Abstract
Preeclampsia is a hypertensive disease of pregnancy characterized by new-onset hypertension, with either proteinuria and/or organ dysfunction. Preeclampsia is a leading cause of maternal morbidity and mortality; however, the underlying cellular and molecular mechanisms are not well understood. There is consensus that the underlying mechanism(s) resulting in preeclampsia is centered around abnormal placentation, inadequate spiral-artery remodeling, and deficiency in trophoblast invasion, resulting in impaired maternal blood flow to the placenta and a release of signals and/or inflammatory mediators into maternal circulation triggering the systemic manifestations of preeclampsia. ER stress, resulting in impaired autophagy and placental release of aggregated proteins, may also confer systemic stress to maternal organs in preeclampsia. Extracellular vesicles, lipid-bilayer enclosed structures containing macromolecules including proteins, miRNA, and other important nucleotides, have been suggested to play an important role in this maternal-fetal communication. Circulating EVs are present in greater quantity in the plasma of preeclampsia subjects compared to normal pregnancy, and the placental derived EVs have been shown to have altered protein and RNA cargo. In this review, we will focus on extracellular vesicles and their role in preeclampsia, specifically their role in immune responses, inflammation, altered angiogenesis, endothelial dysfunction.
Keywords: Extracellular vesicles, placenta, preeclampsia
Introduction: Overview of Preeclampsia
Preeclampsia is a hypertensive disease of pregnancy which affects 3–8% of pregnancies 1–3. This pregnancy syndrome is characterized by new-onset hypertension with either proteinuria and/or organ dysfunction after 20 weeks of gestation 4. It can present early with manifestations necessitating an early delivery (<34 weeks) or a later in gestation with resultant delivery >34 weeks. Preeclampsia is a leading cause of maternal morbidity and mortality and heralds risk for cardiovascular disease later in life for both the mother and child 5–7. Preeclampsia can affect multiple organs, including the peripheral vasculature, liver, kidneys, central nervous system, and the heart 8–12. The underlying cellular and molecular mechanisms that trigger preeclampsia and facilitate its progression are not well understood. Consequently, there are no established early diagnostic tests or effective treatments for preeclampsia. The only treatment option is delivery.
While the precise pathogenesis remains unclear, it is well accepted that the placenta plays a crucial role, as preeclampsia is pregnancy specific, can occur in molar pregnancies or trophoblast tumors, where no fetal tissue is present, and is alleviated after delivery of the placenta 13. The placenta is an important fetal organ that is essential for fetal growth, survival and the maintenance of pregnancy. Placental trophoblast cells are involved in the maintenance of maternal-fetal communications during pregnancy. There are several types of trophoblast cells, including the syncytiotrophoblasts (ST), cytotrophoblasts (CT), and extravillous trophoblasts (EVT). The syncytiotrophoblast layer, which results from the fusion of cytotrophoblast cells, is in direct contact with maternal blood and controls the exchange of gas, nutrients and waste products between the mother and fetus. Extravillous trophoblasts establish the anchoring villi and invade the uterine spiral arteries, inducing vascular remodeling 14.
Despite the unclear etiology of preeclampsia, there is wide-spread acceptance that an important mechanism for development of this pregnancy complication is centered around abnormal placentation, inadequate spiral-artery remodeling, and deficiency in trophoblast invasion 13,15. This results in impaired maternal blood flow to the placenta and high perfusion pressure generating shear stress to the trophoblast 16. It is hypothesized that as a result, the placenta releases signals and/or inflammatory mediators into maternal circulation triggering the systemic manifestations of preeclampsia 13,17. In recent years, extracellular vesicles have been recognized as an important mediator of this maternal-fetal signaling. In this mini-review, we provide a brief overview of placental extracellular vesicles and the potential role they play in the pathogenesis of preeclampsia.
Extracellular Vesicles
Extracellular vesicles (EV) are lipid-bilayer enclosed structures, released from all cells into the extracellular environment, including the systemic circulation 18. They contain macromolecules including proteins, miRNA, mRNA, lipids, growth and apoptotic factors, and other important molecules. Most importantly, there is increasing evidence of their involvement in cell-to-cell communication and signaling 19. EVs are released in both normal and diseased states. They can be found in almost all bodily fluids. Once secreted, the EVs can act in both a paracrine and autocrine manner, interacting with adjacent cells or distantly 20. Once released, EVs interact with recipient cells by fusion with the plasma membrane, via receptor-mediated uptake, by internalization via endocytosis or by micropinocytosis (reviewed in 21).
EVs have been characterized by their size, function, biogenesis and morphology into three major categories (exosomes, microvesicles, and apoptotic bodies). Exosomes range in size from 40 to 120 nm and are formed from the inward budding of the endosomal membrane and the formation of multivesicular bodies. They are then released by cells by exocytosis after the multivesicular body fuses with the plasma membrane 22. Microvesicles, range in size from 100–1000 nm in size and form by direct budding from the plasma membrane 23. Exosomes are enriched with endosomal membrane markers (e.g., CD9, CD63, TSG101, and CD81), while microvesicles are enriched with CD40 protein markers 22, however the specificity of protein markers remains elusive. Apoptotic bodies are formed during apoptosis and are the largest EVs ranging from 1000–5000 nm. These three classes of EVs have been extensively reviewed 18,19,22–24.
As exosomes and microvesicles overlap in size, specific subtypes remain difficult for isolation and characterization. There are currently many strategies for isolation, including differential density ultracentrifugation, immunoaffinity beads directed at surface proteins, size-exclusion chromatography and lateral flow microfluidic separation. Flow cytometry, nano-particle tracking analysis (NTA), and electron microscopy are used to identify and quantify EVs (extensively reviewed in 24).
Placental EVs
Extracellular vesicles are secreted by various placental cells during pregnancy. The syncytiotrophoblast layer is believed to be the primary source of placental derived EVs. The STB releases exosomes, micro-vesicles called STBM, and larger apoptotic bodies 25. As the biogenesis of these subtypes is different, their function and effect on target cells may also vary. There are differences in cargo and function when comparing the exosome to the other vesicle populations. For example, some exosomes exhibit unique proteins and phospholipids. Additionally, viral activity is associated with the C19MC microRNA family present in exosomes 26. However, as noted above, due to difficulty in isolation and characterization, many studies have focused on the EV population as a whole.
The placenta-specific enzyme placental-type alkaline phosphatase (PLAP) has been identified as a unique marker of placenta, trophoblast cell derived, extracellular vesicles 27. PLAP is a membrane protein that is unique to the placenta. Quantitative analysis of PLAP has allowed for placental EVs to be identified in maternal peripheral blood in vivo as early as 6 weeks of gestation 28,29. Significant differences in circulating EVs have been found between pregnant and nonpregnant subjects. The total number of placental EVs has been shown to increase throughout gestation and reaches a maximum at term. The number of placental EVs returns to non-pregnant levels around 48 hours post-delivery 28,30. There is also evidence that the cargo of these EVs change (both miRNA and mRNA) throughout normal pregnancy 18,31. In early pregnancy, hypoxic conditions stimulate cytotrophoblast cells and placental mesenchymal stem cells to release an increased number of EVs with altered vesicle content 32.
Placental EVs and Function in the Maintenance of Pregnancy
Throughout normal pregnancy, placental EVs can act in both an immunosuppressive and immunostimulatory manner, dependent on their cellular origin and cargo (reviewed in 14). EV’s released from the STB, containing placental Fas ligand (FASLG) and CD274 contribute to the inhibition of T-cell activation signals 33,34. Those containing TNSF10 mediate T-cell apoptosis, preventing degradation of invading trophoblast cells, thus resulting in fetal tolerance and increased immune privilege 33. EV’s expressing NKG2D ligands, MHC class 1 chain-related molecules A and B (MIC A and MIC B) and family of six cytomegalovirus UL 16-binding proteins ULBP1-6) (MYC and ULBP), have been shown to activate the NKG2D receptors in natural killer cells, leading to the inhibition of these cells via apoptosis, resulting in fetal immune escape 35,36. Although, in normal pregnancy, only a very low percentage of uterine NK cells (≈70%) express NKG2D, it can be upregulated in response to bacterial and viral infections 37. Members of the B7 family bind to lymphocytes and have been found to co-localize with exosome bio-machinery and are released in exosomes 34. Placental EVs express PD-L1, a member of the program death or check point family, which induces negative regulation of T-cell activation and prevents overstimulation of the immune systems via the CD3-zeta and Jak3 pathway 34. Other immune-suppressive molecules such as HLA-G are expressed in placental EVs during early pregnancy and decrease throughout gestation 38,39.
EV signaling and maternal immune-modulation involve not only the expression of proteins, but also the EV delivery of microRNA. Additional evidence for immunomodulatory effects of placental EVs come from the study of viral resistance in pregnancy. It has been shown that the expression of the C19MC microRNA family in primary human trophoblast EV’s, reduces viral infection in nonplacental cells, and that at least three members of this family (mir517-3p, mir516B-5p, and mir512-3p) have potent antiviral effects. These studies showed that conditioned primary human trophoblasts (PHT), PHT medium, PHT exosomes, and individual C19MC miRNAs confirm this resistance by inducing autophagy 40. The destruction of viral particles could possibly reduce inflammatory responses which could negatively affect placental cell function or lead to systemic inflammation, making autophagy an important mechanism in the maintenance of healthy pregnancy 41. Additionally, miR-517a-3p has been found in high abundance in trophoblast derived EVs and is internalized by both T-cells and NK cells, regulating their activation via the nitric oxide pathway 42. Similarly, miR-141 is contained in the STB-EV cargo and is involved in suppression of T-cells 43.
In conjunction with the immunosuppressive nature of EVs, there is also evidence for pro-inflammatory functions. Trophoblast cells have been shown to release EVs containing fibronectin which recruits and interacts with macrophages and monocytes by binding to α5β1 44. During pregnancy, macrophages are abundant in the decidua and moderate the inflammatory response by the production of cytokines and chemokines. Thus the EV-associated fibronectin results in increased pro-inflammatory cytokine release including IL-1β, 1L-6, serpine-1, and TNFα. Trophoblasts also release EVs with microRNA’s including miR146a-3a which promotes IL-8 production in trophoblasts 45.
In addition to immunosuppression and/or inflammatory functions, placental EVs are involved in vasculogenesis, angiogenesis and endothelial dysfunction. Endothelial dysfunction is a systemic imbalance between vasodilator and vasoconstrictor molecules and is therefore part of the etiology of preeclampsia. MiR-520c-3p in EVs leads to increased invasiveness of EVTs and promotes angiogenesis by targeting CD44 46. Nitric oxide is also a vasodilator and an important regulator of endothelial function. There is evidence that STB-EVs contain eNOS, which is subsequently able to produce nitric oxide 47. Additionally, miR-155 has been found in placental EVs, which is known to inhibit eNOS, resulting in endothelial dysfunction 48.
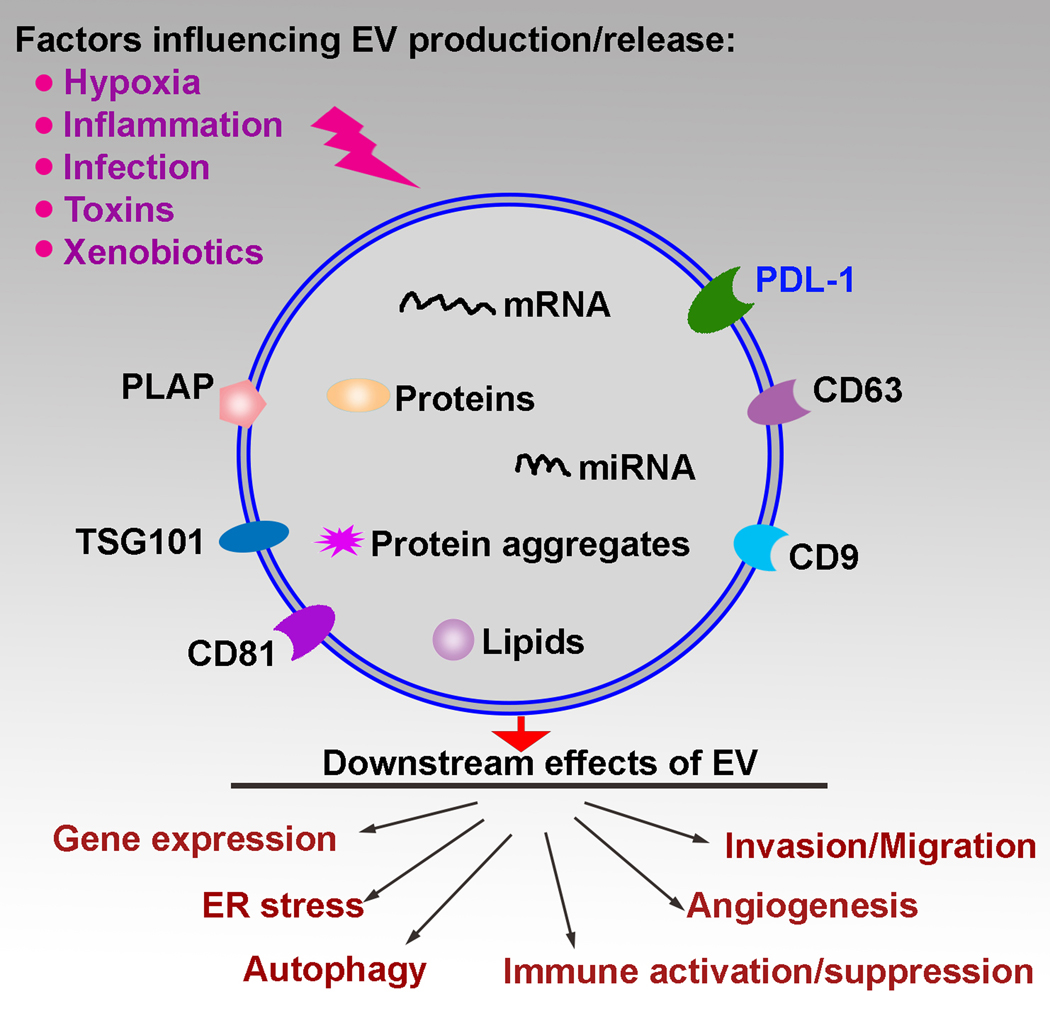
Studies which have looked specifically at STBMs, excluding the smaller exosome subtype, have shown that they can also induce systemic inflammation. These STBM vesicles stimulate leukocytes and monocytes to increase expression of pro-inflammatory cytokines 49,50. Syncytin-1 is shed from the placenta and is a surface molecule on STBMs. It has been shown to modulate immune cell activation and the responses of immune cells to lipopolysaccharide stimulation 51. Varying results have been reported regarding the immunosuppressive nature of STBM’s, depending on the means of isolation of the STBM, suggesting that the function and activities of these vesicles are preparation dependent 52. Given all of the aforementioned data, proteins and microRNAs in placental EVs play an integral role in regulating immune responses, inflammation and angiogenesis during normal pregnancy. A schematic summary is shown in Figure 1.
Figure 1: Placenta extracellular vesicle (EV) production/release and their downstream effects.
Many factors, including hypoxia, infection, and toxins influence the production of EVs. These factors influence their cargo including mRNA, microRNA, proteins, protein aggregates and lipids. The biogenesis of these vesicles also determine their cell surface markers. These include, but are not limited to cell surface molecules such as CD63, CD9, CD81, tumor susceptibility 101 (TSG101), programmed cell death ligand 1 (PDL1), and placental alkaline phosphatase (PLAP). There are numerous downstream effects, including changes in gene expression in target cells, immune activation/suppression, invasion/migration, autophagy, angiogenesis and ER stress.
Placental EVs and Preeclampsia
Given the role of placental EVs in normal pregnancy, as described above, it is believed that EVs may be involved in the pathogenesis of preeclampsia by modulating the immune/inflammatory responses associated with the disorder. During normal pregnancies, placental EVs lead to physiologically adaptive inflammation, whereas excessive inflammation potentially leads to pregnancy disorders such as preeclampsia. There is also growing evidence that both abnormal immune tolerance and hyperactive immune activity can lead to angiogenesis and the onset of preeclampsia 53.
Quantity and cargo of placental EVs in preeclampsia
It is well established that the plasma contains more circulating EVs in preeclampsia compared to normal pregnancy. Moreover, placental EVs production (specifically STB-EVs) has been shown to be enhanced in PE in some studies, while others show a decrease. Much of this difference can likely be attributed to the methods used to measure EVs. Early work in the vesicle field suggested that syncytiotrophoblast derived vesicles (STBM) that are shed from the placenta are significantly increased in early onset preeclampsia 54. Tannetta et al. studied expression of PLAP, Flt-1 and endoglin from two different preparations of STBM and found little changes in both surface and overall expression of all three proteins in mSTBM; however, STBM from perfused placentas from PE subjects released decreased content of PLAP+ EVs and reduced PLAP expression on surface per EV and higher Flt-1 expression 17. Levine et al. recently found a similar increase in overall circulating EVs in preeclampsia patients and a decrease in STB-EVs in maternal plasma samples. These results suggest a possibly lower number of placenta specific EVs, lower amounts of the placenta specific marker on EVs, or a combination of both. 55. This can also reflect the notion that PLAP is not an appropriate marker for the detection of circulating STB-EVs. Despite these observations, PLAP is still commonly used as a measure of placental EVs. Salomon et al. showed an increasing number of total EVs as gestation progressed, with even higher quantities in preeclampsia subjects at each gestational stage. Analysis of PLAP by immunosorbent assay showed significantly higher PLAP content in PE subjects overall, however this difference diminished when normalized to total EV quantity for each gestational age 56. Pillay et al. showed much larger differences in total EV quantity between preeclampsia and normal subjects, with the largest differences seen in early onset preeclampsia samples compared to GA matched controls 57. They also studied PLAP+ EVs, and an increase in PLAP content was found in relation to early onset but not late onset preeclampsia. It is important to note that these results in terms of both quantity and function may be dependent on the PLAP antibody used. There is skepticism implying that commercially derived human PLAP is not pure and that the antibodies may lack specificity 58. This is a limitation that should be recognized in the current studies of EVs and it is thus necessary to validate these antibodies. Additionally, even despite the technology being used for quantification, the number of placental particles detected is still rather limited and using changes in quantity of these EVs as a biomarker for preeclampsia, or other placental related disorders, may be of limited power.
Function of placental EVs in preeclampsia
Inflammation
As noted above, placental EVs interact with maternal immune cells resulting in production of pro-inflammatory cytokines. These trophoblast-derived EVs can lead to vascular inflammation and endothelial injury. The inflammatory response is enhanced in preeclampsia 49. In pregnancies with vascular complications, such as preeclampsia, placental EVs can increase trophoblast apoptosis and decrease migration 59. PP13, placental protein 13, is involved in T-cell and macrophage apoptosis and regulation of maternal immune tolerance. Lower levels of PP13 in maternal serum during the early stages of pregnancy is associated with development of early onset preeclampsia, with increased expression later in pregnancy attributed to an increase in membrane shed vesicles 60,61. However, Sammar et. al reported decreased expression of PP13 in STB-EVs, both membrane bound and intra-vesicular 62. A decrease in Syncytin-2 in placental EVs has also been reported, affecting EV fusion with targeted cells, trophoblast fusion and placental development. This suggests not only a role for the change in cargo but also a change in function of EVs in the etiology of PE 63–65.
Angiogenesis
Anti-angiogenic factors also play an important role in the pathogenesis of PE, leading to an imbalance in angiogenesis and endothelial dysfunction. Soluble fms-like tyrosine kinase-1 (sFlt1) and soluble endoglin (sEng) are known to be increased in PE and to correlate with the severity of the phenotype 66. Administration of human preeclampsia serum in pregnant IL-10−/− mice induced preeclampsia-like symptoms, including elevated sFlt1 and sEng. Additionally, in an invitro model of endovascular activity, preeclampsia serum disrupted communication between trophoblasts and endothelial cells 67. PAI-1 and PAI-2 are also expressed in the placenta and are inhibitors of fibrinolysis. Overexpression of these PAI’s leads to fibrin deposits and inhibits the function of the placental vasculature and spiral arteries, resulting in hypertension 68. sEng and PAI-1 are highly expressed on the surface of STB-EVs, thus potentially adversely affecting placental function in preeclampsia 69. Fms-like tyrosine kinase 1 (Flt-1) from first trimester placental explants, was also upregulated in preeclampsia samples, contributing to the spread of endothelial damage by sequestering vascular endothelial growth factor VEGF in maternal circulation 70. NEP (neprilysin) was also upregulated in STB-EVs from women with preeclampsia 8. NEP, a membrane bound metalloprotease, has many potential signaling functions. NEP, and enhances the degradation of peptides such as vasodilators. Higher expression of NEP has been associated with the onset of hypertension, the main feature of preeclampsia. STB-EVs carry bioactive tissue factor and these EVs from PE patients lead to an increase in platelet tyrosine phosphorylation and aggregation 17. Platelet reactivity is associated with PE in some women and correlates with the increased thrombotic risk associated with PE. Treatment of high-risk PE patient with aspirin to reduce platelet aggregation, also inhibits STBEV-induced aggregation of platelets.
ER Stress, Autophagy and Misfolded Proteins
Recently, evidence has emerged suggesting that PE is a disorder involving protein misfolding, a mechanism occurring potentially upstream of inflammation 71–73. Aggregated, misfolded proteins have been detected in serum and urine of preeclampsia patients 72–74 and have been shown to induce PE-like features in pregnant mice 72. We have attributed this excess protein aggregation in PE to compromised autophagy in trophoblasts, which in turn contributes to poor placentation and hypertension 75,76. Autophagy is a degradation system present in cells that maintains cellular homeostasis by eliminating misfolded or aggregated proteins 77. The lysosomal machinery, involving fusion of autophagosomes and autolysosomes with proteases, such as cathepsin D, degrades the aggregated protein structures and removes damaged organelles 78.
Normal pregnancy induces mild endoplasmic reticulum (ER) stress, which is a necessary component of placental development 76,79. Additionally, as noted above, autophagy is an essential mechanism in the maintenance of healthy pregnancy and EVs derived from trophoblasts have been shown to activate autophagy in non-placental cells via transfer of miRNA conferring viral resistance 40. Excessive or chronic ER stress in preeclampsia leads to placental dysfunction. Nakashima et al. recently elucidated the link between ER stress, autophagy and trophoblasts showing that that ER stress reduces the number of lysosomes, inhibits autophagic flux in trophoblast cells, and results in aggregation of misfolded proteins in PE. It also inhibits lysosomal exocytosis 76 and as a result, leads to a change in EV release from trophoblasts. The placenta produces transthyretin (TTR). In its native state TTR circulates as a homotetramer and serves as a cargo protein for retinol and thyroid hormone. It also scavenges sFlt 80. In response to ER stress, misfolded TTR aggregates accumulate as toxic deposits in the placenta. In preeclampsia, a significant amount of TTR is released into the maternal circulation via placental EVs, in both its natural and aggregated form 71. Preeclamptic EVs may serve as a mechanism for disposal of toxic aggregated protein from the placenta, they may also be delivering aggregated transthyretin to maternal organs, contributing to systemic cellular stress and the pathogenesis of preeclampsia 71. In vitro and in vivo approaches have demonstrated that misfolded transthyretin is a preeclampsia-causing agent and that native transthyretin has the ability to block the onset of preeclampsia-like features 72. Similarly, recent observations from our laboratory suggest that several other proteins, including those associated with Alzheimer’s disease are part of this toxic proteinopathy and may contribute to the EV’s cargo 81. In several neurodegenerative disorders, there is also evidence that EVs containing misfolded pathogenic proteins play a role in the cell-to-cell transmission aggregate proteins leading to the degenerative phenotypes (reviewed in 82). These findings support the early hypotheses that changes in blood flow in the placenta, lead to a release of toxins via extracellular vesicles, which results in maternal preeclampsia symptoms 72.
microRNA
In addition to surface bound proteins and protein cargo, placental extracellular vesicles contain microRNA which can be internalized by maternal cells altering target gene expression 83. microRNA from EV in maternal circulation and the placenta from preeclampsia are the subject of extensive investigations. In preeclampsia, trophoblasts actively secrete exosomal hsa-miR-210. Has-miR-210 is a hypoxia induced microRNA 84. Targeted screening for selected miRNAs showed elevated levels of miR-210, −136, −494, and −495 in peripheral blood exosomes in women with preeclampsia 85. In a separate targeted screen for the expression of C19MC microRNA, miR-517-5p, miR-520a-5p, and miR-525-5p were found to be downregulated in preeclampsia 86. Genome-wide microRNA profiling studies have compared the EV cargo between preeclampsia and normal pregnancy. Salomon et al. identified differentially expressed microRNAs whose targets regulate biological processes such as migration, placenta development, and angiogenesis. They identified hsa-miR486-1-5p and has-miR486-2-5p, which have previously been shown to suppress migration, proliferation and invasion, as potential biomarkers for preeclampsia 56. Li et al. identified 7 differentially expressed microRNAs between women with preeclampsia and controls. They found no differentially expressed microRNA between women with fetal growth restriction and controls. The differences were also not apparent in whole plasma miRNA 87. mir153-3p and mir325-3p are two of the differentially expressed microRNA. The former has been shown to be involved in the inhibition of cell proliferation and increase in apoptosis, and the latter is associated with decreased tube formation and angiogenesis 87. While many of these studies have found similar pathways implicated in the pathogenesis of preeclampsia, the differential expression of specific microRNA is often not reproducible and vary dependent on biospecimen source, isolation procedures and RNA quantification techniques. Thus, there is little agreement whether there are differentially expressed miRNAs between preeclampsia and normal pregnancies 87.
In-Vivo Studies
It is important and valuable to study the effects of EVs in-vivo. Merely identifying their cargo and use of in-vitro cell culture studies does not provide full insight into their function and the manner in which they interact with other cells. In vivo models provide information on the translational significance of EVs. For example, intricate organs such brain have been shown to be repaired by administration of EVS in an experimental model of subcortical stroke 88. However, it has been challenging to establish a reliable animal model of preeclampsia, as it does not occur naturally in laboratory animals and is a systemic condition with a wide array of phenotypic manifestations. Marshall et al. have reviewed many of the current animal models and their limitations 89. Mice are a widely used model as they are easily genetically manipulated; however, there are inherent differences between mouse and human pregnancy, including differences in both the structure and function of the placenta. Models such as endothelial NOS knockout mice (eNOS−/−/Nos3−/−) recapitulate some of the characteristics of preeclampsia, however they develop what can be viewed as chronic hypertension, rather than new onset hypertension which is pregnancy specific 90. Other mouse models, including Catechol-O-methyltransferase (COMT) deficient mice (Comt−/−), the BPH/5 mouse, exogenous s-Flt treatment and others are reviewed in detail 89. There is also evidence that placental Atg7 deficiency in conditional Atg7 knockout mice may result in pregnancy associated hypertension; however, they do not experience the severe pathological features of preeclampsia 91. Work presented from our lab by Kalkunte et al. demonstrated that a single sera injection on gestational day 10 from severely preeclamptic women induces hypertension, proteinuria, and kidney pathological characteristics, as well as intrauterine growth restriction (IUGR), in IL10−/− mice in a pregnancy-specific manner. IL-10 is an anti-inflammatory cytokine and is absence may predispose the mice to inflammatory activities and poor vascularization 67. Our published work has demonstrated this sera also contains EVs with aggregated protein cargo 71. These protein aggregates could aid in inducing the preeclampsia-like pathology in pregnant mice. There is other evidence that placental EVs from preeclamptic placenta can induce a preeclampsia-like phenotype in pregnant mice; however, it is important to note that this result was not necessarily pregnancy specific. The injected EV from human placentas have been found in mouse organs 70 and can be trafficked from mouse amniotic fluid to the placenta and other organs 92. These EVs have been shown to result in damage to the placental vasculature and poor fetal nutrition 93.
Conclusions
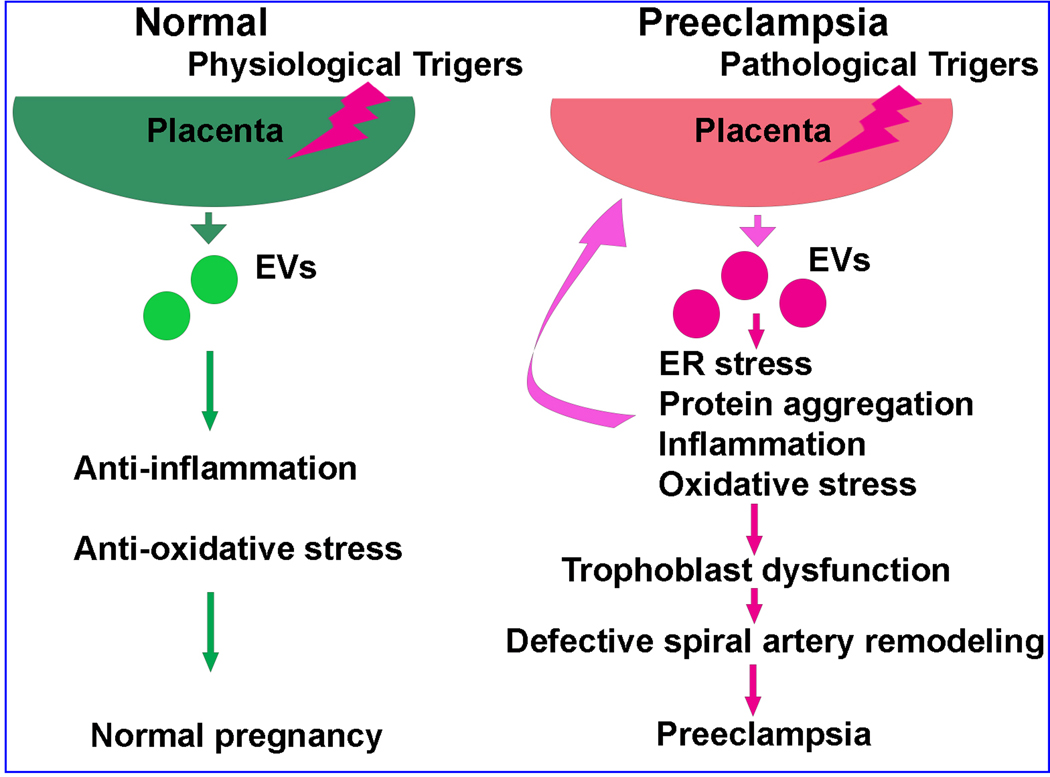
The study of extracellular vesicles is growing and evolving and will aid in understanding the underlying mechanisms for the maintenance of healthy pregnancy and the etiology of preeclampsia. In normal pregnancy, placental derived EVs containing proteins, miRNA and other molecules function in regulation of maternal immune response, inflammation and oxidative stress. Pathological triggers resulting in changes in placental vesicle production and cargo, including excess aggregated proteins, excessive inflammation, altered trophoblast function and defective spinal artery remodeling and the systemic preeclampsia manifestation, a summary of which is represented in Figure 2. Continued investigation of extracellular vesicles, including other RNA cargo and differences that result due to preparation and isolation techniques, will strengthen the understanding of the pathogenesis of preeclampsia, including elucidating new avenues for early diagnosis and treatment.
Figure 2: Extracellular Vesicle production and effects in normal pregnancy and preeclampsia.
In normal pregnancy, placental derived EVs function in regulation of maternal immune response, inflammation and oxidative stress. Pathological triggers resulting in changes in placental vesicle production and cargo, including excess aggregated proteins, results excessive inflammation, altered trophoblast function and defective spinal artery remodeling, resulting in the preeclamptic condition.
Acknowledgments
Funding: This work was supported in part by the NIH P20 GM121298 and P30 GM114750 grants.
Footnotes
Conflicts Declaration: The Authors have no conflicts of interest to declare. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
References
- 1.Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155(3):203–209. [DOI] [PubMed] [Google Scholar]
- 2.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 5.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;286(4):H1389–1393. [DOI] [PubMed] [Google Scholar]
- 6.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56(1):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis EF, Lazdam M, Lewandowski AJ, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–1561. [DOI] [PubMed] [Google Scholar]
- 8.Gill M, Motta-Mejia C, Kandzija N, et al. Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia. Hypertension. 2019;73(5):1112–1119. [DOI] [PubMed] [Google Scholar]
- 9.Hammoud GM, Ibdah JA. Preeclampsia-induced Liver Dysfunction, HELLP syndrome, and acute fatty liver of pregnancy. Clin Liver Dis (Hoboken). 2014;4(3):69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis T, Odutayo A, Keunen J, Hladunewich M. The kidney in normal pregnancy and preeclampsia. Semin Nephrol. 2011;31(1):4–14. [DOI] [PubMed] [Google Scholar]
- 11.Kane SC, Dennis A, da Silva Costa F, Kornman L, Brennecke S. Contemporary clinical management of the cerebral complications of preeclampsia. Obstet Gynecol Int. 2013;2013:985606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130(8):703–714. [DOI] [PubMed] [Google Scholar]
- 13.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12(4):301–308. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Song G, Lim W. Effects of extracellular vesicles on placentation and pregnancy disorders. Reproduction. 2019;158(5):R189–R196. [DOI] [PubMed] [Google Scholar]
- 15.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342–352. [DOI] [PubMed] [Google Scholar]
- 16.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One. 2013;8(2):e56754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell MD, Peiris HN, Kobayashi M, et al. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol. 2015;213(4 Suppl):S173–181. [DOI] [PubMed] [Google Scholar]
- 19.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. [DOI] [PubMed] [Google Scholar]
- 20.Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. [DOI] [PubMed] [Google Scholar]
- 23.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon C, Greening DW, Bolumar D, Balaguer N, Salamonsen LA, Vilella F. Extracellular Vesicles in Human Reproduction in Health and Disease. Endocr Rev. 2018;39(3):292–332. [DOI] [PubMed] [Google Scholar]
- 25.Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol Immunol. 2014;11(6):548–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang Y, Bayer A, Chu T, et al. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta. 2016;47:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014;72(5):440–457. [DOI] [PubMed] [Google Scholar]
- 28.Sarker S, Scholz-Romero K, Perez A, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63(6):520–533. [DOI] [PubMed] [Google Scholar]
- 30.Salomon C, Torres MJ, Kobayashi M, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9(6):e98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong M, Kleffmann T, Pradhan S, et al. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication. Hum Reprod. 2016;31(4):687–699. [DOI] [PubMed] [Google Scholar]
- 32.Salomon C, Kobayashi M, Ashman K, Sobrevia L, Mitchell MD, Rice GE. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS One. 2013;8(11):e79636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191(11):5515–5523. [DOI] [PubMed] [Google Scholar]
- 34.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56(5–6):345–355. [DOI] [PubMed] [Google Scholar]
- 35.Hedlund M, Stenqvist AC, Nagaeva O, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183(1):340–351. [DOI] [PubMed] [Google Scholar]
- 36.Dhar P, Wu JD. NKG2D and its ligands in cancer. Curr Opin Immunol. 2018;51:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thaxton JE, Nevers T, Lippe EO, Blois SM, Saito S, Sharma S. NKG2D blockade inhibits poly(I:C)-triggered fetal loss in wild type but not in IL-10−/− mice. J Immunol. 2013;190(7):3639–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kshirsagar SK, Alam SM, Jasti S, et al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33(12):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truong G, Guanzon D, Kinhal V, et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells - Liquid biopsies for monitoring complications of pregnancy. PLoS One. 2017;12(3):e0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A. 2013;110(29):12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9(12):2173–2174. [DOI] [PubMed] [Google Scholar]
- 42.Kambe S, Yoshitake H, Yuge K, et al. Human exosomal placenta-associated miR-517a-3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biol Reprod. 2014;91(5):129. [DOI] [PubMed] [Google Scholar]
- 43.Ospina-Prieto S, Chaiwangyen W, Herrmann J, et al. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl Res. 2016;172:61–72. [DOI] [PubMed] [Google Scholar]
- 44.Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1beta production by macrophages. Am J Reprod Immunol. 2011;66(4):259–269. [DOI] [PubMed] [Google Scholar]
- 45.Gysler SM, Mulla MJ, Guerra M, et al. Antiphospholipid antibody-induced miR-146a-3p drives trophoblast interleukin-8 secretion through activation of Toll-like receptor 8. Mol Hum Reprod. 2016;22(7):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi H, Ohkuchi A, Kuwata T, et al. Endogenous and exogenous miR-520c-3p modulates CD44-mediated extravillous trophoblast invasion. Placenta. 2017;50:25–31. [DOI] [PubMed] [Google Scholar]
- 47.Motta-Mejia C, Kandzija N, Zhang W, et al. Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity is Reduced in Preeclampsia. Hypertension. 2017;70(2):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen L, Li Y, Li R, et al. Placentaassociated serum exosomal miR155 derived from patients with preeclampsia inhibits eNOS expression in human umbilical vein endothelial cells. Int J Mol Med. 2018;41(3):1731–1739. [DOI] [PubMed] [Google Scholar]
- 49.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178(9):5949–5956. [DOI] [PubMed] [Google Scholar]
- 50.Messerli M, May K, Hansson SR, et al. Feto-maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes. Placenta. 2010;31(2):106–112. [DOI] [PubMed] [Google Scholar]
- 51.Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, Abrahams VM. Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology. 2012;136(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair S, Salomon C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin Immunopathol. 2018;40(5):425–437. [DOI] [PubMed] [Google Scholar]
- 53.Cheng SB, Sharma S. Preeclampsia and health risks later in life: an immunological link. Semin Immunopathol. 2016;38(6):699–708. [DOI] [PubMed] [Google Scholar]
- 54.Goswami D, Tannetta DS, Magee LA, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27(1):56–61. [DOI] [PubMed] [Google Scholar]
- 55.Levine L, Habertheuer A, Ram C, et al. Syncytiotrophoblast extracellular microvesicle profiles in maternal circulation for noninvasive diagnosis of preeclampsia. Sci Rep. 2020;10(1):6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salomon C, Guanzon D, Scholz-Romero K, et al. Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J Clin Endocrinol Metab. 2017;102(9):3182–3194. [DOI] [PubMed] [Google Scholar]
- 57.Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta. 2016;46:18–25. [DOI] [PubMed] [Google Scholar]
- 58.Ravenni N, Weber M, Neri D. A human monoclonal antibody specific to placental alkaline phosphatase, a marker of ovarian cancer. MAbs. 2014;6(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shomer E, Katzenell S, Zipori Y, et al. Microvesicles of women with gestational hypertension and preeclampsia affect human trophoblast fate and endothelial function. Hypertension. 2013;62(5):893–898. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Kusanovic JP, Than NG, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199(2):122 e121–122 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Than NG, Abdul Rahman O, Magenheim R, et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008;453(4):387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sammar M, Dragovic R, Meiri H, et al. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia - A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta. 2018;66:17–25. [DOI] [PubMed] [Google Scholar]
- 63.Vargas A, Moreau J, Landry S, et al. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J Mol Biol. 2009;392(2):301–318. [DOI] [PubMed] [Google Scholar]
- 64.Vargas A, Zhou S, Ethier-Chiasson M, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014;28(8):3703–3719. [DOI] [PubMed] [Google Scholar]
- 65.Vargas A, Toufaily C, LeBellego F, Rassart E, Lafond J, Barbeau B. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod Sci. 2011;18(11):1085–1091. [DOI] [PubMed] [Google Scholar]
- 66.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. [DOI] [PubMed] [Google Scholar]
- 67.Kalkunte S, Boij R, Norris W, et al. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177(5):2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Estelles A, Gilabert J, Keeton M, et al. Altered expression of plasminogen activator inhibitor type 1 in placentas from pregnant women with preeclampsia and/or intrauterine fetal growth retardation. Blood. 1994;84(1):143–150. [PubMed] [Google Scholar]
- 69.Gardiner C, Tannetta DS, Simms CA, Harrison P, Redman CW, Sargent IL. Syncytiotrophoblast microvesicles released from pre-eclampsia placentae exhibit increased tissue factor activity. PLoS One. 2011;6(10):e26313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tong M, Chen Q, James JL, Stone PR, Chamley LW. Micro- and Nano-vesicles from First Trimester Human Placentae Carry Flt-1 and Levels Are Increased in Severe Preeclampsia. Front Endocrinol (Lausanne). 2017;8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tong M, Cheng SB, Chen Q, et al. Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia. Sci Rep. 2017;7(1):6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalkunte SS, Neubeck S, Norris WE, et al. Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model. Am J Pathol. 2013;183(5):1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buhimschi IA, Nayeri UA, Zhao G, et al. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci Transl Med. 2014;6(245):245ra292. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy FP, Adetoba A, Gill C, et al. Urinary congophilia in women with hypertensive disorders of pregnancy and preexisting proteinuria or hypertension. Am J Obstet Gynecol. 2016;215(4):464 e461–467. [DOI] [PubMed] [Google Scholar]
- 75.Nakashima A, Cheng SB, Ikawa M, et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy. 2019:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakashima A, Cheng SB, Kusabiraki T, et al. Endoplasmic reticulum stress disrupts lysosomal homeostasis and induces blockade of autophagic flux in human trophoblasts. Sci Rep. 2019;9(1):11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315(5817):1398–1401. [DOI] [PubMed] [Google Scholar]
- 79.Mizuuchi M, Cindrova-Davies T, Olovsson M, Charnock-Jones DS, Burton GJ, Yung HW. Placental endoplasmic reticulum stress negatively regulates transcription of placental growth factor via ATF4 and ATF6beta: implications for the pathophysiology of human pregnancy complications. J Pathol. 2016;238(4):550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Landers KA, Mortimer RH, Richard K. Transthyretin and the human placenta. Placenta. 2013;34(7):513–517. [DOI] [PubMed] [Google Scholar]
- 81.Cheng SB, Nakashima A, Sharma S. Understanding Pre-Eclampsia Using Alzheimer’s Etiology: An Intriguing Viewpoint. Am J Reprod Immunol. 2016;75(3):372–381. [DOI] [PubMed] [Google Scholar]
- 82.Lim YJ, Lee SJ. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun. 2017;5(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- 84.Biro O, Fothi A, Alasztics B, Nagy B, Orban TI, Rigo J, Jr. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene. 2019;692:138–144. [DOI] [PubMed] [Google Scholar]
- 85.Motawi TMK, Sabry D, Maurice NW, Rizk SM. Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch Biochem Biophys. 2018;659:13–21. [DOI] [PubMed] [Google Scholar]
- 86.Hromadnikova I, Dvorakova L, Kotlabova K, Krofta L. The Prediction of Gestational Hypertension, Preeclampsia and Fetal Growth Restriction via the First Trimester Screening of Plasma Exosomal C19MC microRNAs. Int J Mol Sci. 2019;20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H, Ouyang Y, Sadovsky E, Parks WT, Chu T, Sadovsky Y. Unique microRNA Signals in Plasma Exosomes from Pregnancies Complicated by Preeclampsia. Hypertension. 2020;75(3):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Otero-Ortega L, Laso-Garcia F, Gomez-de Frutos MD, et al. White Matter Repair After Extracellular Vesicles Administration in an Experimental Animal Model of Subcortical Stroke. Sci Rep. 2017;7:44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marshall SA, Hannan NJ, Jelinic M, Nguyen TPH, Girling JE, Parry LJ. Animal models of preeclampsia: translational failings and why. Am J Physiol Regul Integr Comp Physiol. 2018;314(4):R499–R508. [DOI] [PubMed] [Google Scholar]
- 90.Kusinski LC, Stanley JL, Dilworth MR, et al. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am J Physiol Regul Integr Comp Physiol. 2012;303(1):R86–93. [DOI] [PubMed] [Google Scholar]
- 91.Aoki A, Nakashima A, Kusabiraki T, et al. Trophoblast-Specific Conditional Atg7 Knockout Mice Develop Gestational Hypertension. Am J Pathol. 2018;188(11):2474–2486. [DOI] [PubMed] [Google Scholar]
- 92.Sheller-Miller S, Lei J, Saade G, Salomon C, Burd I, Menon R. Feto-Maternal Trafficking of Exosomes in Murine Pregnancy Models. Front Pharmacol. 2016;7:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang X, Yao J, He Q, Liu M, Duan T, Wang K. Exosomes From Women With Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension. 2018;72(6):1381–1390. [DOI] [PubMed] [Google Scholar]