Abstract
Background & Aims:
Adverse events (AEs) including reactivation of herpes zoster (HZ) and venous thromboembolism (VTE) have been reported from clinical trials of tofacitinib in ulcerative colitis (UC). We investigated the incidence rates of AEs in a real-world study of UC patients given tofacitinib.
Methods:
We collected data from 260 patients with UC in the TROPIC consortium study, performed at 6 medical centers in the United States. Patients were followed for a median time of 6 months (interquartile range, 2.7–11.5 months). AEs were captured using a standardized data collection instrument before study initiation and at weeks 8, 16, 26, 39, and 52. Serious AEs were defined as life-threatening or resulting in a hospitalization, disability, or discontinuation of therapy. Logistic regression was performed to examine risk factors for AEs.
Results:
AEs occurred in 41 patients (15.7%); most were infections (N = 13, 5.0%). The incidence rate of any AE was 27.2 [95% CI, 24.4–30.7 per 100 patient-years of follow up (PYF)]. Fifteen were serious AEs (36.6% of AEs), and tofacitinib was discontinued for 12 patients (4.6% of cohort). The incidence rates of serious AE was 10.0 (95% CI, 8.9–11.2 per 100 PYF). Five patients developed HZ infection and 2 developed VTE (all receiving 10 mg tofacitinib, twice per day).
Conclusions:
Real-world safety signals for tofacitinib are similar to those for clinical trials, with AEs reported from almost 16% of patients. HZ infection and VTE occurred in patients receiving 10 mg tofacitinib twice per day. These results support dose de-escalation after induction therapy, to reduce the risk of AEs.
Keywords: IBD, Janus Kinases/antagonists & inhibitors, side effect
Graphical Abstract

Introduction
Tofacitinib is an oral, small-molecule Janus Kinase (JAK) inhibitor approved by the FDA for the treatment of moderately to severely active ulcerative colitis (UC) in adult patients. The safety of tofacitinib for treatment of UC was evaluated in a phase 2 trial 1, three large phase 3 clinical trials2 and an ongoing, open-label, long-term extension (OLE) trial 3–6. In addition to demonstrating the efficacy of tofacitinib as an induction therapy, the two phase 3 induction trials (OCTAVE 1 and 2) also showed a higher overall infection and serious infection rate in the tofacitinib group compared with placebo. In the phase 3 maintenance trial of tofacitinib (OCTAVE Sustain), the treatment group had a higher rate of any infection and reactivation of herpes zoster (HZ) compared with placebo. Across all three trials, tofacitinib was also associated with increased lipid levels2.
A recent study analyzing the pooled data from UC patients in the phase 2 and 3 trials and OLE who received tofacitinib, showed that the safety profile was similar to those reported in the tofacitinib rheumatoid arthritis (RA) clinical trials, and in UC patients treated with other biologic agents, except for a dose-dependent higher IR of HZ infections 3. However, an interim analysis from an ongoing safety clinical trial in RA patients identified a high rate of pulmonary embolism and all-cause mortality with tofacitinib 10mg twice daily as compared to patients treated with TNFα antagonists. As a result, the FDA released a black box warning for tofacitinib in July of 2019 outlining the increased risk of venous thrombo-embolism (VTE) and death with the 10 mg twice daily dose and limiting the approved use of this dose in UC beyond 8 weeks of therapy to specific scenarios, only after a careful evaluation of risks versus benefits.7
There are no real-world data in clinical practice on the safety profile of tofacitinib in UC patients especially with a longer duration of usage at a dose of 10 mg twice/day. We aimed to examine the adverse events (AEs) in a cohort of UC patients in a multi-center consortium in the United States.
Methods:
Study design and Setting
This study is reported according to The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for cohort studies8. IRB approval was obtained at each site to create the Tofacitinib Real-world Outcomes in Patients with ulceratIve colitis and Crohn’s disease (TROPIC) consortium. This included sites from across the United States: Washington University in St. Louis School of Medicine, Icahn School of Medicine at Mount Sinai, Medical College of Wisconsin, University of Chicago, Cedars Sinai Medical Center and the Brooke Army Medical Center.
Participants
Patients were included if they had been prescribed tofacitinib for a diagnosis of UC and had atleast 1 subsequent follow-up visit. Data were collected retrospectively at each site, between May 2018 and July 2019.
Variables
Data collection was performed using a standardized data collection form on REDCap (Research Electronic Data Capture, version 7.3.5: REDCap Consortium, Vanderbilt University, Nashville, TN, U.S.A.), using pre-specified definitions and criteria for coding. Patient’s demographic, clinical, endoscopic and laboratory data were collected prior to the initiation of tofacitinib. Follow up data was abstracted at the following timepoints (+/− 4 weeks) from the date of initiation of tofacitinib: 8, 16, 26, 39 and 52 weeks. At each timepoint, systemic corticosteroid usage (oral or intravenous), AEs, and a need for dose escalation or de-escalation was recorded. Any AE that occurred during the total time of follow-up for an individual patient was collected along with the date of occurrence. Major adverse coronary events (MACE) and VTE events and their risk factors including diabetes, hypertension, peripheral vascular disease, oral contraceptive pills use, and history of VTE were abstracted. Information on UC-related hospitalizations, HZ reactivation and any abdominal surgery-related complications were also recorded. A Charlson comorbidity score was calculated for each patient.
AEs were defined as serious if life-threatening, resulting in a hospitalization, disability or discontinuation of therapy. Anemia was defined as hemoglobin < 13.5 mg/dl in males and < 12 mg/dl in females. Abnormal lipid profile was defined as the presence of any one of the following: total cholesterol ≥200, low density lipoprotein (LDL) ≥130, high density lipoprotein (HDL) <40 or Triglycerides ≥150. Surgical complications after abdominal surgery were graded using the Clavien-Dindo Classification and Comprehensive Complication Index.
Statistical analysis
Descriptive statistics are presented as medians with interquartile range (IQR) for continuous variables and frequencies and percentages for categorical variables. Non-parametric continuous variables were compared using Mann-Whitney U or Kruskall-Wallis or Friedman test as appropriate. Categorical variables were compared using Pearson’s chi-squared or Fisher’s exact tests. All data were analyzed based on observed values, with no imputation for missing values.
A univariate followed by a multivariate logistic regression model was constructed to identify the independent predictors of developing an AE calculated as Odds Ratios (ORs). Variables included in the model were associated with the AEs on univariable regression analysis at p<0.05. The threshold for statistical significance was set at 0.05 for all statistical tests and all p-values were two-sided. Incidence rates (IRs) were calculated based on the unique number of patients with events per 100 patient-years of exposure. Exact Poisson CIs (adjusted for patient-years) are provided. Statistical analysis was performed using Stata version 16.0 (StataCorp, College Station, TX) and GraphPad Prism version 8.3.0 (GraphPad Software; La Jolla, California, USA). Graphpad Prism version 8.3.0 was also used for the graphs.
Results:
Baseline characteristics of the study population
A total of 260 patients with UC were enrolled in this study (table 1) with a median follow-up time of 6 months (IQR, 2.7 – 11.5). The median age at initiation of tofacitinib was 38 years (IQR, 27–49). The majority of participants were male (n = 151, 58.1%), non-Hispanic white (n = 187, 71.9%), and had no smoking history (n = 187, 72.2%). The median disease duration prior to starting tofacitinib was 5 years (IQR 3 – 11) and more than half of the cohort (n = 140, 54%) had extensive UC. Two hundred and seventeen [88.5%] patients had previously used a biologic with 76.5% (n = 146) receiving an anti-TNF agent, 56.2% (n = 146) receiving vedolizumab and 5.8% (n = 5.8) receiving ustekinumab. Tofacitinib induction and maintenance dosing for most patients was 10mg (90.4% and 86.5% respectively), and about 57.3% were on concomitant steroid while on tofacitinib. The efficacy data at an earlier timepoint of this cohort has been reported previously.9 Updated efficacy data will be published in a separate manuscript.
Table 1:
Baseline demographic and clinical characteristics
| Age at induction in years, median (IQR) | 38 (27 – 49) |
| Female sex, no (%) | 151 (58.1) |
| Race, no (%) | |
| Non-Hispanic white | 187 (71.9) |
| African American/Black | 13 (5.0) |
| Hispanic | 25 (9.6) |
| Asians | 17 (6.7) |
| Others/unknown | 18 (6.9) |
| BMI (in kg/m2), median (IQR) | 25 (22 – 30) |
| Smoking Status, no (%) | |
| No smoking history | 187 (72.2) |
| Current or Past Smoker | 68 (26.7) |
| UC extent, no (%) | |
| E1: proctitis | 16 (6.2) |
| E2: left-sided or distal (till splenic Flexure) | 90 (34.8) |
| E3: extensive UC (proximal to splenic Flexure) | 140 (54.1) |
| IBD-related hospitalizations prior to Tofacitinib start, no (%) | 62 (24) |
| Charlson comorbidity score | |
| 0 | 150 (69.8) |
| 1 – 3 | 61 (28.4) |
| >3 | 4 (1.9) |
| Median Mayo score, (IQR) | 3 (2 – 3) |
| Disease duration in years prior to starting tofacitinib, median (IQR) | 5 (3 – 11)) |
| Prior biologic exposure, no (%) | 217 (83.5) |
| Anti-TNF | 199 (76.5) |
| Vedolizumab | 146 (56.2) |
| Ustekinumab | 15 (5.8) |
| Induction dosing, no (%) | |
| 5mg bid | 21 (8.1) |
| 10mg bid | 234 (90.4) |
| 11mg daily | 4 (1.5) |
| Maintenance dosing, no (%) (N=218) | |
| 5mg bid | 25 (12.0) |
| 10mg bid | 180 (86.5) |
| 11mg daily | 3 (1.4) |
| Reason for starting tofacitinib, no (%) (N=259) | |
| Active disease | 235 (90.7) |
| Side effect from other medications | 14 (5.4) |
| Others/Unknown/Not report | 10 (3.9) |
| Concurrent immunomodulator usage, no (%) | 13 (5.2) |
| Concurrent systemic corticosteroid usage, no (%) | 149 (63.1) |
| Follow up in months, median (IQR) | 5.9 (2.7 – 11.5) |
| Total exposure, Patient-years of follow up (years) | 157.8 |
Adverse events
AEs occurred in 15.7% (n =41) of our cohort (Table 2). Overall, the most frequently observed were infections (n = 13, 5.0%), followed by rash (n = 9, 3.5%), of which 5 cases were due to HZ infection. Other commonly reported AEs include joint pain (n = 4, 1.5%), and anemia (n = 4, 1.5%). The IR of any AE was 27.2 (24.4 – 30.7) per 100 Patient-Years Follow-up (PYF). Patients who developed AEs tended to be older when compared to those who did not (mean age at start of tofacitinib in years, 42 vs 37 years respectively, Mann Whitney U test, p=0.02) and less likely to have had a prior anti-TNF exposure (63.4.1% vs 79.8%, Fisher Exact test, p = 0.03) (Supplementary Table 1). On univariate analysis, age, smoking status and prior anti-TNF exposure status were significant significantly associated with the development of an AE (p <0.05) and were included in a multivariate logistic regression where older age was predictive of developing an AE (OR, 1.03 CI 1.00 – 1.05; p = 0.047) while prior anti-TNF exposure status was protective against developing an AE (OR 0.43; CI, 0.21 – 0.92; p = 0.03) (Supplementary Table 2). Of note, concomitant steroid use was not associated with AE on univariate analysis (OR 0.66; CI, 0.32 – 1.36; p = 0.27)
Table 2:
Characteristics of all adverse events.
| Total number of patients with an adverse event, n (%) | 41 (15.7) | |
| Number of days to developing AEs, median [IQR] | 68 [39 – 185.5] | |
| Nature of adverse events | n (%) | IR (/HPY) |
| Infections: | 13 (5.0) | 8.6 |
| Primary or reactivated oral HSV | 5 (1.9) | 3.3 |
| Septic arthritis | 1 (0.4)* | 0.6 |
| Clostridioides difficile infection | 1 (0.4) | 0.6 |
| Others | 6 (2.3) | 3.9 |
| Joint pain | 4 (1.5) | |
| Rash | 9 (3.5) | 6.0 |
| Allergic reaction | - | |
| Malignancy | 2 (0.8) | 1.3 |
| Anemia | 4 (1.5) | 2.7 |
| Major cardiovascular event | - | |
| Venous thrombo-embolic event | 2 (0.8)* | 1.3 |
| GI perforation | - | |
| Others: | 8 (19.5) | 5.3 |
| Neuropathy | 1 (0.4) | 0.6 |
| Migraine exacerbation | 1 (0.4) | 0.6 |
| Acne | 1 (0.4) | 0.6 |
| Cough, headache, chest pain and lightheadedness | 1 (0.4) | 0.6 |
| Urinary frequency and incontinence | 1 (0.4) | 0.6 |
| Fever | 2 (0.8) | 1.3 |
| Muscle cramps | 1 (0.4) | 1.3 |
| Incidence rate of all AEs, per 100 Patients-Year [95%CI] | 27.2 (24.4 – 30.7) | |
One patient had a venous thrombo-embolic event (DVT) and septic arthritis. Percentage adds up to >100 because this patient was counted once in the overall count.
Serious Adverse Events
Fifteen of the AEs were serious AEs (SAEs) (5.8% of the cohort) with discontinuation of therapy occurring in 12 patients (4.6%) (Table 3). Of the three that continued therapy after SAE, 2 patients had a VTE event (see details below). One patient had a hospitalization related to group B streptococcus septic shock. He was maintained on the same dose of tofacitinib for 11 months until the end of study follow-up.
Table 3:
Characteristics of Serious Adverse Events
| Number with Serious AE, n (% of cohort) | 15 (5.8) |
| SAEs requiring hospitalization, n | |
| Infections | 2 |
| Septic arthritisa | 1 |
| Group B Strep bacteremia | 1 |
| Malignancy - Cervical rhabdomyosarcomab | 1 |
| SAEs leading to discontinuation of tofacitinib, n | |
| Rash (Herpes Zoster reactivation) | 4 |
| Anemia | 2 |
| Migraine headache | 1 |
| Cough, headache, chest pain, lightheadedness | 1 |
| Urinary frequency and incontinence | 1 |
| Muscle cramps | 1 |
| Fever | 1 |
| Malignancy – Cervical rhabdomyosarcomab | 1 |
| Venous Thrombo-embolic events: DVTa | 2 |
| Induction dosing, no (%) | |
| 5mg bid or 11mg XR daily | 2 (13.3) |
| 10mg bid | 13 (86.7) |
| Maintenance dosing prior to discontinuation, no (%) | |
| 5mg bid or 11mg XR daily | 2 (13.3) |
| 10mg bid | 13 (86.7) |
| Incidence rate of SAEs, per 100 Patients-Year [95%CI] | 10.0 (8.9 – 11.2) |
AEs: Adverse Events;
One patient had a venous thrombo-embolic event (DVT) and septic arthritis;
One patient with cervical rhabdomyosarcoma was hospitalized for the SAE and discontinued tofacitinib; SAEs: Serious Adverse Events; DVT: Deep venous thrombosis.
The rate of serious AEs was 10.0 [95%CI, 8.9– 11.2] per 100 PYF. The median age of patients with SAEs was 24.9 (IQR, 21.6 – 29.0), the majority were male (n= 11, 73.3%) and approximately half were non-Hispanic whites (53.3%) and were either past or current smokers (53.3%) (Supplementary table 3). Nine patients (69.2%) were on concomitants systemic corticosteroids. Thirteen of the 15 SAEs occurred in patients on 10mg twice a day induction dose. The most frequent SAE was HZ rash (n = 4, 26.7% of SAEs). No major cardiovascular event or gastrointestinal tract perforations were reported in the cohort.
Herpes Zoster reactivation
Five patients in the cohort developed HZ. The IR of HZ was 3.29 per 100 PYF [95%CI, 1.37–7.90]. The median time between starting tofacitinib and reactivation of varicella zoster virus was 7 weeks [IQR, 6–10]. The age range was 27 to 64. Three of the patients were male. One was non-Hispanic White and there were 2 African Americans and Hispanics each. All patients were receiving 10 mg tofacitinib twice daily at the time of HZ reactivation leading to discontinuation in four of them while 1 patient continued at 10 mg twice daily dose on follow-up. Three of the patients had a single dermatomal distribution and 1 had multi-dermatomal lesions. Two patients were on steroid, and 3 had a history of varicella infection. None had a history of HZ before starting tofacitinib and only one of these five patients had received a HZ vaccine before starting tofacitinib.
Venous thrombo-embolism
There were two (0.8%) cases of VTE in males, both non-Hispanic White with extensive UC. The IR of VTE was 1.32 per 100 PYF [95%CI, 0.33 – 5.28] in our cohort. One was a 19-year old with corticosteroid-free clinical response after starting tofacitinib at 10 mg twice a day and no VTE risk factors. He had developed a septic arthritis of the knee requiring hospitalization and he later developed deep venous thrombosis, on Day 70 of tofacitinib, requiring anticoagulation. His dose was de-escalated to 5mg bid after this and continued on tofacitinib at last follow-up.
The second case was a 64 year old obese man (BMI = 30.78kg/m2) with a history of diabetes, hypertension, hyperlipidemia and a prior VTE event. He was on tofacitinib 10 mg twice a day with concomitant immunomodulator. He developed a DVT on day 62 of therapy with tofacitinib and was continued at the same dose after the event but later discontinued due to a lack of clinical response. In the whole cohort, only 4 patients (1.54%) had a previous VTE episode prior to starting tofacitinib, and 81 patients (31.2%) had at least one VTE risk factor at baseline.
Complications after abdominal surgery
In our cohort, 35 patients (13.5%) underwent an IBD-related surgery (Table 4). One patient who was refractory to therapy had emergent subtotal colectomy with end-ileostomy while five patients had a semi-urgent (14.2%) procedure. Overall, the most common surgical procedure was a subtotal colectomy with end ileostomy and Hartmann pouch (n = 18, 51.4%). Twenty patients (57.1%) had surgery within 2 weeks of receiving tofacitinib. Out of 24 patients with data on surgical wound classification, a majority (14, 58.3%) had their surgical wounds classified as clean. Seven out of 35 (20%) experienced as least one Clavien-Dindo grade complication. There were 3 cases of post-op infections reported within 30 days of surgery, two of those were surgical site infection, and one was pneumonia. Six of the 7 patients with post-op complications had surgery within 2 weeks of receiving tofacitinib while four were on corticosteroid prior to surgery. Three patients (8.5%) had re-operations within 30 days of the first surgery, and 6 patients (17%) were re-admitted within 30 days of the surgery. One underwent a distal 20–30cm small bowel resection with new ileostomy for ileal intraluminal ulcers in the distal 30–40 cm of the ileum following an ileoscopy for abdominal pain. The histology from the resected bowel had no discrete characteristics suggestive. The other two patients had drainage of post-op abscesses (one was a peri-anal abscess).
Table 4:
Characteristic of patients who underwent IBD-related surgery while on tofacitinib.
| Number of patients who underwent IBD-related surgery, n (% of cohort) | 35 (13.5) |
| Timing of surgery* | |
| Urgent/emergent | 1 |
| Elective | 5 |
| Semi-elective | 27 |
| Surgery indication | |
| Refractory to medical therapy | 34 |
| Dysplasia or neoplasia | 1 |
| Surgery type | |
| Subtotal Colectomy with End Ileostomy and Hartmann Pouch | 18 |
| Subtotal colectomy with ileostomy. | 2 |
| Total abdominal colectomy with end ileostomy and Hartmann’s pouch | 9 |
| Total abdominal colectomy, with end ileostomy. | 3 |
| Total proctocolectomy, ileal pouch anal anastomosis, diverting ileostomy | 1 |
| Total proctocolectomy and permanent end ileostomy | 1 |
| Ileostomy, incision and drainage of anterior perineal abscess. | 1 |
| Surgical wound classification, n (%)** | |
| Clean | 14 |
| Clean contaminated | 9 |
| Contaminated | 1 |
| Dirty infected | 0 |
| Received tofacitinib within 2 weeks of surgery | 20 |
| Received tofacitinib within 4 weeks of surgery | 22 |
| Surgical Site Infection within 30 days | 2 |
| Experienced as least one Clavien-Dindo grade complication, n (%) | 7 (20) |
| Steroid use prior to surgery | 22 |
| Re-operation within 30 days of surgery | 3 |
| Re-admission within 30 days of surgery | 6 |
| Non-surgical/non-abdominal post-op infection | 1 |
Data on 33 patients
Data on 24 patients
Lipid profile
In our cohort, 38.4 % (71 of 185) had an abnormal lipid profile at baseline, which increased to 48.3% (72 of 149) at the end of induction period (week 8) (Supplementary table 4). Thirty-five patients had complete lipid panel result at all time points. There was a modest increase in all lipid parameters except total cholesterol: HDL-c ratio at the end of year on tofacitinib (Figure 1 and Supplementary table 5). Median percentage increases from baseline in total cholesterol, low-density lipoprotein cholesterol (LDL-c) high-density lipoprotein cholesterol (HDL-c), triglycerides and total cholesterol: HDL-c ratio were 4.5% (IQR, 0 – 11.1), 0 % (−4.8 – 12.5), 0 % (−6.3 – 0), 4.5% (−3.2 – 16.7) and 4.5 (−3.7 – 14.3) respectively at the end of induction (week 8). Corresponding median percentage change at 1 year were 9.5% (IQR, −7.4 – 25.0), 28 % (−4.4 – 50.0), 5.5% (−24.2 – 40.0), 3.4% (−7.1 – 10.3) and – 0.26 (−23.9 – 48.1). No MACE event was reported in 64 patients who were 50 years or above in 42.6 PYF (95% CI, 23.1 – 51.2). None of the patients discontinued tofacitinib due to an abnormal lipid profile. Six patients started a statin while 2 started ezetimibe due to the development of abnormal lipid profile after initiating tofacitinib.
Figure 1: Median percentage change from baseline in lipid parameters:
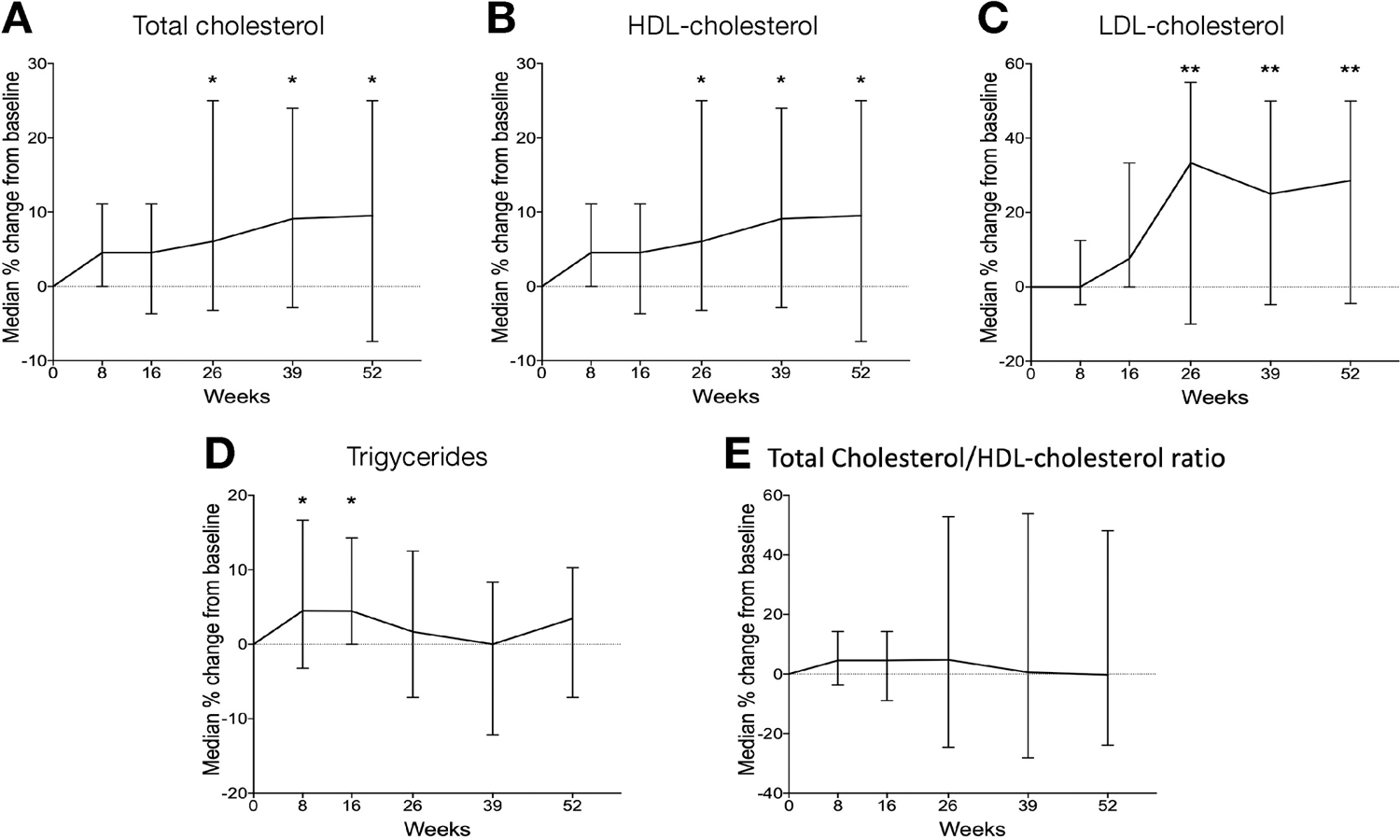
Median percentage change from baseline in levels of a) total cholesterol, b) HDL-cholesterol, c) LDL-cholesterol, d) Triglycerides and e) Total Cholesterol/HDL-cholesterol ratio at weeks 8, 16, 26, 39 and 52 based on paired data of 35 patients with measurements at every time point. *, p <0.05; **, p <0.01.
Hemoglobin
Forty-five percent (107 of 238) of the cohort was anemic at baseline, with a similar prevalence observed in both genders (male 44.5%, female 45.5%). In patients with complete Hgb data throughout the study duration, improvement in Hgb level was evident earlier in females, with a significant improvement noted by week 26 in females (median Hgb level 13.0 g/dL (IQR, 12.5 – 13.8), compared to week 52 in males (median hemoglobin level 13.6 g/dL (IQR, 12.57– 14.0). (Figure 2a–c). At 52 weeks after starting tofacitinib, there was a median 5% (IQR 0 – 11.1%) increase in hemoglobin level in all patients with a higher percentage increase of 7.7% (4.2% – 11.7%) observed in females compared to 2.1% (−0.5% – 11.3%) increase noted in male (figure 2d).
Figure 2: Changes in hemoglobin level over time:
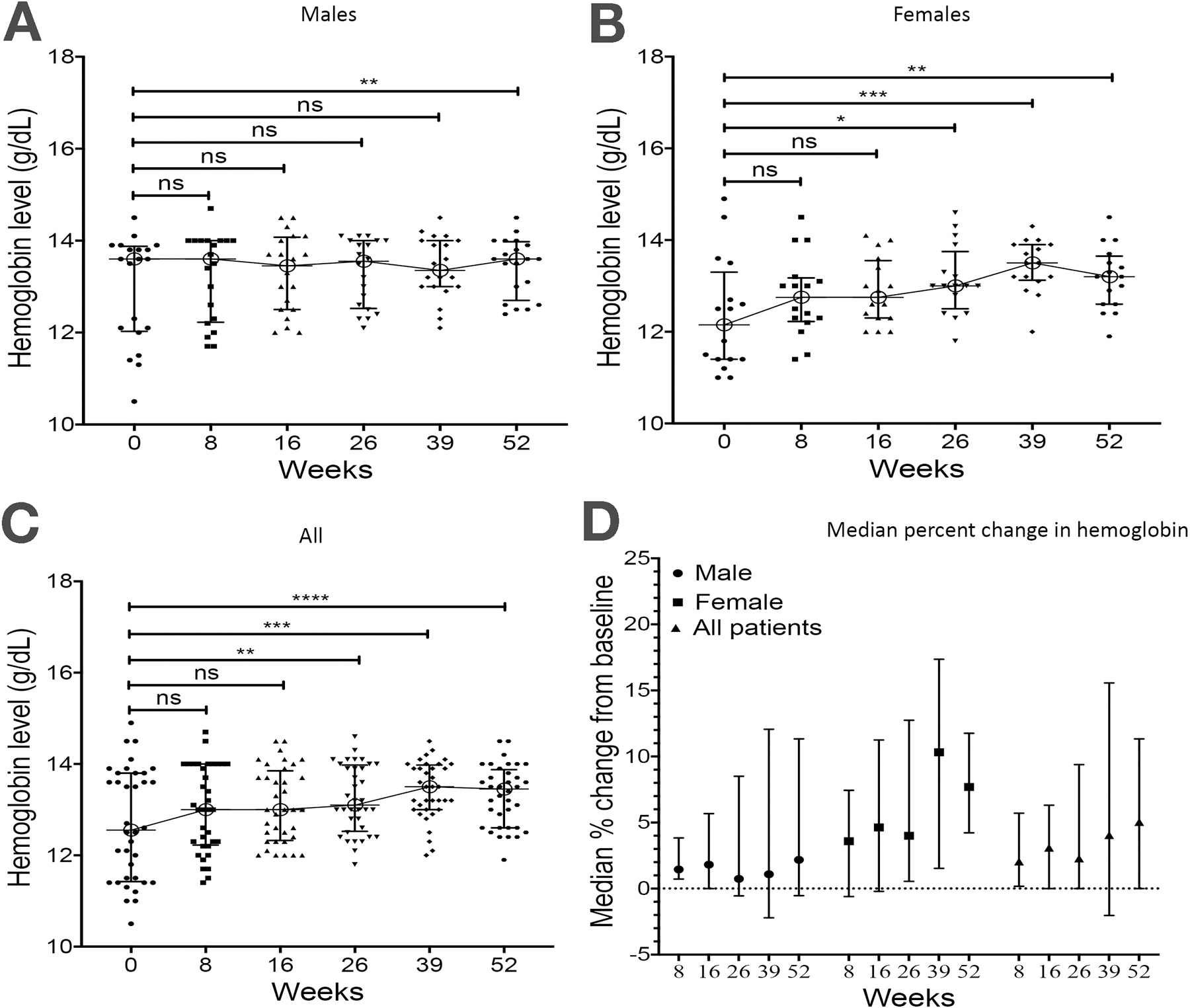
Hemoglobin levels (g/dL) in a) males, b) females and c) all patients at baseline and weeks 8, 16, 26, 39 and 52 of tofacitinib. d) Median percentage change from baseline in hemoglobin levels at weeks 8, 16, 26, 39 and 52. Paired data of 36 patients (20 males and 16 females) with measurements at every time point. *, p <0.05; **, p <0.01; ***, p <0.001; ****, p <0.0001, ns, not significant.
Discussion
In this study, we report the first real-world safety related signals from the TROPIC retrospective multicenter consortium that included 260 patients with UC treated with oral tofacitinib across six IBD centers in the United States. We identified AEs in 15.7% of our cohort with infections being the most common. Serious AEs were reported in 5.8% of the cohort requiring discontinuation of therapy in 4.2% of the cohort. We also found AEs of particular interest including reactivation of HZ with 5 cases after initiation of tofacitinib along with 2 cases of VTE, all of which occurred at the 10 mg twice a day dose. No MACE or gastrointestinal tract perforations were observed in the cohort. We additionally identified reversible changes in lipids. No signals for an increased risk of in complications after abdominal surgery were identified.
While the overall proportion of patients with an AE was lower in this cohort compared to prior publications from the induction (54.9%), maintenance (79.6%) of tofacitinib in UC, this is likely due to differences in the definition and monitoring of AEs between clinical trials and real-world data gathered in the TROPIC consortium.3 Furthermore, patients included in clinical trials can have different characteristics when compared to patients treated in a real world practice. Serious AEs were reported in 5.8% of our cohort similar to prior reports from the 3.6% reported in the tofacitinib arm of induction trial and 5.1–5.6% in the tofacitinib arm of the maintenance trials, possibly because they tend to be more evident and reported.2 The rate of serious AEs in our study was 10.0 [95%CI, 8.9– 11.2] per 100 PYF which was also numerically higher (not statistically significant) than reported from the OCTAVE (4.0 [95% CI, 1.3, 9.4] ongoing, open-label, long-term extension study.5 We additionally found that older age was a risk factor for the development of AEs. The possible protective effect of prior anti-TNF exposure on the risk of AEs may be due to a higher proportion of prior anti-TNF and other biologic exposure compared to the tofacitinib UC clinical trial program and/or the impact of disease activity on the occurrence of AEs.
A dose-dependent increase in the risk of HZ infection through reactivation of Varicella Zoster virus (VZV) has been identified across the clinical trials of patients with UC treated with tofacitinib similar to that previously observed in patients with rheumatoid arthritis (RA) who received tofacitinib.10 The proposed mechanism has been a disruption in the immune response to VZV mediated by type I and II interferons that normally occurs via JAK-STAT pathway signaling.11 The IR of HZ in the TROPIC consortium was 3.29 per 100 PYF [95%CI, 1.37–7.90]. This is consistent with rate reported across overall clinical trial cohort in patients with UC receiving tofacitinib of 4.1 (95%CI, 3.1–5.2) per 100 PYF and among patients with RA who received tofacitinib (4.0 per 100 PYF).3, 12 All patients who developed HZ in the TROPIC were at the 10 mg twice a day dose and the rate of HZ at this dose may be even higher (6.6 (3.2–12.2) per 100 PFY) in clinical trials compared to the 5 mg twice a day dose.3 Prior studies among both patients with RA and UC have identified increasing age, concomitant corticosteroids and Asian ethnicity as additional risk factors for this.12, 13 In our study, 2 of the 5 patients were on concomitant corticosteroids and the age ranged from 27 to 64 years of age. The onset occurred after about 7 weeks of therapy which is within the range of time to develop HZ on Tofacitinib in prior reports (median 324 days, range 13–1185).13 Our data are consistent with those from the tofacitinib UC clinical trials program, highlighting the importance of counseling patients on the risk of HZ reactivation especially in older patients on concomitant corticosteroids and recommending vaccination with an inactivated HZ vaccine.
In the tofacitinib UC program across 1157 patients (2404 patient-years’ exposure; ≤ 6.1 years’ tofacitinib treatment), one patient had DVT (IR 0.04 [0.00–0.23]) while four had PE (0.16 [0.04–0.41]); all of whom had clinical risk factors for VTE alongside UC and had received tofacitinib 10 mg twice a day.4 Similar to this, the two cases of VTE detected in TROPIC occurred in patients with risk factors for VTE and at the 10 mg twice a day dose. However, the IR of VTE was 1.32 per 100 PYF [95%CI, 0.33 – 5.28] which is higher than the IR reported in the tofacitinib UC program either in the overall cohort (0.04 [0.00–0.23]), in those on the predominant dose of 10 mg twice a day (0.05 [0.00–0.30]) and that reported in a population-based study of patients with UC (30.0/10,000 person-years).14 Our data suggests a careful risk-benefit discussion before starting tofacitinib especially in patients with pre-existing risk factors for VTE, dose de-escalation to the lowest clinically feasible dose and monitoring for clinical signs of VTE especially among those who continue on a dose of 10 mg twice a day.
Reassuringly, no signals were noted for lipid profile abnormalities in the subset with regular lipid profile monitoring, which were reversible and consistent with data reported from the tofacitinib UC program.6 Two surgical site infections were reported among the twenty patients who had received tofacitinib within 2 weeks of the surgery. More studies are needed to elucidate the risk of postoperative infections after surgery.
The strengths of the study include the use of a standardized data gathering instrument across a large multicenter cohort. Limitations of the study include the retrospective nature of the data collection and tools including PGA, where data was collected from routine clinical care setting and may have missed AEs that were not adequately captured in the treating clinician’s notes. Additionally, ‘milder’ AEs including mild infections not requiring hospitalization or drug discontinuation may be under-reported in this study. The true impact of tofacitinib on lipids may be under-reported as not all patients had lipid profiles assessed before and after initiation of tofacitinib. However, the data reported here trend similar to a prospective study reported by Sands et al.6
In summary, we report safety signals on a real-world cohort of patients with UC initiated on tofacitinib where increasing age is a risk factor for AEs and consistent with recent reports of a dose-dependent risk of HZ reactivation and VTE events in patients with a risk factor for VTE on the 10 mg twice a day dosing.
Supplementary Material
Acknowledgments
Grant support:
PD is supported by a Junior Faculty Development Award from the American College of Gastroenterology. M.A.C. is supported by DK109384, a Crohn’s and Colitis Foundation Daniel H Present Senior Research Award (Ref. 370763) and philanthropic support from the Givin’ it all for Guts Foundation (https://givinitallforguts.org) and the Lawrence C. Pakula MD IBD Research Innovation and Education Fund. Additional grant support for the REDCap database was provided by the Clinical and Translational Science Award (UL1 TR000448) and Siteman Cancer Center Support Grant (P30-CA091842). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Center for Translational Medicine, the National Center for Advancing Translational Sciences, or the National Institutes of Health. RCU is supported by a Career Development Award from the Crohn’s and Colitis Foundation and an NIH K23 Career Development Award (K23KD111995-01A1). RPH is supported by a Career Development Award from the Crohn’s and Colitis Foundation
Abbreviations:
- AEs
Adverse events
- CI
Confidence interval
- FDA
Food and Drug Administration
- GI
Gastrointestinal
- HDL-c
High density lipoprotein cholesterol
- HZ
Herpes Zoster
- Hgb
Hemoglobin
- IBD
Inflammatory bowel disease
- IL
Interleukins
- IQR
Interquartile range
- IRs
Incidence rates
- JAK
Janus Kinase
- LDL-c
low density lipoprotein cholesterol
- MACE
Major adverse coronary events
- OLE
Open label extension
- OR
Odds ratio
- PE
Pulmonary embolism
- PYF
Patient-Years Follow-up
- RA
Rheumatoid arteritis
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- TNF
Tumor Necrosis Factor
- TROPIC
Tofacitinib Real-world Outcomes in Patients with ulceratIve colitis and Crohn’s disease
- UC
Ulcerative colitis
- VTE
Venous thromboembolism
Footnotes
Disclosures:
Quazim A. Alayo, Aava Khatiwada, Bixuan Lin, Marc Fenster, Christina Dimopoulos, Geoffrey Bader, Roni Weisshof, Michael Jacobs, Anish Patel, Gaurav Syal, George P. Christophi and Poonam Beniwal-Patel disclose no relevant conflicts of interest.
Parakkal Deepak: PD has served as a consultant or advisory board member for Janssen and Pfizer, speaker’s bureau for AbbVie; research grants from Takeda.
Alexandra Gutierrez: AG has served as a speaker for Janssen.
Matthew A. Ciorba: MAC has done consultancy for Takeda and has been on the speaker bureau for AbbVie, Pfizer, Takeda, and UCB.
Jean-Frederic Colombel: JFC has served as an advisory board member or consultant for AbbVie, Amgen, Boehringer-Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Inc., Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, Theradiag. Speaker for AbbVie, Ferring, Takeda, Celgene Corporation; stock options for Intestinal Biotech Development, Genefit; and research grants from AbbVie, Takeda, Janssen and Janssen.
David T. Rubin: DTR is a consultant and has received grant support from Abbvie, Merck & Co., Janssen, Takeda and Pfizer. R
Christina Ha: CH has served as a consultant or on advisory boards of AbbVie, Genentech, Janssen, Pfizer, Samsung Bioepis, and Takeda.
Ryan C. Ungaro: RCU has served as an advisory board member or consultant for Janssen, Pfizer, and Takeda; research grants from Abbvie, Boehringer Ingelheim, and Pfizer.
Robert P. Hirten: RPH has served as a speaker, a consultant, or an advisory board member for Takeda and Janssen and has received research support from Intralytix, Inc.
Joel Pekow: JP has received grants from Takeda and Abbvie, and serves as a consultant for Verastem. He was on the advisory board for Pfizer and Janssen.
Benjamin L. Cohen: BLC has served as a speaker, a consultant or, an advisory board member for Abbvie, Alfasigma, Allergan, Celltrion, Ferring, Grifols, Janssen, and Sublimity Therapeutics
Andres Yarur: AY has received consulting fees from Takeda Pharmaceuticals and Prometheus Laboratories and has served on the speakers bureau for AbbVie, Takeda Pharmaceuticals, and Prometheus Laboratories.
Reference
- 1.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 2.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Panes J, D’Haens GR, et al. Safety of Tofacitinib for Treatment of Ulcerative Colitis, Based on 4.4 Years of Data From Global Clinical Trials. Clin Gastroenterol Hepatol 2019;17:1541–1550. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn WJ, Panes J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther 2020;51:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib Treatment Is Associated With Modest and Reversible Increases in Serum Lipids in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2020;18:123–132 e3. [DOI] [PubMed] [Google Scholar]
- 7.U. S. Food and Drug Administration. (July-26-2019). FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR): FDA Drug Safety Communication. Washington, DC: Author. Retrieved from https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and. [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bmj 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel A, Fenster M, Bader G, et al. 796; Real-World Effectiveness of Tofacitinib in Ulcerative Colitis; a Multi-Center Study. Gastroenterology 2019;156:S-168–S-169. [Google Scholar]
- 10.Colombel JF. Herpes Zoster in Patients Receiving JAK Inhibitors For Ulcerative Colitis: Mechanism, Epidemiology, Management, and Prevention. Inflamm Bowel Dis 2018;24:2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009;228:273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winthrop KL, Curtis JR, Lindsey S, et al. Herpes Zoster and Tofacitinib: Clinical Outcomes and the Risk of Concomitant Therapy. Arthritis Rheumatol 2017;69:1960–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winthrop KL, Melmed GY, Vermeire S, et al. Herpes Zoster Infection in Patients With Ulcerative Colitis Receiving Tofacitinib. Inflamm Bowel Dis 2018;24:2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein CN, Blanchard JF, Houston DS, et al. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost 2001;85:430–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


