 and STAT3 in B cells via heterodimeric receptors comprising of IL-12Rβ2 and IL-27Rα (Figure 2(a)) [43]. However, additional studies are required to understand why in CD4+ T cells, IL-35 signals through heterodimeric receptor chains IL12Rβ2 and gp130 as well as unconventional receptor complexes comprised of IL-12Rβ2/IL-12Rβ2 or gp130/gp130 homodimers [49]. It is therefore intriguing that gp130 homodimerization as a functional signaling receptor has only been observed in viral IL6 signaling or during IL-6 trans-signaling via IL-6: IL6R:gp130 complexes [57,58]. However, similar to B cells, viral IL-6 activates STAT1 and STAT3, while STAT1 and STAT4 are activated by IL-35 in T cells (Figure 2(b)) [59,60]. Nevertheless, it is still unclear whether the differential receptor and STAT utilization in response to IL-35 signaling derives from intrinsic differences between IL-35 signaling mechanisms of T and B cells or due to differences in the quality of the IL-35 preparations used by various labs.
Figure 2.
Interleukin 35 (IL-35) signaling pathways in B and T lymphocytes.
Regulation of i35-Breg Development and Functions during Inflammation
Inflammatory cytokines produced by gut microbiota promote the differentiation of Breg cells while the treatment of mice with antibiotics decreases Breg numbers, suggesting that cytokines may play important roles in regulating the development and/or expansion of Breg cells [61]. However, chronic exposure of B cells to elevated levels of proinflammatory cytokines substantially reduces numbers of functional Bregs in autoimmune disease patients [62]. Additionally, mice challenged with pathogens that induce IFNs activate effector B cells that produce IFN-γ or induce rapid accumulation of effector B cells at sites of inflammation [63,64]. These studies suggest that proinflammatory cytokines antagonize Breg development. The seminal work leading to discovery of i35-Bregs by Wang et al. was derived from studies to determine whether immune-suppressive cytokines have diametrically opposite effects from proinflammatory cytokines. As noted above, they induced uveitis in mice and demonstrated that treatment of the mice with rIL-35 ameliorated uveitis by inducing the expansion of i35-Breg cells [43,65]. Another report indicated that rIL-35 could induce the expansion of i35-Breg cells in vitro if the B cells are pre-activated with TLR agonists, anti-CD40 or BCR. These and other studies established that elevated levels of immune-suppressive cytokine such as IL-35 in the lymph nodes during priming of naïve B cells might serve as physiological signals for the differentiation and expansion i35-Bregs. However, major cell types that produce IL-35 in vivo and mechanisms that regulate IL-35 production by B cells are poorly understood. Thus, while CD4+ T cells and myeloid cells are the dominant cells recruited into the retina during ocular inflammation [66,67], analysis of the blood, draining LNs, and spleen of mice with EAU revealed ∼7-fold increase of IL-10-producing Bregs compared to Tregs or IL-10-secreting myeloid cells [68]. The level of IL-35-expressing Bregs was even higher with significant proportion of the Bregs producing both IL-10 and IL-35 [68]. Analysis of IL-35-stimulated B cells by EMSA and ChIP assays revealed that transcription of il12a and ebi3 require IL-35-induced activation of BATF-IRF4-IRF8 complex, and B cells that do not express high levels of IRF4 and IRF8 transcription factors do not efficiently secrete IL-10. These observations indicate that co-recruitment of IRF4 and IRF8 to immune suppressive genetic loci of B cells may be required for differentiation to Bregs that produce IL-10 and IL-35 [68].
i35-Bregs in Immune-Related Disease
Reduced numbers and functional defects of Bregs correlate with disease severity in several autoimmune diseases including multiple sclerosis, rheumatoid arthritis and systemic lupus erythematosus [19–21,29,69]. These observations are consistent with results from animal models of uveitis and MS showing that mice deficient in IL-35 develop exacerbated EAU and EAE [43,44]. Role of IL-35 in suppressing autoimmune diseases is further corroborated by studies showing that reduced levels of IL-35-producing Treg cells (iTR35) develop exacerbated disease in mouse models of autoimmune diseases including inflammatory bowel disease (IBD) [45,70,71]. In contrast to autoimmune diseases, robust immune response is required to clear infectious agents and cytokine-mediated immune responses play important role in host protection from infectious diseases. However, chronically infected individuals harboring asymptomatic levels of the pathogen also require peripheral immune tolerance mechanisms mediated by regulatory Tregs and Bregs to prevent reactivation of the virus or parasite. Dual role of i35-Breg cells in immunity is further illustrated by report showing that mice lacking functional IL-35 in B cells develop exacerbated EAE while the mice are resistant to infection with intracellular bacterial pathogen (Salmonella enterica serovar Typhimurium) [44]. In hepatitis B virus infection, it has also been suggested that IL-35 plays the role of modulating the balance between regulatory and effector lymphocytes with disease outcome depending on the stage of the disease [72]. Thus, in acute hepatitis B virus infection, elevated IL-35 suppresses liver inflammation and hepatocyte damage [73]; while in chronic hepatitis patients, persistent elevation of IL-35 levels promotes liver inflammation and necrosis, and inhibits liver function [74].
Similarly, IL-35 plays diametrically opposite functions in autoimmunity and cancer as i35-Breg cells are upregulated in a number of neoplastic diseases and has been implicated in contributing to the progression of gastric cancer [75]. In a mouse model of breast cancer, Breg cells promoted lung metastasis by inducing the differentiation of conventional T cells to Treg cells that suppress tumor immunity [76]. Mechanistically, the acquisition of immune-suppressive properties by B cells in the tumor microenvironment correlates with upregulation of TGF-β, PD-L1, CD86, and IL-10 expression [77]. However, phenotype of the tumor evoked Bregs that suppress antitumor immune responses has not been fully characterized and understanding of factors that promote their recruitment to tumor microenvironment would aid in developing strategies to target them and enhance antitumor immunity. Besides inducing the differentiation of Treg cells that inhibit tumor-infiltrating anti-tumor T cells, paracrine effects of the IL-35 produced by the Bregs upregulates anti-apoptotic and cell cycle genes that promote cell growth (survivin, FAS, Bcl-2 cyclin D1). IL-35 also promotes carcinogenesis by upregulating expression of inhibitory receptors (Pd1, Lag3, TIM1) on the anti-tumor CD4+ and CD8+ tumor-infiltrating lymphocytes and inducing intra-tumoral T-cell exhaustion [24].
IL-35 Therapy
Since its discovery immune suppressive functions of IL-35 has been of interest as a biologic for treatment of autoimmune diseases. However, its therapeutic use has been hampered by difficulty of isolating sufficient amounts for clinical trials. Each of its subunits, IL-12p35 and Ebi3, are secreted independently and they associate to form the heterodimeric IL-35 cytokine during inflammation. However, the two subunits form a weakly associated non-covalent heterodimer that exist at extremely low concentrations in vivo, making it difficult to isolate the native IL-35 in vivo. Besides the instability of the IL-12p35/Ebi3 heterodimer, another reason very low abundance of IL-35 in vivo is that both subunit proteins must be simultaneously expressed in the same cells. Synthesis of the IL-12p35 protein is the limiting factor for production of IL-35 as in the absence of IL-12p35 substantial amount of the synthesized Ebi3 is degraded in the endoplasmic reticulum, thereby contributing to low IL-35 levels in vivo. Thus, therapeutic use of IL-35 required genetic engineering of the “native” IL-35. Recombinant mouse IL-35 protein was generated using a bicistronic vector containing IRES (internal ribosomal entry site) that allowed stoichiometric expression of the Ebi3 and IL-12p35 [43,45] in HEK293T cells [45] or insect cells [43]. Despite high level expression of the two proteins, less than 5% of the proteins in the insect cell cultures formed the desired IL-35 heterodimer, with majority of the secreted proteins existing as Ebi3:Ebi3 and IL-12p35:IL-12p35 homodimers [43]. Preferential formation of homodimers is attributed in part to the fact that EBI3 has 3 methionine and 4 cysteine residues, whereas IL-12p35 has 10 methionine and 7 cysteine residues [78]. Nonetheless, significant amounts of the highly purified heterodimeric rIL-35 can be generated following 2 cycles of FPLC chromatography on Supercryl S-200 followed by 2 cycles of fractionation on Superose-6 columns [43,79]. Confirmation that the purified rIL-35 was indeed a heterodimer was established by sedimentation equilibrium ultracentrifugation [43]. The rIL-35 suppressed ocular inflammation in the EAU model indicating that the rIL-35 is biologically active and that human rIL-35 can potentially be used to treat human uveitis [43]. In EAU, the rIL-35 suppressed disease by inhibiting Th17 and Th1 cells that mediate the disease and inducing the expansion of i35-Breg cells [43]. Although rIL-35 produced as IL-12p35-Ebi3 fusion protein also exhibits immune suppressive functions in vitro, compared to the “native” heterodimeric rIL-35, it is less effective in suppressing disease (unpublished data).
i35-Breg Therapy
Major impediment to i35-Breg therapy is the relatively low levels of i35-Bregs and technical difficulties associated with isolating large quantities of i35-Breg cells. Approximately 8% of the Bregs in the spleen of mice injected with LPS or rIL-35 produce both IL-10 and re-stimulation of the cells in vitro with rIL-35 increases their percentage to ~35% with ~18% of the cells co-producing IL-10 and IL-35 [43]. However, prolonged propagation of the cells in culture leads to progressive loss of Breg cells co-producing IL-10 and IL-35 and expansion of Bregs exclusively producing IL-35. This suggests that B cells that co-produce IL-10 and IL-35 (i35-Breg) or those that exclusively produce IL-10 or IL-35 overlap and may represent Breg subsets at different stages of differentiation or development. It is, however, unclear whether IL-35 directly induces the differentiation of B cells into i35-Bregs or merely expands a rare i35-Breg population. Nonetheless, in vitro culture conditions for generating mouse or human i35-Breg cells include stimulation with anti-CD40, BCR agonist (anti-IgM + anti-CD40), or TLR agonist (LPS, CpG) and the agonists can be supplemented with cytokines (BAFF, APRIL, IL-2, IL-15, IL-35). For treatment of mice with EAU, EAE or other organ-specific autoimmune diseases, the i35-Breg therapy cells can be initiated 5 days after disease induction and in most cases disease suppression often correlate with inhibition of proinflammatory Th17 and Th1 responses. The i35-Breg cells also suppress disease by promoting infectious tolerance by transferring suppressive function onto a conventional T- and B-cell populations.
Adaptive Plasticity of i35-Bregs
Adoptive transfer studies using CD45.1+ and CD45.2+ congenic mice have been used to trace the fate i35-Bregs during inflammation. In the EAU model, CD45.2+ i35-Bregs were adoptively transferred to CD45.1+ mice with EAU and the CD45.2+ B cells were found to proliferate in tissues of the recipient CD45.1+ mice. In addition, the CD45.1+ mice contained not only CD45.1+ and CD45.2+ B cells co-expressing IL-10 and IL-35 (i35-Bregs) but the mice also contained Breg cells exclusively producing IL-10 or IL-35, suggesting that in vivo i35-Bregs might comprise of functionally distinct subsets of Breg cells at different stages of B-cell development [43]. It is notable that while IL-10 or IL-35 can suppress the proliferation of conventional B cells in vitro, only IL-35 induces the expansion of IL-10 and/or IL-35-producing Bregs [43]. This suggests that IL-10-producing B cells may function to suppress inflammatory lymphocytes and orchestrate peripheral tolerance mechanisms while i35-Bregs may serve to induce infectious tolerance mechanisms that expand the pool of Breg cells required to suppress inflammation in vivo.
Emerging i35-Breg Therapies
Although administration of autologous i35-Breg has promise as a potentially effective immunotherapy for CNS autoimmune and neurodegenerative diseases, some challenges must be overcome before it can be brought to the clinic. A major limitation of i35-Breg therapy is the issue of dosing as biologically active IL-35 is a weakly associated (non-covalent) heterodimer of IL12p35 and Ebi3 that readily dissociate, making it difficult to ascertain the bioavailability of the IL-35 secreted by i35-Breg. A second obstacle pertinent to CNS diseases is that trafficking of cells, including lymphocytes, to the brain, retina or spinal cord is highly restricted by the blood–brain barrier (BBB) and blood–ocular barrier (BOB). Thus, entry of Bregs into the CNS must be preceded by proinflammatory Th17 and CD8+ T cells that initiate breakdown of the BBB or BOB through perforin-mediated disruption of tight junctions. Resolving these issues are important to effective i35-Breg immunotherapy. We describe below alternative approaches that therapeutically exploit the immunoregulatory functions of IL-35 and its subunits (Figure 3).
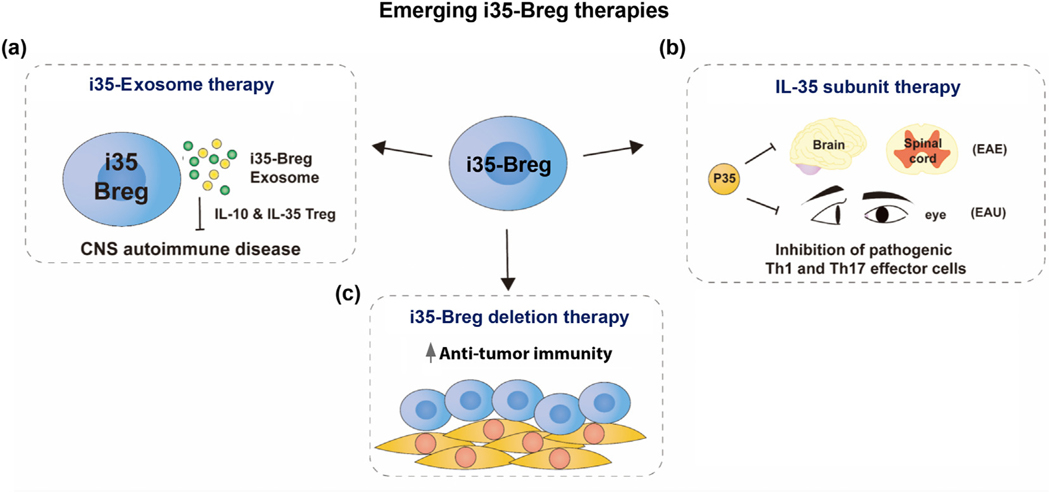
Figure 3.
Emerging i35-Breg therapies. (a) IL-35 containing exosomes (i35-Exosomes) suppress neuroinflammation and CNS autoimmune diseases in mice including experimental autoimmune encephalomyelitis (EAE) and experimental autoimmune uveitis (EAU). (b) IL-12p35 antagonizes pathogenic Th1 and Th17 responses and suppresses uveitis and encephalomyelitis in mice. (c) IL-35 produced by regulatory lymphocytes induces intra-tumor anti-tumor CD4+ and CD8+ T cells to undergo T-cell exhaustion. Targeted i35-Breg depletion therapy might promote anti-tumor immunity.
i35-Breg-Exosome therapy
The BBB and the BOB limit the size of vehicles for delivering compounds into the brain or the retina and is a major impediment to use of cell therapy in treatment of neuroinflammatory diseases. Exosomes are 30- to 150-nm nanosized extracellular vesicles shed by almost all cell types, they are enriched in bioactive molecules and exosomes from immune cells modulate immune responses that regulate the development or suppression of autoimmune diseases. Exosomes readily penetrate the BBB or BOB and have highly desirable qualities as drug delivery vehicles into CNS tissues [80,81]. They exhibit minimal toxicity or immunogenicity and are therefore ideal natural nanocarriers that for delivering biologics to CNS tissues as treatment for neurogenerative and CNS autoimmune diseases. A recent report demonstrating that i35-Bregs release exosomes that contain IL-35 (i35-Exosomes) indicates that i35-Exosomes can be exploited for delivery of IL-35 into the CNS. In fact, mice treated with i35-Exosomes were protected from developing severe uveitis and disease protection correlated with expansion of IL-10 and IL-35 secreting Treg cells with concomitant suppression of Th17 responses (Figure 3(a)) [82]. i35-Exosome therapy has several important advantages over i35-Breg therapy: (i) The ease of isolating large amounts of i35-Exosomes circumvents the technically difficult and labor-intensive effort entailed in producing sufficient amounts of i35-Bregs for therapeutic use The authors showed that 3 days after injection of mice with anti-CD40/anti-IgM, as much as 1 mg exosomes (~32 × 1010/ml) can be isolated from a mouse of which 1 × 1010 i35-exosomes contained 15 μg IL-35 [82]; (ii) i35-exosomes containing IL12p35/Ebi3 heterodimers are compartmentalized in a vesicle obviating the dosing issue of ascertaining the precise amount of bioactive IL-35 delivered to the subject; (iii) although exosomes have been shown to express immunostimulatory markers including MHC-II [83,84], they appear to exhibit low immunogenic potential in the immune privileged environment of the neuroretina as stem cell or astrocyte derived exosomes have been used to inhibit laser-induced choroidal neovascularization or uveoretinitis [85–87]. If indeed exosomes delivered in the retina are not allogeneic, delivery of IL-35 to CNS tissues with exosomes would allay the concerns of HLA mismatched i35-Breg cell therapy that might be rejected or induce graft-versus-host-like disease in the recipient. Moreover, as exosomes are non-cellular particles, they have an advantage over cell immunotherapy since they would be refractory to effects of cytokines [88,89]. However, there has been limited research on the latent effects of exosomes loaded with exogenous functional cargoes in eye diseases, and additional studies are needed to develop such therapies in ophthalmology. It is also notable that Bregs that are used for immunotherapy in mouse models of IBD and arthritis as well as in EAE or EAU are generally elicited with the immunogen used for disease induction. However, it has not been rigorously investigated whether these Bregs act in antigen-dependent manner or if Bregs that suppress arthritis can also suppress EAE. This is not an issue with i35-exosome therapy because as nanocarriers merely functioning as IL-35 delivery vehicles, they can be used to treat diverse inflammatory disease. Taken together, absence of toxicity or alloreactivity makes i35-Exosomes an attractive therapeutic option for delivering IL-35 into CNS tissues.
IL-35 subunit therapy
Producing sufficient quantities of the functional IL-35 heterodimeric cytokine is challenging, is very labor intensive, and has been a major obstacle to ex vivo production of large-scale i35-Bregs for therapy. This led to interest to investigate whether single-chain IL-12p35 or Ebi3 might also possesses intrinsic immune-regulatory activities that could be exploited therapeutically. This led to realization of IL-12p35 subunit possesses immunoregulatory properties hitherto attributed to IL-35. It suppresses lymphocyte proliferation, antagonizes pathogenic Th17 responses and the suppression of inflammation correlated with increase of Breg cells (Figure 3(b)) [23,79]. However, these immune suppressive effects were not observed in IL-12Rβ2 deficient mice, suggesting that its anti-inflammatory effects require signals downstream of the IL-12Rβ2. Surprisingly, IL-12p35 does not activate STAT1 or STAT3 pathway but inhibits these STAT pathway and cell-cycle regulatory proteins, suggesting that it antagonizes signals downstream of the IL-35 receptor [23]. The Ebi3 subunit exerts a more potent growth inhibitory effect than IL-12p35, and unlike IL-12p35, it does induce expression of IL-10 or IL-35, suggesting that inhibition of T lymphocyte proliferation by IL-35 derives in part from synergistic effects of IL-12p35 and Ebi3, while transcriptional activation of Il10, Il12a, and ebi3 mainly requires IL12p35 [79]. Studies of the individual IL-35 subunit proteins thus show that IL-12p35 and Ebi3 may exert distinct and overlapping effects that can be exploited therapeutically.
i35-Breg depletion therapy
Modulating IL-35 levels or depleting i35-Bregs is a therapeutic approach that can be beneficial in chronic bacterial/parasitic infections and cancer, which require robust proinflammatory response to eliminate or neutralize the pathogen or tumor cells. Despite the obvious appeal of Breg depletion therapy in these diseases, Breg depletion therapy may also render the patient susceptible to autoinflammatory or autoimmune diseases. Moreover, the lack of Breg-specific markers may lead to off-target effects resulting indiscriminate depletion of effector B cells that mediate sterile immunity to pathogens and promote anti-tumor immunity (Figure 3(c)). Recent reports show that unlike conventional effector B cells, Breg cells including i35-Bregs exhibit enhanced expression of the inhibitory receptors Lag3, PD-L1, and PD-L2 that can be exploited to selectively deplete i35-Breg cells or suppress their capacity to induce T-cell exhaustion [22–24]. Observation that the IL-12p35 subunit antagonizes IL-35 signaling pathway downstream of the IL-12Rβ2 also provides another strategy to selectively inhibit activity of i35-Bregs.
Conclusion Perspective
Discovery of i35-Breg cell expands the growing number of regulatory B-cell types and indicates that additional Breg with unique cytokine expression profiles would be identified in the future. Despite the relatively low abundance of i35-Breg cells in PBMCs or the mouse spleen, their numbers increase dramatically during inflammatory disease, and they have the distinctive capacity of inducing infectious tolerance and thus amplify the repertoire of regulatory B and T cells that confer protection against autoimmune diseases. Although the role and phenotypes of IL-10-producing Breg cells have been extensively studied, the number of studies of i35-Bregs in mouse models of human disease is limited, and by necessity in this review, we concentrated the induction and role of i35-Breg in mouse models of CNS autoimmune diseases. The take-home message from studies of the immunobiology of i35-Bregs and their roles in the EAU, the mouse model of human uveitis and EAE, mouse model of multiple sclerosis are as follows: (i) Compared to Tregs or IL-10-secreting myeloid cells, Bregs are the major producers of IL-35 in the blood, draining LNs and spleen of mice with EAU and most of the Bregs produced both IL-10 and IL-35. (ii) IL-35 signal downstream of its receptor (IL12Rβ2/IL27Rα) in B cells is mediated through Jak1/Jak2 and STAT1/STAT3, and the production of IL-10 and IL-35 by i35-Bregs requires the recruitment of BATF-IRF4-IRF-8 transcription factor complex to il10, il12a and ebi3 genetic loci. (iii) i35-Breg represents three overlapping Bregs at different stages of differentiation or development and comprises cells that co-produce IL-10 and IL-35 and those that exclusively produce IL-10 or exclusively produce IL-35. (iv) i35-Breg cells suppress encephalomyelitis and uveitis in mice by inhibiting Th17 and Th1 cells that mediate EAE or EAU, inducing infectious tolerance that and reprogram conventional B and T lymphocytes into IL-10 and/or IL-35-producing cells. Dysregulated expression of IL-35 is observed in inflammatory autoimmune diseases, including marked reduction in primary Sjogren’s syndrome, Behçet’s disease, multiple sclerosis, and type 1 diabetes while elevated in gastric cancer, hepatitis, myositis and systemic sclerosis. Thus, i35-Breg, IL-35 and emerging IL-35 therapies were discussed in this review and can be potential immunomodulatory approaches to increase or deplete IL-35. However, before i35-Breg or IL-35 therapy can brought to the clinic, a number of unresolved issues relating to the immunobiology and bioavailability of the enigmatic IL-35 cytokine that need to be addressed, and a few are listed below: (i) It is still a very daunting task to generate sufficient amounts of rIL-35 for therapeutic use or for ex vivo production of large amounts of i35-Bregs for clinical work. Highly purified “native” IL-35 is not commercially available, and this has impeded research. While IL-35 fusion proteins are available, they do not fully recapitulate the functions of the native IL-35. (ii) IL-35-specific antibodies and ELISA kits that detect both IL-12p35 and Ebi3 with high fidelity in human samples are not available. Analysis is confounded by the fact that IL-12 also has IL-12p35 as one of its subunits and Ebi3 is utilized by IL-27 and IL-39. (iii) A limitation of i35-Breg therapy is the issue of dosing as heterodimer of IL12p35 and Ebi3 readily dissociate, making it difficult to ascertain the bioavailability of the IL-35 secreted by i35-Breg. Among the emerging IL-35 therapy is the efficacy of i35-containing exosomes that readily cross the brain or retina barrier and suppress uveitis and encephalitis. This may solve the problem of dosing, and the absence of toxicity or alloreactivity also makes i35-Exosomes an attractive therapeutic option for delivering IL-35 into CNS tissues. Other unresolved questions include the following: (iv) What factors regulate stability of IL-12p35/Ebi3 heterodimer or their dissociation in vivo? What are the physiological inducers of i35-Bregs? (v) What physiological conditions or factors induce the differentiation and expansion of i35-Breg cells? Are i35-Breg that produce both IL-10 and IL-35 distinct Breg subsets or merely Breg cells at distinct stages of B-cell development? Finally, what is the teleological explanation for the necessity of Breg and Treg cells and do they activate distinct regulatory mechanisms?
While it is difficult to delineate distinct or overlapping roles of Tregs and Bregs, numerical and functional Breg or Treg defects contributes to immune-related pathologies, suggesting that both cell types are required to suppress inflammation. As IL-35 mediates immune suppression by propagating infectious tolerance, it may well be that IL-35-producing Breg or Treg cells play the unique role of concurrently, and sequentially reprogramming conventional B and T cells to acquire regulatory function [90]. It is likely that answers to these questions would open exciting new avenues of research leading to the development of therapeutics.
Acknowledgments
The authors thank Evaristus Mbanefo, Ph.D. (NEI, NIH) and Sahar Alhakeem, M.Sc. (NEI, NIH) for critical reading of the manuscript.
Abbreviations used:
- Bregs
regulatory B cells
- IL-35
interleukin 35, i35-Breg, IL-35-producing B cell
- CNS
central nervous system
- EAU
experimental autoimmune uveitis
- EAE
experimental autoimmune encephalomyelitis
- STAT
signal transducer and activator of transcription
- JAK
Janus kinase
Footnotes
Additional Information
The authors have no competing financial and non-financial interests. The Intramural Research Programs of the National Eye Institute and National Institutes of Health, Bethesda, Maryland provided funding for this research.
References
- 1.Nishizuka Y, Sakakura T, (1969)Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science, 166, 753–755. [DOI] [PubMed] [Google Scholar]
- 2.Gershon RK, Kondo K, (1970)Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology, 18, 723–737. [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon RK, Kondo K, (1971)Infectious immunological tolerance. Immunology, 21, 903–914. [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor H, Hugenberger J, McVay-Boudreau L, Eardley DD, Kemp J, Shen FW, et al. , (1978)Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J. Exp. Med, 148, 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M, (1995)Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol, 155, 1151–1164. [PubMed] [Google Scholar]
- 6.Thornton AM, Shevach EM, (1998)CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med, 188, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH, (2001)Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med, 193, 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shevach EM, (2000)Regulatory T cells in autoimmmunity*. Annu. Rev. Immunol, 18, 423–449. [DOI] [PubMed] [Google Scholar]
- 9.Shevach EM, (2001)Certified professionals: CD4(+)CD25(+) suppressor T cells. J. Exp. Med, 193, F41–F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK, (2001)Type 1 T regulatory cells. Immunol. Rev, 182, 68–79. [DOI] [PubMed] [Google Scholar]
- 11.Weiner HL, (2001)Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev, 182, 207–214. [DOI] [PubMed] [Google Scholar]
- 12.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol.11:1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris A, Moller G, (1968)Regulation of cellular antibody synthesis effect of adoptively transferred antibody-producing spleen cells on cellular antibody synthesis. J. Immunol, 101, 439–445. [PubMed] [Google Scholar]
- 14.Shimamura T, Hashimoto K, Sasaki S, (1982)Feedback suppression of the immune response in vivo. I. Immune B cells induce antigen-specific suppressor T cells. Cell. Immunol, 68, 104–113. [DOI] [PubMed] [Google Scholar]
- 15.L’Age-Stehr J, Teichmann H, Gershon RK, Cantor H, (1980)Stimulation of regulatory T cell circuits by immunoglobulin-dependent structures on activated B cells. Eur. J. Immunol, 10, 21–26. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy MW, Thomas DB, (1983)A regulatory role for the memory B cell as suppressor-inducer of feedback control. J. Exp. Med, 157, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauri C, Blair PA, (2014)The incognito journey of a regulatory B cell. Immunity, 41, 878–880. [DOI] [PubMed] [Google Scholar]
- 18.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol.30:221–41. [DOI] [PubMed] [Google Scholar]
- 19.Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, et al. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol.239:80–6. [DOI] [PubMed] [Google Scholar]
- 20.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity.32:129–40. [DOI] [PubMed] [Google Scholar]
- 21.Mauri C, Menon M, (2017)Human regulatory B cells in health and disease: therapeutic potential. J. Clin. Invest, 127, 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lino AC, Dang VD, Lampropoulou V, Welle A, Joedicke J, Pohar J, et al. , (2018)LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity, 49, 120–133 (e9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JK, Dambuza IM, He C, Yu CR, Uche AN, Mattapallil MJ, et al. , (2017)IL-12p35 inhibits Neuroinflammation and ameliorates autoimmune encephalomyelitis. Front. Immunol, 8, 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. , (2019)Adaptive plasticity of IL-10(+) and IL-35(+) Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol, 20, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, et al. , (2014)Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity, 41, 1040–1051. [DOI] [PubMed] [Google Scholar]
- 26.Rosser EC, Mauri C, (2015)Regulatory B cells: origin, phenotype, and function. Immunity, 42, 607–612. [DOI] [PubMed] [Google Scholar]
- 27.Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr., (1996)Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med, 184, 2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK, (1997)Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J. Exp. Med, 186, 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM, (2002)B cells regulate autoimmunity by provision of IL-10. Nat. Immunol, 3, 944–950. [DOI] [PubMed] [Google Scholar]
- 30.Evans MK, Yu CR, Lohani A, Mahdi RM, Liu X, Trzeciak AR, et al. , (2007)Expression of SOCS1 and SOCS3 genes is differentially regulated in breast cancer cells in response to proinflammatory cytokine and growth factor signals. Oncogene, 26, 1941–1948. [DOI] [PubMed] [Google Scholar]
- 31.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, et al. , (2009)Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J. Immunol, 182, 3492–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN, (2007) B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J. Immunol, 178, 3447–3456. [DOI] [PubMed] [Google Scholar]
- 33.Carter NA, Vasconcellos R, Rosser EC, Tulone C, MunozSuano A, Kamanaka M, et al. Mice lacking endogenous IL-10producing regulatory B cells develop exacerbated disease and preent with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol.186:5569–79. 988 [DOI] [PubMed] [Google Scholar]
- 34.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN, (2012)A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol, 188, 3188–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauri C, Menon M, (2015)The expanding family of regulatory B cells. Int. Immunol, 27, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tedder TF, (2015)B10 cells: a functionally defined regulatory B cell subset. J. Immunol, 194, 1395–1401. [DOI] [PubMed] [Google Scholar]
- 37.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF, (2009)The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol, 182, 7459–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest.121:3645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG, (2015)PD-L1hi B cells are critical regulators of humoral immunity. Nat. Commun, 6, 5997. [DOI] [PubMed] [Google Scholar]
- 40.Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, et al. , (2015)Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature, 521, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaku H, Cheng KF, Al-Abed Y, Rothstein TL, (2014)A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J. Immunol, 193, 5904–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol.188:1036–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. , (2014)Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med, 20, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. , (2014)IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature, 507, 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. , (2007)The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature, 450, 566–569. [DOI] [PubMed] [Google Scholar]
- 46.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol.13:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egwuagu CE, Yu CR, Sun L, Wang R, (2015)Interleukin 35: critical regulator of immunity and lymphocyte-mediated diseases. Cytokine Growth Factor Rev, 26, 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, et al. , (2016)A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in lupus-like mice. Eur. J. Immunol, 46, 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, et al. The composition and signaling of the IL- 35 receptor are unconventional. Nat. Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaffen SL, Jain R, Garg AV, Cua DJ, (2014)The IL-23IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol, 14, 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villarino AV, Kanno Y, O’Shea JJ, (2017)Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol, 18, 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC, (2003)Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science, 300, 2101–2104. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, et al. , (2010)Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/ soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J. Immunol, 184, 1543–1551. [DOI] [PubMed] [Google Scholar]
- 54.Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J, (2009)Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr. Pharm. Des, 15, 2095–2103. [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Park SM, Lee H, Kim YS, (2016)Membrane-bound p35 subunit of IL-12 on tumor cells is functionally equivalent to membrane-bound Heterodimeric single chain IL-12 for induction of anti-tumor immunity. Immune Netw, 16, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chehboun S, Labrecque-Carbonneau J, Pasquin S, Meliani Y, Meddah B, Ferlin W, et al. , (2017)Epstein– Barr virus-induced gene 3 (EBI3) can mediate IL-6 trans-signaling. J. Biol. Chem, 292, 6644–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adam N, Rabe B, Suthaus J, Grotzinger J, Rose-John S, Scheller J, (2009)Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J. Virol, 83, 5117–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suthaus J, Adam N, Grotzinger J, Scheller J, Rose-John S, (2011)Viral Interleukin-6: structure, pathophysiology and strategies of neutralization. Eur. J. Cell Biol, 90, 495–504. [DOI] [PubMed] [Google Scholar]
- 59.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J, (2011)The soluble interleukin 6 receptor: generation and role in inflammation and cancer. Eur. J. Cell Biol, 90, 484–494. [DOI] [PubMed] [Google Scholar]
- 60.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S, (1813)The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta, 2011, 878–888. [DOI] [PubMed] [Google Scholar]
- 61.Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. , (2014)Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat. Med, 20, 1334–1339. [DOI] [PubMed] [Google Scholar]
- 62.Huang A, Cheng L, He M, Nie J, Wang J, Jiang K, (2017)Interleukin-35 on B cell and T cell induction and regulation. J Inflamm (Lond), 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waffarn EE, Hastey CJ, Dixit N, Soo Choi Y, Cherry S, Kalinke U, et al. , (2015)Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat. Commun, 6, 8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bao Y, Liu X, Han C, Xu S, Xie B, Zhang Q, et al. , (2014)Identification of IFN-gamma-producing innate B cells. Cell Res, 24, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menon M, Blair PA, Isenberg DA, Mauri C, (2016)A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity, 44, 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan CC, Caspi RR, Ni M, Leake WC, Wiggert B, Chader GJ, et al. , (1990)Pathology of experimental autoimmune uveoretinitis in mice. J. Autoimmun, 3, 247–255. [DOI] [PubMed] [Google Scholar]
- 67.Nussenblatt RB, (1990)The natural history of uveitis. Int. Ophthalmol, 14, 303–308. [DOI] [PubMed] [Google Scholar]
- 68.Yu CR, Choi JK, Uche AN, Egwuagu CE, (2018) Production of IL-35 by Bregs is mediated through binding of BATF-IRF-4-IRF-8 complex to il12a and ebi3 promoter elements. J. Leukoc. Biol, 104, 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. , (2013)CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med, 5, 173ra23. [DOI] [PubMed] [Google Scholar]
- 70.Choi J, Leung PS, Bowlus C, Gershwin ME, (2015)IL-35 and autoimmunity: a comprehensive perspective. Clin. Rev. Allergy Immunol, 49, 327–332. [DOI] [PubMed] [Google Scholar]
- 71.Su LC, Liu XY, Huang AF, Xu WD, (2018)Emerging role of IL-35 in inflammatory autoimmune diseases. Autoimmun. Rev, 17, 665–673. [DOI] [PubMed] [Google Scholar]
- 72.Yang L, Jia S, Shao X, Liu S, Zhang Q, Song J, et al. , (2019)Interleukin-35 modulates the balance between viral specific CD4(+)CD25(+)CD127(dim/−) regulatory T cells and T helper 17 cells in chronic hepatitis B virus infection. Virol. J, 16, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teng DK, Liu Y, Lv YF, Wang L, Zhang W, Wang JP, et al. , (2019)Elevated interleukin-35 suppresses liver inflammation by regulation of T helper 17 cells in acute hepatitis B virus infection. Int. Immunopharmacol, 70, 252–259. [DOI] [PubMed] [Google Scholar]
- 74.Shi YY, Dai MJ, Wu GP, Zhou PP, Fang Y, Yan XB, (2015)Levels of interleukin-35 and its relationship with regulatory T-cells in chronic hepatitis B patients. Viral Immunol, 28, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang K, Liu J, Li J, (2018)IL-35-producing B cells in gastric cancer patients. Medicine (Baltimore), 97, e0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4 T cells to T-regulatory cells. Cancer Res.71:3505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y, Gallastegui N, Rosenblatt JD, (2015)Regulatory B cells in anti-tumor immunity. Int. Immunol, 27, 521–530. [DOI] [PubMed] [Google Scholar]
- 78.Devergne O, Birkenbach M, Kieff E, (1997)Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc. Natl. Acad. Sci. U. S. A, 94, 12041–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dambuza IM, He C, Choi JK, Yu CR, Wang R, Mattapallil MJ, et al. , (2017)IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat. Commun, 8, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Batrakova EV, Kim MS, (2015)Using exosomes, naturallyequipped nanocarriers, for drug delivery. J. Control. Release, 219, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li N, Zhao L, Wei Y, Ea VL, Nian H, Wei R, (2019) Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res Ther, 10, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang M, Choi JK, Jittayasothorn Y, Egwuagu CE, (2020)Interleukin 35-producing exosomes suppress neuroinflammation and autoimmune uveitis. Front. Immunol, 11, 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. , (1996)B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med, 183, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devhare PB, Ray RB, (2017)A novel role of exosomes in the vaccination approach. Ann. Transl. Med, 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, et al. , (2013)Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem, 288, 28058–28067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, et al. , (2017)Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci. Rep, 7, 4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, et al. , (2017)MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep, 8, 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quah BJ, O’Neill HC, (2005)The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol. Dis, 35, 94–110. [DOI] [PubMed] [Google Scholar]
- 89.Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, et al. , (2004)Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J. Immunol, 172, 2137–2146. [DOI] [PubMed] [Google Scholar]
- 90.Olson BM, Sullivan JA, Burlingham WJ, (2013)Interleukin 35: a key mediator of suppression and the propagation of infectious tolerance. Front. Immunol, 4, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]