Abstract
Oncogenic DNA viruses establish lifelong infections in humans, and they cause cancers, often in immunocompromised patients, despite anti-viral immune surveillance targeted against viral antigens. High-throughput sequencing techniques allowed the field to identify novel viral non-coding RNAs (ncRNAs). ncRNAs are ideal factors for DNA viruses to exploit; they are non-immunogenic to T cells, thus viral ncRNAs can manipulate host cells without evoking adaptive immune responses. Viral ncRNAs may still trigger the host innate immune response, but many viruses encode decoys/inhibitors to counter-act and evade recognition. In addition, ncRNAs can be secreted to the extracellular space and influence adjacent cells to create a pro-viral microenvironment. In this review, we present recent progress in understanding interactions between oncoviruses and ncRNAs including small and long ncRNAs, microRNAs, and recently identified viral circular RNAs. In addition, potential clinical applications for ncRNA will be discussed. Extracellular ncRNAs are suggested to be diagnostic and prognostic biomarkers and, with the realization of the importance of viral ncRNAs in tumorigenesis, approaches to target critical viral ncRNAs are emerging. Further understanding of viral utilization of ncRNAs will advance anti-viral therapeutics beyond conventional medication and vaccination.
Keywords: Non-coding RNA, microRNA, circular RNA, herpesvirus, tumorigenesis, immune evasion
1. Introduction
Oncoviruses such as Epstein-Barr virus (EBV, HHV4), Kaposi’s sarcoma herpesvirus (KSHV, HHV8), Human papillomavirus (HPV) 16 and 18, and Human T-cell lymphotropic virus type 1 (HTLV-1), are associated with 16% (~2 million) of cancer cases every year [1]. DNA viruses (EBV, KSHV, HPV) in particular are direct carcinogens causing proliferative phenotypes to infected cells. Even though the human T cell repertoire contains significant amounts of T cell clones specific to the viral antigens, infection of these oncoviruses persist for life. Most infections, however, are asymptomatic; viruses stay in latent phase, for the most part evading the host immune surveillance system. This immune evasion by virus-infected cells have been achieved by various ways: reducing viral gene expression to limit the amount of viral antigen presentation, evolving critical proteins so that they are harder to process for presentation, and expressing various viral immunoevasins that manipulate host cells to reduce immunogenicity against innate and adaptive immunity ([2,3] for reviews).
Non-coding RNAs (ncRNAs) are particularly useful tools for DNA viruses since ncRNA are not presented via Major histocompatibility complex (MHC), thus they are non-immunogenic to the adaptive immune system. ncRNAs can potentially, therefore, manipulate host cells without evoking host adaptive immune responses. At least nine animal virus families have been reported to encode viral ncRNAs including human herpesviruses [4–6]. In particular, the above-mentioned onco-herpesviruses like EBV and KSHV encode various ncRNAs that are also found in humans, such as long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) [4]. These viruses have a latent phase during which most viral genes are not expressed, with the exception of ncRNAs, as discussed below. Humans evolved pattern recognition receptors (PRRs) that detect foreign nucleic acids (such as viral RNAs), and these include the Toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I), and protein kinase R (PKR) [3]. Various DNA viruses have, however, developed ncRNA decoys to escape detection. For example, a double-stranded RNA-dependent PKR can be activated in response to dsRNA of viral origin, or to TLR stimulation, which results in type I interferon (IFN) response, the first line of anti-viral defense ([7] for review). Diverse viral ncRNAs like adenoviral VAI/II, EBV’s Epstein–Barr virus-encoded small RNAs (EBERs), and HIV’s trans-activation response (TAR) RNA work as decoys to inhibit PKR activation [4,8] As such, oncogenic DNA viruses have been reported to utilize various type of ncRNAs. Aside from short and long non-coding RNAs like EBV’s EBERs or KSHV’s polyadenylated nuclear (PAN) RNA, emergence of high-throughput deep-sequencing techniques in this century allowed the discovery of virus-encoded ncRNAs including microRNAs, small nucleolar RNAs, and circular RNAs.
In this review, we discuss recent progress in the contributions of non-coding RNAs during infection with oncoviruses including EBV, KSHV, and HPV. We mainly focus on three groups of ncRNAs. (i) Small and long ncRNAs, such as EBERs and PAN, are the most abundant transcripts in infected cells, but their functions are still not fully understood. (ii) microRNAs (miRNAs), in herpesviruses, are especially equipped with enormous number of miRNAs and they are critical for infection. (iii) Circular RNAs (circRNAs) are novel gene-regulatory ncRNAs and many oncoviruses were recently found to encode them. The circRNA field is new and knowledge in functions is limited, but their potential functions are intriguing. Furthermore, we will discuss the medical potential of ncRNAs as diagnostic and prognostic markers and as therapeutic targets.
2. Viruses utilize ncRNAs to manipulate hosts
2.1. Small and long non-coding RNAs
Oncogenic herpesviruses such as EBV and KSHV encode small and long ncRNAs, though features, functions, and modus operandi differ depending on the species. In this section, we will describe multi-functional ncRNAs encoded in oncogenic herpesviruses, such as EBERs from EBV and PAN from KSHV, focusing on recently revealed functions.
2.1.1. EBERs
EBV encodes two small RNAs called EBERs (Epstein-Barr virus-encoded small RNAs): EBER1 and EBER2 are single-stranded non-polyadenylated RNAs transcribed by human pol III with the length of 167 and 172 nucleotides, respectively. Both EBERs are highly expressed in latently infected cells and accumulate in the nucleus. In an early report, EBERs were often not detected in lytic cells in vivo and downregulation of EBERs were reported as an early lytic event, suggesting that their role is in maintaining latent infection [9]. Recent studies showed that, however, certain tonsillar cells are positive for both EBER and BZLF1, a viral early lytic gene, and virions and extracellular vesicles also contain EBERs, suggesting expression of EBERs during lytic cycle [10–12]. EBER-deleted mutant EBV strains were tested for their effects on the EBV life cycle but the results were controversial; Gregorovic et al reported little change in EBV’s ability to transform primary B cells [13] whereas Wu et al showed EBER2 deletion reduced the transformation efficiency by 100-fold [14]. The reason for the discrepancy is unknown but differences in EBER sequences may provide some clue: Gregorovic et al used the B95–8 strain as opposed to the Akata strain used by Wu et al. Recent high-throughput sequencing of various EBV strains revealed high levels of polymorphism in EBER2, with Akata-type sequences appearing in approximately 70% strains whereas B95–8 type appeared only in 7% of all strains [15]. Further phenotypical analysis is needed to determine contributions of EBERs but differences in strains, sequences, and context of infection should be taken into account before drawing general conclusions.
EBERs are highly structured and are known to form ribonucleoprotein (RNP) complexes. EBERs were identified to associate with La, human RNA chaperone, ribosomal protein L22, and RNA-binding protein AUF1, which binds to AU-rich elements (ARE) to destabilize transcripts. BJAB cells ectopically expressing EBERs showed upregulated transcripts that contain AREs in their 3′ UTRs [16], suggesting EBER’s regulatory mechanism via selective mRNA degradation. EBERs are involved not only in post-transcriptional regulation, but also in transcriptional control. EBER2 was found to recruit the transcription factor PAX5 to potentially regulate viral late gene expression [17]. This suggests a function of EBERs as a scaffold to maintain latency.
Host immune sensors also recognize EBERs and activate interferon-stimulated genes. RIG-I recognizes EBERs and elicit type I IFN response [18]. Although it is counterintuitive that viruses intentionally induce innate immune responses, it has been reported that activation of RIG-I pathway results in increase of IL-6 and IL-10, which enhance viral cell growth associated with hematological malignancies and nasopharyngeal carcinomas (NPCs) [18,19]. EBERs are thought to be exported to the extracellular space [20,21] and recognized by TLR3 in dendritic cells resulting in secretion of type I IFNs [20], as in the case of RIG-I. Recently, TLR7 in B cells was also revealed to recognize EBERs, which leads to CXCL8/IL-8 secretion that promotes lytic induction [15]. Together, these data suggest that EBV expresses EBERs to utilize the host innate immune system for its benefit during the latent phase. A conundrum is how EBV survives anti-viral effect of these ISGs, but viral microRNAs seem to be at least partially responsible for this (see 2.2.2 and 2.2.3 for detail).
Recently, it was reported that EBER1 can replace similar sequences, such as TMER4 of MHV68, promoting the transition of infected B cells from lymph nodes into the circulation. [22]. Similarly, Herpesvirus telomerase RNA (vTR) in Marek’s disease virus (MDV) can be substituted by either EBER1 or EBER2 in tumor formation caused by MDV infection [23]. Using such functional conservation among viruses related to EBV, in addition to conventional mutant study, may lead to a fruitful strategy to decipher EBER’s relevant functions.
2.1.2. BARTs
EBV encodes long ncRNAs called BamHI-A rightward transcripts (BARTs). BARTs are multi-splicing transcripts coded in the BART loci and are highly expressed in NPC and gastric carcinoma (GaCa), compared to B cells in vitro and in vivo [24,25]. Two TATA-less promoters, P1 and P2, have been reported. The P2 promoter contains multiple putative binding sites for C/EBPβ, which is highly expressed in epithelial cells. This has been proposed to be responsible for rich BART transcript levels in NPC [24]. The BART locus is around 22kb and harbors 7 exons where alternative splicing produces various transcripts. Some of them, namely RPMS1, A73, BART0, RK-BARF0, have coding potential but natural products of these transcripts have not been detected to date [26,27]. The BART locus also encodes clusters of viral miRNAs (see viral miRNA section for detail) as well as v-snoRNA1 [28] in intronic regions, and circRNAs as back-splicing variants (see circRNA section for detail).
The contributions of BARTs to EBV biology is largely unknown. The B95–8 EBV strain is widely used to immortalize primary B cells even though it lacks majority (~20kb) of the BART locus, suggesting that the BART locus is not necessary in the context of B cells in cell culture. Since BARTs are localized in the nuclei in EBV-infected cells, the potential of BARTs as long nRNAs (lncRNAs) have been investigated. [25,29]. Ectopic expression of an isoform of BARTs in EBV-negative AGS cell line showed transcriptomic changes, during which the BART cDNA largely localized in the nucleus, implying that BARTs are predominantly non-transcribed lncRNAs rather than mRNAs. Furthermore, knock-down of BARTs in EBV-positive NPC cell line C666–1 lead to increased type I IFN responses [25]. Since BARTs also contain BART miRNAs, which are involved in suppressing immune responses, the extent of contributions of BARTs to immune evasion is yet to be fully determined. Still, as EBV seems to utilize BART transcripts as immunoevasins, it is an attractive clinical target for EBV-infected solid tumors like NPC.
2.1.3. PAN
During KSHV reactivation, a 1.1kb polyadenylated nuclear (PAN) RNA comprises up to 92% of viral transcripts in the host cell [30]. This KSHV PAN RNA (also called T1.1 and nut-1, nuclear transcript) possesses a 5′ 7-methylguanosine cap and an expression and nuclear retention element (ENE). The ENE is a 79-nucleotide sequence near the 3′ end of the PAN RNA and is responsible for its nuclear accumulation and prevents its deadenylation [31].
KSHV PAN RNA binds to poly(A)-binding protein C1 (PABPC1) in the nucleus. Normally, PABPC1 is found in the cytoplasm, binding to most poly-A tails of mRNA to regulate mRNA translation and stability. During KSHV lytic reactivation, PABPC1 re-localizes to the nucleus through the action of the viral shutoff exonuclease (SOX) protein [32,33]. PAN RNA and nuclear PABPC1 interaction has been demonstrated [34,35]. While SOX expression was shown to upregulate PAN RNA, presumably through PABPC1, PAN RNA is not required for host shutoff due to SOX [34]. This was demonstrated by knockdown of PAN using anti-PAN oligonucleotides.
On the other hand, knockdown of KSHV PAN resulted in significantly reduced virion production and downregulation of K8.1 (late gene). Knockout of PAN [36] showed a general decrease in viral mRNA accumulation of representative immediate early, early and late genes. KSHV Latency Associated Nuclear Antigen (LANA) expression was unaffected, suggesting that PAN RNA is not required for latency establishment and maintenance.
In deep sequencing experiments combined with ribosome profiling, Arias et al. [30] reported short open reading frames (sORFs) in segments of PAN that have been designated PAN1.1 (37 aa), PAN1.2 (44 aa) and PAN1.3 (25 aa), with PAN1.1 predicted to be a putative signaling peptide. There is, however, some overlap with the main ORF of K7 and additional work needs to be done to characterize these PAN sORFs. Most recently, viral circRNAs have been found encoded in the PAN loci (see 2.3 for detail, [37,38]). Since PAN is the most abundant viral transcript during KSHV lytic reactivation and is necessary for lytic gene expression, PAN’s function/s point towards supporting production of nascent virions during reactivation. Alternatively, PAN has been proposed to sequester or inhibit the function of cellular factors that may otherwise inhibit KSHV infection [31].
2.1.4. Other viral ncRNAs
In addition to EBERs and BARTs, EBV encodes multiple stable intronic sequence RNAs (sisRNA). The EBV genome contains repeat sequences of ~3kb called W repeat, which are transcribed and spliced to form a part of viral transcripts coding for EBNA-LP and EBNA2 [39]. Since each W repeat contains two exons for EBNA-LP, short (81nt) and long (~3kb) introns exist in the unspliced transcripts. These exons are stable and not degraded rapidly thus termed ebv-sisRNA-1 and −2 [40]. Mutations of either sisRNAs suppressed EBV’s ability to transform naïve B cells [41,42]. The mechanism is unclear but human RNA-binding proteins like LIN28 or NONO, which were shown to bind EBV sisRNAs, may play a role [43]. Another EBV ncRNA that binds NONO is reported at the EBV latency origin of replication (OriP) [44]. During reactivation, OriP region is transcribed to transcripts in bidirectional manner [45] resulting in transcripts, OriPtL and OriPtR. Both are predicted to shape short-haripin of ~250 bp in length and, one of them, OriPtL can bind to NONO[44]. NONO is a key component of paraspeckle, which can induce antiviral IL8 response against herpes simplex virus 1 [46]. It is, therefore, possible that these EBV ncRNAs are involved in regulating antiviral innate immune responses.
High-throughput sequencing techniques allowed the discovery of viral transcripts with relatively low expression levels. One such recent example was the antisense-to-latency transcript (ALT) lncRNA in KSHV. ALT was originally identified with tiling microarray and later isolated from KSHV-positive B cell lines [47,48]. ALT is a very long ncRNA (~10,000 nt) and was found to be an early lytic gene and transcribed from the antisense strand at the locus coding for the KSHV latent gene LANA [48]. Therefore, it is possible that ALT may bind to the LANA transcript to regulate its expression, though further research is needed. Such promiscuous transcription as seen with ALT have been reported in EBV [45] and MHV68 [49] indicating the existence of previously unknown viral transcripts including ncRNAs.
2.2. microRNAs
This decade has seen numerous reports on the functional analysis of virus-encoded miRNAs. miRNAs are small (21~23nt) single-stranded RNAs that can regulate gene expression by destabilizing and degrading target transcripts. Binding of miRNAs to target RNAs occur mainly in the 3′UTR of mRNAs in a sequence-specific manner. Seed sequences (2′~7′ of miRNAs) are often critical for the binding specificity. However, unconventional seed matching has been reported, indicating a more complicated system of gene regulation by miRNAs [50]. Since the discovery of viral miRNAs in herpesviruses in 2004, dozens of gene targets have been identified ([51,52] for reviews). Though there is scarce evolutionary conservation among different viruses, some functions are similar e.g. pro-growth and immune evasion. Here, we discuss recent findings on viral miRNAs, focusing on common themes on how to enhance infection.
2.2.1. Relevance of viral miRNAs in EBV and KSHV in tumorigenesis
Gamma herpesviruses like EBV and KSHV encode many miRNAs, often in clusters. The EBV genome harbors 25 pre-miRNAs and at least 44 mature miRNAs have been confirmed to be expressed. Similarly, KSHV encodes 12 pre-miRNAs and 25 mature miRNAs. Most of these viral miRNAs are clustered (Bam HI fragment H rightward open reading frame 1(BHRF1) and BART clusters for EBV, and one cluster in the latency locus for KSHV) and are thought to be expressed simultaneously. In the case of EBV, expression patterns of viral miRNAs depend on latency stage and cell types. In tumor cells from EBV-positive NPC and GaCa, all 44 viral miRNAs are found to be expressed [53]. In EBV-positive B cells in latency III, during which all latent genes are seen, lymphoblastoid cell lines (LCLs) or AIDS-related diffuse large B-cell lymphoma express miRNAs from both BHRF1 and BART locus, though 10 or more BART miRNAs out of 40 have low or no expression [53,54]. In latency 0/I/II cells (EBV-infected germinal center B cell and memory B cells, or EBV-positive diffuse large B-cell lymphoma), the number of BART miRNAs expressed is further reduced and all of the BHRF1 miRNAs are no longer expressed [53–55]. In a large cohort study with peripheral blood samples from more than 129 patients (includes primary infection, reactivation, and healthy carriers of EBV) showed that three out of eight EBV miRNAs tested are not detected in any samples [56]. The mechanism by which EBV miRNAs are regulated is unclear but since all BART miRNAs originate from same primary transcript, there may be a cell and infection-specific post-transcriptional regulations, that are disrupted in tumors where all EBV miRNAs are expressed [53]. Since viral miRNAs are implicated in immune evasion and pro-growth phenotypes, these observations signify the importance of viral miRNAs in oncogenesis.
Each species of miRNAs can, in theory, regulate hundreds of gene transcripts. Therefore, expression of clusters of viral miRNAs alone can pose huge transcriptomic regulation, which may lead to significant phenotypic changes in infected cells. Studies utilizing miRNA mutants have been conducted to evaluate relevance of viral miRNAs. Oncogenic viruses EBV and KSHV give a growth advantage in infected cells and viral miRNAs contribute to this phenotype in vitro and in vivo [57–60]. In EBV, lack of the BHRF1 cluster miRNA caused severe defects in primary B cell transformation [58–60]. Pro-apoptotic genes like CASP3 and PUMA are EBV miRNA targets [61,62]. CASP3 is also a reported target of KSHV miRNAs to reduce apoptosis [63]. Furthermore, mTOR signaling, a key pathway for KSHV to induce transformation [64], is activated when KSHV miRNAs target mTOR inhibitory factor CASTOR1 [65]. Mutagenesis of each KSHV miRNA was performed to assess their contributions to cellular transformation and tumorigenesis using ex vivo nude mice system and KSHV-infected primary rat mesenchymal precursor cells [66]. Specific KSHV miRNAs are critical in regulating cell cycle and apoptosis regulating NF-κB pathway [66,67]. One of which, miR-K12–11 is of particular interest to the field since it is a molecular mimic of a human oncogenic miRNA (See 2.2.2 for detail). Cell transformation and immortalization of infected cells is a critical feature of herpesviruses during tumorigenesis, and viral miRNAs contribute to this process.
2.2.2. Molecular mimicry and functional conservation of viral miRNAs
Most of herpesviral miRNAs utilizes human miRNA biogenesis process [68] but, there are other viruses that mimic tRNA for biogenesis of small RNAs specifically expressed during infection. MHV68 miRNAs are generated from structures mimicking tRNAs, termed tRNA-miRNA-encoding RNAs (TMERs). This is in contrast to EBV and KSHV miRNAs, which utilize the human miRNA biogenesis system [69–71]. Each TMER is transcribed by Pol III and separated to a tRNA and two miRNAs by RNase Z, followed by cleavage by Dicer1 [69–71]. Functions of t-RNA-like residuals of TMERs are unclear, but the recent finding in Respiratory syncytial virus (RSV) raises an interesting possibility. Upon infection, RSV activates ribonuclease angiogenin, resulting in cleavage of human tRNAs and formation of tRNA-derived RNA Fragments (tRFs). One of the tRFs, tRF5-GluCTC, binds to the 3′UTR of apolipoprotein E receptor 2 (APOER2), which leads to better RSV replication [72]. Another virus using a noncanonical miRNA generation pathway is herpes virus simiri (HVS) Sm class U RNAs (HSURs), which are transcribed by Pol II. Their similarity to small nucleolar RNA (snRNA) allows HSURs to recruit the host Integrator complex, which is responsible for host snRNA processing, to produce pre-miRNAs [73]. These examples in MHV68 and HVS serves a precaution for future study that search of viral small RNAs should not be restricted to human miRNA-like structures but should also include tRNA-like structures and tRNA-derived fragments that specifically appears upon infection.
When comparing pre-miRNAs, viral miRNA sequences are hardly conserved among related viruses or to hosts [74]. An exception would be EBV and closely related Rhesus lymphocryptovirus (rLCV), between which ~65% of sequence and one third of miRNAs are conserved [74,75]. Functionally, however, similarities have been reported among viral miRNAs as well as between viral and human miRNAs. For example, both EBV and KSHV miRNAs share targets such as CASP3 [61,63] to inhibit apoptosis and MICB to evade NK cell killing[52]. Targets of EBV, KSHV, and MHV68 miRNAs are thus partially shared, though seed sequences differ. In comparing BC-1 (EBV+, KSHV+) and BC-3 cell lines (EBV-, KHSV+), 58% of targets are shared between EBV and KSHV miRNAs [76]. Similarly, in recent research utilizing EBV, KSHV, and MHV68 (encodes 14 pre-miRNAs), 64% of targets are shared among viruses [77]. These shared targeted transcripts showed enrichment in important translation and protein modification pathways, including ubiquitination pathway, EIF2 signaling, and mTOR signaling [77]. Similarly, NF-κB pathway is regulated by miRNAs of both KSHV and EBV. Negative regulator of NF-κB pathway are targets of viral miRNAa; kshv-miR-K1 targets IκBɑ [66,67] whereas EBV suppress RNF38 (ebv-miR-BART8–3p) and NKIRAS2 (ebv-miR-BART13) [78,79]. Though direct targets are different, these miRNAs contribute cell expansion, tumorigenesis, and metastasis of infected cells. This functional, but not necessarily sequential, conservations suggest that viral miRNAs of EBV and KSHV evolved independently, but to the same purpose: regulating host gene transduction networks to impose pro-growth, transformative phenotypes in infected cells.
Mimicry of viral miRNAs to human miRNAs have been observed. The seed sequence for KSHV miR-K12–11 is identical to hsa-miR-155, which is encoded in the locus of BIC (B-cell integration cluster), a noncoding RNA that is highly expressed in lymphoid organs (spleen, thymus). Both BIC and hsa-miR-155 are highly expressed in B cell lymphomas and other solid cancers. Intriguingly, hsa-miR-155 is not detected in several primary effusion lymphoma (PEL) cell lines. Instead, these cell lines express KSHV miR-K12–11. Validation work by Skalsky et al. [80] showed that both miRNAs target the BACH1 3′UTR. BACH1 is a transcriptional repressor of HMOX1 (heme oxygenase 1). Indeed, during normoxia, our group has reported that miR-K12–11 represses BACH1, with a concurrent upregulation of HMOX1 protein expression [81]. miR-K12–11 was, like human miR-155, found to induce B cell expansion in mice [82,83]. The expanded CD19+ B cell population with exogenous miR-K12–11 or hsa-miR-155 expression also demonstrated an invasive phenotype in the mice spleen. This indicates a role for KSHV viral miRNAs in contributing towards lymphomagenesis due to KSHV. Interestingly, EBV does not encode direct miR-155 homologue but EBV infection induces miR-155 expression to contribute to B-cell immortalization[84], partly driven by EBV’s LMP1 and LMP2A latent genes [85]. Thus, functionality of miR-155 is crucial for transformation by KSHV and EBV, though they were evolved to have different strategies.
Another example of molecular mimicry is how KSHV miR-K12–3 and miR-K12–6 have similar seed sequences to human miR-23 and miR-15/16 family miRNAs, respectively. Thus, they overlap in the gene regulation of a significant fraction of target transcripts [86,87]. Since miR-15/16 family inhibits BCL2 and induce apoptosis, it is counterintuitive for the virus to encode a mimic of miR-15/16. Further investigation is needed to evaluate miR-K12–6’s contribution in this instance. Taken together, viral miRNAs have evolved to share targets among viruses and their hosts to benefit the virus.
2.2.3. Immune evasion and collaborative inhibition by human and viral miRNAs
Herpesviruses encode numerous proteins called immunoevasins to evade host surveillance [3]; however, miRNAs have several advantages over these. First, miRNAs are, by their nature, non-immunogenic to T cells. This is particularly ideal feature for viruses to manipulate hosts during latency when most of immunoevasins, which themselves are immunogenic, are not expressed. Second, since multiple miRNAs are expressed simultaneously, viral miRNAs can work on many targets involved in several steps of the same pathway leading to a significant effect. A class of viral miRNAs’ targets consist of immune-regulatory gene transcripts. Viral miRNAs directly target genes involved in innate immunity such as TLRs, MICB (NK cell ligand), NLRP3 (core of NLRP3 inflammasome), and type I IFN. For adaptive immunity, transporter associated with antigen processing (TAP2, peptide transporter for MHC-I), type II IFN, and viral genes, which are sources of antigens presented to T cells, are downregulated by EBV miRNAs ([88] for review).
Viruses collectively modulate related pathways and different targets in the same pathway to benefit the virus. An example of a host target pathway shared by viruses is the cascade that involves interleukin-1 receptor-associated kinase 1 (IRAK1) and Myeloid differentiation primary response protein (MyD88) (Fig 1A). They are both key components of complexes (Toll-like receptor, IL-1 receptor signaling) that drive downstream events which ultimately results in the upregulation of pro-inflammatory cytokines [89]. EBV has been demonstrated to upregulate the anti-inflammatory hsa-miR-146a in LCLs [90] and Burkitt lymphoma cell lines [91] through the viral protein LMP1. Likewise, HSV1 induces miR-146a during infection of primary neural cells [92] and monocytes [93] via NF-κB activation. The viral-induced upregulation of miR-146a correlates with tight control of IRAK1 mRNA (Fig 1A). Similarly, hsa-miR-21 was demonstrated to directly inhibit MYD88 and IRAK1 mRNA levels in HCV [94] (Fig 1A). miR-21 is highly expressed in HCV+ hepatocellular carcinomas and HCV viral proteins NS5A and NS3/4A were shown to upregulate AP-1, stimulating miR-21 expression. miR-21 has also been shown to be upregulated 3-to 7-fold by EBV in LCLs [90,95].
Fig. 1.
Collaborative control of inflammatory pathways by viral microRNAs. (A) Infection of different viruses result in upregulation of miRNAs to down-regulate genes in the TLR/IRAK1 cascade. (B) Multiple viral miRNAs can target same transcripts or genes in same pathway. Multiple KSHV miRNAs control genes in the same pathway.
KSHV-encoded miRNAs also target the same TLR/IL-1R signaling cascade in collaborative way. Abend et al. [96] reported how miR-K12–9 can target both IRAK1 and MYD88 transcripts (Fig 1B), and both contribute to the Toll-like receptor (TLR)/IL-1R signaling cascade. In parallel, miR-K12–5 also down regulates MyD88, and stronger repression of MYD88 mRNA is seen when both miR-K12–5 and miR-K12–9 mimics are co-transfected in HUVEC. The result downstream is the general suppression of pro-inflammatory cytokines IL-6 and IL-8 (Fig 1B). Concurrently, miR-K12–10a also represses the cytokine receptor TWEAKR (tumor necrosis factor (TNF)-like weak inducer of apoptosis receptor) [96], which also results in the down regulation of pro-inflammatory cytokines (IL-6, IL-8). Additionally, miR-K12–10a-mediated targeting of TWEAKR transcript also blocks apoptosis induction.
Another example of collaborative inhibition by KSHV viral miRNAs is the modulation of the mevalonate/cholesterol biogenesis pathway. Gene expression of key enzymes HMGCS1 (3-hydroxy-3-methylglutaryl-coenzyme A (CoA) synthase 1), HMGCR (3-Hydroxy-3-methylglutaryl-CoA reductase), and squalene synthase (encoded by gene FDFT1, farnesyl-diphosphate farnesyltransferase 1) are modulated by several viral miRNAs [81,97]. Some miRNAs target more than one enzyme in the same pathway. For example, miR-K12–9 and miR-K12–11 target both HMGCS1 and HMGCR transcripts. The end result is down regulation of total cholesterol in de novo infected HUVEC. Together, these demonstrate that viral miRNAs can now be recognized as another class of immunoevasins and that viral miRNAs inhibit targets in collaborative manner.
2.2.4. Extracellular Viral miRNAs
In addition to these autocrine functions, viral miRNAs may have a paracrine effect to create a pro-viral niche. Extracellular miRNAs are miRNAs that are circulating in body fluids as well as supernatant in cell culture. They often co-purify with extracellular vesicles (EV) and are resistant to exonucleases, suggesting internalization of miRNAs in exosomes or multivesicular bodies [98]. Most of EBV-encoded miRNAs are secreted extracellularly from EBV-positive B cells [12,99] and released in target monocyte-derived dendritic cells [99] or epithelial cells [100]. KSHV-encoded miRNAs are also observed in circulation in patients of KSHV-associated malignancies such as Kaposi’s sarcoma (KS) and PEL [101]. Though the extent of contributions by EV-bound viral miRNAs are unclear, those KSHV-EV preparation from patients and PEL-cell line stimulate the ERK pathway and induce IL-6 secretion and cell migration in immortalized HUVECs [101,102].
Incorporation of viral miRNAs in EVs and delivery to host cells have to be, however, carefully considered. For human miRNAs, the majority of circulating miRNAs are associated with proteins, not EVs, and copy numbers of given miRNAs are less than 1 per 100 EVs [103–105]. Regardless of EVs involvement, extracellular EBV miRNAs were shown to suppress target transcripts including CXCL11, which reduces antiviral innate immune responses [99], and KSHV-EV can pose a pro-viral effect by evading type I IFN response [102]. Thus, herpesviruses employ viral miRNAs to create a favorable microenvironment by affecting neighboring cells.
2.2.5. Tug-of-war in controlling human and viral miRNAs
Virus infection can drastically change miRNA expression profiles in infected cells. Mature miRNA sequences from KSHV miRNAs are highly conserved among clinical isolates and cell lines whereas levels of their expression vary depending on stage of viral life cycle and cell types [51]. Viral infection changes miRNA expression profiles in several ways: expression of viral miRNAs and transcriptional regulation. Viral miRNAs expressed after infection of primary B cells with EBV accounts for 14~21% of total miRNAs [75,106]. Expression levels of viral miRNA can vary widely across miRNAs in the same gene cluster and also across different patient-derived cell lines [107]. The functional consequences of these different viral expression levels and how these expression levels are controlled by host and viral factors remains to be determined.
Like other gene expression, miRNA expression is also regulated at the transcriptional level. As discussed in 2.2.2, human miR-155 is not only mimicked by KSHV but also induced by EBV infection to promote cell expansion partly by EBV’s latent oncogene LMP1 [84,85]. During primary infection and transformation of infected B cells, many other human oncomiRs such as miR-21, and miR-146 are overexpressed whereas anti-oncomiRs like miR-15a is suppressed via EBV’s latent genes LMP1 and EBNA2 ([5] for review). Another EBV latent protein, EBNA1 induces the expression of let-7a targeting the Dicer that facilitate EBV reactivation, thereby promoting latent infection [108]. KSHV also induces oncomiRs (miR-17–92 cluster) via vFLIP and vCyclin, which results in pro-growth phenotype [109]. In addition, KSHV upregulates the expression of miR-132 through the transcription factor, cyclic AMP-response-element-binding protein (CREB), resulting in inhibiting antiviral innate immunity [110]. The viral life cycle stage can be another switch for transcriptional regulation of host miRNAs. Hypoxia and hypoxia-inducible factors (HIFs) play essential roles in KSHV life cycle and KSHV associated diseases. In response to hypoxia, expression of KSHV mature miRNAs is not affected although pri-miRNA expression is increased [111,112]. However, silencing HIF-causes dramatic reduction in both KSHV pri- and mature miRNAs under both normoxia and hypoxia [111]. Thus, EBV and KSHV modulate human miRNAs transcriptionally to facilitate successful infection.
Post-transcriptional regulation of miRNA biogenesis is another layer of regulation. All KSHV miRNAs are generated from common pri-miRNAs located within the latent locus. Therefore, post-transcriptional regulation is a key to delineate differential expression patterns of individual mature miRNAs. Biogenesis of miRNAs involves various RNA-binding proteins encoded by the host, such as Dicer, Drosha, and Argonaute (AGO) proteins. Viral miRNAs are, therefore, difficult for the host to distinguish from human miRNAs. However, the human host has a system to recognize and degrade viral miRNAs. Monocyte chemotactic protein-induced protein 1 (MCPIP1), also known as Regnase-1 (Regulatory RNase 1), is an RNA-binding protein with RNA cleavage activity. Our recent study reported novel mechanisms by which the host regulates KSHV miRNA biogenesis [113]. MCPIP1 degrades specific KSHV and EBV pre-miRNAs through its RNase activity [113]. MCPIP1 expression is induced by proinflammatory cytokines including TNF-α, MCP-1, and IL-1β, some of which are secreted following KSHV infection [113–115]. We hypothesize that specificity for a stronger effect of MCPIP1 regulation comes from two issues: (i) Temporal distinction between viral and cellular pre-miRNAs. (ii) A motif that is enriched in KSHV pre-miRNAs. First, at the time of infection there already exists a large pool of stable mature miRNAs. At the time of new viral infection, the viral miRNA biogenesis process begins, and thus newly synthesized viral pre-miRNAs would be subjected to MCPIP1-mediated degradation. Even if cellular pre-miRNAs are degraded by MCPIP1, the steady-state levels of cellular mature miRNAs will likely be changed less than viral mature miRNAs because the ratio of mature miRNA to pre-miRNA is high for the cellular miRNAs and lower for the viral miRNAs [113]. Second, it is still unclear how MCPIP1 recognizes specific pre-miRNAs and degrades them. One area of investigation is a motif that is enriched in KSHV pre-miRNAs, but not cellular pre-miRNAs [113].
MCPIP1 protein targets MCPIP1 transcripts for degradation, suggesting the degradation action of MCPIP1 on pre-miRNAs would temporarily occur after inflammatory induction [114]. On the other hand, KSHV pre-miRNA, mir-K6, which is resistant to MCPIP1 mediated degradation, generates a mature miRNA that decreases MCPIP1 expression by directly targeting the MCPIP1 3′ UTR [113]. Additional studies will be needed to uncover details of these mechanisms and applicability to additional viral infections.
Host RNA binding proteins (RBPs) involved in miRNA biogenesis are a point of control for both humans and viruses. Drosha, which cleaves the primary miRNA transcript to its pre-miRNA form, is differentially expressed during KSHV infection. During latency, Drosha is highly expressed and KSHV mir-K12–10 and mir-K12–12 are processed into their mature forms [116]. During lytic reactivation, Drosha levels decrease, and so do mir-K12–10 and mir-K12–12 levels. This Drosha-mediated cleavage may presumably extend to the global regulation of other KSHV miRNAs [117]. Additionally, single nucleotide polymorphisms (SNP) in KSHV miRNA coding sequences may inhibit their accessibility to miRNA processing enzymes, thereby altering the level of mature miRNA expression [118,119] (SNPs discussed further below). These observations emphasize the importance of RBPs involved in miRNA processing and genetic variations of miRNAs in the control of viral miRNA biogenesis.
Conversely, viral ncRNAs are reported to degrade specific host miRNAs. Conventionally, miRNAs are known to down-regulate target gene expression but recently, target-directed miRNA degradation (TDMD) has been reported. In TDMD, miRNAs bind to targets in a sequence-specific, seed-dependent manner. Rather than host miRNAs degrading the viral targets, the viral target displaces the 3′ end of host miRNAs from AGO, thereby making human miRNAs susceptible to exonuclease-mediated degradation [120]. Herpesvirus saimiri’s HSUR RNA is the first example of viral factor to induce TDMD by degrading hsa-miR-27a/b [121]. Interestingly, these targets are somewhat conserved and MCMV’s m169 transcript can also degrade mouse miR-27/1/b [122]. Similarly, though not a ncRNA, HCMV’s UL144–145 (polycistronic transcript consists of UL144 ORF, intergenic region, and UL145 ORF) can degrade hsa-miR-17 and hsa-miR-20 binding sites in the intergenic region, which can degrade the human miRNAs [123]. These examples open up an interesting question that whether any EBV/KSHV ncRNAs or mRNAs cause TDMD. In conclusion, viruses facilitate expression of miRNAs either by mimicking host miRNAs or encoding their own while regulating host miRNAs that are detrimental to viruses.
2.3. Circular RNAs
2.3.1. Discovery of viral circRNAs
Circular RNAs are a novel class of ncRNAs with gene regulatory functions ([124] for review). circRNAs are formed a back-splicing process and therefore lack polyadenylation (Fig 2). In humans, CDR1as is the first example of regulatory circRNA and it harbors dozens of binding sites targeted toward hsa-miR-7, preventing the miRNA’s binding to other molecules [125,126]. Such inhibitory function or “sponge” effect is particularly suitable for circRNAs since they are resistant to exonuclease and long-lived compared to their linear mRNA counterpart [124]. As described previously, DNA viruses, particularly herpesviruses, encode various types of ncRNAs like lncRNAs and miRNAs. It is, therefore, not a surprise to assume they also encode circRNAs. In fact, several evidences were reported recently to indicate the existence of viral circRNAs encoded in EBV, KSHV, rLCV, MHV68, and HPV [37,38,127–130]. Identification of novel circRNAs were performed by deep-sequencing of RNAs from virus-infected cells with two twists: detection of back-spliced junctions, which are unique to circRNAs and not detected in the linear counterparts, and circRNA enrichment. As circRNAs are exonuclease-resistant and non-polyadenylated, treatment with RNase R and/or depletion of adenylated RNA of total RNA was employed to identify viral circRNAs in EBV and KSHV [37,38,127], though it is not necessary to identify at least the most abundant circRNAs in EBV and HPV [128,129].
Fig. 2.
Generation of circular RNAs. Primary transcripts are either forward-spliced to form mRNAs or back-spliced (dotted line) to form circular RNAs. Back-spliced circRNAs are thus devoid of 5′ cap and poly A tail.
Gamma-herpesviruses were found to encode multiple circRNAs. More than 30 EBV circRNAs across 8 genes were detected, in which the majority of these viral genes encode several circRNA back-splicing isoforms. In particular, one of the BARTs, RPMS1-derived circRNA, was confirmed in three studies [37,127,128] and can express more than 10 circRNAs in at least one cell line for each latency types [127]. Similarly, the LMP2 locus was found to encode multiple circRNAs. Both RPMS1 and LMP2 ORF harbor multiple exons, thus have many well-defined splicing donor/acceptors (site of back-spliced junctions). In KSHV, at least 10 viral ORFs are reported to express circRNAs in KSHV-infected lymphocytes, epithelial and endothelial cells [37,38,130]. In particular, circRNAs encoded in v-IRF4 were confirmed to be expressed during latent stage in three independent studies [37,38,130]. Akin to miRNAs, the only conservation reported to date is an isoform of circRPMS1 between EBV and rLCV [130]. It is possible, though, that viral circRNAs are conserved in targets, structures, or functions rather than sequences.
Expression of viral circRNAs depend on cell types, latency stage, and circRNA species. In EBV, circEBNA1s are widely expressed in Latency III cell lines, but almost missing in Latency I cells or gastric carcinoma cell lines [127]. In KSHV, Abere et al. compared 6 KSHV-positive cell line for linear and circular vIRF4 and found that 4 of them have higher linear vIRF4 transcript levels than circular forms, but the remaining 2 cell lines showed more circular forms than linear forms indicating abundance of circRNAs [131]. We found that circ-vIRF4 has relatively low expression in KSHV-infected primary human endothelial cells [38]. In addition to these cell type-specific circRNA expression, lytic induction stimulates majority of viral circRNAs such as EBV’s circRPMS1, circBHBLF1, and circRNAs from KSHV lncRNA PAN in cell culture [37,38,127] and in a KS patient with high levels of lytic gene expression [38]. Interestingly, back-spliced junctions of these lytically induced circRNAs are variable [37,38], suggesting either different isoforms or lack of precise control in back-splicing in some circRNAs. In such lytic conditions, we observed that several KSHV circRNAs are expressed as high as viral gene transcripts like LANA [38]. In contrast, circ-vIRF4 shows a different pattern; when comparing various KSHV-positive cell lines, lytic stimulation reduces the ratio of linear vIRF4:circ-vIRF4 significantly. Since forward- and back-splicing are reported to be in competition in general [132], lytic induction apparently shifted the balance between linear vIRF4 and circ-vIRF4 by favoring forward-splicing. Together, depending on cell type, virus life cycle, and circRNAs, viral circRNAs can be as abundant as linear RNA products.
There are several features in these viral circRNAs uncommon to human circRNAs. KSHV’s vIRF4 consists of two exons, but back-splicing does not occur at the splicing donor and acceptor of these exons; instead, it happens within previously annotated exons [37]. Such circRNA-specific splicing donor/acceptor is also found in HPV’s circE7 [129]. Oncoproteins E6 and E7 are transcribed as bicistronic mRNAs and the splicing acceptor for circE7 locates within E6 in such a way that circE7 contains part of E6 and full-length E7 [129]. Furthermore, such cross-ORFs were observed in EBV and KSHV. EBV’s RPMS1 and LMP2 are in close proximity and some circRNA contains exons from RPMS1 and LMP2 [127]. We also observed one circRNA that harbors parts of KSHV ORF35 and 36 [38]. Though the relevance is unclear, these chimeric circRNAs can be a particular feature of ORF-packed viral genomes. One aspect of these ‘in-exon’ splicing events is that these splicing junctions have not been previously reported often due to the reliance on poly-A RNA material (which excludes circRNAs) in cDNA library preparation for short-read RNA-Seq of viral transcripts. Emerging direct RNA sequencing techniques allow identification of previously unknown splicing isoforms in herpesviruses [49,133].
Coding capacity of circRNAs is intriguing. Herpesviral circRNAs tested so far had no association with ribosomes [37] but this does not rule out the existence of other coding circRNAs in herpesviruses. Interestingly, circE7 contains full length HPV E7 coding sequence with m6A modifications, which allowed translation via a cap-dependent manner in 293T cells ectopically expressed with circE7-harboring plasmid [129]. The coding capacity of viral circRNAs has an intriguing potential suggesting an unknown landscape of viral proteins, but it also defies the apparent advantage of ncRNAs: non-immunogenicity. Furthermore, as in the case for KSHV circRNAs and HPV circE7, certain viral circRNAs are back-spliced at positions conventionally known to be within exons [37,38,129]. If peptides are coded in such circRNAs, the translational frame can be shifted from the linear coding transcript, thus producing different antigens compared to the ones from the linear transcript. Viral circRNA-encoded proteins should be, if existing in nature, evaluated for their contributions to viruses considering the need to avoid the host surveillance system.
2.3.2. Functions of viral circRNAs and its implication
Though the field is still in its early stage, viral circRNAs have been implicated in tumorigenesis [128,129,134]. The aforementioned BART locus was found to encode various circRNAs, termed circRPMS1, one of the circRNA isoforms from the BART locus, and they are expressed in several EBV-positive B cell lines of any latency and gastric carcinoma cell lines [127,128], latency I EBV-positive gastric cancer tissues [128], Post-transplant Lymphoproliferative Disorders (PTLD) samples [37], and NPC cell lines [128]. Ectopic expression of a variant of circRPMS1 in EBV-negative AGS cells showed reduction of human miRNAs that are predicted to bind circRPMS1, suggesting that circRPMS1 can work as a sponge [135]. Further, circRPMS1 increased the migration of AGS cells. Similarly, in an independent study of an NPC cell line C666–1, different groups of human miRNAs, namely miR-31, 203, and 451, were found to bind circRPMS1. Knock-down of circRPMS1 lead to more apoptosis as well as reduced invasiveness. Inhibitors of those miRNAs could partially rescue the phenotype [134], suggesting that circRPMS1 sponge human miRNAs to elicit tumorigenic functions. circRPMS1 was also found to be highly expressed in metastatic, EBV-positive NPC tissues vs adjacent tissues [134]. Together, circRPMS1 is implicated to be an oncogenic factor in adherent cells.
KSHV-encoded circular RNAs also have oncogenic potential; We reported that several KSHV circRNAs can promote cell proliferation when ectopically expressed in KSHV-infected SLK epithelial cell line [38]. Thus, viral circRNAs seem to share a common objective i.e. positively regulate cells infected with oncogenic viruses like EBV, KSHV, and HPV. The question, however, is why circRNAs are employed for this purpose though all these viruses encode robust proteins to do the similar job. The non-immunogenic nature of ncRNAs may be a part of the answer.
Unless a protein is encoded, as in the case of circE7 in HPV [129], circRNAs are non-coding, thus non-immunogenic to the adaptive immune system. Chen et al., showed that artificial GFP-coding circRNAs are not recognized by innate immune receptors like RIG-I and do not elicit type I IFN response [136]. Since EBV, KSHV, and HPV form episomes in the nucleus and express viral genes by co-opting host machinery, it is conceivable that viral circRNAs are recognized as “self” to evade innate immune system. There is a further possibility that viral circRNAs are actively suppressing the antiviral immune system. Recently, certain human circRNAs with 16~26 bp duplex structures were shown to inhibit functions of PKR [137]. This raises the possibility that viral circRNAs may work as decoy dsRNAs for PKR, as in the case of EBERs [8].
Extracellular circRNAs have a fascinating potential, in addition to de novo transcribed circRNAs in infected cells. Recently, KSHV circRNAs were reported to be incorporated in virions [131]. These virion- or EV-incorporated circRNAs may be transferred to infected cells and can create a favorable niche for viruses ([138] for review). There has been, however, conflicting reports as to whether human circRNAs are recognized by innate immune receptors such as RIG-I or TLR and invoke type I IFN responses. Chen et al showed that exogenously introduced circRNAs are recognized by RIG-I [136] whereas Wesselhoeft et al. claims that indeed exogenous linear RNAs, but not exogenous circRNAs, are functional ligands of RIG-I [139]. Further investigation is needed to determine whether extracellular circRNAs are indeed an efficient way for viruses to enhance tropism or whether the host’s antiviral sensing mechanism can detect circRNAs.
3. Clinical potential of ncRNAs
3.1. ncRNAs as biomarkers
EBERs are highly expressed through all latency stages and EBER in situ hybridization is the standard method to diagnose EBV-positive tumors [140]. Viral mRNAs are hardly expressed during EBV latency and secondary diagnosis has been limited. Therefore, viral miRNAs are candidate surrogate biomarkers. EBV miRNAs were found differentially expressed in various EBV-infected neoplastic tissues [53]. Like human miRNAs, viral miRNAs are found in circulation. Recent cohort studies revealed the association of viral miRNA expression with decreased survival in adult acute myeloid leukemia (AML) patients [141] and differences in EBV miRNA levels and contents in blood samples depending on infection stages [56]. EBV miRNAs were detected at high expression levels in the plasma of pediatric patients with infectious mononucleosis at the start of illness [142]. A significant positive correlation between viral load and EBV miRNAs in plasma was also demonstrated.
Chronic active EBV infection (CAEBV) is an intractable lymphoproliferative disorder wherein patients have elevated levels of EBV DNA circulating in their blood. Using targeted-capture sequencing of the EBV genome, it was shown that some CAEBV patients (31 out of 80 cases) harbor EBV with deletions in BART1 and BART2 microRNA clusters in the EBV genome [143]. Several of these BART miRNAs modulate the immediately early genes BZLF1 and BRLF1, with deregulation of these genes contributing to increased lymphomagenesis [144]. In another study, they found several BART miRNAs were expressed at significantly higher levels in the plasma of symptomatic CAEBV patients (active disease), compared to those with asymptomatic (inactive) CAEBV disease [145]. The expression levels of certain BART miRNA (miR-BART2–5p, miR-BART4, miR-BART7, miR-BART13, miR-BART15, and miR-BART22) were significantly decreased after treatment and when complete remission was achieved [145], exhibiting another instance of EBV miRNAs as biomarkers. Also, these two contrasting studies highlight the importance of perturbations in viral miRNA levels and how they impact disease.
Nasopharyngeal cancer (NPC) is an EBV-associated tumor with abundant expression of EBV noncoding RNAs. EBER1 and 2 in situ hybridization is currently used for clinical diagnosis of NPC [146–148], and is considered the gold standard assay. All BART miRNAs are expressed in NPC biopsies, while BHRF1 miRNAs are not [53,149]. Patients with more advanced NPC staging had higher levels of miR-BART7 and miR-BART13 [150]. Also, miR-BART7 [150] and miR-BART17 (in patient serum, [151]) have been demonstrated as markers for poorer prognosis due to tumor progression, metastasis, or relapse after treatment.
EBV-associated gastric carcinoma (GaCa) represents ~10% of gastric cancers [152]. Similar to NPC, GaCa exhibited high expression of BART miRNAs, BHRF1 undetectable [153]. This pattern of expression in epithelial-type cells may be due to C/EBP (CCAAT/enhancer-binding protein), a transcription factor in epithelial cells and which has a binding site in the promoter region of the BART transcript [152]. Together, EBV ncRNAs show potential as diagnostic and prognostic biomarkers.
Due to their resistance to RNase degradation, viral circRNAs can potentially be utilized as stable biomarkers. Abere et al. [131] demonstrated that newly obtained sera from patients with AIDS-associated Kaposi’s sarcoma had detectable levels of circ-vIRF4 and circPAN in 4 out of 4 samples (100% detection rate). Remarkably, archived sera from 25 years ago also had detectable viral circRNAs in 5 out of 10 samples (50% detection rate). Additionally, viral circRNAs were detected via in situ hybridization in KS tumor tissues at higher rates (66%) compared to LANA mRNA (38%). It would be interesting to test whether patient samples that were obtained in less than ideal conditions (for example, in resource-poor settings) would also exhibit a high detection rate. A stable biomarker such as viral circRNAs could be an alternative to the current gold standard which is LANA immunohistochemistry.
3.2. Sequence variation and regulation of viral miRNAs
After the discovery of viral miRNAs, independent groups have reported sequence variants of KSHV viral miRNAs. Gottwein et al. [118] showed that BCBL-1 was less efficient than BC1 in down regulating a luciferase reporter for miR-K12–5. On further analysis, they found that miR-K12–5 expression was much lower in BCBL-1 compared to BC1. This correlated with a single nucleotide polymorphism (SNP) in the stem-loop structure of the miR-K12–5 precursor in BCBL-1 cells. This SNP introduced a larger bulge in the pri-miRNA that may affect processing efficiency by Drosha, and this was demonstrated using an in vitro maturation assay. Multiple reports have found that mature miRNAs (cellular and viral) can vary by a few bases (isomirs) at the 3′ [107] and 5′ ends [154], which can alter the target specificity of these miRNAs. These variations increase the number of genes that can be repressed by the same starting miRNA gene.
In a survey of PEL cell lines and patient samples, Marshall et al. also showed that miR-K12–5 had a single nucleotide polymorphism [155]. Additionally, they found 3 different sequence variants for miR-K12–9. Subsequently, the same group showed that SNPs in the pre-microRNA region of miR-K12–5 and −9sv (sequence variant) correlate with changes in mature microRNA expression [156]. For instance, the BC1 variant of miR-K12–5 (with a SNP in the pre-microRNA) had higher pre-microRNA expression and was processed more efficiently by Drosha. However, the BCBL1 variant of miR-K12–9 was more highly expressed, while the BC3 and BCP1 variants (with multiple SNPs in the mature miRNA) has lower expression. Not all SNPs resulted in significant changes in expression, since the BC1 variant of miR-K12–10 (SNP in the terminal loop) did not affect miRNA expression [156].
While viral miRNAs in general are well-conserved in patient populations, Ray et al. [157]. proposed that viral microRNA polymorphisms may differentiate patients with inflammatory symptoms (MCD, Multicentric Castleman Disease or KICS, KSHV-associated Inflammatory Cytokine Syndrome) from those without (HIV/KSHV-coinfected patients with no inflammatory symptoms).They reasoned that miRNAs with known SNPs either help down regulate RTA (miR-K12–9, miR-K12–7, and miR-K12–5) or are involved in the expression of IL-6 and IL-10 (miR-K12–7). Indeed, using both phylogenetic and statistical analyses, they showed that in 72 of the 77 SNP loci, a SNP was present at a higher percentage in sequences from KSHV-MCD and KICS patients. Another study in KS skin lesions also showed that a single SNP in the pre-miRNA K12–3 has a global effect on the mature viral miRNA levels (downregulation of K12–8-3p and 9–5p but an upregulation of K12–5-5p [119].
SNPs, also called single nucleotide variants (SNVs) in EBV miRNAs were described in clinical NPC biopsies as having a non-random pattern, with extremely low variation frequency within the miRNA seed region (nt 2–7) [158]. This suggests strong conservation of the miRNA seed region and miRNA targets. A comparison of EBV genomic sequence and EBV miRNA sequences revealed differences that attributed SNVs to post-transcriptional editing [158]. Subsequently, A-to-I editing, most likely by ADAR1 (adenosine deaminase acting on RNA 1) was reported in EBV pri-miRNAs found in EBV-infected cell lines [159] SNPs in the BART promoter region have also been described, with increased frequency in NPC, but not GaCa, and may account for the increased BART miRNA expression in NPC [160]. SNVs were also enriched in EBER1/2 [143]. from patient samples but were not seen to impact CAEBV development. In summary, sequence variation in viral miRNAs may or may not impact clinical manifestations of disease. However, single nucleotide changes may result in changes in the efficiency of mature miRNA processing or of targeting human or viral mRNAs.
3.3. Targeting ncRNAs
Treating latent herpesvirus infections is difficult, since latent infections express few gene products. However, many herpesvirus miRNAs are expressed during latency at elevated levels. Specifically targeting these viral miRNAs is an exciting therapeutic strategy since viral miRNAs are distinct from cellular miRNAs. A recent paper has pursued this approach by targeting three KSHV miRNAs with antisense inhibitors. These viral miRNA inhibitors suppressed the growth of KSHV lymphoma cells in mouse xenograft models [161]. Likely obstacles include delivery of the antisense inhibitors to the correct tissues and infected cells, but this approach could be applicable against multiple viruses that express their own viral non-coding RNAs.
4. Conclusion
Studying the functions of viral ncRNAs is challenging due to a number of realities. First, the lack of sequence conservation of non-coding RNAs across related viruses hinders certain bioinformatic approaches to look for associations. Second, genetic mutations in non-coding RNAs usually have unintended secondary effects due to the dense nature of transcripts in viral genomes. Third, redundancy of multiple ncRNAs performing overlapping functions forces loss-of-function approaches to simultaneously inhibit multiple ncRNAs. Fourth, lack of representative animal models of viral infection limits many studies to cell culture-based assays, which lack multiple cell types, including immune cells. Multiple reports have shown little or no phenotype due to deleting a viral ncRNA in cell culture, but when studied in vivo, a defect in infection was observed [162]. New methods for visualizing single RNA molecules (such as RNAscope [163]) may aid in correlating ncRNA expression in infected and diseased tissue with biomarkers of immune responses to better understand these connections in humans. Direct RNA-sequencing techniques (PacBio or Nanopore sequencing) will aid in determining full sequences of ncRNAs, especially circRNAs. However, one advantage of studying viral ncRNAs is that uninfected cells are indispensable controls to investigate the roles of individual or combinations of viral non-coding RNAs.
In spite of difficulties, the research on ncRNAs in the context of viral infection is progressing quickly in this decade, particularly with the help of high-throughput sequencing and targeted gene knock-out/knock-down techniques. The field has just started to understand the depth of involvement of ncRNAs in peculiar ways compared to viral proteins. Unlike conventional one-ORF one-product mechanisms, dozens of ncRNAs can be produced from the same loci simultaneously, as in the case of the BART locus. Post-transcriptional regulation at RNA processing/splicing is the default for many ncRNAs like circRNAs or miRNAs; the same ncRNAs can work as RNA as well as proteins if translated; some ncRNAs like EBERs and PAN can be dominant transcripts over coding transcripts. These features may allow viral ncRNAs to function on cell growth and immune evasion in collaborative and robust manner, though the approach can vary depending on the virus and the species of ncRNAs. Interactions of viral ncRNAs can be even more convoluted in malignancies like PEL, with most tumor cells dually infected with EBV and KSHV, and they are a fascinating model to study this complex interaction of ncRNAs. As we start to see promising research exploring their potential as biomarkers and therapeutic targets, further understanding of viral ncRNAs will advance the treatment of disease caused by oncogenic viruses.
Fig. 3.
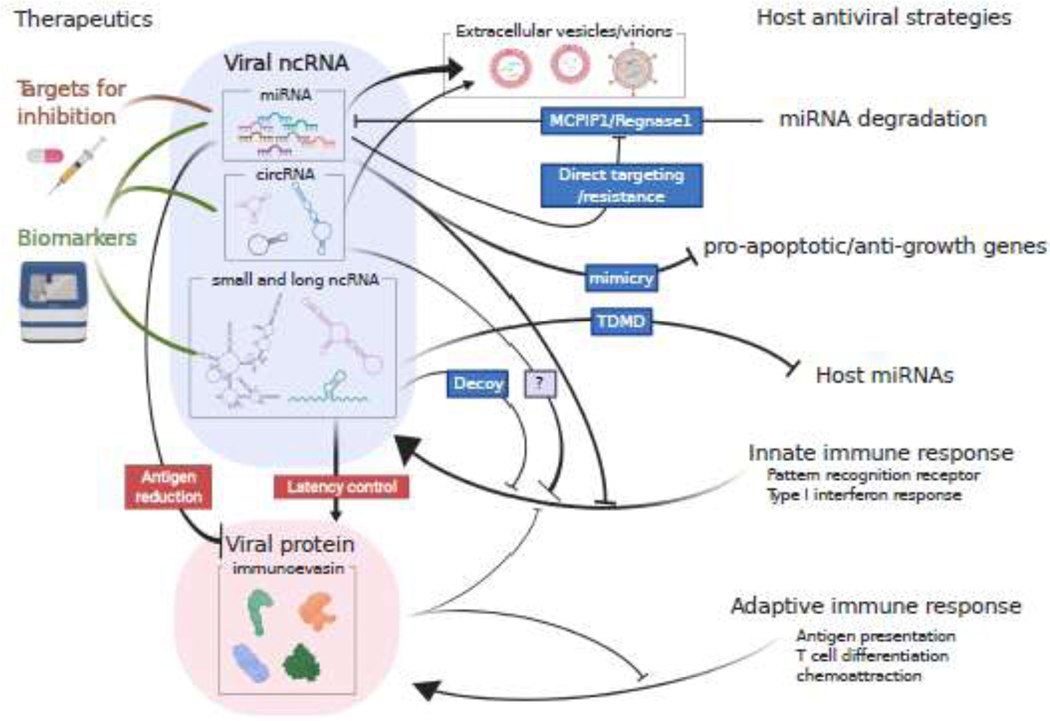
Graphical summary of this review. Non-coding RNAs are utilized by viruses to evade various host anti-viral mechanisms. Blue boxes depict examples for host and virus to commence the anti/pro-viral functions. MCPIP1, Monocyte chemotactic protein-induced protein 1; TDMD, target-directed miRNA degradation.
ACKNOWLEDGEMENTS:
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH (1ZIABC011176). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Figures were created with BioRender.com.
ABBREVIATIONS:
- ncRNA
non-coding RNA
- lncRNA
long non-coding RNA
- miRNA
microRNA
- circRNA
circular RNA
- EBV
Epstein-Barr virus
- KSHV
Kaposi’s sarcoma herpesvirus
- KS
Kaposi’s sarcoma
- PEL
primary effusion lymphoma
- NPC
nasopharyngeal carcinoma
- PTLD
Post-transplant lymphoproliferative disorders
- GaCa
gastric carcinoma
- MCD
Multicentric Castleman Disease
- KICS
KSHV-associated Inflammatory Cytokine Syndrome
- EBER
Epstein–Barr virus-encoded small RNAs
- BART
BamHI-A rightward transcripts
- LANA
Latency Associated Nuclear Antigen
- PAN
polyadenylated nuclear RNA
- ORF
open reading frame
- UTR
untranslated region
- RBP
RNA-binding proteins
- IFN
interferon
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. , Global burden of cancers attributable to infections in 2008: a review and synthetic analysis, Lancet Oncol. 13 (2012) 607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- [2].Lilley BN, Ploegh HL, Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond, Immunol. Rev. 207 (2005) 126–144. doi: 10.1111/j.0105-2896.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- [3].Carriere J, Rao Y, Liu Q, Lin X, Zhao J, Feng P, Post-translational Control of Innate Immune Signaling Pathways by Herpesviruses, Front Microbiol. 10 (2019) 5234. doi: 10.3389/fmicb.2019.02647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Withers JB, Mondol V, Pawlica P, Rosa-Mercado NA, Tycowski KT, Ghasempur S, et al. , Idiosyncrasies of Viral Noncoding RNAs Provide Insights into Host Cell Biology, Annu. Rev. Virol. 6 (2019) 297–317. doi: 10.1146/annurev-virology-092818-015811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zuo L, Yue W, Du S, Xin S, Zhang J, Liu L, et al. , An update: Epstein-Barr virus and immune evasion via microRNA regulation, Virol Sin. 32 (2017) 175–187. doi: 10.1007/s12250-017-3996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mishra R, Kumar A, Ingle H, Kumar H, The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection, Front Immunol. 10 (2020) 1537. doi: 10.3389/fimmu.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sadler AJ, Williams BRG, Interferon-inducible antiviral effectors, Nature Rev. Immunol. 8 (2008) 559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharp TV, Schwemmle M, Jeffrey I, Laing K, Mellor H, Proud CG, et al. , Comparative analysis of the regulation of the interferon-inducible protein kinase PKR by Epstein-Barr virus RNAs EBER-1 and EBER-2 and adenovirus VAI RNA, Nucleic Acids Res. 21 (1993) 4483–4490. doi: 10.1093/nar/21.19.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Greifenegger N, Jäger M, Kunz-Schughart LA, Wolf H, Schwarzmann F, Epstein-Barr virus small RNA (EBER) genes: differential regulation during lytic viral replication, J. Virol. 72 (1998) 9323–9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hudnall SD, Ge Y, Wei L, Yang N-P, Wang H-Q, Chen T, Distribution and phenotype of Epstein-Barr virus-infected cells in human pharyngeal tonsils, Mod. Pathol. 18 (2005) 519–527. doi: 10.1038/modpathol.3800369. [DOI] [PubMed] [Google Scholar]
- [11].Baglio SR, van Eijndhoven MAJ, Koppers-Lalic D, Berenguer J, Lougheed SM, Gibbs S, et al. , Sensing of latent EBV infection through exosomal transfer of 5’pppRNA, Proc Natl Acad Sci USA. 113 (2016) 201518130–E596. doi: 10.1073/pnas.1518130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jochum S, Ruiss R, Moosmann A, Hammerschmidt W, Zeidler R, RNAs in Epstein-Barr virions control early steps of infection, Proc Natl Acad Sci USA. 109 (2012) E1396–404. doi: 10.1073/pnas.1115906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gregorovic G, Bosshard R, Karstegl CE, White RE, Pattle S, Chiang AKS, et al. , Cellular gene expression that correlates with EBER expression in Epstein-Barr Virus-infected lymphoblastoid cell lines, J. Virol. 85 (2011) 3535–3545. doi: 10.1128/JVI.02086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu Y, Maruo S, Yajima M, Kanda T, Takada K, Epstein-Barr virus (EBV)-encoded RNA 2 (EBER2) but not EBER1 plays a critical role in EBV-induced B-cell growth transformation, J. Virol. 81 (2007) 11236–11245. doi: 10.1128/JVI.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Z, Tsai M-H, Shumilov A, Baccianti F, Tsao SW, Poirey R, et al. , Epstein–Barr virus ncRNA from a nasopharyngeal carcinoma induces an inflammatory response that promotes virus production, Nat. Microbiol. 4 (2019) 2475–2486. doi: 10.1038/s41564-019-0546-y. [DOI] [PubMed] [Google Scholar]
- [16].Pimienta G, Fok V, Haslip M, Nagy M, Takyar S, Steitz JA, Proteomics and Transcriptomics of BJAB Cells Expressing the Epstein-Barr Virus Noncoding RNAs EBER1 and EBER2, PLoS ONE. 10 (2015) e0124638. doi: 10.1371/journal.pone.0124638. Lee N, Moss WN, Yario TA, Steitz JA, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].EBV Noncoding RNA Binds Nascent RNA to Drive Host PAX5 to Viral DNA, Cell. 160 (2015) 1–13. doi: 10.1016/j.cell.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Samanta M, Iwakiri D, Kanda T, Imaizumi T, Takada K, EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN, Embo J. 25 (2006) 4207–4214. doi: 10.1038/sj.emboj.7601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duan Y, Li Z, Cheng S, Chen Y, Zhang L, He J, et al. , Nasopharyngeal carcinoma progression is mediated by EBER-triggered inflammation via the RIG-I pathway, Cancer Letters. 361 (2015) 67–74. doi: 10.1016/j.canlet.2015.02.037. [DOI] [PubMed] [Google Scholar]
- [20].Iwakiri D, Zhou L, Samanta M, Matsumoto M, Ebihara T, Seya T, et al. , Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3, J. Exp. Med. 206 (2009) 2091–2099. doi: 10.1084/jem.20081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ahmed W, Tariq S, Khan G, Tracking EBV-encoded RNAs (EBERs) from the nucleus to the excreted exosomes of B-lymphocytes, Sci. Rep. 8 (2018) 15438–11. doi: 10.1038/s41598-018-33758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoffman BA, Wang Y, Feldman ER, Tibbetts SA, Epstein-Barr virus EBER1 and murine gammaherpesvirus TMER4 share conserved in vivo function to promote B cell egress and dissemination, Proc Natl Acad Sci USA. 116 (2019) 25392–25394. doi: 10.1073/pnas.1915752116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kheimar A, Kaufer BB, Epstein-Barr virus-encoded RNAs (EBERs) complement the loss of Herpesvirus telomerase RNA (vTR) in virus-induced tumor formation, Sci. Rep. 8 (2018) 209. doi: 10.1038/s41598-017-18638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen H, Huang J, Wu FY, Liao G, Hutt-Fletcher L, Hayward SD, Regulation of expression of the Epstein-Barr virus BamHI-A rightward transcripts, J. Virol. 79 (2005) 1724–1733. doi: 10.1128/JVI.79.3.1724-1733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Verhoeven RJA, Tong S, Mok BW-Y, Liu J, He S, Zong J, et al. , Epstein-Barr Virus BART Long Non-coding RNAs Function as Epigenetic Modulators in Nasopharyngeal Carcinoma, Front. Oncol. 9 (2019) 2655. doi: 10.3389/fonc.2019.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Al-Mozaini M, Bodelon G, Karstegl CE, Jin B, Al-Ahdal M, Farrell PJ, Epstein-Barr virus BART gene expression, Journal of General Virology. 90 (2009) 307–316. doi: 10.1099/vir.0.006551-0. [DOI] [PubMed] [Google Scholar]
- [27].Marquitz AR, Raab-Traub N, The role of miRNAs and EBV BARTs in NPC, Semin. Cancer Biol. 22 (2012) 166–172. doi: 10.1016/j.semcancer.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hutzinger R, Feederle R, Mrazek J, Schiefermeier N, Balwierz PJ, Zavolan M, et al. , Expression and processing of a small nucleolar RNA from the Epstein-Barr virus genome, PLoS Pathog. 5 (2009) e1000547. doi: 10.1371/journal.ppat.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marquitz AR, Mathur A, Edwards RH, Raab-Traub N, Host Gene Expression is Regulated by Two Types of Noncoding RNAs Transcribed from the Epstein-Barr Virus BART Region, J. Virol. 89 (2015) JVI.01492–15–11268. doi: 10.1128/JVI.01492-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arias C, Weisburd B, Stern-Ginossar N, Mercier A, Madrid AS, Bellare P, et al. , KSHV 2.0: a comprehensive annotation of the Kaposi’s sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features, PLoS Pathog. 10 (2014) e1003847. doi: 10.1371/journal.ppat.1003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Conrad NK, Steitz JA, A Kaposi’s sarcoma virus RNA element that increases the nuclear abundance of intronless transcripts, Embo J. 24 (2005) 1831–1841. doi: 10.1038/sj.emboj.7600662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Glaunsinger B, Ganem D, Lytic KSHV Infection Inhibits Host Gene Expression by Accelerating Global mRNA Turnover, Molecular Cell. 13 (2004) 713–723. doi: 10.1016/S1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- [33].Kumar GR, Glaunsinger BA, Nuclear Import of Cytoplasmic Poly(A) Binding Protein Restricts Gene Expression via Hyperadenylation and Nuclear Retention of mRNA, Molecular and Cellular Biology. 30 (2010) 4996–5008. doi: 10.1128/MCB.00600-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Borah S, Darricarrère N, Darnell A, Myoung J, Steitz JA , A viral nuclear noncoding RNA binds re-localized poly(A) binding protein and is required for late KSHV gene expression, PLoS Pathog. 7 (2011) e1002300. doi: 10.1371/journal.ppat.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Borah S, Nichols LA, Hassman LM, Kedes DH, Steitz JA, Tracking expression and subcellular localization of RNA and protein species using high-throughput single cell imaging flow cytometry, Rna. 18 (2012) 1573–1579. doi: 10.1261/rna.033126.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rossetto CC, Pari G, KSHV PAN RNA associates with demethylases UTX and JMJD3 to activate lytic replication through a physical interaction with the virus genome, PLoS Pathog. 8 (2012) e1002680. doi: 10.1371/journal.ppat.1002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Toptan T, Abere B, Nalesnik MA, Swerdlow SH, Ranganathan S, Lee N, et al. , Circular DNA tumor viruses make circular RNAs, Proc. Natl. Acad. Sci. U.S.a. 115 (2018) E8737–E8745. doi: 10.1073/pnas.1811728115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tagawa T, Gao S, Koparde VN, Gonzalez M, Spouge JL, Serquiña AP, et al. , Discovery of Kaposi’s sarcoma herpesvirus-encoded circular RNAs and a human antiviral circular RNA, Proc Natl Acad Sci USA. 115 (2018) 12805–12810. doi: 10.1073/pnas.1816183115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kalla M, Hammerschmidt W, Human B cells on their route to latent infection – Early but transient expression of lytic genes of Epstein-Barr virus, Eur. J. Cell Biol. 91 (2012) 65–69. doi: 10.1016/j.ejcb.2011.01.014. [DOI] [PubMed] [Google Scholar]
- [40].Moss WN, Steitz JA, Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA, BMC Genomics. 14 (2013) 543–16. doi: 10.1186/1471-2164-14-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bridges R, Correia S, Wegner F, Venturini C, Palser A, White RE, et al. , Essential role of inverted repeat in Epstein–Barr virus IR-1 in B cell transformation; geographical variation of the viral genome, Phil. Trans. R. Soc. B. 374 (2019) 20180299. doi: 10.1098/rstb.2018.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Szymula A, Palermo RD, Bayoumy A, Groves IJ, Ba abdullah M, Holder B, et al. , Epstein-Barr virus nuclear antigen EBNA-LP is essential for transforming naïve B cells, and facilitates recruitment of transcription factors to the viral genome, PLoS Pathog. 14 (2018) e1006890. doi: 10.1371/journal.ppat.1006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tompkins VS, Valverde DP, Moss WN, Human regulatory proteins associate with non-coding RNAs from the EBV IR1 region, BMC Res Notes. 11 (2018) 1062–8. doi: 10.1186/s13104-018-3250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cao S, Moss W, O’Grady T, Concha M, Strong MJ, Wang X, et al. , New Noncoding Lytic Transcripts Derived from the Epstein-Barr Virus Latency Origin of Replication, oriP, Are Hyperedited, Bind the Paraspeckle Protein, NONO/p54nrb, and Support Viral Lytic Transcription, J. Virol. 89 (2015) 7120–7132. doi: 10.1128/JVI.00608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].O’Grady T, Cao S, Strong MJ, Concha M, Wang X, Splinter Bondurant S, et al. , Global bidirectional transcription of the Epstein-Barr virus genome during reactivation, J. Virol. 88 (2014) 1604–1616. doi: 10.1128/JVI.02989-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, et al. , Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli, Molecular Cell. 53 (2014) 393–406. doi: 10.1016/j.molcel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- [47].Chandriani S, Xu Y, Ganem D, The lytic transcriptome of Kaposi’s sarcoma-associated herpesvirus reveals extensive transcription of noncoding regions, including regions antisense to important genes, J. Virol. 84 (2010) 7934–7942. doi: 10.1128/JVI.00645-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schifano JM, Corcoran K, Kelkar H, Dittmer DP, Expression of the Antisense-to-Latency Transcript Long Noncoding RNA in Kaposi’s Sarcoma-Associated Herpesvirus, J. Virol. 91 (2017) 2080. doi: 10.1128/JVI.01698-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].O’Grady T, Feswick A, Hoffman BA, Wang Y, Medina EM, Kara M, et al. , Genome-wide Transcript Structure Resolution Reveals Abundant Alternate Isoform Usage from Murine Gammaherpesvirus 68, Cell Reports. 27 (2019) 3988–4002.e5. doi: 10.1016/j.celrep.2019.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Grosswendt S, Filipchyk A, Manzano M, Klironomos F, Schilling M, Herzog M, et al. , Unambiguous Identification of miRNA:Target Site Interactions by Different Types of Ligation Reactions, Molecular Cell. 54 (2014) 1042–1054. doi: 10.1016/j.molcel.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Qin J, Li W, Gao S-J, Lu C, KSHV microRNAs: Tricks of the Devil, Trends Microbiol. 25 (2017) 648–661. doi: 10.1016/j.tim.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Skalsky RL, Cullen BR, EBV Noncoding RNAs, Curr. Top. Microbiol. Immunol. 391 (2015) 181–217. doi: 10.1007/978-3-319-22834-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Qiu J, Cosmopoulos K, Pegtel M, Hopmans E, Murray P, Middeldorp J, et al. , A novel persistence associated EBV miRNA expression profile is disrupted in neoplasia, PLoS Pathog. 7 (2011) e1002193. doi: 10.1371/journal.ppat.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sakamoto K, Sekizuka T, Uehara T, Hishima T, Mine S, Fukumoto H, et al. , Next-generation sequencing of miRNAs in clinical samples of Epstein-Barr virus-associated B-cell lymphomas, Cancer Med. 6 (2017) 605–618. doi: 10.1002/cam4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Imig J, Motsch N, Zhu JY, Barth S, Okoniewski M, Reineke T, et al. , microRNA profiling in Epstein-Barr virus-associated B-cell lymphoma, Nucleic Acids Res. 39 (2011) 1880–1893. doi: 10.1093/nar/gkq1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hartung A, Makarewicz O, Egerer R, Karrasch M, Klink A, Sauerbrei A, et al. , EBV miRNA expression profiles in different infection stages: A prospective cohort study, PLoS ONE. 14 (2019) e0212027. doi: 10.1371/journal.pone.0212027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dahlke C, Maul K, Christalla T, Walz N, Schult P, Stocking C, et al. , A microRNA encoded by Kaposi sarcoma-associated herpesvirus promotes B-cell expansion in vivo, PLoS ONE. 7 (2012) e49435. doi: 10.1371/journal.pone.0049435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Seto E, Moosmann A, Grömminger S, Walz N, Grundhoff A, Hammerschmidt W, Micro RNAs of Epstein-Barr virus promote cell cycle progression and prevent apoptosis of primary human B cells, PLoS Pathog. 6 (2010) e1001063. doi: 10.1371/journal.ppat.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Feederle R, Linnstaedt SD, Bannert H, Lips H, Bencun M, Cullen BR, et al. , A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus, PLoS Pathog. 7 (2011) e1001294. doi: 10.1371/journal.ppat.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Feederle R, Haar J, Bernhardt K, Linnstaedt SD, Bannert H, Lips H, et al. , The members of an Epstein-Barr virus microRNA cluster cooperate to transform B lymphocytes, J. Virol. 85 (2011) 9801–9810. doi: 10.1128/JVI.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vereide DT, Seto E, Chiu Y-F, Hayes M, Tagawa T, Grundhoff A, et al. , Epstein–Barr virus maintains lymphomas via its miRNAs, Oncogene. 33 (2013) 1258–1264. doi: 10.1038/onc.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O, Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells, Cell Host Microbe. 5 (2009) 376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [63].Suffert G, Malterer G, Hausser J, Viiliäinen J, Fender A, Contrant M, et al. , Kaposi’s sarcoma herpesvirus microRNAs target caspase 3 and regulate apoptosis, PLoS Pathog. 7 (2011) e1002405. doi: 10.1371/journal.ppat.1002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang L, Damania B, Kaposi’s sarcoma-associated herpesvirus confers a survival advantage to endothelial cells, Cancer Res. 68 (2008) 4640–4648. doi: 10.1158/0008-5472.CAN-07-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Li T, Ju E, Gao S-J, Kaposi sarcoma-associated herpesvirus miRNAs suppress CASTOR1-mediated mTORC1 inhibition to promote tumorigenesis, J. Clin. Invest. 129 (2019) 3310–3323. doi: 10.1172/JCI127166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Moody R, Zhu Y, Huang Y, Cui X, Jones T, Bedolla R, et al. , KSHV microRNAs mediate cellular transformation and tumorigenesis by redundantly targeting cell growth and survival pathways, PLoS Pathog. 9 (2013) e1003857. doi: 10.1371/journal.ppat.1003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lei X, Bai Z, Ye F, Xie J, Kim C-G, Huang Y, et al. , Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA, Nat. Cell Biol. 12 (2010) 193–199. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Skalsky RL, Cullen BR, Viruses, microRNAs, and Host Interactions, Annu. Rev. Microbiol. 64 (2010) 123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bogerd HP, Karnowski HW, Cai X, Shin J, Pohlers M, Cullen BR, A mammalian herpesvirus uses noncanonical expression and processing mechanisms to generate viral MicroRNAs, Molecular Cell. 37 (2010) 135–142. doi: 10.1016/j.molcel.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Diebel KW, Smith AL, van Dyk LF, Mature and functional viral miRNAs transcribed from novel RNA polymerase III promoters, Rna. 16 (2010) 170–185. doi: 10.1261/rna.1873910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Reese TA, Xia J, Johnson LS, Zhou X, Zhang W, Virgin HW, Identification of novel microRNA-like molecules generated from herpesvirus and host tRNA transcripts, J. Virol. 84 (2010) 10344–10353. doi: 10.1128/JVI.00707-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Deng J, Ptashkin RN, Chen Y, Cheng Z, Liu G, Phan T, et al. , Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism, Mol Ther. 23 (2015) 1622–1629. doi: 10.1038/mt.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cazalla D, Xie M, Steitz JA, A primate herpesvirus uses the integrator complex to generate viral microRNAs, Molecular Cell. 43 (2011) 982–992. doi: 10.1016/j.molcel.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Walz N, Christalla T, Tessmer U, Grundhoff A, A global analysis of evolutionary conservation among known and predicted gammaherpesvirus microRNAs, J. Virol. 84 (2010) 716–728. doi: 10.1128/JVI.01302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Skalsky RL, Kang D, Linnstaedt SD, Cullen BR, Evolutionary conservation of primate lymphocryptovirus microRNA targets, J. Virol. 88 (2014) 1617–1635. doi: 10.1128/JVI.02071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, et al. , Viral MicroRNA Targetome of KSHV-Infected Primary Effusion Lymphoma Cell Lines, Cell Host Microbe. 10 (2011) 515–526. doi: 10.1016/j.chom.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bullard WL, Kara M, Gay LA, Sethuraman S, Wang Y, Nirmalan S, et al. , Identification of murine gammaherpesvirus 68 miRNA-mRNA hybrids reveals miRNA target conservation among gammaherpesviruses including host translation and protein modification machinery, PLoS Pathog. 15 (2019) e1007843. doi: 10.1371/journal.ppat.1007843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lin C, Zong J, Lin W, Wang M, Xu Y, Zhou R, et al. , EBV-miR-BART8–3p induces epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2 pathways, Journal of Experimental & Clinical Cancer Research. 37 (2018) 283–14. doi: 10.1186/s13046-018-0953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Xu Y-J, Zhou R, Zong J-F, Lin W-S, Tong S, Guo Q-J, et al. , Epstein-Barr virus-coded miR-BART13 promotes nasopharyngeal carcinoma cell growth and metastasis via targeting of the NKIRAS2/NF-κB pathway, Cancer Letters. 447 (2019) 33–40. doi: 10.1016/j.canlet.2019.01.022. [DOI] [PubMed] [Google Scholar]
- [80].Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, et al. , Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of miR-155, J. Virol. 81 (2007) 12836–12845. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gallaher AM, Das S, Xiao Z, Andresson T, Kieffer-Kwon P, Happel C, et al. , Proteomic Screening of Human Targets of Viral microRNAs Reveals Functions Associated with Immune Evasion and Angiogenesis, PLoS Pathog. 9 (2013) e1003584–14. doi: 10.1371/journal.ppat.1003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Boss IW, Nadeau PE, Abbott JR, Yang Y, Mergia A, Renne R, A Kaposi’s sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rγnull mice, J. Virol. 85 (2011) 9877–9886. doi: 10.1128/JVI.05558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dahlke C, Maul K, Christalla T, Walz N, Schult P, Stocking C, et al. , A microRNA Encoded by Kaposi Sarcoma-Associated Herpesvirus Promotes B-Cell Expansion In Vivo, PLoS ONE. 7 (2012) e49435. doi: 10.1371/journal.pone.0049435.g006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR, Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus, J. Virol. 84 (2010) 11670–11678. doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Du Z-M, Hu L-F, Wang H-Y, Yan L-X, Zeng Y-X, Shao J-Y, et al. , Upregulation of MiR-155 in Nasopharyngeal Carcinoma is Partly Driven by LMP1 and LMP2A and Downregulates a Negative Prognostic Marker JMJD1A, PLoS ONE. 6 (2011) e19137. doi: 10.1371/journal.pone.0019137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Morrison K, Manzano M, Chung K, Schipma MJ, Bartom ET, Gottwein E, The Oncogenic Kaposi’s Sarcoma-Associated Herpesvirus Encodes a Mimic of the Tumor-Suppressive miR-15/16 miRNA Family, CellReports. 29 (2019) 2961–2969.e6. doi: 10.1016/j.celrep.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Manzano M, Shamulailatpam P, Raja AN, Gottwein E, Kaposi’s Sarcoma-Associated Herpesvirus Encodes a Mimic of Cellular miR-23, J. Virol. 87 (2013) 11821–11830. doi: 10.1128/JVI.01692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Albanese M, Tagawa T, Buschle A, Hammerschmidt W, MicroRNAs of Epstein-Barr Virus Control Innate and Adaptive Antiviral Immunity, J. Virol. 91 (2017) e01667–16. doi: 10.1128/JVI.01667-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Singer JW, Fleischman A, Al-Fayoumi S, Mascarenhas JO, Yu Q, Agarwal A, Inhibition of interleukin-1 receptor-associated kinase 1 (IRAK1) as a therapeutic strategy, Oncotarget. 9 (2018) 33416–33439. doi: 10.18632/oncotarget.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mrazek J, Kreutmayer SB, Grässer FA, Polacek N, Hüttenhofer A, Subtractive hybridization identifies novel differentially expressed ncRNA species in EBV-infected human B cells, Nucleic Acids Res. 35 (2007) e73–e73. doi: 10.1093/nar/gkm244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Motsch N, Pfuhl T, Mrazek J, Barth S, Grässer FA, Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a, RNA Biology. 4 (2007) 131–137. doi: 10.4161/rna.4.3.5206. [DOI] [PubMed] [Google Scholar]
- [92].Hill JM, Zhao Y, Clement C, Neumann DM, Lukiw WJ, HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling, Neuroreport. 20 (2009) 1500–1505. doi: 10.1097/WNR.0b013e3283329c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Venuti A, Musarra-Pizzo M, Pennisi R, Tankov S, Medici MA, Mastino A, et al. , HSV-1\EGFP stimulates miR-146a expression in a NF-κB-dependent manner in monocytic THP-1 cells, Sci. Rep. 9 (2019) 5157–14. doi: 10.1038/s41598-019-41530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chen Y, Chen J, Wang H, Shi J, Wu K, Liu S, et al. , HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1, PLoS Pathog. 9 (2013) e1003248. doi: 10.1371/journal.ppat.1003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Forte E, Salinas RE, Chang C, Zhou T, Linnstaedt SD, Gottwein E, et al. , The Epstein-Barr virus (EBV)-induced tumor suppressor microRNA MiR-34a is growth promoting in EBV-infected B cells, J. Virol. 86 (2012) 6889–6898. doi: 10.1128/JVI.07056-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Abend JR, Uldrick T, Ziegelbauer JM, Regulation of tumor necrosis factor-like weak inducer of apoptosis receptor protein (TWEAKR) expression by Kaposi’s sarcoma-associated herpesvirus microRNA prevents TWEAK-induced apoptosis and inflammatory cytokine expression, J. Virol. 84 (2010) 12139–12151. doi: 10.1128/JVI.00884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sarker O, Viral MicroRNAs Repress the Cholesterol Pathway, and 25-Hydroxycholesterol Inhibits Infection, mBio. 8 (2017). doi: 10.1128/mBio.00576-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jahagirdar D, Purohit S, Jain A, Sharma NK, Export of microRNAs: A Bridge between Breast Carcinoma and Their Neighboring Cells, Front. Oncol. 6 (2016) 553. doi: 10.3389/fonc.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, et al. , Functional delivery of viral miRNAs via exosomes, Proc Natl Acad Sci USA. 107 (2010) 6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nanbo A, Katano H, Kataoka M, Hoshina S, Sekizuka T, Kuroda M, et al. , Infection of Epstein–Barr Virus in Type III Latency Modulates Biogenesis of Exosomes and the Expression Profile of Exosomal miRNAs in the Burkitt Lymphoma Mutu Cell Lines, Cancers. 10 (2018) 237. doi: 10.3390/cancers10070237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chugh PE, Sin S-H, Ozgur S, Henry DH, Menezes P, Griffith J, et al. , Systemically Circulating Viral and Tumor-Derived MicroRNAs in KSHV-Associated Malignancies, PLoS Pathog. 9 (2013) e1003484. doi: 10.1371/journal.ppat.1003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].McNamara RP, Chugh PE, Bailey A, Costantini LM, Ma Z, Bigi R, et al. , Extracellular vesicles from Kaposi Sarcoma-associated herpesvirus lymphoma induce long-term endothelial cell reprogramming, PLoS Pathog. 15 (2019) e1007536. doi: 10.1371/journal.ppat.1007536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Turchinovich A, Weiz L, Langheinz A, Burwinkel B, Characterization of extracellular circulating microRNA, Nucleic Acids Res. 39 (2011) 7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, et al. , Quantitative and stoichiometric analysis of the microRNA content of exosomes, Proc. Natl. Acad. Sci. U.S.a. 111 (2014) 14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. , Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma, Proc Natl Acad Sci USA. 108 (2011) 5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tagawa T, Albanese M, Bouvet M, Moosmann A, Mautner J, Heissmeyer V, et al. , Epstein-Barr viral miRNAs inhibit antiviral CD4 +T cell responses targeting IL-12 and peptide processing, J. Exp. Med. 213 (2016) 2065–2080. doi: 10.1084/jem.20160248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M, et al. , The Viral and Cellular MicroRNA Targetome in Lymphoblastoid Cell Lines, PLoS Pathog. 8 (2012) e1002484. doi: 10.1371/journal.ppat.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mansouri S, Pan Q, Blencowe BJ, Claycomb JM, Frappier L, The Epstein-Barr Virus EBNA1 Protein Regulates Viral latency Through Effects on Let-7 microRNA and Dicer, J. Virol. (2014). doi: 10.1128/JVI.01785-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Choi HS, Jain V, Krueger B, Marshall V, Kim CH, Shisler JL, et al. , Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17–92 Cluster and Down-Regulates TGF-β Signaling, PLoS Pathog. 11 (2015) e1005255. doi: 10.1371/journal.ppat.1005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RSB, et al. , miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator, Nat. Cell Biol. 12 (2010) 513–519. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- [111].Shrestha P, Davis DA, Veeranna RP, Carey RF, Viollet C, Yarchoan R, Hypoxia-inducible factor-1 alpha as a therapeutic target for primary effusion lymphoma, PLoS Pathog. 13 (2017) e1006628. doi: 10.1371/journal.ppat.1006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Viollet C, Davis DA, Tekeste SS, Reczko M, Ziegelbauer JM, Pezzella F, et al. , RNA Sequencing Reveals that Kaposi Sarcoma-Associated Herpesvirus Infection Mimics Hypoxia Gene Expression Signature, PLoS Pathog. 13 (2017) e1006143. doi: 10.1371/journal.ppat.1006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Happel C, Ramalingam D, Ziegelbauer JM, Virus-Mediated Alterations in miRNA Factors and Degradation of Viral miRNAs by MCPIP1, PLoS Biol. 14 (2016) e2000998–23. doi: 10.1371/journal.pbio.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Iwasaki H, Takeuchi O, Teraguchi S, Matsushita K, Uehata T, Kuniyoshi K, et al. , The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1, Nature Immunology. 12 (2011) 1167–1175. doi: 10.1038/ni.2137. [DOI] [PubMed] [Google Scholar]
- [115].Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, et al. , Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction, Circulation Research. 98 (2006) 1177–1185. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lin Y-T, Sullivan CS, Expanding the role of Drosha to the regulation of viral gene expression, Proc Natl Acad Sci USA. 108 (2011) 11229–11234. doi: 10.1073/pnas.1105799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhu Y, Huang Y, Jung JU, Lu C, Gao S-J, Viral miRNA targeting of bicistronic and polycistronic transcripts, Current Opinion in Virology. 7 (2014) 66–72. doi: 10.1016/j.coviro.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gottwein E, Cai X, Cullen BR, A novel assay for viral microRNA function identifies a single nucleotide polymorphism that affects Drosha processing, J. Virol. 80 (2006) 5321–5326. doi: 10.1128/JVI.02734-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Marshall VA, Labo N, Sztuba-Solinska J, Cornejo Castro EM, Aleman K, Wyvill KM, et al. , Polymorphisms in KSHV-encoded microRNA sequences affect levels of mature viral microRNA in Kaposi Sarcoma lesions, Oncotarget. 9 (2018) 35856–35869. doi: 10.18632/oncotarget.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].la Mata M, Gaidatzis D, Vitanescu M, Stadler MB, Wentzel C, Scheiffele P, et al. , Potent degradation of neuronal mi RNAs induced by highly complementary targets, EMBO Rep. 16 (2015) 500–511. doi: 10.15252/embr.201540078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cazalla D, Yario T, Steitz JA, Down-Regulation of a Host MicroRNA by a Herpesvirus saimiri Noncoding RNA, Science. 328 (2010) 1563–1566. doi: 10.1126/science.1187197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Marcinowski L, Tanguy M, Krmpotic A, Rädle B, Lisnić VJ, Tuddenham L, et al. , Degradation of Cellular miR-27 by a Novel, Highly Abundant Viral Transcript Is Important for Efficient Virus Replication In Vivo, PLoS Pathog. 8 (2012) e1002510. doi: 10.1371/journal.ppat.1002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lee S, Song J, Kim S, Kim J, Hong Y, Kim Y, et al. , Selective Degradation of Host MicroRNAs by an Intergenic HCMV Noncoding RNA Accelerates Virus Production, Cell Host Microbe. 13 (2013) 678–690. doi: 10.1016/j.chom.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [124].Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J, The biogenesis, biology and characterization of circular RNAs, Nat Rev Genet. 20 (2019) 675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- [125].Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. , Natural RNA circles function as efficient microRNA sponges, 495 (2013) 384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- [126].Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. , Circular RNAs are a large class of animal RNAs with regulatory potency, Nature. 495 (2013) 333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- [127].Ungerleider N, Concha M, Lin Z, Roberts C, Wang X, Cao S, et al. , The Epstein Barr virus circRNAome, PLoS Pathog. 14 (2018) e1007206. doi: 10.1371/journal.ppat.1007206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Huang J-T, Chen J-N, Gong L-P, Bi Y-H, Liang J, Zhou L, et al. , Identification of virus-encoded circular RNA, Virology. 529 (2019) 144–151. doi: 10.1016/j.virol.2019.01.014. [DOI] [PubMed] [Google Scholar]
- [129].Zhao J, Lee EE, Kim J, Yang R, Chamseddin B, Ni C, et al. , Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus, Nat Commun. 10 (2019) 18. doi: 10.1038/s41467-019-10246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ungerleider NA, Jain V, Wang Y, Maness NJ, Blair RV, Alvarez X, et al. , Comparative Analysis of Gammaherpesvirus Circular RNA Repertoires: Conserved and Unique Viral Circular RNAs, J. Virol. 93 (2019) 10110–15. doi: 10.1128/JVI.01952-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Abere B, Li J, Zhou H, Toptan T, Moore PS, Chang Y, Kaposi’s Sarcoma-Associated Herpesvirus-Encoded circRNAs Are Expressed in Infected Tumor Tissues and Are Incorporated into Virions, mBio. 11 (2020) 1865. doi: 10.1128/mBio.03027-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. , circRNA Biogenesis Competes with Pre-mRNA Splicing, Molecular Cell. 56 (2014) 55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- [133].Depledge DP, Srinivas KP, Sadaoka T, Bready D, Mori Y, Placantonakis DG, et al. , Direct RNA sequencing on nanopore arrays redefines the transcriptional complexity of a viral pathogen, Nat Commun. 10 (2019) 754–13. doi: 10.1038/s41467-019-08734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liu Q, Shuai M, Xia Y, Knockdown of EBV-encoded circRNA circRPMS1 suppresses nasopharyngeal carcinoma cell proliferation and metastasis through sponging multiple miRNAs, Cmar. 11 (2019) 8023–8031. doi: 10.2147/CMAR.S218967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Huang J-T, Chen J-N, Gong L-P, Bi Y-H, Liang J, Zhou L, et al. , Identification of virus-encoded circular RNA, Virology. 529 (2019) 144–151. doi: 10.1016/j.virol.2019.01.014. [DOI] [PubMed] [Google Scholar]
- [136].Chen YG, Kim MV, Chen X, Batista PJ, Aoyama S, Wilusz JE, et al. , Sensing Self and Foreign Circular RNAs by Intron Identity, Molecular Cell. 67 (2017) 228–238.e5. doi: 10.1016/j.molcel.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Liu C-X, Li X, Nan F, Jiang S, Gao X, Guo S-K, et al. , Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity, Cell. 177 (2019) 865–880.e21. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- [138].Shi X, Wang B, Feng X, Xu Y, Lu K, Sun M, circRNAs and Exosomes: A Mysterious Frontier for Human Cancer, Molecular Therapy - Nucleic Acids. 19 (2020) 384–392. doi: 10.1016/j.omtn.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wesselhoeft RA, Kowalski PS, Parker-Hale FC, Huang Y, Bisaria N, Anderson DG, RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo, Molecular Cell. 74 (2019) 508–520.e4. doi: 10.1016/j.molcel.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tsuchiya S, Diagnosis of Epstein-Barr virus-associated diseases, Critical Reviews in Oncology/Hematology. 44 (2002) 227–238. doi: 10.1016/s1040-8428(02)00114-2. [DOI] [PubMed] [Google Scholar]
- [141].Movassagh M, Oduor C, Forconi C, Moormann AM, Bailey JA, Sensitive detection of EBV microRNAs across cancer spectrum reveals association with decreased survival in adult acute myelocytic leukemia, Sci. Rep. 9 (2019) 20321–10.doi: 10.1038/s41598-019-56472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gao L, Ai J, Xie Z, Zhou C, Liu C, Zhang H, et al. , Dynamic expression of viral and cellular microRNAs in infectious mononucleosis caused by primary Epstein-Barr virus infection in children, Virol J. 12 (2015) 208–11. doi: 10.1186/s12985-015-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Okuno Y, Murata T, Sato Y, Muramatsu H, Ito Y, Watanabe T, et al. , Defective Epstein–Barr virus in chronic active infection and haematological malignancy, Nat. Microbiol. 4 (2019) 404–413. doi: 10.1038/s41564-018-0334-0. [DOI] [PubMed] [Google Scholar]
- [144].Lin X, Tsai M-H, Shumilov A, Poirey R, Bannert H, Middeldorp JM, et al. , The Epstein-Barr Virus BART miRNA Cluster of the M81 Strain Modulates Multiple Functions in Primary B Cells, PLoS Pathog. 11 (2015) e1005344. doi: 10.1371/journal.ppat.1005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Kawano Y, Iwata S, Kawada J-I, Gotoh K, Suzuki M, Torii Y, et al. , Plasma viral microRNA profiles reveal potential biomarkers for chronic active Epstein-Barr virus infection, J. Infect. Dis. 208 (2013) 771–779. doi: 10.1093/infdis/jit222. [DOI] [PubMed] [Google Scholar]
- [146].Tsai ST, Jin YT, Mann RB, Ambinder RF, Epstein-Barr virus detection in nasopharyngeal tissues of patients with suspected nasopharyngeal carcinoma, Cancer. 82 (1998) 1449–1453. [PubMed] [Google Scholar]
- [147].Wu TC, Mann RB, Epstein JI, MacMahon E, Lee WA, Charache P, et al. , Abundant expression of EBER1 small nuclear RNA in nasopharyngeal carcinoma. A morphologically distinctive target for detection of Epstein-Barr virus in formalin-fixed paraffin-embedded carcinoma specimens, The American Journal of Pathology. 138 (1991) 1461–1469. [PMC free article] [PubMed] [Google Scholar]
- [148].Maohuai C, Chang AR, Shikyuen L, Roles of DNA cytometry and detection of EBERs in predicting a diagnosis of nasopharyngeal carcinoma, Anal. Quant. Cytol. Histol. 23 (2001) 207–212. [PubMed] [Google Scholar]
- [149].Cosmopoulos K, Pegtel M, Hawkins J, Moffett H, Novina C, Middeldorp J, et al. , Comprehensive profiling of Epstein-Barr virus microRNAs in nasopharyngeal carcinoma, J. Virol. 83 (2009) 2357–2367. doi: 10.1128/JVI.02104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhang G, Zong J, Lin S, Verhoeven RJA, Tong S, Chen Y, et al. , Circulating Epstein-Barr virus microRNAs miR-BART7 and miR-BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment, Int. J. Cancer. 136 (2014) E301–E312. doi: 10.1002/ijc.29206. [DOI] [PubMed] [Google Scholar]
- [151].Hirai N, Wakisaka N, Kondo S, Aga M, Moriyama-Kita M, Ueno T, et al. , Potential Interest in Circulating miR-BART17–5p As a Post-Treatment Biomarker for Prediction of Recurrence in Epstein-Barr Virus-Related Nasopharyngeal Carcinoma, PLoS ONE. 11 (2016) e0163609. doi: 10.1371/journal.pone.0163609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Morales-Sanchez A, Fuentes-Panana EM, Epstein-Barr Virus-associated Gastric Cancer and Potential Mechanisms of Oncogenesis, Curr Cancer Drug Targets. 17 (2017) 534–554. doi: 10.2174/1568009616666160926124923. [DOI] [PubMed] [Google Scholar]
- [153].Kim DN, Chae H-S, Oh ST, Kang J-H, Park CH, Park WS, et al. , Expression of viral microRNAs in Epstein-Barr virus-associated gastric carcinoma, J. Virol. 81 (2007) 1033–1036. doi: 10.1128/JVI.02271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Manzano M, Forte E, Raja AN, Schipma MJ, Gottwein E, Divergent target recognition by coexpressed 5’-isomiRs of miR-142–3p and selective viral mimicry, Rna. 21 (2015) 1606–1620. doi: 10.1261/rna.048876.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Marshall V, Parks T, Bagni R, Wang CD, Samols MA, Hu J, et al. , Conservation of virally encoded microRNAs in Kaposi sarcoma--associated herpesvirus in primary effusion lymphoma cell lines and in patients with Kaposi sarcoma or multicentric Castleman disease, J. Infect. Dis. 195 (2007) 645–659. doi: 10.1086/511434. [DOI] [PubMed] [Google Scholar]
- [156].Han S-J, Marshall V, Barsov E, Quiñones O, Ray A, Labo N, et al. , Kaposi’s sarcoma-associated herpesvirus microRNA single-nucleotide polymorphisms identified in clinical samples can affect microRNA processing, level of expression, and silencing activity, J. Virol. 87 (2013) 12237–12248. doi: 10.1128/JVI.01202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Marín RM, Voellmy F, von Erlach T, Vaníček J, Analysis of the accessibility of CLIP bound sites reveals that nucleation of the miRNA:mRNA pairing occurs preferentially at the 3’-end of the seed match, Rna. 18 (2012) 1760–1770. doi: 10.1261/rna.033282.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chen S-J, Chen G-H, Chen Y-H, Liu C-Y, Chang K-P, Chang Y-S, et al. , Characterization of Epstein-Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing, PLoS ONE. 5 (2010) e12745. doi: 10.1371/journal.pone.0012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Iizasa H, Wulff BE, Alla NR, Maragkakis M, Megraw M, Hatzigeorgiou A, et al. , Editing of Epstein-Barr Virus-encoded BART6 MicroRNAs Controls Their Dicer Targeting and Consequently Affects Viral Latency, Journal of Biological Chemistry. 285 (2010) 33358–33370. doi: 10.1074/jbc.M110.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kim H, Burassakarn A, Kang Y, Iizasa H, Yoshiyama H, A single nucleotide polymorphism in the BART promoter region of Epstein-Barr virus isolated from nasopharyngeal cancer cells, Biochem Biophys Res Commun. 520 (2019) 373–378. doi: 10.1016/j.bbrc.2019.10.028. [DOI] [PubMed] [Google Scholar]
- [161].Ju E, Li T, Liu Z, da Silva SR, Wei S, Zhang X, et al. , Specific Inhibition of Viral MicroRNAs by Carbon Dots-Mediated Delivery of Locked Nucleic Acids for Therapy of Virus-Induced Cancer, ACS Nano. 14 (2020) 476–487. doi: 10.1021/acsnano.9b06333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Feldman ER, Tibbetts SA, Emerging Roles of Herpesvirus microRNAs During In Vivo Infection and Pathogenesis, Curr Pathobiol Rep. 3 (2015) 209–217. doi: 10.1007/s40139-015-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, et al. , RNAscope, The Journal of Molecular Diagnostics. 14 (2012) 22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]