Abstract
Background:
More than 20% of patients with cirrhosis do not receive semi-annual hepatocellular carcinoma (HCC) surveillance as recommended. Few studies have evaluated the effects of patient-level factors on surveillance receipt.
Methods:
We administered a telephone survey to a large cohort of patients with cirrhosis from 3 health systems (a tertiary care referral center, a safety-net health system, and Veterans Affairs) to characterize patient knowledge, attitudes, and perceived barriers of HCC surveillance. Multinomial logistic regression was performed to identify factors associated with HCC surveillance receipt (semi-annual and annual vs none) during the 12-month period preceding survey administration.
Results:
Of 2871 patients approached, 1020 (35.5%) completed the survey. Patients had high levels of concern about developing HCC and high levels of knowledge about HCC. However, patients had knowledge deficits, including believing surveillance was unnecessary when physical examination and laboratory results were normal. Nearly half of patients reported barriers to surveillance, including costs (28.9%), difficulty scheduling (24.1%), and transportation (17.8%). In the year before the survey, 745 patients (73.1%) received 1 or more surveillance examination; 281 received on-schedule, semi-annual surveillance and 464 received annual surveillance. Semi-annual HCC surveillance (vs none) was significantly associated with receipt of hepatology subspecialty care (odds ratio, 30.1; 95% CI, 17.5 – 51.8) and inversely associated with patient-reported barriers (odds ratio, 0.62; 95% CI, 0.41 – 0.94). Patterns of associations comparing annual vs no surveillance were similar although the magnitude of effects were reduced.
Conclusions:
Patient-reported barriers such as knowledge deficits, costs, difficulty scheduling, and transportation are significantly associated with less-frequent receipt of HCC surveillance, indicating a need for patient-centered interventions, such as patient navigation.
Keywords: liver cancer, screening, knowledge, barriers, early detection
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the fastest increasing causes of cancer-related death in the U.S. and a leading cause of death in patients with cirrhosis.1,2 The high mortality rate attributed to HCC is largely driven by late stage diagnoses, for which treatment options are limited and median survival is typically 1–2 years.3 Given the association between early tumor detection and improved survival, the AASLD and EASL recommend semi-annual HCC surveillance using abdominal ultrasound with or without alpha fetoprotein (AFP) for at-risk populations, including those with cirrhosis.4–7
Despite improvement over time, fewer than 30% of patients with cirrhosis receive HCC surveillance, with underuse particularly prevalent among racial-ethnic minorities and patients of low socioeconomic status.8,9 Although lack of provider recommendation is one of the most common reasons for surveillance underuse, patient-level factors also play an important role.10–12 Most studies evaluating HCC surveillance have focused on the association between patient demographics or clinical characteristics and surveillance receipt.8 However, models of health behavior highlight the importance of considering patient knowledge, attitudes, and perceived barriers.13,14 These factors are important to understand because they are modifiable factors that could be targeted for intervention. A prior survey among patients with cirrhosis reported patient-level barriers including scheduling process difficulties, costs of surveillance tests, and transportation; however, the study was conducted using a convenience sample from a single health system, with three-fourths of patients recruited at the time of a clinic appointment.12 Therefore, it is unclear if these results would be generalizable to broader patient populations followed in different health systems and with different patterns of healthcare utilization.
Our study’s aim was to characterize patient knowledge, attitudes, and barriers of HCC surveillance and their association with surveillance receipt in a racially and socioeconomically diverse cohort of patients with cirrhosis followed in three different health systems.
METHODS
Study Population
The study was conducted at three health systems: Parkland Health and Hospital System, UT Southwestern Medical Center, and Michael E. DeBakey Veterans Affairs Medical Center.
Parkland is a publicly funded integrated safety-net health system with a network of twelve primary care clinics in Dallas County, specialty hepatology clinics and radiology suites; UT Southwestern is an academic tertiary-care referral center with a large liver transplant program; and the Michael E. DeBakey VA Medical Center is one of the largest VA systems in the U.S. and a designated VA liver transplant center.
Electronic medical records of patients were screened to identify adult patients (age >18 years) with cirrhosis. Patients were identified using a validated set of ICD-9/ICD-10 codes for cirrhosis (571.2 or 571.5; K70.30 or K74.6) or cirrhosis complications (456.0, 456.1, 456.2, 456.21, 567.23, 572.2, 572.3, 572.4; K65.2, K72.9, K72.91, K76.6, K76.7, I85.0, I85.1).15 The presence of cirrhosis was confirmed by chart review by two authors (A.S. or R.H.) prior to survey administration, with cirrhosis diagnosis based on consistent histology, non-invasive markers of fibrosis demonstrating F4 fibrosis, or imaging showing a cirrhotic-appearing liver with signs of portal hypertension including splenomegaly, varices, or thrombocytopenia. We excluded patients with Child Pugh C cirrhosis, uncontrolled hepatic encephalopathy, personal history of HCC, and history of liver transplantation, as HCC surveillance is not recommended in these groups. We excluded patients who were unable to complete the survey (e.g. low literacy) or those with language other than English or Spanish. The study was approved by the IRB of UT Southwestern Medical Center and Michael E. DeBakey VA Medical Center.
Survey Development and Administration
Eligible patients were recruited to complete a telephone survey between April and December 2018, with up to six call attempts. We used a theoretical model of patient behavior for HCC surveillance, based on the Health Behavior Framework, to guide selection of survey measures.13,14 The survey was divided into the following sections: knowledge about HCC surveillance, attitudes regarding surveillance benefits, barriers to surveillance, financial burden of medical care, and demographics. The 6-item knowledge section covered HCC risk, need for surveillance in patients with cirrhosis, surveillance logistics, effectiveness, HCC treatment, and prognosis. For attitudes, we used 5 items, adapted from the Psychological Consequences Questionnaire33, to measure patients’ perceived risk, worries, and fears of developing or dying from HCC. We assessed potential barriers to HCC surveillance including lack of desire, lack of time to complete surveillance, difficulties with scheduling, financial or transportation concerns, and fear of finding cancer. We included 10 questions, adapted from the Medical Expenditure Panel Survey34, to assess financial burden including ability to cover medical bills, need to make financial sacrifices or borrow money, and need to delay medical care. Demographic information included marital status, living situation, level of education, employment status and household income. After initial development of the survey, we pretested it using cognitive interviewing methods with 10 English-speaking and 10 Spanish-speaking patients at which point saturation of feedback was achieved.
Electronic Medical Record Data Collection
Additional correlates including demographics, clinical history, and laboratory data were obtained through the EMR. Patient age, sex, race and ethnicity, preferred language, and insurance type were obtained. Patients were classified according to liver disease etiology using laboratory data and ICD-9/ICD-10 codes in the following hierarchy: hepatitis C virus, hepatitis B virus, alcohol-related liver disease, nonalcoholic steatohepatitis (NASH), and other. NASH was assigned if patient had evidence of metabolic syndrome without other known liver disease. Data regarding presence of decompensation (ascites or hepatic encephalopathy) were classified as none, mild or controlled, and severe or uncontrolled. Medical comorbidity was assessed using the Cirrhosis Comorbidity (CirCom) score using ICD-9/ICD-10 codes.16 Laboratory data of interest near survey administration (+/−3 months) included platelet count, bilirubin, albumin, and international normalized ratio (INR).
Study Outcomes
We recorded all imaging (ultrasound, contrast-enhanced CT, or contrast-enhanced MRI) and AFP results during the 12-month period preceding survey administration. Studies performed at external institutions were included if results were uploaded in the EMR. For our primary outcome of interest, we classified participants into 3 categories based on frequency and interval of imaging studies per recommended guidelines: semi-annual surveillance (≥1 imaging study during each 6-month period), annual surveillance (≥1 imaging study during the 12-month period but not meeting criteria for semiannual surveillance), and no surveillance (no imaging during the period).
Statistical Analysis
Distributions of patient characteristics and survey responses were reported with descriptive statistics, including stratified descriptions by health system. For survey questions with missing or unknown data, we performed single imputation using the mean of the patient’s responses from similar survey items. Univariate and multivariable multinomial logistic regression models were performed to identify factors associated with HCC surveillance receipt (semi-annual and annual surveillance groups versus no surveillance). Predictor variables with p<0.10 in univariate analyses and those of a priori interest (e.g. health system, liver disease etiology) were included in multivariable models to minimize type II error. Statistical significance was defined as p< 0.05 for multivariable analyses. Data were assessed for clustering by site; however, hierarchical models with patients clustered by site offered no improvement in model fit. All data analysis was performed using SAS 9.4.
RESULTS
Patient Characteristics
Of 2871 patients approached, 1020 (35.5%) completed the survey. Of non-responders, 1303 (45.4%) could not be contacted by phone and 534 (18.5%) were contacted but declined or could not complete the survey. Patient characteristics of respondents are detailed in Table 1. Nearly two-thirds (61.7%) of respondents were from Parkland, 23.1% from the VA, and 15.2% from UT Southwestern. Median age was 61 years, and 649 (63.9%) were male. As expected, patients from the VA system were older and more male than the other sites. The cohort was racially and ethnically diverse, with 35.2% non-Hispanic white, 33.4% Hispanic, and 28.6% non-Hispanic Black, with greatest diversity at Parkland. The most common cirrhosis etiologies were HCV infection (59.1%), alcohol-related (17.8%), and NASH (13.3%). Most patients had compensated cirrhosis, with 73.9% having Child Pugh A cirrhosis. Hepatic decompensation was present in 41.3% of patients, with 18.2% having hepatic encephalopathy alone, 6.2% ascites alone, and 7.4% having both ascites and hepatic encephalopathy. Based on CirCom scores, most patients had no or one comorbidity (34.5% and 27.9%, respectively). In the year prior to survey administration, patients attended a median of 5 primary care visits (IQR 3–8), and 68.0% had received hepatology care. Nearly all patients at UT Southwestern had received hepatology care, compared to nearly three-fourths of patients at the VA and half of patients at Parkland. Overall, survey respondents had similar demographics and clinical characteristics than non-respondents (Supplemental Table).
Table 1:
Characteristics of patients who completed baseline survey, overall and by health system
| Parkland (n=629) | UTSW (n=155) | DeBakey VA (n=236) | Total (n=1020) | |
|---|---|---|---|---|
| Age (years) | ||||
| 21–50 | 105 (16.7) | 23 (14.8) | 15 (6.4) | 143 (14.0) |
| 51–60 | 260 (41.3) | 37 (23.9) | 37 (15.7) | 334 (32.7) |
| 61–90 | 264 (42.0) | 95 (61.3) | 184(78.0) | 543 (53.2) |
| Sex (% male) | 364 (57.9) | 65(41.9) | 220 (93.2) | 649 (63.6) |
| Race/Ethnicity (%) | ||||
| Non-Hispanic White | 113 (18.0) | 102 (65.8) | 144 (61.0) | 359 (35.2) |
| Non-Hispanic Black | 219 (34.8) | 16 (10.3) | 57 (24.2) | 292 (28.6) |
| Hispanic | 287 (45.6) | 26 (16.8) | 28(11.9) | 341 (33.4) |
| Other | 10 (1.6) | 11(7.1) | 7 (3.0) | 28 (2.7) |
| Etiology of Liver Disease (%) | ||||
| Hepatitis C | 383 (60.9) | 41 (26.5) | 179 (75.8) | 603 (59.1) |
| Alcohol-related | 123 (19.6) | 30 (19.4) | 26 (11.0) | 179 (17.5) |
| Nonalcoholic steatohepatitis | 74 (11.8) | 47 (30.3) | 15 (6.4) | 136 (13.3) |
| Hepatitis B | 20 (3.2) | 7 (4.5) | 1 (0.4) | 28 (2.7) |
| Other | 29 (4.6) | 30 (19.4) | 15 (6.4) | 74 (7.3) |
| Child Pugh Class (% Child A) | 443 (70.4) | 126 (81.3) | 185 (78.4) | 754 (73.9) |
| CirCom Score (%)1 | ||||
| 0 | 196 (31.2) | 103 (66.5) | 53 (22.5) | 352 (34.5) |
| 1 | 185 (29.4) | 21 (13.6) | 79 (33.5) | 285 (27.9) |
| 2 | 92 (14.6) | 15 (9.7) | 66 (28.0) | 173 (17.0) |
| 3 | 32 (5.1) | 1 (0.7) | 5 (2.1) | 38 (3.7) |
| 4 | 124 (19.7) | 15 (9.7) | 33 (14.0) | 172 (16.9) |
| Insurance coverage2 | ||||
| Medicare | 126 (20.0) | 77 (49.7) | 55 (23.3) | 258 (25.3) |
| Medicaid | 150 (23.9) | 0 (0) | 17 (7.2) | 167 (16.4) |
| Private Insurance | 32 (5.1) | 71 (45.8) | 24 (10.2) | 127 (12.5) |
| VA Benefits | 0 (0) | 2 (1.3) | 137 (58.1) | 139 (13.6) |
| Parkland subsidy plan | 228 (36.2) | 0 (0) | 0 (0) | 228 (22.3) |
| Missing/none | 93 (14.8) | 5 (3.2) | 3 (1.3) | 101 (9.9) |
| Education2 | ||||
| Did not graduate high school | 282 (44.8) | 10 (6.5) | 26 (11.0) | 318 (31.2) |
| Graduated high school/vocational | 208 (33.1) | 38 (24.5) | 98 (41.5) | 344 (33.7) |
| At least some college | 133 (21.1) | 107 (69.0) | 108 (45.8) | 348 (34.1) |
| Missing | 6 (1.0) | 0 (0) | 4 (1.7) | 10 (1.0) |
| Household income2 | ||||
| < $15,000 | 340 (54.1) | 18 (11.6) | 42 (17.8) | 390 (38.2) |
| $15,000 – $25,000 | 93 (14.8) | 11 (7.1) | 33 (14.0) | 137 (13.4) |
| $25,000 – $50,000 | 55 (8.7) | 32 (20.6) | 32 (13.6) | 119 (11.7) |
| >$50,000 | 11 (1.7) | 75 (48.4) | 28 (11.9) | 114 (11.2) |
| Missing | 130 (20.7) | 19 (12.3) | 101 (42.8) | 250 (24.5) |
| Receipt of hepatology care1 | 364 (57.9) | 145 (93.5) | 185 (78.4) | 694 (68.0) |
During year prior to randomization
Self-reported from survey responses
Patient Knowledge of HCC Surveillance
Patient knowledge regarding HCC surveillance was high across health systems, with >85% of patients answering at least two-thirds of questions correctly. The percent of correct responses for each knowledge question ranged from 58.8% to 96.7% (Figure 1). Over 80% knew patients with cirrhosis are at high risk for developing HCC, ultrasound is used for surveillance, surveillance should be performed semi-annually, and surveillance increases early HCC detection; however, there were notable knowledge deficiencies. Over one-fourth of patients did not know early stage HCC could be cured and >40% reported surveillance was not necessary if their physical exam and laboratory results were normal. We noted site-level differences in patient knowledge, with patients at the safety-net system more likely to know ultrasound is used for HCC surveillance, surveillance should be done semi-annually, and early stage HCC can be cured; however, they were more likely to incorrectly believe surveillance is not needed with normal exam and labs.
Figure 1:
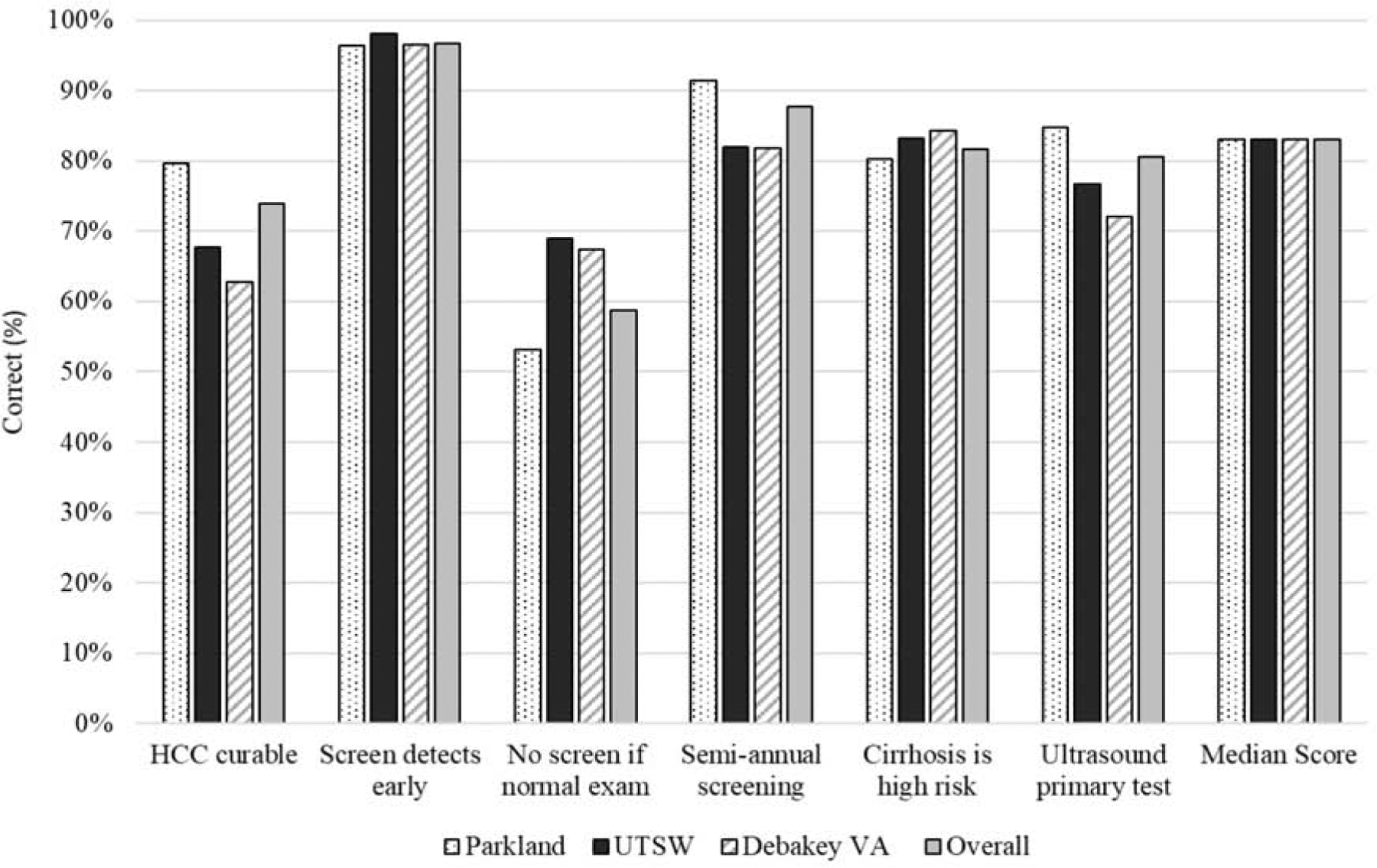
Patient Knowledge regarding HCC Surveillance, stratified by site
Patient Attitudes about HCC Surveillance
Patients reported concerns about developing HCC, with 529 (51.9%) believing they were at least somewhat likely and 259 (25.4%) believing they were very likely to develop HCC in their lifetime. Only 159 (15.6%) believed they were unlikely to develop HCC in their lifetime. In fact, 165 (16.2%) of patients expressed fear of having HCC most of the time and an additional 214 (21.0%) having fear sometimes. Patients also expressed worry about dying from HCC, with 150 (14.7%) and 146 (14.3%) being afraid of dying from HCC sometimes and most of the time, respectively. Although concern for developing HCC was consistent across health systems, we noted variation in worry about dying from HCC (sometimes or most of the time: 47% of safety-net vs. 16% of VA patients). Over half of patients (59.0%) reported receiving HCC surveillance reduced their worry about dying from HCC.
Patient-Reported Barriers to HCC Surveillance
Patient-reported barriers to HCC surveillance are illustrated in Figure 2. The most common barriers to HCC surveillance were costs of surveillance tests (n=295, 28.9%), scheduling process difficulties for ultrasound exams (n=246, 24.1%), difficulty with transportation (n=182, 17.8%), and uncertainty of where to receive surveillance (n=174, 17.1%). Overall, 522 (51.2%) patients reported at least one of these reported barriers. Although barriers were more common at the safety-net health system, over 30% of patients at each health system reported at least one of these barriers. An additional 182 (17.8%) reported their doctor recommended surveillance but they remained uncertain about its necessity. Few patients reported length of the ultrasound examination (9.6%) or fear of finding cancer (13.0%) as barriers to HCC surveillance.
Figure 2:
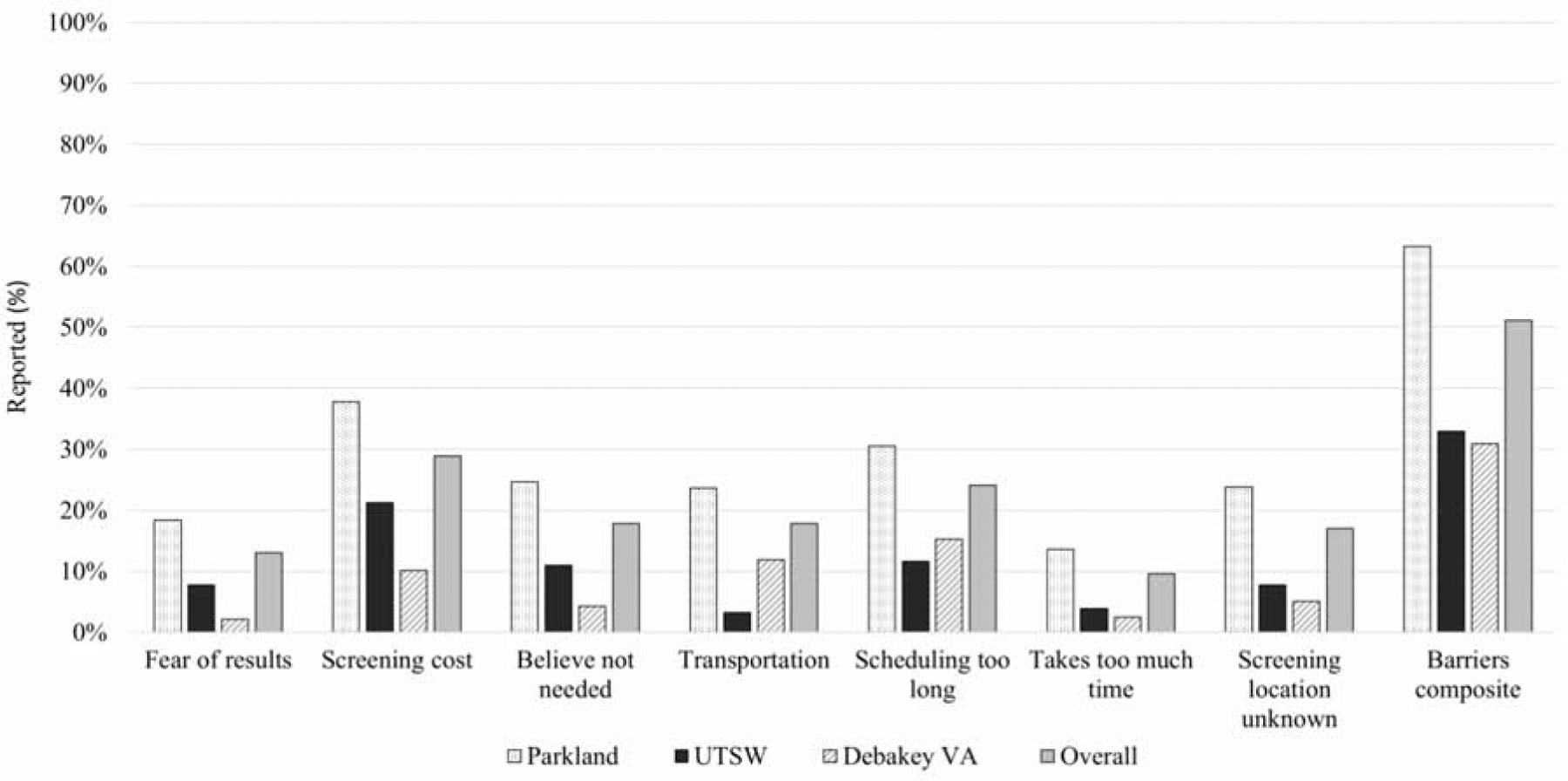
Patient Reported Barriers to HCC Surveillance, stratified by site
Financial Burden of HCC Surveillance
In addition to surveillance barriers, patients reported medical-related financial distress, in part related to costs of cirrhosis management and HCC surveillance. Despite nearly all (91.8%) patients reporting medical coverage, 42.8% reported being worried about ability to pay medical bills and 24.4% were unable to cover co-payments or deductibles. Nearly one-fifth (17.5%) of patients reported making financial sacrifices, including 11.8% whose family had to borrow money or acquire debt to cover costs of care. Although nearly two-thirds of those who borrowed money incurred <$10,000 of debt, 2.3% had to file bankruptcy. Nearly one-fourth (24.4%) of patients reported delays in medical care including HCC surveillance, related to financial distress in the year prior to the survey. Financial distress appeared similar between patients at the safety-net system and tertiary care referral center but lower among those at the VA system, potentially related to differences in delivery models for health care and medical coverage stability. Nearly half of patients at Parkland and UT Southwestern reported concern about their ability to pay medical bills, compared to only one-fourth of patients at the VA system.
Receipt of HCC surveillance
Surveillance imaging was performed in 745 (73.1%) patients during the study period, with 281 (27.5%) having semi-annual surveillance and 464 (45.4%) annual surveillance. In multivariable analysis, receipt of semi-annual surveillance (compared to no surveillance) was associated with Hispanic ethnicity (aOR 1.91, 95%CI 1.09 – 3.35), HBV etiology (aOR 7.31, 95%CI 1.66 – 32.3), and receipt of Hepatology care (aOR 30.1, 95%CI 17.5 – 51.8); semi-annual surveillance was inversely associated with financial distress resulting in delayed medical care (aOR 0.60, 95%CI 0.38 – 0.99) and presence of ≥1 most common patient-reported barriers (aOR 0.62, 95%CI 0.41 – 0.94) (Table 2). Semi-annual surveillance was higher in patients with Hepatology care (36.7% vs. 8.0%) and lower in those reporting financial distress resulting in delayed care (19.7% vs. 30.2%) or those with commonly reported barrier (25.6% vs. 29.5%).
Table 2:
Correlates of HCC surveillance in prior year, multinomial logistic regression analysis
| Multivariable Analysis (OR, 95% CI) |
||
|---|---|---|
| Annual Screening | Semi-annual Screening | |
| Race/ethnicity | ||
| Non-Hispanic White | Reference | Reference |
| Black | 1.34 (0.84 – 2.12) | 1.11 (0.65 – 1.91) |
| Hispanic | 1.45 (0.90 – 2.36) | 1.91 (1.09 – 3.35) |
| Other | 1.15 (0.42 – 3.18) | 1.10 (0.33 – 3.66) |
| Etiology | ||
| Hepatitis C | Reference | Reference |
| Alcohol-related | 1.89 (1.15 – 3.10) | 1.53 (0.84 – 2.79) |
| Nonalcoholic steatohepatitis | 2.52 (1.46 – 4.37) | 1.53 (0.77 – 3.03) |
| Hepatitis B | 6.12 (1.64 – 22.9) | 7.31 (1.66 – 32.3) |
| Other | 1.76 (0.92 – 3.38) | 1.62 (0.72 – 3.64) |
| Child Pugh Class | ||
| Child Pugh A | Reference | Reference |
| Child Pugh B | 1.41 (0.94–2.13) | 1.59 (0.99 – 2.56) |
| Receipt of hepatology care | 6.56 (4.44 – 9.71) | 30.1 (17.5 – 51.7) |
| Health status | ||
| Excellent/very good | Reference | Reference |
| Good | 1.05 (0.63 – 1.75) | 1.24 (0.67 – 2.32) |
| Fair/poor | 0.96 (0.59 – 1.57) | 1.40 (0.77 – 2.55) |
| Knowledge score | ||
| ≤50% | Reference | Reference |
| 66.7% | 1.38 (0.77 – 2.48) | 2.10 (1.01 – 4.37) |
| 83.3% | 1.10 (0.67 – 1.82) | 1.47 (0.77 – 2.82) |
| 100% | 1.32 (0.76 – 2.29) | 1.82 (0.91 – 3.64) |
| Delayed Medical Care | 1.02 (0.70 – 1.48) | 0.60 (0.38 – 0.97) |
| ≥1 Patient-reported Barrier** | 0.70 (0.49 – 0.99) | 0.62 (0.41 – 0.94) |
Adjusted for health system, age, sex, and health status
Included the most common reported barriers (costs of surveillance tests, difficulty with ultrasound scheduling, transportation difficulties, and uncertainty where to get surveillance performed)
DISCUSSION
To the best of our knowledge, this study represents one of the largest and first multi-center efforts to characterize patient knowledge and barriers for HCC surveillance. Patients at each site expressed fear of developing HCC and demonstrated high knowledge of surveillance. However, patient-reported barriers, including medical-related financial distress, were common across health systems and appeared to translate into lower HCC surveillance. These correlates of HCC surveillance represent modifiable factors that can be targeted to increase HCC surveillance utilization.
Consistent with prior studies, we found underuse of HCC surveillance across health systems.8,27,28 Although nearly three-fourths of patients received some imaging during the study period, less than one-third received semi-annual surveillance. This utilization pales in comparison to screening adherence in other cancer screening programs including breast and colorectal cancer screening.17, 18 Semi-annual surveillance receipt is a primary driver of early HCC detection and mortality reduction, highlighting a need for interventions.4,7 Surveillance underuse is related to a combination of patient-, provider-, and system-level factors, and interventions will likely have to target each level to have the greatest effect.
The importance of knowledge as a determinant of cancer screening has been mixed with some finding an association and others not.19–21 Although there are few data regarding this association in HCC surveillance, existing studies have similarly had mixed findings. A survey study among 160 patients from a tertiary care center found patient involvement in healthcare decisions, but not knowledge, was associated with surveillance receipt.22 In contrast, a survey among 541 patients from a safety-net health system found patient knowledge regarding the at-risk population was associated with increased surveillance receipt.12 In our study, we demonstrated patients had high knowledge about HCC surveillance including the at-risk population, recommended testing, and the benefits of surveillance. Patients with lower knowledge had lower surveillance receipt in univariable analysis although this was no longer significant in multivariable analysis. This may due to timing of our data collection (knowledge was measured after surveillance behavior) or because other attitudes and barriers are more important determinants of behavior. Irrespective, patient-centered interventions should cognitively engage patients and combat misconceptions on when and why to screen, such as surveillance not being necessary with a normal exam and labs. Patient education can be delivered by a variety of means including providers or nurses in clinic, patient-to-patient communication, or smartphone apps.
Nearly half of patients reported barriers to HCC surveillance, including concerns of cost, scheduling, and transportation. These barriers had been reported in a prior single-center study, but it was unclear if these barriers could be generalized to others.12 Results from our study reinforce these barriers are experienced by patients across different health settings, with differing healthcare coverage and care delivery models, and highlight the need for interventions. Some barriers, such as scheduling and transportation, are likely related to surveillance being ultrasound-based and often performed on a separate date and separate location than the patient’s clinic visit. Blood-based biomarkers, with sufficient accuracy to supplant ultrasound as a primary surveillance modality, could be performed the same day as a visit, thereby reducing barriers to surveillance completion and increasing surveillance effectiveness.24,29 However, we are likely years from such biomarkers being available, so data are needed to evaluate other interventions, such as patient navigation services, in the interim. A study evaluating population health mailed outreach did not find a benefit of adding patient navigation when examining one-time screening within 6 months but did find a significant increase in semi-annual surveillance over an 18-month period.25,26 Of note, patient navigation in that study only consisted of reminder calls for surveillance and help with rescheduling. It is possible more intensive navigation such as transportation assistance or help with ultrasound scheduling could further increase surveillance receipt.
Additionally, we found nearly half of patients expressed worry about ability to pay medical bills, nearly one-fourth being unable to cover co-payments, and nearly one-fourth reported delays in medical care including HCC surveillance related to financial distress. Although ultrasound is cheap compared to other screening tests such as colonoscopy35, it is subject to co-payments, which can place significant stress on patients. Several studies have demonstrated financial toxicity related to cancer care, resulting in non-adherence and treatment delays, but most of this literature has focused on cancer treatment instead of screening.30–32 Our study extends these findings by showing financial toxicity of medical care can also impact adherence to cancer screening programs. These findings highlight a potential need for policy reforms, including expansion of covered benefits and limits on out-of-pocket spending, to reduce financial burden in this population and improve receipt of surveillance. In the interim, interventions such as patient navigation, combined with subsidizing out-of-pocket costs, may increase HCC surveillance.
We acknowledge our study had limitations. First, patients may have had imaging at external institutions without results being captured in the EMR. Although this is less likely among patients at the VA or safety-net health system given their integrated nature, this is likely for some patients at the tertiary care referral center who were referred from long distances and may have surveillance performed locally. Second, we assessed surveillance over a one-year time frame, and this may not reflect surveillance over longer periods. Third, there is a possibility of response bias, in which patients provide responses they perceived as desirable instead of actual attitudes and barriers. Finally, survey studies are inherently limited by non-response bias, in which more compliant patients and/or those interested in surveillance were more likely to respond. We believe these limitations are outweighed by the study’s strengths including its large sample size representing a diverse cohort followed in different types of health systems, confirmation of cirrhosis diagnosis by chart review, and use of validated survey measures.
In summary, we found patient-reported barriers were significantly associated with lower HCC surveillance receipt in a large diverse cohort of patients followed in different healthcare settings. These data highlight the potential for interventions, such as patient navigation, to increase HCC surveillance utilization.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
More than 20% of patients with cirrhosis do not receive semi-annual hepatocellular carcinoma (HCC) surveillance as recommended, but little is known about the effects of patient-level factors on surveillance receipt
Findings:
Patient-reported barriers such as knowledge deficits, costs, difficulty scheduling, and transportation are significantly associated with less-frequent receipt of HCC surveillance.
Implications for patient care:
There is a need for patient-centered interventions, such as patient navigation, for patients requiring HCC surveillance.
Financial Support
This work was conducted with support from National Cancer Institute R01 CA212008 and Cancer Prevention Research Institute of Texas (CPRIT) RP150587. The work is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338) and by the Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413), Michael E. DeBakey VA Medical Center, Houston, TX. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health, Department of Veterans Affairs or the United States government, or Cancer Prevention Research Institute of Texas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Singal has served as a consultant for Wako Diagnostics, Glycotest, Exact Sciences, Roche, GRAIL, Bayer, and TARGET Pharmasolutions. None of the other authors have any relevant conflicts of interest to disclose
REFERENCES
- 1.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477–491 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:976–987 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 7.Costentin C, Layese R, Bourcier V, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-time Adjusted Survival of Patients With Compensated Viral Cirrhosis. Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- 8.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Utilization of hepatocellular carcinoma surveillance in patients with cirrhosis: A systematic review and meta-analysis. Hepatology (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AG, Li X, Tiro J, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med 2015;128:90.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons OL, Feng Y, Parikh ND, et al. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clin Gastroenterol Hepatol 2019;17:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farvardin S, Patel J, Khambaty M, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 2017;65:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastani R, Glenn BA, Taylor VM, et al. Integrating theory into community interventions to reduce liver cancer disparities: The Health Behavior Framework. Prev Med 2010;50:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell AE, Bastani R, Chen MS Jr, et al. Constructing a theoretically based set of measures for liver cancer control research studies. Prev Med 2010;50:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehra MS, Ma Y, Clark C, et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol 2013;47:e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology 2014;146:147–56; quiz e15–6. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease C, Prevention. Cancer screening - United States, 2010. MMWR Morb Mortal Wkly Rep 2012;61:41–5. [PubMed] [Google Scholar]
- 18.White A, Thompson TD, White MC, et al. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DP, Jr, CD Brownlee, TP McCoy, et al. The effect of health literacy on knowledge and receipt of colorectal cancer screening: a survey study. BMC Fam Pract 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anhang Price R, Koshiol J, Kobrin S, et al. Knowledge and intention to participate in cervical cancer screening after the human papillomavirus vaccine. Vaccine 2011;29:4238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearlman DN, Clark MA, Rakowski W, et al. Screening for breast and cervical cancers: the importance of knowledge and perceived cancer survivability. Women Health 1999;28:93–112. [DOI] [PubMed] [Google Scholar]
- 22.Singal AG, Volk ML, Rakoski MO, et al. Patient involvement in healthcare is associated with higher rates of surveillance for hepatocellular carcinoma. J Clin Gastroenterol 2011;45:727–32. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy NA, Rodgers A, Altus R, et al. Optimisation of hepatocellular carcinoma surveillance in patients with viral hepatitis: a quality improvement study. Intern Med J 2013;43:772–7. [DOI] [PubMed] [Google Scholar]
- 24.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol 2016;14:875–886 e6. [DOI] [PubMed] [Google Scholar]
- 25.Singal AG, Tiro JA, Marrero JA, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology 2017;152:608–615.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singal AG, Tiro JA, Murphy CC, et al. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients With Cirrhosis: A Randomized Clinical Trial. Hepatology 2019;69:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 2010;52:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular Carcinoma Surveillance Among Cirrhotic Patients With Commercial Health Insurance. J Clin Gastroenterol 2016;50:258–65 [DOI] [PubMed] [Google Scholar]
- 29.Parikh ND, Mehta A, Singal AG, Block T, Marrero JA, Lok AS. Biomarkers for the Diagnosis of Hepatocellular Carcinoma. Cancer Epi Biomarkers Prevention (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chino F, Peppercorn JM, Rushing C, Kamal A, Attomare I, Samsa G, et al. Out-of-pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncol 2017; 3(11): 1582–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zafar SY. Financial toxicity of cancer care: It’s time to intervene. J National Cancer Institute 2016; 108(5): djv370. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey S, Bansal A, Fedorenko CR, Blough D, Overstreet K, Shankaran V, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol 2016; 34(9): 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making 2008;28:917–25. [DOI] [PubMed] [Google Scholar]
- 34.Medical Expenditure Panel Survey (MEPS). Content last reviewed August 2018. Agency for Healthcare Research and Quality, Rockville MD. https://www.ahrq.gov/data/meps.html [PubMed] [Google Scholar]
- 35.Singal AG, Parikh ND, Hutton DW, Tapper EB. Cost effectiveness of hepatocellular carcinoma surveillance: An assessment of benefits and harms. Am J Gastro (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


