Abstract
Background:
US iodine intake, estimated from the median urinary iodine concentration of population representative data, has declined by half since the 1970s which is problematic because maternal iodine intake is critical for fetal neurodevelopment. Relying on median urinary concentrations to assess iodine intake of populations is standard practice but does not describe the number of individuals with insufficient intake. Prevalence estimates of inadequate and excessive intake are better for informing public health applications but require multiple urine samples per person; such estimates have been generated in pediatric populations but not yet among pregnant women.
Objective:
Our aims were: 1) to assess median urinary iodine concentrations across pregnancy for comparison to national data; and 2) to estimate the prevalence of inadequate and excessive iodine intake among pregnant women in mid-Michigan.
Study Design:
Data were collected in 2008–2015 as part of a prospective pregnancy cohort where women were enrolled at their first prenatal clinic visit. Few exclusion criteria (<18 y, or non-English speaking) resulted in a sample of women generally representative of the local community, unselected for any specific health conditions. Urine specimens were obtained as close as practicable to at least one specimen per trimester during routine prenatal care throughout pregnancy (n=1–6 specimens/woman) and stored at −80°C until urinary iodine was measured to estimate iodine intake (n=1,014 specimens from 464 women). We assessed urinary iodine across pregnancy by each gestational week of pregnancy and by trimester. We used multiple urine specimens per woman, accounted for within-person variability, performed data transformation to approximate normality, and estimated the prevalence of inadequate and excessive iodine intake using a method commonly employed for assessment of nutrient status.
Results:
Maternal characteristics reflected the local population in racial and ethnic diversity and socio-economic status: 53% non-Hispanic White, 22% non-Hispanic Black and 16% Hispanic; 48% had ≤ high school education and 71% had an annual income < $25,000. Median urinary iodine concentrations in the 1st, 2nd, and 3rd trimester—including some women contributing more than one specimen per trimester—were 171 μg/L (n=305 specimens), 181 μg/L (n=366 specimens), and 179 μg/L (n=343 specimens), respectively, with no significant difference by trimester (p=.50, Kruskall-Walllis test for equality of medians). The estimated prevalence of inadequate and excessive iodine intake was 23%, and <1%, respectively.
Conclusions:
Median urinary iodine concentrations in each trimester were above the World Health Organization cut-off of 150 μg/L indicating iodine sufficiency at the group level across pregnancy. However, the estimated prevalence of inadequate iodine intake was substantial at 23%, while prevalence of excessive intake was <1%, indicating a need for at least some women to increase consumption of iodine during pregnancy. The American Thyroid Association, the Endocrine Society, and the American Academy of Pediatrics recommend that all pregnant and lactating women receive a daily multivitamin/mineral supplement that contains 150 μg of iodine. The data presented here should encourage the collection of similar data from additional US population samples for the purpose of informing the American College of Obstetricians and Gynecologists own potential recommendations for prenatal iodine supplementation.
Keywords: ARCH, Archive for Research in Child Health, birth cohort, ECHO, Environmental influences on Child Health Outcomes, maternal nutrition, nutrient deficiency, pregnancy diet, UIC, urinary iodine concentration
Condensation:
Prevalence of inadequate and excessive iodine intake, estimated from iodine concentrations of 1–6 urine samples per woman obtained throughout pregnancy, was 23% and <1%, respectively.
Introduction
Iodine is an Essential Nutrient:
Maternal iodine deficiency has long been known to lead to offspring neuromotor, behavioral, and cognitive impairment (1–4), yet it is still the leading preventable cause of intellectual deficiency worldwide (5). Iodine is essential for production of thyroid hormone, a key driver of normal brain development, and even mild to moderate iodine deficiency may lead to neurological deficits (6–8). Because of the known severe adverse health effects caused by insufficient maternal iodine, the American Thyroid Association (9), the Endocrine Society (10), and the American Academy of Pediatrics (11) recommend that all pregnant and lactating women receive a daily multivitamin/mineral supplement that contains 150 μg of iodine. However, as is the case for virtually all essential nutrients, excessive intake of iodine may potentially also lead to adverse health outcomes (12,13), which may be the reason that the American College of Obstetricians and Gynecologists does not have a similar recommendation.
Trends in US Iodine Status:
Endemic iodine deficiency was historically prevalent in several parts of the US—including Michigan and the entire Great Lakes region—but following voluntary salt iodization in the 1920s, US iodine status was thought to be adequate (14). However, data from the National Health and Nutrition Examination Surveys (NHANES) show a sharp decline in estimated iodine intake in recent decades with the decline attributed largely to changes in commercial processing techniques of milk and bread (15–17). At the US population level, it appears that iodine levels remain sufficient, but some subgroups, including pregnant women and especially non-consumers of dairy products—a major source of iodine in US diets—have been shown to be mildly iodine deficient (18–20). There is no evidence of excessive iodine intake at the US population level (17).
Monitoring Iodine Status in Populations:
Iodine intake is difficult to accurately assess because of the extremely high day-to-day variation in US iodine consumption. The iodine content of food and beverages is highly variable because it is dependent on the iodine content of soil and food processing conditions (21). Urinary iodine concentration (UIC) is a good indicator of recent iodine intake because more than 90% of ingested iodine is excreted in urine within 48 hours of consumption, but the substantial variation in daily iodine intake is thought to make spot urine specimens inappropriate for determining iodine status in individuals (22–24). Accordingly, the World Health Organization (WHO) recommends using median UIC to assess iodine status at the population level and sets the lower criteria for population iodine sufficiency at a median UIC of 100 μg/L among non-pregnant adults and 150 μg/L for pregnant women (25). However, this approach does not describe the number of individuals with insufficient or excessive intake. Prevalence estimates of inadequate and excessive intake are better for informing public health applications but require multiple urine samples per person. Using repeat urine samples in large national iodine studies in five non-US countries, prevalence estimates have been generated in pediatric populations (26) but not yet in the US, and not yet among pregnant women.
Our aims in this study were to: 1) assess group-level iodine status (i.e., median UIC) across pregnancy among women in a US pregnancy cohort; and 2) estimate the prevalence of inadequate and excessive iodine intake among pregnant women living in a historically iodine deficient region of the US.
Materials and Methods
Study Participants:
The Archive for Research in Child Health (ARCH) is a pregnancy cohort in which women were enrolled and interviewed at their first prenatal care visit. ARCH was established to be a low-cost, low-participant-burden study, relying on archived information (medical records, birth certificates, newborn blood spots), brief interviews, and clinically obtained specimens (i.e., extra tubes of blood and urine when collected for routine clinical purposes). Recruitment occurred from 2008–2015 in three clinics in Lansing, Michigan, enrolling 801 pregnant women (mean gestational age at enrollment = 13.4 weeks). Few exclusion criteria (<18 y, or non-English speaking) resulted in a sample of women generally representative of the local community, unselected for any specific health conditions. Follow-up is ongoing and includes phone surveys at 1 month and 1 year postpartum and annually thereafter.
ARCH urine specimens were obtained, aliquoted, and stored at −80°C at enrollment and at subsequent routine prenatal care visits, which did not occur at the same gestational age in all study participants. Urine aliquots were collected prior to dipstick measurements because some test strips contain iodine as a reagent for detecting blood and glucose in urine, which would alter results (27, 28).
For this study, stored urine specimens were selected from participants recruited between 2008—2015, prioritizing two partially overlapping groups of participants:
Those with simultaneously archived serum (for future testing of thyroid hormones) and with index children age five years or younger (limited to this age because of resource/funding limitations) at time of urine specimen measurement (n=427); and
Mother-infant dyads who participated in a sub-study that obtained detailed neurodevelopmental data at ages 4–6 y (n=128).
After excluding 13 participants whose pregnancies did not result in a live birth, and 17 who left the state or could not be linked to a birth certificate, the final number of participants is 464 (Figure 1). The 464 women provided a total of 1,014 urine specimens including multiple specimens from most women (136 women provided only one specimen, 116 women provided two specimens, 200 women provided three specimens, 10 women provided four specimens, and 2 women provided five or more specimens). The Institutional Review Boards of Michigan State University, Sparrow Hospital, and the Michigan Department of Health and Human Services approved all study procedures.
Figure 1:
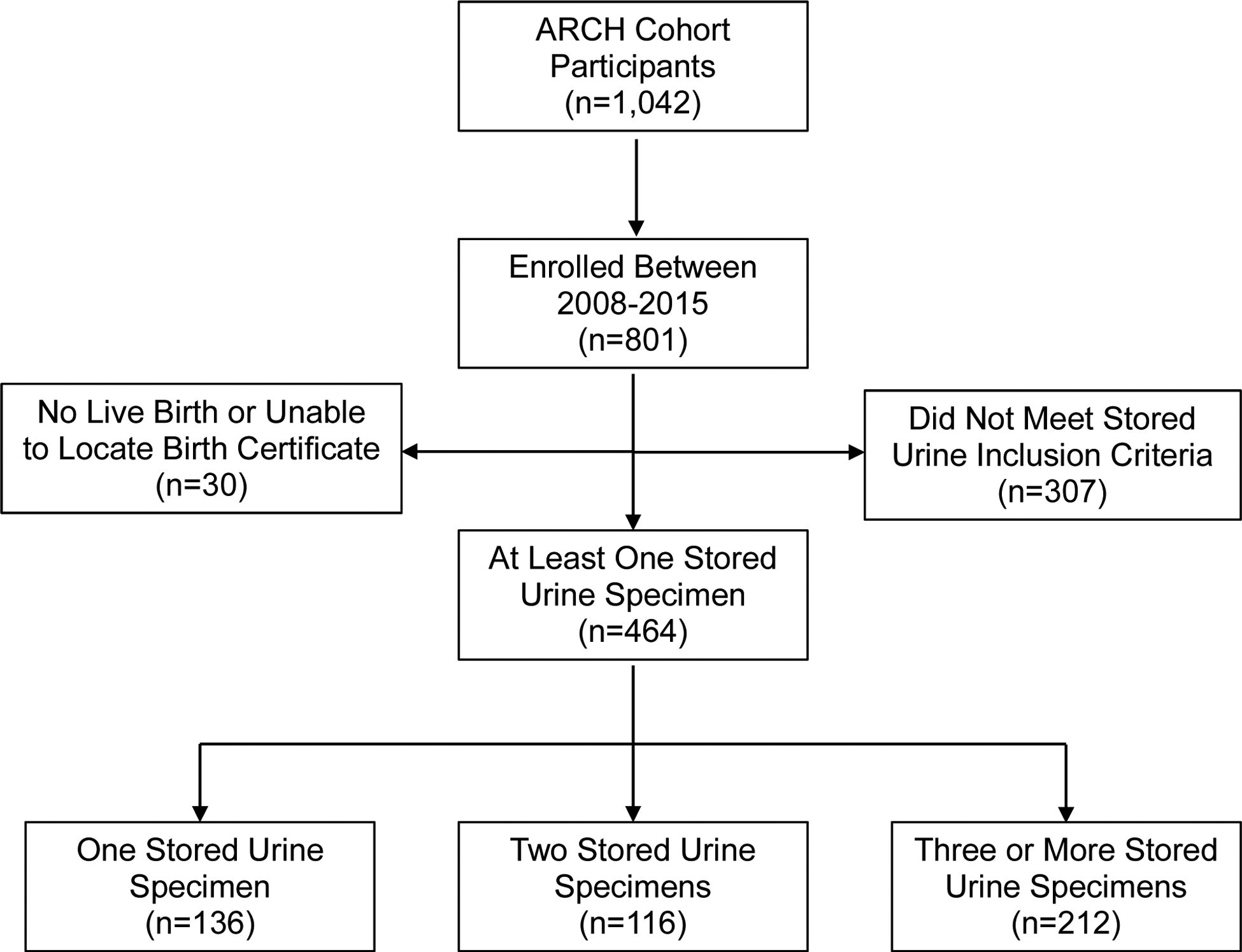
Flow Chart of ARCH Participants Eligible for the Iodine Assessment Analysis Abbreviation: ARCH=Archive for Research in Child Health
Laboratory Analyses:
UICs were measured using ion chromatography/mass spectrometry (29) in the Iodine and Thyroid Function Laboratory at Boston University School of Medicine, which participates in the CDC’s Ensuring the Quality of Urinary Iodine Procedures (EQUIP) certification program. The limit of detection for iodine is 0.5 μg/L, and the inter-assay coefficient of variation for iodine measurement in this laboratory is 2.2–7.6%.
Statistical Analyses:
Descriptive statistics were calculated for all variables of interest. Age was divided into three categories based on the distribution of the data; pre-pregnancy BMI was categorized as either underweight, normal weight, overweight, or obese; all other variables are presented as they were asked of participants or as obtained from the birth certificate.
Because specimens were collected at routine prenatal care visits, which did not occur at the same gestational age in all study participants, we estimated UIC across pregnancy by each gestational week of pregnancy and by trimester (i.e. three 13-week increments). Gestational week of pregnancy at the time of urine specimen collection was calculated from the gestational age at birth on the birth certificate and the date of specimen collection. The birth certificate measure used was the obstetric estimate or “estimated weeks gestation” which refers to the number of weeks from the beginning to the end of the pregnancy, as estimated by the physician. The National Center for Health Statistics transitioned to using this “obstetric estimate” for measuring gestational age in vital statistics data beginning in 2014 because of evidence of greater validity compared to estimates based on the last normal menses (30). Trimester categories were based on guidelines from the American College of Obstetricians and Gynecologists, which include gestational weeks 1–13, 14–27, and 28–40 as trimesters one, two, and three, respectively (31).
To account for the multiple measures per women, a version of the Kruskal-Wallis test was implemented that accounted for the repeated sampling within women (32). We used a quantile regression analysis to estimate the median (50th percentile) UIC by week of pregnancy and thus provide a robust estimate of UIC across pregnancy in the presence of outliers; an estimate of variance of the median regression parameter was computed using a bootstrap procedure, with 200 with-replacement samples of women rather than observations to account for the within-woman clustering. Quantile regression was conducted using the rq function in the R statistical software package (http://www.r-project.org/).
We estimated the prevalence of inadequate and excessive iodine intake using the Estimated Average Requirement/Tolerable Upper Intake Level cut-point method as described in detail elsewhere (12). Briefly, this method is commonly employed for assessing the nutrient intakes of groups. The Estimated Average Requirement represents the daily intake value of a nutrient that is estimated to meet the nutrient requirement of half of the healthy individuals in a life stage and gender group. The Upper Intake Level is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. Estimated Average Requirements and Upper Intake Levels are established for each nutrient by the US Institute of Medicine (IOM) (12). The Estimated Average Requirement for iodine for pregnant women (all ages) is 160 μg/d, whereas the Upper Intake Level for iodine is 900 μg/d for for pregnant women aged 14–18 y and 1100 μg/d for pregnant women aged 19–50 y (12).
Guided by the techniques described by Zimmerman, et al. (26, 33), we first estimated the prevalence of inadequate, adequate, and excessive iodine intake by the number of urine specimens per woman to assess the impact of the within-person variance on our sample. Using multiple UIC measures per woman, we then assessed the within- versus between-woman variance and applied internal within-person variance proportions. These important steps are required because the Estimated Average Requirement cut-point method for assessing nutrient inadequacy (12) overestimates the prevalence of inadequate intake unless the within-person variance is accounted for (34). In the final step to utilize the estimated proportion of total variance in iodine intake that corresponds to within-person variability, we used the Software for Intake Distribution Estimation (SIDE), V1.0 (http://www.side.stat.iastate.edu/). This software computes a complex transformation of the iodine data to approximate normality and allows for a more accurate estimation of the prevalence of inadequate intake (33). Details for the interested reader are available in Nusser et al. (33).
Results
Maternal characteristics of the analytic sample are shown in Table 1 and reflect the local population including diversity in race/ethnicity (52.6% non-Hispanic White; 22% non-Hispanic Black; 15.7% Hispanic), nearly half with high school education or less (48%), and most with annual incomes under $25,000 USD (71%). Most were ≤ 25 y (58.2%), unmarried (71.4%), and reported their pregnancy as unplanned (65.5%). Approximately 30% reported smoking during pregnancy, 8% were underweight, and 55% were overweight or obese (pre-pregnancy).
Table 1.
| Characteristic | n | % |
|---|---|---|
| Age (Yrs) | ||
| ≤25 | 270 | 58.2 |
| 26–30 | 125 | 26.9 |
| 31+ | 69 | 14.9 |
| Race/Ethnicity | ||
| Non-Hispanic White | 244 | 52.6 |
| Non-Hispanic Black | 102 | 22.0 |
| Hispanic | 73 | 15.7 |
| Other Races/Ethnicities Combined3 | 45 | 9.7 |
| Education Level | ||
| <High School Graduate | 74 | 16.3 |
| High School Graduate or GED | 144 | 31.7 |
| Some College | 158 | 34.7 |
| ≥College Graduate | 79 | 17.4 |
| Annual Household Income (US Dollars) | ||
| <25,000 | 323 | 71.0 |
| 25,000 to 49,999 | 87 | 19.1 |
| ≥50,000 | 45 | 9.9 |
| Accumulated Wealth | ||
| Own a Car (Yes) | 282 | 61.6 |
| Own a Home (Yes) | 88 | 19.4 |
| Own stocks or Bonds (Yes) | 33 | 7.5 |
| Marital Status | ||
| Married, Living with Baby’s Father | 109 | 23.5 |
| Married | 24 | 5.2 |
| Unmarried, Living with Baby’s Father | 177 | 38.2 |
| Unmarried | 154 | 33.2 |
| Planned Pregnancy (Yes) | 160 | 34.5 |
| Pre-pregnancy BMI (kg/m2) | ||
| <18.5 | 38 | 8.2 |
| 18.5 to 24.9 | 170 | 36.6 |
| 25 to 29.9 | 111 | 23.9 |
| ≥30 | 145 | 31.3 |
| Smoking During Pregnancy (Yes) | 130 | 29.1 |
Abbreviations: ARCH=Archive for Research in Child Health; GED=Graduate Equivalency Diploma; BMI=Body Mass Index
Missing Values: Education (n=9); Income (n=9); Own a Car (n=6); Own a Home (n=10); Owns Stocks or Bonds (n=23); Tobacco Use (n=17)
Includes American Indian/Alaska Native (n=2), Native Hawaiian/Pacific Islander (n=1), Asian (n=12), those who selected more than one racial category (n=22), those whose race and/or ethnicity was missing (n=8)
As previously noted, urine specimens were collected at routine prenatal care visits, which did not occur at planned study intervals and were not the same gestational age in all study participants, so we report results across pregnancy at the group level first by gestational week and then by trimester, noting that individual women contributed 1–6 urine samples across pregnancy. Figure 2 represents a complete illustration of median UICs across each gestational week of pregnancy. No difference was found across gestational weeks as evidenced by regressing the median UIC on gestational week which yielded an estimated slope of 0.25 ± 0.38 per week (p=0.51). Table 2 describes median UICs by trimester, including some women who contributed more than one specimen per trimester. The overall median UIC in our cohort was 176 μg/L. Median UICs for the 1st, 2nd, and 3rd trimester were 171 μg/L (n=305 specimens), 181 μg/L (n=366 specimens), and 179 μg/L (n=343 specimens), respectively, with no significant difference by trimester (p=.50, Kruskall-Walllis test for equality of medians).
Figure 2:
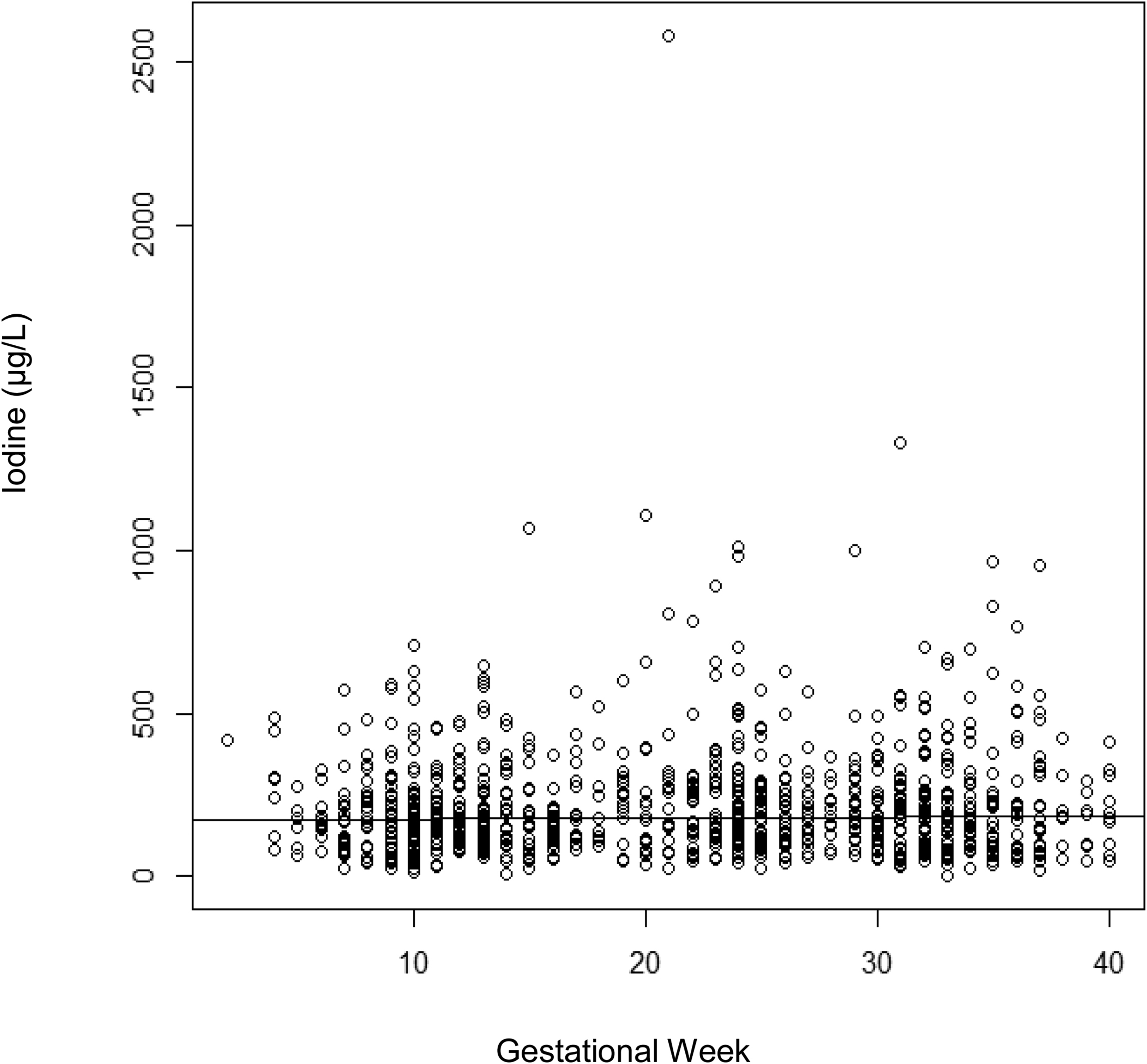
Quantile Regression of Median (50th percentile) Urinary Iodine Concentration (μg/L) on Gestational Week of Pregnancy; slope=0.25 (p=0.51). Regression based on 1,014 samples from 464 women.
Table 2.
Urinary Iodine Concentration (μg/L) by Trimester Among Eligible ARCH Participants (n=464 women, n=1,014 urine specimens)1
| Trimester | n2 | Median3 | Mean | Std Dev | Min | Max |
|---|---|---|---|---|---|---|
| First | 305 | 171 | 199 | 130 | 15 | 712 |
| Second | 366 | 181 | 227 | 206 | 6 | 2580 |
| Third | 343 | 179 | 222 | 174 | 4 | 1330 |
Abbreviations: ARCH=Archive for Research in Child Health
Number of specimens; some women contributed more than one specimen per trimester
Kruskall-Walllis test for equality of medians shows no significant difference by trimester (p=.50)
Illustrating the principle that the prevalence of inadequate intake is overestimated unless multiple urine specimens are used to estimate within-person variation (34), our data show that fewer specimens led to higher prevalence estimates of inadequate iodine intake. When using data from only one urine specimen, the unadjusted prevalence estimate of inadequate iodine intake was 47.2%, but the estimate decreased to 41.6% with 2 specimens, and 39.2% with 3 specimens, and did not change appreciably with an increasing number of specimens over 3, thus indicating that taking the means of three urine specimens stabilized the estimation of urinary iodine in this cohort. The prevalence of excessive iodine intake was extremely low regardless of the number of urine specimens per woman or within-person variability and ranged from 0.2–0.4%.
Finally, using multiple urine specimens per woman, performing a data transformation to approximate normality using the SIDE software, and accounting for the final within-person variability (0.248), the adjusted prevalence estimate of inadequate iodine was 23.1% ± 5.5%, whereas the estimate of excessive iodine was <1%. Figure 3 depicts the distribution of UICs by showing a density plot using estimated percentiles and indicating the cut-off for inadequate iodine with a red line. The 5th, 25th, 50th, 75th, and 95th percentiles of iodine intake were 118 ± 7, 163 ± 6, 203 ± 6, 252 ± 9, and 339 ± 19 μg/L. respectively.
Figure 3:
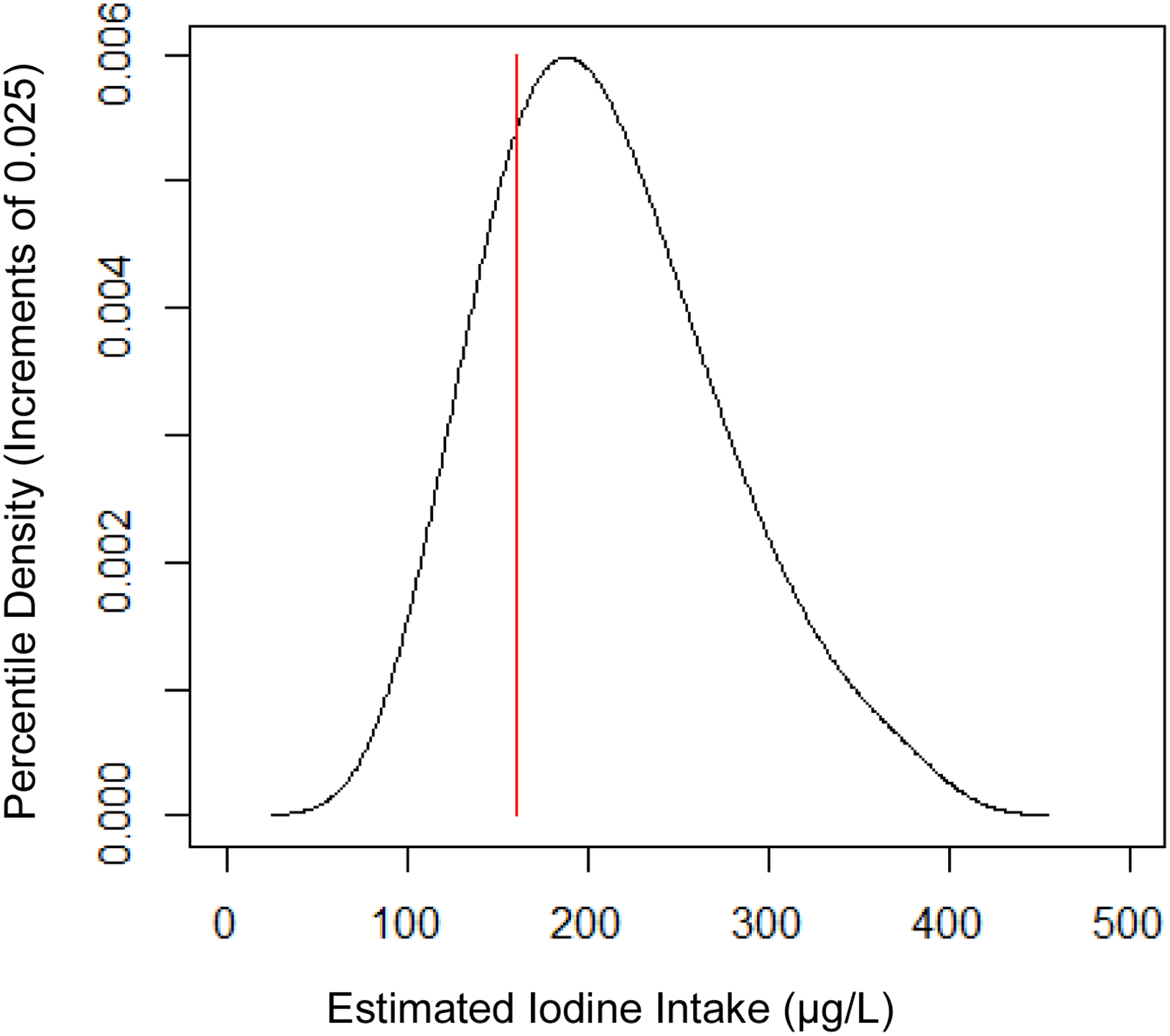
Smoothed Density Plot of Estimated Iodine Intake (μg/L) using Percentile Increments from 0.025 to 0.975. Red line indicates adequate iodine (160 μg/L).
Structured Discussion/Comment:
1. Principal Findings:
Using median UIC as a biomarker of population-level iodine intake in this pregnancy cohort located in a US geographic region known to be historically iodine deficient, we found iodine intake to be sufficient at the population-level. The overall median UIC in our cohort was 176 μg/L, which is above the threshold set by the WHO for sufficient median UIC of 150 μg/L for pregnant women (5, 25). However, we also found cause for concern in that nearly a quarter (23%) of pregnant women and their offspring were estimated to be at risk of iodine deficiency, while the prevalence of excessive iodine intake was <1%. Our data confirm that the common practice of relying on population-level estimates of iodine status can fail to reveal the number of individual pregnant women who may be deficient in iodine—a critical nutrient involved in fetal brain development.
Results:
Maternal characteristics of this cohort reflect the local population in Lansing, Michigan where women were recruited, including 22% non-Hispanic Black, 16% Hispanic, and the rest non-Hispanic White. Only 19% reported owning a home and a full 71% reported annual household incomes of <$25,000, yet 52% reported having at least some college. Women were unselected for any specific health condition and 8% were underweight, 55% were overweight or obese (pre-pregnancy), and smoking rates were high at 29%. Results may vary in other US population groups.
Using data from multiple urine specimens per woman, and guided by the techniques used by Zimmermann et al. 2016 (26), we replicated a series of statistical methods including a complex transformation of the data to approximate normality and accounting for within-person variability to obtain a better estimate of the prevalence of inadequate and excess iodine intake.
2. Clinical Implications:
These findings are important because damage to the fetal brain resulting from the maternal hypothyroxinemia associated with iodine deficiency is irreversible by mid-gestation (4). A recent meta-analysis suggests that the influence of iodine nutrition on fetal neurodevelopment is most critical by 14 weeks gestation (35). Iodine deficiency is noted by the World Health Organization to be “the single most important preventable cause of brain damage” (5) and has been associated with impaired psychomotor development and cognitive outcomes in deficient or even only mildly iodine deficient geographic regions of the world (6–8). Iodine status of the US population is monitored by the ongoing NHANES which is cross-sectional and collects only one urine specimen per person (16–20). Thus, even though this surveillance shows that some population subgroups are at higher risk for iodine deficiency than others (19, 36), the iodine status of individuals is not assessed in NHANES and is not assessed clinically because urinary iodine concentration only explains recent iodine intake, which may not be indicative of habitual iodine intake.
The finding that taking the means of 3 urine specimens stabilized the estimation of urinary iodine in the ARCH cohort is important because it challenges the notion that urinary iodine can never be used as a clinical indicator of usual iodine intake. It is reasonable to assume that recent iodine intake must to some extent reflect usual iodine intake. Although the iodine content of foods can be highly variable, the largest contributor of iodine to US diets is dairy products, with grains and eggs also serving as important food sources (37–39). To the extent that dairy, grain, and egg consumption are part of a habitual dietary pattern, it is likely that recent iodine intake reflects usual iodine intakes. Women who are planning pregnancy, pregnant, or lactating, especially if they exclude dairy and eggs, may especially benefit from iodine supplementation. The American Thyroid Association, the Endocrine Society, and American Academy of Pediatrics recommend daily supplementation of 150 μg of Iodine for all pregnant women (9–11). A recent survey of the iodine content of US prenatal multivitamin supplements found that only 58% contained any iodine (40), and NHANES results from 1999–2012 indicate that only about 20% of US pregnant women were taking iodine-containing prenatal supplements (41). Simply prescribing any prenatal multivitamin supplement does not guarantee adequate iodine intake.
3. Research Implications:
Dietary sources of iodine vary by differences in food choices, fortification and food processing practices, and soil conditions, which may explain why 10–12 spot urine specimens were needed to estimate usual iodine intake in two European studies (23, 24). However, we find that as few as 2–3 urine specimens may provide a reasonable estimate of within-person variability in our mid-Michigan cohort.
Urinary iodine excretion depends to some extent on glomerular filtration rate (GFR), and some have speculated that pregnancy-related increases in GFR may result in decreased urinary iodine with advancing gestation (42). Evidence is conflicting, with decreasing median urinary iodine across trimesters found in Iran, an iodine replete region (43), and Tasmania, an iodine deficient region (42), but the opposite was found—increasing urinary iodine across the course of gestation—in the UK, a mildly iodine deficient population (44). In our mid-Michigan pregnancy cohort, considered iodine-replete at the population level, we found stable median UICs over the course of gestation. Differences across studies may be due to the underlying iodine status of the population or other unaccounted differences. We are unaware of any other results with multiple urine specimens per woman across pregnancy from US samples.
It would be useful to characterize iodine status across pregnancy in different regions of the US among different population subgroups with varied dietary intake patterns. More importantly, associations should be assessed between maternal iodine levels, maternal and infant thyroid function, and childhood neurodevelopmental outcomes. The ECHO program (https://www.nih.gov/echo) is in a position to perform such analyses on a large scale, thereby informing the public and providers whether more active interventions are needed to ensure adequate iodine intake among all pregnant women.
4. Strengths and Limitations:
First, in our estimation of within-person variability, only 10 women had four or more iodine measures, and only 2 women had five or more. It is possible that women with more observations may differ systematically from those with fewer, and if so, that may have influenced our prevalence estimates. Second, we did not collect information on dietary intake during pregnancy for the participants included in these analyses, so we cannot assess food or supplemental sources of iodine. Measures of dietary intake of ARCH enrollees were instituted in 2015, allowing for future analyses of diet in relation to biomarkers of iodine intake and thyroid function in other study participants from this pregnancy cohort.
5. Conclusions:
The high prevalence of inadequate iodine intake coupled with the low prevalence of excessive iodine intake in this US pregnancy cohort indicate a need for at least some women to increase their iodine intake. This may be best achieved by increased awareness among obstetricians and other health care professionals, as well as women of childbearing age, about the importance of consuming adequate prenatal iodine from foods and the existing recommendations for prenatal iodine supplementation.
Supplementary Material
AJOG at a Glance:
A. Why was this study conducted?
Maternal iodine intake is essential for thyroid hormone production and fetal neurodevelopment.
Both low and high iodine intakes can potentially lead to adverse health outcomes, but US trends indicate declining urinary iodine concentrations—a biomarker of iodine intake.
Iodine intake is commonly estimated from urinary iodine excretion, but requires multiple urine samples to assess individual intake, and no prevalence estimates of low or high iodine status have been reported from US pregnancy samples.
B. What are the key findings?
In this pregnancy cohort (n=1,014 urine specimens from 464 women) recruited from a general population, the prevalence of inadequate iodine intake was high (23%) whereas excessive intake was low (<1%).
C. What does this study add to what is already known?
The high prevalence of inadequate iodine, coupled with the low prevalence of excessive iodine in this mid-Michigan pregnancy cohort, indicate a need for increased awareness about prenatal iodine intake from foods and prenatal supplements.
Acknowledgements
This work was supported in part by the Michigan State University (MSU) Office of the Vice President for Research, MSU College of Human Medicine, MSU Center for Research in Autism, Intellectual and Neurodevelopmental Disabilities (C-RAIND), the Michigan Health Endowment Fund (G-1608-140432, R-1605-14007) and the NIH ECHO program (UG3OD023285, UH3OD023285). Environmental influences on Child Health Outcomes (ECHO) is a nationwide research program supported by the National Institutes of Health (NIH), Office of the Director to enhance child health. No funding source had any involvement in any aspect of the study design, collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
With much appreciation to the ARCH and CHARM (Child Health Advances from Research with Mother) study teams, the Iodine Research Laboratory at Boston University, and all the mothers and children who participated.
Sources of Support: Supported in part by: the Office of the Director at the National Institutes of Health under award numbers UG3OD023285, UH3OD023285; the Michigan Health Endowment Fund under award numbers G-1608-140432, R-1605-14007; the Michigan State University (MSU) Center for Research in Autism, Intellectual and Neurodevelopmental Disabilities (C-RAIND), the MSU Office of the Vice President for Research, and the MSU College of Human Medicine. No funding source had any involvement in any aspect of the study design, collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors report no conflicts of interest.
Disclaimers: The authors have no disclaimers or conflicts of interest to declare.
Contributor Information
Jean M KERVER, Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI;; Department of Pediatrics and Human Development, Michigan State University, East Lasing, MI;
Elizabeth N PEARCE, Section of Endocrinology, Diabetes, and Nutrition, Boston University School of Medicine;.
Mr. Tengfei MA, Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI;.
Ms. Monica GENTCHEV, Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI;.
Michael R ELLIOTT, Department of Biostatistics, University of Michigan, Ann Arbor, MI.
Nigel PANETH, Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI;; Department of Pediatrics and Human Development, Michigan State University, East Lasing, MI;
References
- 1.Pharoah POD, Buttfield IH, Hetzel BS. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. The Lancet 1971;297:308–310. [DOI] [PubMed] [Google Scholar]
- 2.Leung AM, Pearce EN, Braverman LE. Iodine Nutrition in Pregnancy and Lactation. Endocrinol Metab Clin North Am 2011;40(4):765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glinoer D Importance of iodine nutrition during pregnancy. Publ Health Nutr 2007;10:1542–6. [DOI] [PubMed] [Google Scholar]
- 4.Morreale de Escobar G, Jesus Obregon M, and Escobar del Rey F. Iodine deficiency and brain development in the first half of pregnancy. Publ Health Nutr 2007;10(12A):1554–1570. [DOI] [PubMed] [Google Scholar]
- 5.WHO, UNICEF, ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva: World Health Organization; 2007. [Google Scholar]
- 6.Costeira MJ, Oliveira P, Correia Santos N, Ares S, Saenz-Rico B, Morreale de Escobar G, Almeida Palha J. Psychomotor development of children from an iodine-deficient region. J Pediatr 2011;159:447–53. [DOI] [PubMed] [Google Scholar]
- 7.Bath SC, Steer CD, Goldin J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive otucomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013;382:331–37. [DOI] [PubMed] [Google Scholar]
- 8.Hynes KL, Otahal P, Burgess JR, Oddy WH, Hay I.Reduced Educational Outcomes Persist into Adolescence Following Mild Iodine Deficiency in Utero, Despite Adequacy in Childhood: 15-Year Follow-Up of the Gestational Iodine Cohort Investigating Auditory Processing Speed and Working Memory. Nutrients 2017. December 13;9(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017. March;27(3):315–389. [DOI] [PubMed] [Google Scholar]
- 10.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of Thyroid Dysfunction During Pregnancy and Postpartum: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 97:2543–2565. [DOI] [PubMed] [Google Scholar]
- 11.Council on Environmental Health, Rogan WJ, Paulson JA, Baum C, Brock-Utne AC, Brumberg HL, Campbell CC, Lanphear BP, Lowry JA, Osterhoudt KC, Sandel MT, Spanier A, Trasande L. Iodine Deficiency, Pollutant Chemicals, and the Thyroid: New Information on an Old Problem. Pediatrics. 2014. June;133(6):1163–6 [DOI] [PubMed] [Google Scholar]
- 12.Otten JJ, Pitzi Hellwig J, and Meyers LD, eds. Food and Nutrition Board, Institute of Medicine Dietary reference intakes: the essential guide to nutrient requirements. Washington, D.C.: National Academy Press; 2006; Iodine pp. 320–7. [Google Scholar]
- 13.Shi X, Han C, Li C, Mao J, Wang W, Xie X, Li C, Xu B, Meng T, Du J, Zhang S, Gao Z, Zhang X, Fan C, Shan Z, Teng W 2015. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7,190 pregnant women in China. J Clin Endocrinol Metab 100:1630–1638. [DOI] [PubMed] [Google Scholar]
- 14.Leung AM, Pearce EN, Braverman LE. History of US Iodine Fortification and Supplementation. Nutrients 2012;4:1740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollowell JG, Staehling NW, Hannon WH et al. : Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from NHANES I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab 1998;83:3401–8. [DOI] [PubMed] [Google Scholar]
- 16.Hollowell JG, Haddow JE. The prevalence of iodine deficiency in women of reproductive age in the United States of America. Publ Health Nutr 2007;10:1532–9. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell KL, Makhmudov AA, Ely EK, Jones RL, Wang RY. Iodine Status of the U.S. Population, NHANES 2005–2006 and 2007–2008. Thyroid 2011;21(4):419–27. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell KL, Pan Y, Mortensen ME, Makmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children’s Study & in US women (15–44 years), NHANES 2005–2010. Thyroid 2013;23:927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrine CG, Herrick KA, Serdula MK, Sullivan KM. Some subgroups of reproductive age women in the US may be at risk for iodine deficiency. J Nutr 2010:140:1489–94. [DOI] [PubMed] [Google Scholar]
- 20.Perrine CG, Herrick KA, Gupta PM, Caldwell KL. Iodine Status of Pregnant Women and Women of Reproductive Age in the United States. Thyroid 2019. January;29(1):153–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann MB, Jooste PL, Panday C. Iodine deficiency disorders. Lancet 2008;372:1251–62. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen LB, Ovesen L, Christianses E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr 1999;53(5):401–7. [DOI] [PubMed] [Google Scholar]
- 23.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011;141(11):2049–54. [DOI] [PubMed] [Google Scholar]
- 24.Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr 2008;99:813–18. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Urinary iodine concentrations for determining iodine status deficiency in populations Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2013. (https://apps.who.int/iris/bitstream/handle/10665/85972/WHO_NMH_NHD_EPG_13.1_eng.pdf;jsessionid=ED9E1DEF0DD56EE4254EAB8995C01FBC?sequence=1, accessed [5/2/2020]). [Google Scholar]
- 26.Zimmermann MB, Hussein I, Al Ghannami S, El Badawi S, Al Hamad NM, Abbas Hajj B, et al. Estimation of the Prevalence of Inadequate and Excessive Iodine Intakes in School-Age Children from the Adjusted Distribution of Urinary Iodine Concentrations from Population Surveys. J Nutr 2016. June;146(6):1204–11. [DOI] [PubMed] [Google Scholar]
- 27.Chanoine JP, Bourdoux P, Vo Thi NB, Ermans AM : Iodine contamination of urine samples by test strips. Clin Chem 1987;33:1935. [PubMed] [Google Scholar]
- 28.Pearce EN, Lazarus JH, Smyth PP, He X, Smith DF, Pino S, Braverman LE. Urine test strips as a source of iodine contamination. Thyroid 2009;19:919. [DOI] [PubMed] [Google Scholar]
- 29.Valentin-Blasini L, Mauldin JP, Maple D, Blount BC: Analysis of perchlorate in human urine using ion chromatography and electrospray tandem mass spectrometry. Analytical chemistry 2005, 77(8):2475–2481. [DOI] [PubMed] [Google Scholar]
- 30.Martin JA, Osterman MJK, Kirmeyer SE, and Gregory ECW. Measuring gestation age in vital statistic data: Transitioning to the obstetric estimate. National Vital Statistics Reports 2015;64(5):1–20. [PubMed] [Google Scholar]
- 31.The American college of Obstetricians and Gynecologists. Methods for estimating the due date. Committee Opinion No. 700. Obstet Gyneco 2017;129:e150–4. [DOI] [PubMed] [Google Scholar]
- 32.Jung SH, & Ying Z Rank-based regression with repeated measurements data. Biometrika 2003, 90(3), 732–740.) [Google Scholar]
- 33.Nusser SM, Carriquiry AL, Dodd KW, & Fuller WA. A semiparametric transformation approach to estimating usual daily intake distributions. Journal of the American Statistical Association 1996;91:1440–1449. [Google Scholar]
- 34.Carriquiry AL. Estimation of usual intake distributions of nutrients and foods. J Nutr 2003;133:601S–8S. [DOI] [PubMed] [Google Scholar]
- 35.Levie D, Korevaar TIM, Bath SC, Murcia M, Dineva M, Llop S, Espada M, van Herwaarden AE, de Rijke YB, Ibarluzea JM, Sunyer J, Tiemeier H, Rayman MP, Guxens M, Peeters RP.Association of Maternal Iodine Status With Child IQ: A Meta-Analysis of Individual Participant Data. J Clin Endocrinol Metab. 2019. December 1;104(12):5957–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan Y, Caldwell KL, Li Y, Caudill SP, Mortensen ME, Makhmudov A, Jones RL. Smoothed urinary iodine percentiles for the US population and pregnant women: National Health and Nutrition Examination Survey, 2001–2010. Eur Thyroid J 2013;2:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung AM, Lamar A, He X, Braverman LE, Pearce EN.Iodine status and thyroid function of Boston-area vegetarians and vegans. J Clin Endocrinol Metab 2011;96(8):E1303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abt E, Spungen J, Pouillot R, Gamalo-Siebers M, Wirtz M. Update on dietary intake of perchlorate and iodine from U.S. food and drug administration’s total diet study: 2008–2012. J Expo Sci Environ Epidemiol. 2018. Jan; 28(1):21–30. [DOI] [PubMed] [Google Scholar]
- 39.Lee KW, Shin D, Cho MS, Song WO. Food Group Intakes as Determinants of Iodine Status among US Adult Population. Nutrients. 2016. May 26; 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel A, Lee SY, Stagnaro-Green A, MacKay D, Wong AW, Pearce EN. Iodine Content of the Best-Selling United States Adult and Prenatal Multivitamin Preparations. Thyroid. 2019;29(1):124–127. [DOI] [PubMed] [Google Scholar]
- 41.Jun S, Gahche JJ, Potischman N, Dwyer JT, Guenther PM, Sauder KA, Bailey RL.Dietary Supplement Use and Its Micronutrient Contribution During Pregnancy and Lactation in the United States. Obstet Gynecol. 2020 Mar;135(3):623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stilwell G, Reynolds PJ, Parameswaran V, Blizzard L, Greenaway TM, Burgess JR. The influence of gestational stage on urinary iodine excretion in pregnancy. J Clin Endocrinol Metab 2008;93:1737–1742. [DOI] [PubMed] [Google Scholar]
- 43.Ainy E, Ordookhani A, Hedayati, Azizi F. Assessment of intertrimester and seasonal variations of urinary iodine concentration during pregnancy in an iodine-replete area. Clin Endocrin 2007;67:577–581. [DOI] [PubMed] [Google Scholar]
- 44.Bath SC, Furmidge-Owen VL, Redman CWG, Rayman MP. Gestational changes in iodine status in a cohort study of pregnant women from the United Kingdom: season as an effect modifier. Am J Clin Nutr 2015;101:1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


