Abstract
Objective:
To assess quality of life (QOL) in patients who developed lower-extremity lymphedema (LLE) after radical gynecologic cancer surgery on prospective clinical trial GOG 244.
Methods:
The prospective, national, cooperative group trial GOG-0244 determined the incidence of LLE and risk factors for LLE development, as well as associated impacts on QOL, in newly diagnosed patients undergoing surgery for endometrial, cervical, or vulvar cancer from 6/4/2012–11/17/2014. Patient-reported outcome (PRO) measures of QOL (by the Functional Assessment of Cancer Therapy [FACT]), body image, sexual and vaginal function, limb function, and cancer distress were recorded at baseline (within 14 days before surgery), and at 6, 12, 18, and 24 months after surgery. Assessments of LLE symptoms and disability were completed at the time of lower limb volume measurement. A linear mixed model was applied to examine the association of PROs/QOL with a Gynecologic Cancer Lymphedema Questionnaire (GCLQ) total score incremental change ≥4 (indicative of increased LLE symptoms) from baseline, a formal diagnosis of LLE (per the GCLQ), and limb volume change (LVC) ≥10%.
Results:
In 768 evaluable patients, those with a GCLQ score change ≥4 from baseline had significantly worse QOL (p<0.001), body image (p<0.001), sexual and vaginal function (p<0.001), limb function (p<0.001), and cancer distress (p<0.001). There were no significant differences in sexual activity rates between those with and without LLE symptoms.
Conclusions:
LLE is significantly detrimental to QOL, daily function, and body image. Clinical intervention trials to prevent and manage this chronic condition after gynecologic cancer surgery are needed.
Keywords: lymphedema, quality of life, endometrial cancer, cervical cancer, vulvar cancer, GOG 244
INTRODUCTION
Advances in oncologic treatment have led to an ever-increasing number of cancer survivors over time. These treatments, however, are often associated with side effects that can be detrimental to quality of life (QOL). Cancer treatment, for example, is the leading cause of secondary lymphedema, or an abnormal accumulation of lymph fluid, which can be a chronic, debilitating condition [1]. The majority of lymphedema research has focused on upper-extremity lymphedema in breast cancer patients. Lower-extremity lymphedema (LLE) has been studied to a much lesser degree, mostly in descriptive studies or studies with small sample sizes, despite its prevalence in gynecologic cancer patients [2–8]. Research has demonstrated that LLE can negatively impact QOL [2,5,6], physical function [4,8], socialization, and patient finances [7]. Here, we address the knowledge gap with regard to gynecologic cancer treatment and associations with LLE through a longitudinal evaluation of estimated incidence and QOL consequences of LLE.
Gynecologic Oncology Group (GOG) 244 was a national, cooperative group trial designed to prospectively estimate the incidence of LLE, and investigate potential risk factors for LLE development and its impact on QOL, in a large sample of newly diagnosed patients undergoing radical surgery for endometrial, cervical, or vulvar malignancy. The primary trial results indicated that in gynecologic cancer, limb volume change (LVC) decreased with age >65 years and increased with removal of >8 lymph nodes [9]. Patient-reported lymphedema symptoms were able to differentiate those with and without an LLE diagnosis and LVC [10]. Specifically, significantly more patients with a ≥4-point increase in Gynecologic Cancer Lymphedema Questionnaire (GCLQ) total score were diagnosed with LLE (p<0.001) [10]. Here, we examine the effects of LLE on QOL, psychological adjustment, physical function, and disability.
METHODS
Study Population.
The Lymphedema and Gynecologic cancer (LeG) study (GOG 244) was a prospective, multi-institutional study of women with newly diagnosed endometrial, cervical, or vulvar cancer who underwent surgery as their primary intervention from 6/4/2012–11/17/2014, with 2 years of follow-up. Eligible participants included those planned for: 1) hysterectomy/bilateral salpingo-oophorectomy (BSO) and pelvic lymphadenectomy +/− paraaortic node sampling via an open or laparoscopic approach for clinical stage I-II uterine carcinoma; 2) radical hysterectomy or trachelectomy and pelvic lymphadenectomy +/− paraaortic node sampling via an open or laparoscopic approach for clinical stage IA-IIA cervical carcinoma; or 3) definitive surgery with radical vulvectomy or radical local excision with concurrent unilateral or bilateral inguinal or inguinal-femoral lymphadenectomy for primary stage I-IV vulvar cancer. Participants were able to receive radiation and/or chemotherapy after primary surgery.
Participants completed assessments of QOL, psychological adjustment, and patient-reported outcome (PRO) functions at baseline (within 14 days before surgery) and at 6, 12, 18, and 24 months post-surgery. Assessments of lymphedema symptoms and disability were completed at the time of lower limb volume measurement at baseline, 4-6 weeks, and 3, 6, 9, 12, 18, and 24 months post-surgery. All patients signed written informed consent. This study was funded by NCI GOG and NIH R01 CA162139.
Measures.
Participant demographics and medical and cancer treatment information, as well as known, suspected, and possible risk factors for LLE development, were collected. The PRO surveys consisted of measures of health-related QOL (Functional Assessment of Cancer Therapy [FACT]), sexual activity (PROMIS Sexual Function and Satisfaction screener items [PROMIS-SexFS]), cancer distress (Impact of Event Scale [IES]), limb function (Lower Extremity Functional Scale [LEFS]), and lymphedema symptom assessment (Gynecological Cancer Lymphedema Questionnaire [GCLQ]).
The FACT-General (FACT-G) and disease-specific subscales (FACT-Cervix [FACT-Cx], FACT-Endometrial [FACT-En], and FACT-Vulva [FACT-V]) were used to assess health-related QOL. The FACT-G is a 27-item scale measuring QOL in patients with cancer [11]. It includes four subscales: 1) physical well-being (PWB), with 7 items; 2) functional well-being (FWB), with 7 items; 3) social well-being (SWB), with 7 items; and 4) emotional well-being (EWB), with 6 items. Participants rate each item on a 5-point Likert-type scale (0=not at all; 1=a little bit; 2=somewhat; 3=quite a bit; 4=very much). The subscales are summed for a total score ranging from 0-108, with a higher score indicative of better QOL. The FACT-Cx consists of the FACT-G plus a 15-item cervical cancer-specific subscale. The FACT-En consists of the FACT-G plus a 16-item endometrial cancer subscale. The FACT-V consists of the FACT-G plus a 19-item vulvar cancer-specific subscale. The FACT-SWB was examined for its associations with LVC, a formal LLE diagnosis (reported on the GCLQ), and with a GCLQ total score increment ≥4 to investigate potential influences for those with and without LLE.
FACT disease-specific items addressing body image, such as “I like the appearance of my body” (FACT-Cx/FACT-V), “I feel sexually attractive” (FACT-Cx), and “I am unhappy about the change in my appearance” (FACT-En), were standardized and summed across the three cancer groups. Two PROMIS screener questions were included to determine if a participant was in a sexual relationship and/or currently sexually active. Sexual function was assessed by the FACT-G item, “I am satisfied with my sex life”. Items addressing sexual and vaginal function were included in the disease-specific subscales but not queried uniformly across subscales. The following items to examine sexual and vaginal function were standardized and summed across all groups: Cx3–“I am afraid to have sex”; Cx4–“My vagina feels too narrow or short”; B14–“I am interested in sex”; ES6–“I have vaginal bleeding or spotting”; ES1–“I have hot flashes”; ES8–“I have pain or discomfort with intercourse”; and V6–“l am bothered by pain or numbness in my vulva area”.
Psychological adjustment, specifically psychosocial distress, was measured by the IES, a 15-item self-report measure focusing on intrusive and avoidant thoughts associated with a stressor such as cancer. Participants rate how true each statement has been for them during the past month on a 4-point Likert-type scale. Intrusion and Avoidance subscale scores are computed. Internal consistency estimates are good for both the Intrusion subscale (Cronbach’s alpha=.87) and the Avoidance subscale (alpha=.86). The IES has been used extensively in patients at high risk for cancer development [12] and in cancer populations [13–15], including breast cancer patients with lymphedema [16]. The overall score (0-75) is calculated based on individual responses. Higher scores indicate more stress.
The LEFS measures lower-limb disability. The LEFS is a 20-item instrument that uses a 5-point scale to rate difficulty (0=extreme difficulty to 4=no difficulty) in performing activities, such as usual work, hobbies, walking, climbing, standing, sitting, getting out of a car, rolling over in bed, lifting, running, and putting on shoes or socks. Scores range from 0-80, with a lower score indicative of poorer function. Test-retest reliability of LEFS score is r=.94 (95% CI: .89), with correlations between LEFS and Medical Outcomes Study (MOS) 36-Item Short Form Survey (SF-36) physical function subscale of r=.80 (95% CI: .73). LEFS has been found to have strong reliability and construct validity compared with the SF-36 [17] and other measures [18]. Sensitivity to change on the LEFS was found to be superior to that of the SF-36 [17].
The GCLQ was used to assess 20 symptoms associated with LLE present within the past 4 weeks. Scores for total current symptoms and clustered symptoms were calculated to describe the most prominent symptoms associated with an LLE diagnosis and LVC over time. Items were combined into symptom clusters of heaviness (H1), swelling (general; Sg2), swelling (limb; SL2), infection-related (I4), aching (A1), numbness (N4), and physical functioning (Pf6). A total GCLQ score was the summation of seven cluster scores, which were calculated only if all seven clusters provided valid scores. A 4-point GCLQ increment from baseline was used based on a validation study [19], as this cut-off score yielded >60% sensitivity and specificity, an optimal cut-off for identifying LLE. Exploratory supplemental items documented patients’ awareness of a formal LLE diagnosis and the use of lymphedema-specific treatment to evaluate interventions. The GCLQ has been validated [19], demonstrating its ability to distinguish between those with and without an LLE diagnosis and its predictive value to detect early onset and those at risk for LLE development [10].
Limb Volume Measurement.
LVC was measured by taking circumferential measurements at 10-cm intervals starting 10 cm above the bottom of the heel and continuing to the inguinal crease using a Jobst measurement board. Leg volume was the summation of truncated cone volumes. All participating sites had a representative complete in-person limb volume measurement training at a GOG meeting. Training sessions consisted of a 1-hour didactic session followed by a 1-hour clinical practicum including 2 measurements, the first by a trained GOG professional (allowing for a 1.0-cm variance) and the second by the trainee.
Statistical Considerations.
We explored the associations between PRO/QOL outcomes and a formal patient-reported LLE diagnosis (GCLQ), a GCLQ total score change (increment) ≥4 from baseline, and an LVC increment ≥10%. The GCLQ and leg volume measurements were performed at baseline (within 14 days before surgery), at 6 weeks, and at 3, 6, 9, 12, 18, and 24 months post-surgery. Only the assessment time points (baseline and 6, 12, 18, and 24 months post-surgery) at which QOL was measured were included in the analysis. For the purpose of the analysis, a formal LLE diagnosis, a GCLQ total score increment ≥4, and LVC increment ≥10% were treated as time-dependent variables and considered negative (“No”) until the time point when they were identified (“Yes”), and remained ”Yes” at subsequent assessment time points. The associations between the PRO/QOL scores and a formal LLE diagnosis, a GCLQ score increment ≥4, or LVC increment ≥10% were evaluated with a linear mixed model with adjustment for baseline scores, assessment time, and disease sites. The assessment time points were treated as categorical. Due to multiple testing, a 99% confidence interval is provided with the estimated least squares mean differences, and the p values were adjusted with Sisak correction for multiple testing as Padjusted = 1-(1 - Punadjusted)m, where m is the number of multiple endpoints for this report [20].
Item analysis was conducted for FACT subscale items addressing: 1) satisfaction with sex life, 2) body image, and 3) sexual/vaginal function standardized across all groups. These items were summed, and differences were estimated from a fitted linear mixed model adjusting for baseline score, disease sites, and assessment time when patients reported an LLE diagnosis on the GCLQ or experienced a GCLQ total score increment ≥4 from baseline.
RESULTS
Compliance Status of QOL Data.
Of 1054 patients enrolled on the GOG 244 study, 914 were evaluable (had lymphadenectomy and valid leg volume measurements). The compliance rate for PRO/QOL data was calculated for patients who were alive at the assessment time points. There were 36 deaths across the duration of the study. Compliance rates per assessment time point were as follows: 96% (874/914) at baseline; 79% (721/909) at 6 months; 73% (652/896) at 12 months; 65% (572/884) at 18 months; and 68% (532/778) at 24 months. Among these 914 evaluable patients, 768 (115 cervical cancer, 619 endometrial cancer, and 34 vulvar cancer) provided data at baseline and at least one follow-up assessment time point (84% response rate); these patients were evaluable for the PRO/QOL outcomes analyses (Figure 1; Consort Diagram).
Figure 1.
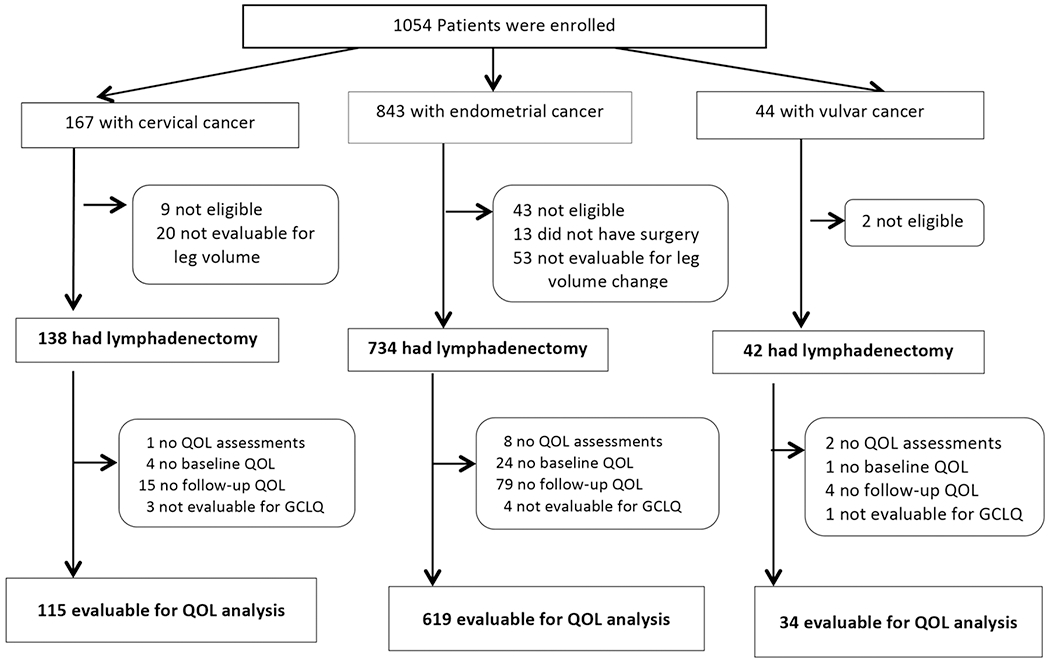
Consort diagram
Patient Characteristics and Disease Status.
Patient and disease characteristics for the 768 PRO/QOL evaluable patients are presented in Table 1. The mean ages were 46 years for those with cervical cancer, 61 years for those with endometrial cancer, and 60 years for those with vulvar cancer. The majority had excellent performance status at enrollment (93%, n=711) and stage I disease (83%, n=639).
Table 1.
Patient and disease characteristics
| Characteristic | Category | Cervix n=115 | Endometrial n=619 | Vulvar n=34 | All N=768 | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age, years | <30 | 9 | 8 | 1 | 0 | 0 | 0 | 10 | 1 |
| 30-39 | 31 | 27 | 16 | 3 | 1 | 3 | 48 | 6 | |
| 40-49 | 39 | 34 | 41 | 7 | 4 | 12 | 84 | 11 | |
| 50-59 | 16 | 14 | 221 | 36 | 15 | 44 | 252 | 33 | |
| 60-69 | 16 | 14 | 235 | 38 | 7 | 21 | 258 | 34 | |
| 70-79 | 4 | 3 | 94 | 15 | 5 | 15 | 103 | 13 | |
| >=80 | 0 | 0 | 11 | 2 | 2 | 6 | 13 | 2 | |
| Race | Asian | 10 | 9 | 16 | 3 | 0 | 0 | 27 | 3 |
| Black | 5 | 4 | 56 | 9 | 2 | 6 | 63 | 8 | |
| Other/unspecified | 19 | 17 | 309 | 5 | 2 | 6 | 51 | 7 | |
| White | 81 | 70 | 517 | 84 | 30 | 88 | 628 | 82 | |
| Ethnicity | Hispanic | 14 | 12 | 33 | 5 | 2 | 6 | 49 | 6 |
| Non-Hispanic | 98 | 85 | 579 | 94 | 31 | 91 | 708 | 92 | |
| Other/Unspecified | 3 | 3 | 7 | 1 | 1 | 3 | 11 | 1 | |
| Performance Status | 0 | 110 | 96 | 575 | 93 | 26 | 76 | 711 | 93 |
| 1 | 5 | 4 | 43 | 7 | 8 | 24 | 56 | 7 | |
| 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| Stage of Disease | I | 112 | 97 | 504 | 81 | 23 | 68 | 639 | 83 |
| II | 2 | 2 | 31 | 5 | 3 | 9 | 36 | 5 | |
| III | 1 | 1 | 78 | 13 | 8 | 24 | 87 | 11 | |
| IV | 0 | 0 | 6 | 1 | 0 | 0 | 6 | 1 | |
Analyses of the PRO/QOL data after adjustment for baseline score, disease sites, and assessment time in patients with a GCLQ total score increment ≥4 (increased LLE symptoms) from baseline, a formal diagnosis of LLE (reported on GCLQ), and LVC increment ≥10% indicated that the GCLQ was the most sensitive to change (Table 2).
Table 2.
Patient-reported outcomes/quality of life analyses
| PRO/QOL instruments | Difference with vs without lymphedema diagnosis (99% CI)* | Padjustedvalue ** |
|---|---|---|
| FACT-G | −2.8 (−5.7 ~ 0.1) | 0.08 |
| IES | 2.4 (−0.1 ~ 5.0) | 0.09 |
| Body Image subscale | −0.4 (−1.0 ~ 0.1) | 0.29 |
| Sexual and Vaginal subscale | −0.5 (−1.3 ~ 0.3) | 0.56 |
| LEFS | −5.7 (−9.3 ~ −2.3) | <0.001 |
| Social Well-Being | −0.4 (−1.3 ~ 0.5) | 0.83 |
| Difference between GCLQ total score increment ≥4 vs <4 (99% CI)* | Padjusted** | |
| FACT-G | −4.4 (−6.3 ~ −2.5) | <0.001 |
| IES | 3.0 (1.2 ~ 4.8) | <0.001 |
| Body Image subscale | −0.5 (−0.8~ −0.1) | <0.001 |
| Sexual and Vaginal subscale | −0.8 (−1.3 ~ −0.3) | <0.001 |
| LEFS | −7.3 (−9.6 ~ −5.0) | <0.001 |
| Social Well-Being | −0.5 (−1.1 ~ 0.1) | 0.27 |
| Difference between leg volume increment ≥10% vs <10% (99% CI)* | Padjusted** | |
| FACT-G | −0.5 (− 2.4 ~ 1.3) | 0.98 |
| IES | −0.5 (− 2.2~ 1.2) | 0.98 |
| Body Image subscale | −0.3 (−0.7 ~ 0.04) | 0.12 |
| Sexual and Vaginal subscale | −0.1 (−0.6 ~ 0.3) | 0.96 |
| LEFS | −1.1 (−3.4 ~ 1.2) | 0.76 |
| Social Well-Being | 0.1 (−0.5 ~ 0.8) | 0.99 |
QOL, quality of life; PRO, patient-reported outcome; FACT-G, Functional Assessment of Cancer Therapy-General; IES, Impact of Event Scale; LEFS, Lower Extremity Functional Scale
differences in QOL/PRO scores estimated from a fitted linear mixed model adjusting for baseline score, disease sites, and assessment time when patients had leg volume change increment ≥10% from baseline.
: Padjusted = 1-(1-Punadjusted)6.
Association between GCLQ Total Score Increment ≥4 and PROs
A total of 338 patients had a GCLQ total score increment ≥4 from baseline (or increased LLE symptoms). Of these patients, 247 (73%) had a GCLQ total score increment ≥4 within 6 months from baseline, 52 (15%) within 6-12 months, 27 (8%) within 12-18 months, and 12 (4%) within 18-24 months. For patients with a GCLQ score change ≥4, the FACT-G score was 4.4 points lower (99% CI: −6.3 ~ −2.5; adjusted p<0.001), the body image subscale total score was 0.5 points lower (99% CI: −0.8 ~ −0.1; adjusted p<0.001), and the sexual and vaginal subscale total score was 0.8 points lower (99% CI: −1.3 ~ −0.3; adjusted p<0.001). LEFS score (limb function) was 7.3 points lower (99% CI: −9.6 ~ −5.0; adjusted p<0.001) compared to those who had a GCLQ score change <4 from baseline (no LLE symptoms). IES score (cancer distress) was 3.0 points higher (99% CI: 1.2 ~ 4.8; adjusted p<0.001) among those with a GCLQ score change ≥4 from baseline.
Association between a Formal LLE Diagnosis Reported on the GCLQ and PROs
A total of 114 patients reported an LLE diagnosis on the GCLQ over the 2 years following lymphadenectomy. Of those, 64 patients (56%) reported an LLE diagnosis within 6 months from baseline, 30 (26%) within 6-12 months, 11 (10%) within 12-18 months, and 9 (8%) within 18-24 months. Table 2 presents the data after adjustment for baseline score, disease sites, and assessment time. Only LEFS (limb function) was found to be significant (p<0.001) when comparing those with and without an LLE diagnosis reported on the GCLQ (Figure 2).
Figure 2. Patient-reported outcomes by lymphedema diagnosis on the Gynecologic Cancer Lymphedema Questionnaire (GCLQ).
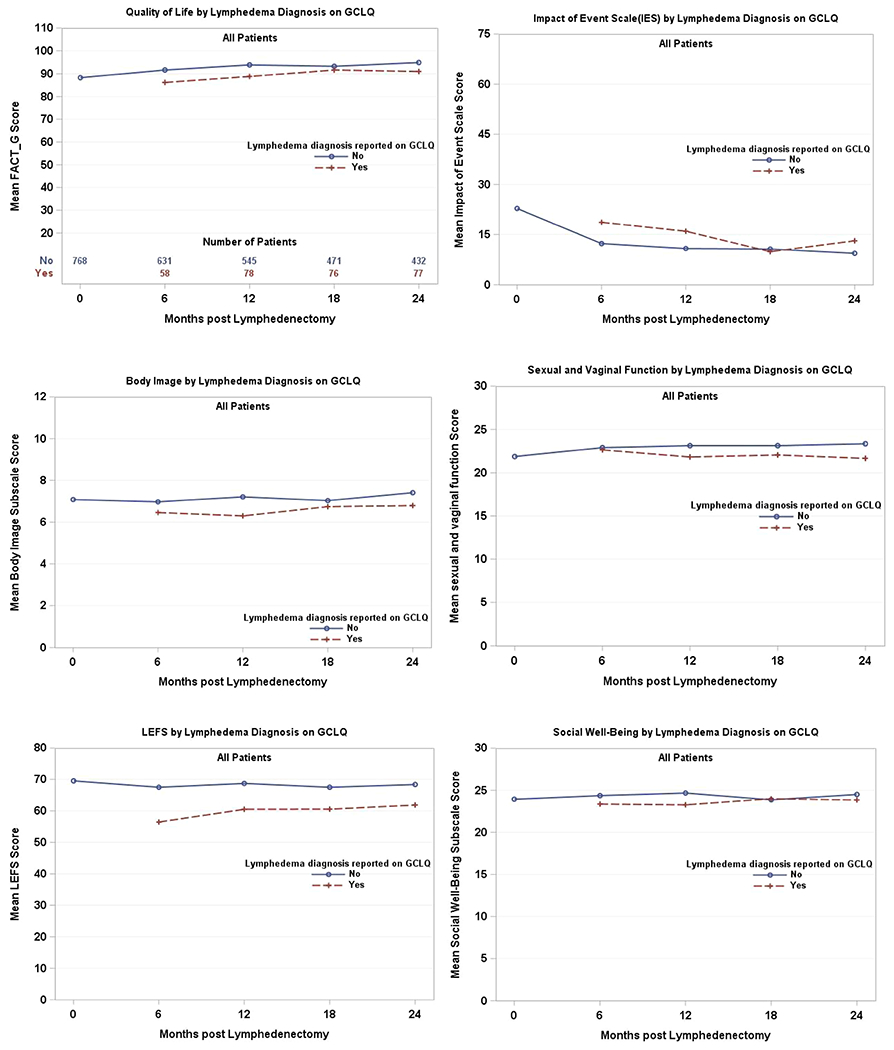
A higher score indicates better quality of life on the Functional Assessment of Cancer Therapy-General (FACT-G), better sense of body image on the subscale, and better sexual and vaginal function. A higher Impact of Event Scale (IES) score indicates more anxiety or worry.
The Association between LVC Increment ≥10% and PROs.
Within the 2 years of lymphadenectomy, 277 patients had an LVC increment ≥10%. Of these patients, 131 (47%) experienced the change within 6 months, 84 (30%) within 6-12 months, 30 (11%) within 12-18 months, and 32 (12%) within 18-24 months. After adjustment for baseline score, disease sites, and assessment time, there were no significant findings for the PROs (Table 2).
FACT Subscale Items Standardized Across All Groups.
Sexual and vaginal function, satisfaction with sexual life, and body image were compared between patients with and without a formal LLE diagnosis, in addition to patients with a GCLQ total score increment ≥4 versus <4 from baseline, and disease sites. Analyses for sexual and vaginal function items were conducted in patients reporting a sexual relationship. The differences and their 99% confidence intervals are presented in Table 3. No differences were found for vaginal and sexual function items or satisfaction with sexual life. The only significant difference was found for a body image item between those with increased LLE symptoms and those without change.
Table 3.
Analysis for the standardized supplemental Functional Assessment of Cancer Therapy (FACT) items
| Estimated difference (99% CI)* | ||
|---|---|---|
| Sexual and vaginal function items | With vs without lymphedema diagnosis | GCLQ total score increment ≥4 vs <4 |
| I am afraid to have sex | −0.19 (−0.52 ~ 0.15) | −0.11 (−0.29 ~ 0.07) |
| My vaginal feels too narrow or short | −0.14 (−0.41 ~ 0.13) | −0.09 (−0.24 ~ 0.07) |
| I am interested in sex | 0.09 (−0.21 ~ 0.38) | −0.05 (−0.26 ~ 0.16) |
| I have hot flashes | −0.09 (−0.39 ~ 0.22) | −0.11 (−0.31 ~ 0.09) |
| I have vaginal bleeding or spotting | −0.02 (−0.12 ~ 0.09) | −0.03 (−0.10 ~ 0.03) |
| I have pain or discomfort with intercourse | −0.18 (−0.49 ~ 0.13) | −0.19 (−0.40 ~ 0.02) |
| I am bothered by pain or numbness in my vulvar | −0.03 (−0.20 ~ 0.14) | −0.12 (−0.25 ~ 0.02) |
| Estimated difference (99% CI)* | ||
| With vs without lymphedema diagnosis | GCLQ total score increment ≥4 vs <4 | |
| I am satisfied with my sexual life (GS7) | −0.33 (−0.80 ~ 0.14) | −0.24 (−0.52 ~ 0.04) |
| Estimated difference (99% CI)* | ||
| Body image Item | With vs without lymphedema diagnosis | GCLQ total score increment ≥4 vs <4 |
| I like the appearance of my body | −0.08 (−0.31 ~ 0.16) | −0.1 (−0.26 ~ 0.06) |
| I feel sexually attractive | −0.06 (−0.16 ~ 0.29) | −0.08 (−0.25 ~ 0.08) |
| I am unhappy about a change in my appearance | −0.4 (−0.64 ~ −0.16) | −0.31 (−0.44 ~ −0.17) |
Differences in sexual and vaginal function item scores were estimated from a fitted linear mixed model adjusting for baseline score, disease sites, and assessment time when patients had a lymphedema diagnosis on the GCLQ or when patients had a GCLQ increment change ≥4.
difference was estimated from a fitted linear mixed model adjusting for baseline score, disease sites, and assessment time when patients had a lymphedema diagnosis on the GCLQ or when patients had a GCLQ increment change ≥4.
differences in body image item scores were estimated from a fitted linear mixed model adjusting for baseline score, disease sites, and assessment time when patients had a lymphedema diagnosis on the GCLQ or when patients had a GCLQ increment change ≥4.
GCLQ, Gynecologic Cancer Lymphedema Questionnaire
Sexual Function.
Preoperatively, 62% (n=441/717) of participants indicated being “in a sexual relationship that could involve sexual activity”. Of them, 44% (n=195) were sexually active within the past 30 days. By the 24-month assessment, for those remaining on study, 67% (n=312/469) reported being in a sexual relationship, with sexual activity rates increasing to 59% (n=185/312) (Table 4). There were no significant differences between sexual activity rates for those with LLE symptoms and those without symptoms.
Table 4.
Sexual relationships and activity rates
| PROMIS Screener Item #1 - Are you in a relationship that could include sexual activity? | |||
|---|---|---|---|
| Time point | No Sexual Relationship | Yes – Sexual Relationship | Total Sample |
| Baseline | 38% (n=276) | 62% (n=441) | 717 |
| 6 months | 35% (n=219) | 65% (n=413) | 632 |
| 12 months | 35% (n=192) | 65% (n=364) | 556 |
| 18 months | 35% (n=173) | 65% (n=321) | 494 |
| 24 months | 33% (n=157) | 67% (n=312) | 469 |
| PROMIS Screener Item #2 - In the past 30 days, have you had any type of sexual activity with another person (including your partner)? | |||
| Time point | No Sexual Activity | Yes – Sexual Activity | Total Sample |
| Baseline | 56% (n=246) | 44% (n=195) | 441 |
| 6 months | 47% (n=194) | 53% (n=219) | 413 |
| 12 months | 44% (n=159) | 56% (n=205) | 364 |
| 18 months | 41% (n=132) | 59% (n=189) | 321 |
| 24 months | 41% (n=127) | 59% (185) | 312 |
Group Differences by Disease Site.
Additional analyses were conducted to examine potential PRO outcomes differences between those with a GCLQ total score increment ≥4. Within the cervical cancer group, there was a significant difference (p<0.04) on the sexual and vaginal function items in comparison to other groups, while all other PRO/QOL outcomes differences were not significant. Within the endometrial cancer group, associations of GCLQ total score increment with FACT-En (p<0.001), IES (p<0.001), LEFS (<0.001), body image Items (p=0.015), and sexual and vaginal function items (p<0.001) were all significant in comparison to the other groups. Only FACT-SWB subscale differences were not significant. No significant differences were noted for the vulvar group for any PRO outcomes in terms of GCLQ total score increment.
By disease site, there were no significant differences for any PRO outcomes for individuals with an LVC increment ≥10% and PRO/QOL. For patients reporting a formal diagnosis of LLE (on the GCLQ), there were no significant differences for the PRO/QOL within the cervical and vulvar groups; however, within the endometrial group, the LEFS was significantly associated (p<0.001) with patient-reported LLE.
Group comparisons revealed that more patients with cervical cancer than with endometrial or vulvar cancer were sexually active within the past 30 days (49% at baseline to 61% at 24 months). The vulvar cancer group reported the lowest sexual activity rates (33% at baseline and 38% at 24 months). After adjusting for patients’ age and assessment time, more patients with endometrial cancer compared with vulvar cancer reported sexual activity (tukey adjusted p=0.012). There were no statistically significant differences between patients with cervical and endometrial cancer (tukey adjusted = 0.73) or between patients with cervical and vulvar cancer (tukey adjusted p=0.16) in terms of sexual activity.
DISCUSSION
This prospective, national, cooperative group study was able to demonstrate that symptoms of LLE were associated with poorer outcomes on PRO/QOL measures. A GCLQ total score incremental change ≥4 (increased LLE symptoms) was more informative than LVC in elucidating the impact of LLE. For this trial, LVC was determined by circumferential measurements taken by trained professionals, and an LVC ≥10% was used as a proxy for LLE. However, LVC was not found to be significant for PRO/QOL measures. The GCLQ as a PRO LLE symptom assessment efficiently distinguished between those with and without LLE [10] and assisted us in understanding what this condition truly means to women living with it. Women experiencing LLE symptoms reported lower health-related QOL, higher cancer distress (IES), and more lower limb dysfunction (LEFS), highlighting how this condition translates into everyday life for these women. For example, women with LLE symptoms (e.g., heaviness, swelling, and numbness) reported more difficulty with emotional, physical, functional and social well-being per the health-related QOL measure. These symptoms had a negative impact on daily life, specifically usual activities, walking, putting on shoes or socks, and/or getting out of the car per the LEFS. Women with LLE symptoms also experienced increased cancer distress. It is possible that LLE serves as a chronic reminder of their cancer experience, heightening distress and creating challenges in coping and adjusting during cancer survivorship. Our findings also revealed that women with LLE symptoms experienced poor body image and worse sexual/vaginal health.
This is the largest prospective trial to collect sexual partner and sexual activity status from preop through 2 years of follow-up. Prior to surgical intervention, approximately one-third of our patients reported a lack of a sexual relationship that could involve sexual activity. Of note, if we did not query preoperative sexual partner status, we could have mistakenly attributed lack of sexual activity to cancer treatment rather than lack of partner. Of those in a sexual relationship, less than half reported sexually activity at their preoperative assessment. We recognize that many women can be symptomatic from their cancer and treatment, creating challenges to sexual function; therefore, it is noteworthy that sexual activity rates increased to 59% over the duration of the study. We also examined whether there were group differences by disease site. Patients with cervical cancer reported more sexual activity than those with endometrial cancer. Patients with vulvar cancer reported the lowest level of sexual activity, which may have been associated with age. No differences in rates of sexual activity were noted for those with and without LLE symptoms.
The findings of this trial demonstrate that LLE symptoms have a significant negative impact on QOL, daily life activities, and self-image. Advancements in surgical practice to minimize risk of lymphedema, such as minimally invasive surgery and sentinel lymph node biopsy, have been successful. The next phase of research should target strategies for the treatment and management of this chronic condition. The GCLQ proved to be an effective, time-efficient clinical measure in identifying women at risk or with early LLE development. However, clinical trials are greatly needed to identify strategies to improve limb function, QOL, and adjustment in survivorship for those living with this chronic, debilitating condition.
Research Highlights.
This prospective cooperative group study demonstrated LLE symptoms were associated with poorer outcomes on PRO/QOL measures
Women with LLE symptoms reported lower health-related QOL, higher cancer distress (IES), and negative impact on daily life
Prospective trial to collect and follow sexual partner/activity status in newly diagnosed gynecologic cancer patients
Acknowledgments
This study was supported by NCI grants to NRG Oncology (U10CA180822), NRG Operations (U10CA180868) and UG1CA189867 (NCORP). Drs. Carter, Zivanovic, and Barakat are supported in part by the NIH/NCI Memorial Sloan Kettering Cancer Center support grant P30 CA008748. Dr. Nolte is supported by NCI grant to Institution R01CA162139.
The following Gynecologic Oncology institutions participated in this study: University of Oklahoma, Women’s Cancer Center of Nevada, Cancer Research for the Ozarks NCORP, University of North Carolina at Chapel Hill, Women and Infants Hospital, Memorial Sloan Kettering Cancer Center, Georgia Center for Oncology Research and Education (CORE), Metro-Minnesota CCOP, Abington Memorial Hospital, University of California at Los Angeles Health System, Ohio State University Comprehensive Cancer Center, Froedhert and the Medical College of Wisconsin, University of New Mexico, Roswell Park Comprehensive Cancer Center, University of New Mexico, Mayo Clinic, The Hospital of Central Connecticut, University of Minnesota Medical Center-Fairview, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Delaware/Christiana Care CCOP, Virginia Commonwealth University, University of Alabama at Birmingham, Hartford Hospital, Emory University School of Medicine, Washington University School of Medicine, Gynecologic Oncology of West Michigan PLLC, Lewis Cancer and Research Pavilion at St. Joseph’s/Candler, Stony Brook University Medical Center, Saint Joseph’s Hospital and Medical Center, University of Arkansas for Medical Sciences, Cancer Research Consortium of West Michigan NCORP, Michigan Cancer Research Consortium Community Clinical Oncology Program, Main Medical Center – Scarborough Campus, William Beaumont Hospital, University of Iowa Hospitals and Clinics, University of California Medical Center at Irvine-Orange Campus, Case Western Reserve University, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Baystate Medical Center, Carle Cancer Center, Mainline Health CCOP, Southeast Cancer Control Consortium CCOP, University of Texas Southwestern Medical Center, MD Anderson Cancer Center, City of Hope, Wichita CCOP and Northside Hospital, Avera Cancer Institute, Upstate Carolina CCOP, Tulane University MBCCOP, New Hanover Regional Medical Center/Zimmer Cancer Center and Cleveland Clinic Foundation.
Conflict of Interest Statement
Dr. Carter, Helen Huang, Dr. Carlson, Dr. Lockwood, Dr. Wenzel, Mr. Kauderer, Dr. Hutson, Dr. Walker, Dr. Fleury, Dr. Bonebrake, Dr. Soper, Dr. Mathews, Dr. Zivanovic, Dr. Richards, Dr. Tan, Dr. Alberts, Dr. Barakat and Dr. Wenzel report no conflicts of interest. Dr. Armer reports grant funding received from the NCI LEG study to their institution. Dr. Nolte reports salary support to institution from NCI R01CA162139 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Cemal Y, Jewell S, Albornoz CR, Pusic A, Mehrara BJ . Systematic review of quality of life and patient reported outcomes in patients with oncologic related lower extremity lymphedema. Lymphat Res Biol. 2013. March;11(1): 14–9. 10.1089/lrb.2012.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Biglia N, Zanfagnin V, Daniele A, Robba E, Bounous V. Lower body lymphedema in patients with gynecologic cancer. Anticancer Res. 2017;27:4005–4015. https://doi:10.21873/anticanres.11785. [DOI] [PubMed] [Google Scholar]
- [3].Abu-Rustum NR, Alektiar K, lasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. 2006. Gynecol Oncol. 103:714–718. https://doi:10.1016/j.ygyno.2006.03.055. [DOI] [PubMed] [Google Scholar]
- [4].Dunberger G, Lindquist H, Waldenstrom AC, Nyberg T, Steineck G and Avall-Lundquist E. Lower limb lymphedema in gynecological cancer survivors – effect on daily life functioning. Support Care Cancer. 2013;21:3063–3070. 10.1007/s00520-013-1879-3. [DOI] [PubMed] [Google Scholar]
- [5].Rowlands IJ, Beesley VL, Janda M, et al. Quality of life of women with lower limb swelling or lymphedema 3-5 years following endometrial cancer. Gynecol Oncol 2014. 133:314–318. https://doi:10.1016/j.ygyno.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [6].Leitao MM, Zhou QC, Gomez-Hidalgo NR et al. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol Oncol. 2020; 156:147–153. https//doi:10.1016/j.ygyno.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim SI, Lim MC, Lee JS, et al. Impact of lower limb lymphedema om quality of life in gynecologic cancer survivors after pelvic lymph node dissection. Eur J Obstet Gynecol Reprod Biol. 2015;192:31–36. https://doi:10.1016/j.ejogrb.2015.06.011. [DOI] [PubMed] [Google Scholar]
- [8].Watson CH, Lopez-Acevedo M, Broadwater G, et al. A pilot study of lower extremity lymphedema, lower extremity function, and quality of life in women after minimally invasive endometrial cancer staging surgery. Gynecol Oncol.2019; 153(2):399–404. https://doi:10.1016/j.ygyno.2019.02.021. [DOI] [PubMed] [Google Scholar]
- [9].Carlson JW, Kauderer J, Hutson A, et al. GOG 244-The lymphedema and gynecologic cancer (LEG) study: Incidence and risk factors in newly diagnosed patients. Gynecol Oncol. 2020;156:467–474. https://doi:10.1016/j.ygyno.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carter J, Huang HQ, Armer J, et al. GOG 244 - The LymphEdema and Gynecologic cancer (LEG) study: The association between the gynecologic cancer lymphedema questionnaire (GCLQ) and lymphedema of the lower extremity (LLE). Gynecol Oncol. 2019. December;155:452–460. https://10.1016/j.ygyno.2019.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cella D, Wiklund I, Shumaker SA, et al. Integrating health-related quality of life into crosssectional clinic trials. Qual Life Res. 1993;2:433–440. https://doi:10.1007/BF00422217. [DOI] [PubMed] [Google Scholar]
- [12].Valdimarsdottir HB, Bovbjerg DH, Kash KM, et al. Psychological distress in women with a familial risk of breast cancer. Psycho-Oncology. 1995;4:133–141. https://doi:10.1002/pon.2960040207. [Google Scholar]
- [13].Lesko LM, Ostroff JS, Mumma GH, et al. Long-term psychological adjustment of acute leukemia survivors: impact of bone marrow transplantation versus conventional chemotherapy. Psychosom Med. 1992;54:30–47. https://doi:10.1097/00006842-199201000-00006. [DOI] [PubMed] [Google Scholar]
- [14].Cordova MJ, Andrykowski MA, Kenady DE, et al. Frequency and correlates of posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. J Consult Clin Psychol. 1995;63:981–986. https://doi:10.1037//0022-006x.63.6.981. [DOI] [PubMed] [Google Scholar]
- [15].Cella DF, Tross S. Psychological adjustment to survival from Hodgkin’s disease. J Consult Clin Psychol. 1986;54:616–622. https://doi:10.1037//0022-006x.54.5.616. [DOI] [PubMed] [Google Scholar]
- [16].Passik SD, Newman M, Brennan M, et al. Predictors of psychological distress, sexual dysfunction and physical functioning among women with upper-extremity lymphedema related to breast cancer. Psycho-oncology. 1995;4:255–263. 10.1002/pon.2960040402. [DOI] [Google Scholar]
- [17].Binkley JM, Stratford PW, Lott SA, et al. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79:371–383. [PubMed] [Google Scholar]
- [18].Watson CJ, Propps M, Ratner J, et al. Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35:136–146. https://doi:10.2519/jospt.2005.35.3.136. [DOI] [PubMed] [Google Scholar]
- [19].Carter J, Raviv L, Appollo K, Baser RE, Iasonos A, Barakat RR. A Pilot Study Using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a Clinical Care Tool to Identify Lower Extremity Lymphedema in Gynecologic Cancer Survivors. Gynecol Oncol. 2010;117:317– 23. https://doi:10.1016/j.ygyno.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sidak ZK (1967. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62: 626–633. https://doi:10.2307/2283989. [Google Scholar]


