Abstract
Microglia, the immune cells of the brain, have a canonical role in regulating responses to neurological disease or injury, but have also recently been implicated as regulators of neurophysiological processes such as learning and memory. Given these dual immune and physiological roles, microglia are a likely mechanism by which external toxic stimuli are converted into deficits in neuronal circuitry and subsequently function. However, while it is well established that exposure to environmental toxicants negatively affects the peripheral immune system, it remains unknown whether and how such exposure causes neuroinflammation which, in turn, may negatively impact microglial functions in vivo. Here, we examined how acute 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in adulthood, which negatively impacts immune cells in the periphery, affects microglial characteristics in the cortex of the mouse. We found that microglia density, distribution, morphology, inflammatory signaling, and response to a secondary, pathological activation were unaffected by acute TCDD exposure. These results suggest that acute, peripheral TCDD exposure in adulthood is not sufficient to induce an overt inflammatory phenotype in cortical microglia.
Keywords: Dioxin, Aryl hydrocarbon receptor, Microglia, Neurotoxicity, Visual cortex
1. Introduction
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is an environmental pollutant that is primarily generated through industrial processes. It is highly toxic and bioaccumulates up the food chain, where it persists in humans with a half-life of up to 10 years. Despite precautions to limit human exposure, the pervasive nature of TCDD ensures that every person experiences some level of background exposure [1]. Once exposed, TCDD causes pathological changes in the function of multiple organ systems, including the brain [2]. TCDD toxicity is mediated by binding to the aryl hydrocarbon receptor (AhR), for which it is the strongest agonist [3]. AhR is a ubiquitously expressed ligand-activated transcription factor which monitors environmental cues and sends physiological signals that permit normal cellular function by regulating a large number of intracellular processes [4,5]. Subsequently, when TCDD activates this receptor, a cascade of negative responses can be induced, including increased expression of metabolic enzymes and inflammatory factors [6,7].
While TCDD is known to be neurotoxic [8,9], the cellular pathways through which TCDD and other dioxins affect brain function are still unclear. Several lines of evidence suggest that disruption of immune cell function may be a mechanism through which TCDD enacts its neurotoxic effects. In the peripheral immune system, TCDD negatively impacts both the development and function of various peripheral immune cell types. Exposure to TCDD results in dysregulated cellular differentiation, altered antigen presentation, and increased production of inflammatory cytokines, as well as priming a defective response to an immune challenge [10–14]. The action of TCDD on peripheral immune cells is mediated by the AhR receptor, as the immunosuppressive effects of TCDD exposure are absent in AhR knockout mice [15] and activation of AhR by TCDD has been demonstrated to alter the inflammatory response in macrophages [12,16]. Given similarities between peripheral immune cells and microglia, the immune cells of the brain, it is possible that microglia function is similarly negatively affected by TCDD exposure. The AhR receptor is also expressed in microglia [17], giving microglia and peripheral immune cells a common mechanism of TCDD vulnerability. Supporting this, in vitro studies show that the microglial immune response is reduced by activation of AhR by its agonists [17] and microglial exposure to TCDD results in the release of several neurotoxic inflammatory signals [18,19]. Therefore, it is possible that TCDD’s neurotoxic effect is a result of aberrant microglial activation and subsequent negative impacts on neuronal remodeling and survival.
To determine whether microglia are negatively impacted by environmental TCDD exposure in a way that could underlie the neurotoxic effects of TCDD, we examined the effects of acute TCDD exposure on microglial response profiles, which are known to change with inflammatory state [20]. We found that acute TCDD exposure in adulthood did not alter microglial density, distribution, morphology, expression of cytokines, or response to a secondary, pathological insult in the form of lipopolysaccharide (LPS) administration. This demonstrates that neither microglial baseline characteristics nor their inflammatory response are overtly altered by a TCDD dose known to alter peripheral immune function. Our findings indicate that environmental TCDD exposure has differential impacts on the function of the central and peripheral immune systems.
2. Materials and methods
2.1. Animals
Experimental protocols were carried out in strict accordance with the University of Rochester Committee on Animal Resources (UCAR) and conformed to the National Institute of Health’s “Guide for the Care and Use of Laboratory Animals, 8th Edition, 2011." All experiments were performed on C57Bl/6 J mice (Jackson Labs) exposed to a standard light cycle of 12 h of light and 12 h of dark (6AM lights on). Chow and water were provided ad libitum.
2.2. TCDD exposure
The sample size for quantification of microglia density, distribution, and morphology consisted of 32 mice divided into four experimental groups. Each group contained at least 6 mice and at least two mice of each sex between the ages of P60 and P142. Mice were first given a direct dose of 10 μg/kg of TCDD or olive oil as a control via oral gavage [10,21]. This exposure paradigm was chosen because it has been demonstrated to alter peripheral immune cell responses [22] and because TCDD administered via this dose [23] and administration route [24] has been demonstrated to cross the blood brain barrier (BBB) in mice. Twenty-four hours later the mice received a challenge of 0.75 mg/kg of LPS or saline through intraperitoneal (IP) injection. A moderate LPS dose has been demonstrated to induce a shift in the microglial transcriptome towards an inflammatory profile without approaching septic levels [25]. A second cohort of mice was generated for RT-qPCR experiments. Sample size consisted of 24 mice divided into four experimental groups, each containing 3 males and 3 females between the ages of P120-P130. Mice were first given a direct dose of 10 μg/kg of TCDD or olive oil via oral gavage. Twenty-four hours later the mice received a challenge of 4 mg/kg of LPS or saline (IP). While moderate doses of LPS have been shown to induce pro-inflammatory shifts in the microglial transcriptome [25], a higher dose of LPS was used in this cohort to ensure the microglia pro-inflammatory response was sufficiently robust to detect priming or suppression resulting from TCDD exposure. Forty-eight hours after LPS administration, brains were harvested and flash frozen for RT-qPCR.
2.3. Histology
For quantification of microglia density, distribution, and morphology, brains were harvested 24 h after mice received IP injection of LPS or saline, as alterations in the microglial transcriptome and increased expression of microglial pro-inflammatory markers can be detected after 24 h [25,26]. All mice were first deeply anesthetized using Euthasol, perfused intracardially with 0.1 M PBS with heparin and 4% paraformaldehyde. Brains were removed and coronal sections including primary visual cortex (V1) were taken at 50 μm thickness using a standard freezing microtome.
Sections were processed free-floating at room temperature (RT), except where noted. Briefly, sections were rinsed in 0.1 M PBS, incubated in a 10 mM sodium citrate buffer at 80 °C for 30 min, and brought to RT before rinsing in 0.1 M PBS. Sections were then incubated in a peroxidase block (10 % Methanol, 30 % Hydrogen Peroxide) for 20 min, rinsed in 0.1 PBS, and transferred to an additional blocking solution (0.2 % Triton-X, 5% Bovine Serum Albumin) for one hour. After this block, sections were incubated for 24 h in a humidified chamber at 4 °C in primary antibody (1:2500 rabbit polyclonal anti-Iba-1, Wako Pure Chemical Industries Inc. #019–19741; 0.5 % Bovine Serum Albumin). Sections were allowed to acclimate to RT for 30 min and subsequently incubated for 4 h in secondary antibody (1:500 donkey anti-rabbit Alexa Fluor 488, Invitrogen, Catalogue# A21206; 0.5 % BSA). Sections were mounted onto slides and fluorescence preserved with Prolong Gold Mounting Media (Invitrogen; #P36934).
2.4. Image analysis
Following histological processing, V1 sections (3 per animal) were imaged using a standard confocal microscope (Zeiss LSM 510 META) at 20× and 40× (objective magnification) at a digital scan resolution of 1024 by 1024 pixels. Image analysis was performed offline in ImageJ (freeware, NIH) on max intensity z-projected stacks.
Microglial Density: V1 was identified using stereotactic coordinates (Paxinos, Elsevier). Each microglial cell body was identified, and the number of microglia and their X,Y positions were recorded. Cell density was calculated as number of microglia/mm2. Data points represent average cellular density per animal across three sections.
Microglial Spacing: X,Y positions obtained in ImageJ were analyzed using a custom algorithm implemented in Matlab (Mathworks) in order to determine the average distance between each microglia and its nearest neighbor (nearest neighbor distance). A spacing index was generated as: (average nearest neighbor distance)2* microglial density. Data points represent average spacing per animal across three sections.
Microglial Morphology: Sholl Analysis was conducted with an automated ImageJ Sholl analysis plugin (kindly provided by the Anirvan Ghosh Laboratory, UCSD) to assay microglial process arbor complexity by quantifying the number of microglial processes at increasing distances from the soma. For quantitative analysis between groups, the maximum number of intersections was calculated, along with the full width at half maximum (estimated by interpolating the data points on the Sholl graphs for each animal).
2.5. Gene expression analyses
RNA was extracted using the RNeasy Mini Plus kit (Qiagen) according to the manufacturer’s instructions. RNA was quantified using the Nano Drop spectrophotometer. 1 μg of RNA was reverse transcribed to cDNA using Superscript IV reverse transcriptase and random hexamers (Invitrogen). To test that RNA was free of genomic DNA contamination, a control without reverse transcriptase was included.
Real-time quantitative polymerase chain reaction (RT-qPCR) analysis was conducted using an Applied Biosystems QuantStudio 3 Real-Time PCR system with the following reaction set-up: 5 μL of Power SYBR Green PCR Master Mix (Applied Biosystems), 0.08 μL of reverse and forward primers each (20 μM), 3.84 μL of RNase-free water, and 1 μL of cDNA (5 ng). A water-only control was also included in each reaction. The qPCR cycling conditions were: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Optimization of primer concentrations and primer efficiencies was carried out prior to the commencement of quantification experiments. Samples were set up in duplicate and analyzed using QuantStudio design and analysis software (Applied Biosystems). Melt curve analysis was also performed on all samples. Calculations used the comparative CT (ΔΔCT) method (Livak and Schmittgen 2001 Methods) and measurements were normalized to the expression of two reference genes, Gapdh and β-actin. Primer 3 software was used to design primers and sequences are listed in Table 1.
Table 1.
List of primer sequences used for QPCR.
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | 5′-CCTCTATGCCAACACAGTGC-3′ | 5′-CCTGCTTGCTGATCCACATC-3′ |
| Gapdh | 5′-CAACTCCCACTCTTCCACCT-3′ | 5′-GAGTTGGGATAGGGCCTCTC-3′ |
| Il-1β | 5′-CAACAGAGAGCAACCAGAGC-3′ | 5′-TCATCTTTTGGGGTCCGTCA-3′ |
| Tnf-α | 5′-GACCCCTTTACTCTGACCCC-3′ | 5′-AGGCTCCAGTGAATTCGGAA-3′ |
| Il-6 | 5′-TACCACTCCCAACAGACCTG-3′ | 5′-ACTCCAGAAGACCAGAGGAA-3′ |
| Cyp1a1 | 5′-TTTGGAGCTGGGTTTGACAC-3′ | 5′-CTGCCAATCACTGTGTCTA-3′ |
| Ahrr | 5′-GAGGCCAGGTCCCAGAGATGAGAGA-3′ | 5′-GGGGCGCAGAAGATCGGGCG-3′ |
2.6. Statistical analysis
Statistical analysis was conducted using the GraphPad Prism statistical software. All data is shown as the mean ± standard error of the mean (SEM) and significance was determined using α = 0.05. Two-way or three-way ANOVAs were used to determine significance with Bonferroni post hoc tests.
3. Results
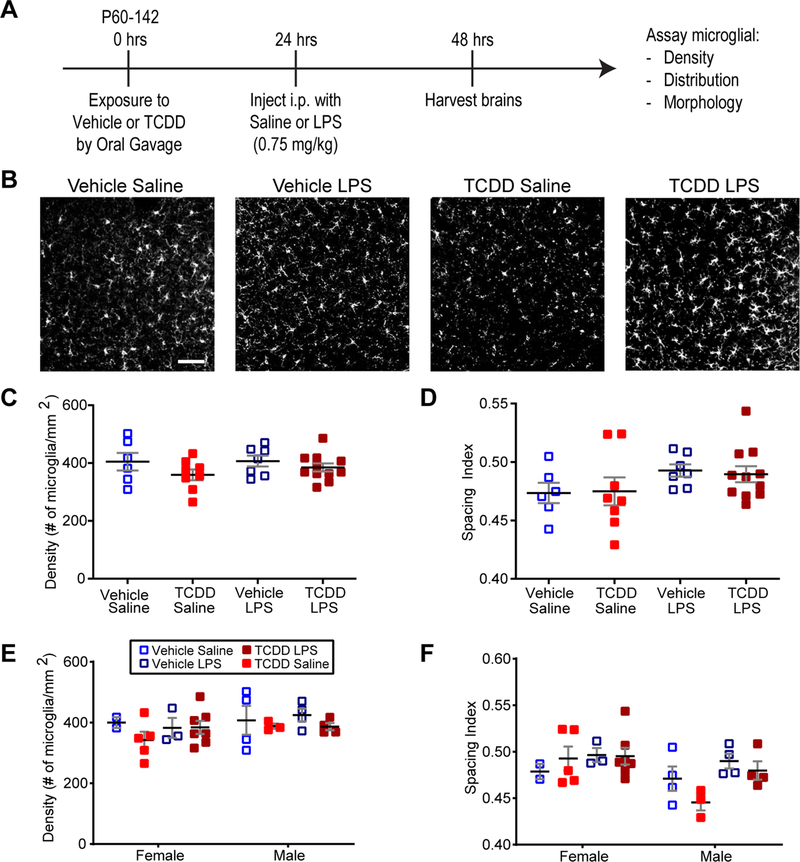
Acute exposure to TCDD has been demonstrated to have a negative effect on the peripheral immune system [22], priming a defective response to an immune challenge [10]. To examine whether similar effects occur in the brain, we examined baseline microglial characteristics after we exposed adult mice to vehicle or TCDD (10 μg/kg) through oral gavage, an exposure paradigm that has been demonstrated to be immunosuppressive in the peripheral immune system [22]. Additionally, to determine whether TCDD dysregulated microglial responses to insult as it does peripheral immune responses [10,11], we treated the same mice with saline or LPS (0.75 mg/kg, IP) 24 h later, and harvested brains 24 h after the last treatment (Fig. 1A). Fixed sections were immunostained against the microglia-specific marker Iba1 and Iba1 positive microglia in primary visual cortex were assayed. Because the microglial response to pathological insult typically involves cell proliferation and clustering at the site of injury [27], we assayed both microglia density and spacing. Microglia appeared evenly distributed across the cortical region in all groups, and we found no significant effect of acute TCDD exposure or subsequent LPS treatment on the density or spacing of microglia (Fig. 1B–D). Additionally, given known sex-specific differences in microglial behavior both at baseline and in response to a pathological insult, we looked for sex-specific differences in density and spacing. We found no sex-specific treatment-induced differences in microglial response from either treatment, although there was a significant main effect of sex on microglial clustering but not density (Fig. 1B, E–F, three-way ANOVA, p = 0.0287, Bonferroni post hoc test, p > 0.05). It is important to note that our experiments were not powered to detect sex-specific differences, and these results are preliminary and will need to be replicated with a larger sample size. However, the study suggests that acute TCDD exposure does not cause a pathological proliferation of cortical microglia, nor does it prime them to respond adversely to a secondary insult as it does peripheral immune cells.
Fig. 1.
Acute TCDD exposure does not affect microglia density and distribution, alone or in combination with LPS exposure. (A) Timeline of acute exposure to TCDD and LPS. (B) Representative images showing Iba1+ microglia in binocular primary visual cortex of C57Bl/6 J mice acutely exposed to vehicle or TCDD followed by acute exposure to saline or LPS. Scale bar =50 μm. (C,D) There is no significant difference in microglia density or distribution in binocular primary visual cortex across treatment groups. (E,F) There is no sex-specific effect of either treatment on microglial density or distribution across treatment groups, although there is a significant main effect of sex on microglial distribution (p > 0.05).
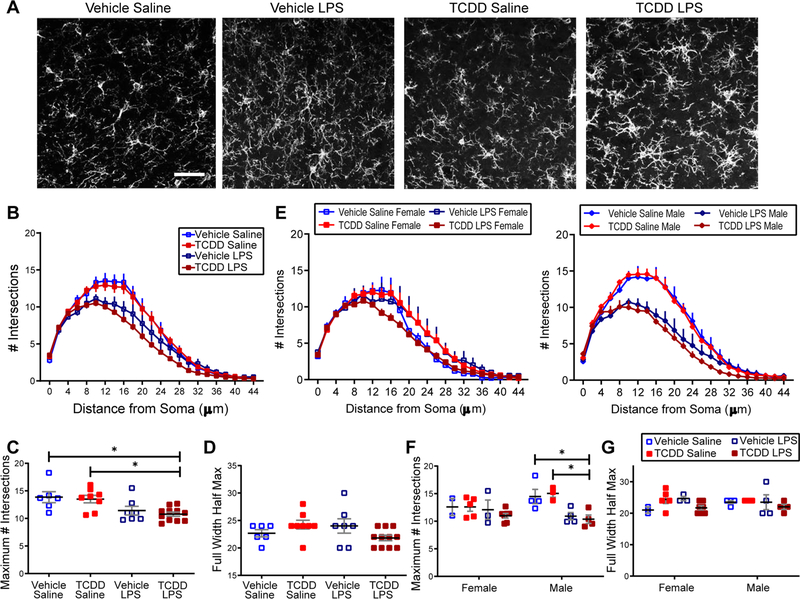
While the lack of microglial proliferation and clustering indicates a lack of large scale TCDD-induced microglial activation, microglia activation exists on a spectrum and subtler morphological alterations can indicate a change in the state of microglial function. To determine whether acute TCDD exposure alone or with an LPS “second hit” impacted microglial morphology, we used Sholl analysis to assay microglial arbor complexity in either treatment alone or in combination (Fig. 2A). We found that compared to vehicle-treated controls, adult mice with acute exposure to TCDD exhibit no significant differences in microglial process arbor complexity as assayed by comparing the height and width of the Sholl distributions (Fig. 2B,C,D). When comparing conditions combined with a “second hit” LPS treatment, we found an expected significant main effect of treatment with LPS, but no effect of TCDD on process ramification. While there is a significant reduction in the maximum number of intersections in the TCDD and LPS combined condition when compared to non-LPS exposed mice, this group is not significantly different than the vehicle and LPS combined condition, demonstrating the effect of LPS rather than TCDD on microglial morphology (Fig. 2B,C, two-way ANOVA, TCDD: p = 0.4635, LPS: p = 0.0007; Bonferroni post hoc test, p < 0.05). The full width at half maximum of the Sholl distribution remains similar across all groups (Fig. 2B, D). When the data was broken down by sex, the effect of LPS on process ramification was specific to males, with no sex-specific effect of TCDD (Fig. 2E,F,G, three-way ANOVA, TCDD: p = 0.6873, LPS: p = 0.0008, Sex * LPS: p = 0.0321, Bonferroni post hoc test, p < 0.05), demonstrating a sexually dimorphic microglial response to LPS which is not additionally affected by TCDD. These results suggest that acute TCDD exposure in adulthood does not cause overt changes in microglia morphological characteristics or inflammatory response.
Fig. 2.
Acute TCDD exposure does not affect microglial baseline morphology, alone or in combination with LPS exposure. (A) Representative images of Iba1+ microglia in binocular primary visual cortex in C57Bl/6 J mice acutely exposed to vehicle or TCDD followed 24 h later by saline or LPS (0.75 mg/kg) treatment. Scale bar =20 μm. (B) Both vehicle and TCDD acute exposure groups appear to have less complex microglial process arbors after LPS treatment. (C) Microglia process ramification is reduced by LPS treatment. Microglia exposed to TCDD and LPS in combination had significantly fewer maximum process intersections than those exposed to vehicle or TCDD alone, but did not differ significantly from the LPS control condition (n = 4–6 per group, *p < 0.05) (D) Microglia exhibit no difference in the width of the Sholl distribution across conditions. (E,F,G) Process arbor complexity is affected by LPS treatment only in males, as the height but not width of the Sholl distribution is decreased after combined TCDD and LPS exposure while not differing from LPS controls (n = 2–5 per group, *p < 0.05).
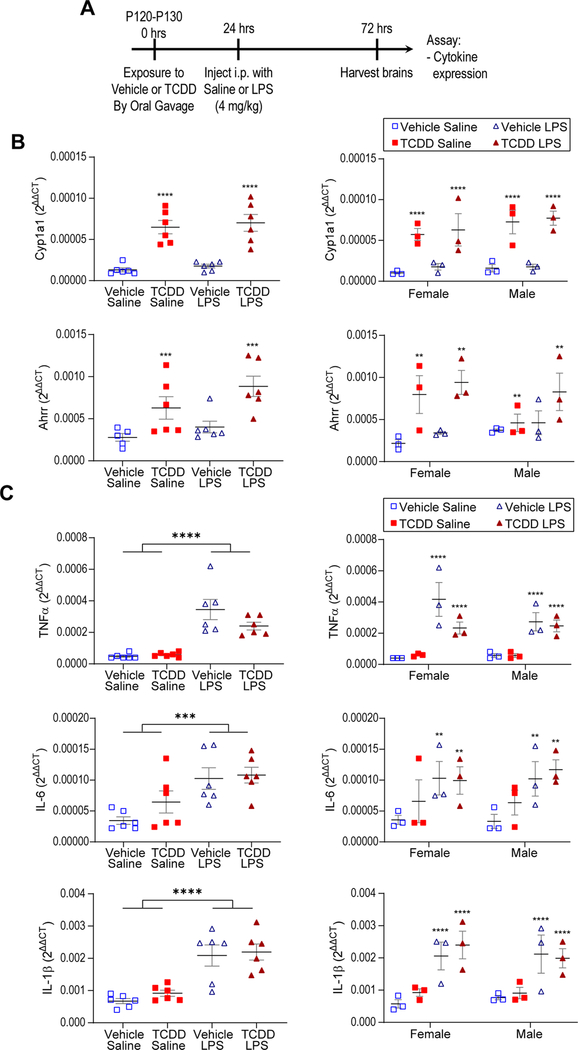
To ensure that TCDD exposure caused AhR activation in the brain, we performed RT-qPCR to assay expression of the AhR downstream target genes Cyp1A1 and Ahrr. We found a significant increase in both Cyp1A1 and Ahrr across sex in both TCDD conditions, with no effect from LPS alone (note that a larger dose of 4 mg/kg was used in this cohort) and no interaction between TCDD and LPS (Fig. 3A,B, two-way ANOVA, Cyp1A1: TCDD: p < 0.0001, LPS: p = 0.4785; Ahrr: TCDD: p < 0.0005, LPS: p = 0.0814), demonstrating that TCDD successfully activates AhR in this paradigm. To assay the molecular impact of TCDD exposure on microglial inflammatory state, we performed RT-qPCR for the pro-inflammatory cytokines TNF-α, IL-6, and IL-1β. We found LPS induced a significant increase in all three cytokines across sex (Fig. 3C, two-way ANOVA, TNF-α: p < 0.0001; IL-6: p = 0.0009' IL-1β: p < 0.0001), while there was no effect of TCDD either alone or in combination with LPS (Fig. 3B), demonstrating that acute, adult TCDD exposure neither induces nor primes or suppresses the microglial inflammatory response. These results confirm at the molecular level our findings that TCDD exposure in adulthood does not cause overt changes in the microglial inflammatory response.
Fig. 3.
Acute TCDD exposure does not affect cytokine expression, alone or in combination with LPS exposure. (A) Timeline of acute exposure to TCDD and LPS. (B) RT-qPCR shows upregulation of AhR downstream target genes Cyp1A1 and Ahrr following TCDD exposure with no effect of sex (n = 6 per group, ****p < 0.0001, ***p < 0.0005, **p < 0.005). (C) RT-qPCR demonstrates LPS-induced upregulation of TNF-α, IL-6, and IL-1β but no effect of TCDD exposure or sex (****p < 0.0001; IL-6: ***p < 0.001, **p < 0.005). Graphs show individual data points as well as mean ± SEM.
4. Discussion and conclusions
While the impact of TCDD on peripheral immune cells has been studied, it is still unknown how TCDD affects CNS immune cells. With their newly established dual role as mediators of both neuropathology and neurocognition [28], microglia are a likely substrate by which external toxic stimuli are converted into deficits in neuronal circuitry and, subsequently, function. In this set of experiments, we asked whether acute exposure to TCDD negatively impacts microglial baseline characteristics and functional response to stimuli. Our results suggest that acute exposure to TCDD in adulthood is not sufficient to cause overt changes in microglial density, distribution or morphology indicative of pathological activation, nor does it induce cytokine expression or prime microglia to have an altered inflammatory response to a secondary insult. Given this contrast between the impact of acute TCDD exposure in the peripheral and central immune systems, our findings suggest that mature CNS immune cells may indeed enjoy the benefit of a privileged immune environment which buffers the impact of external toxic stimuli such as TCDD.
4.1. Impact of acute TCDD exposure on microglia as compared to peripheral immune cells
While in the periphery acute TCDD exposure induces both direct effects on peripheral immune cell development [8] and indirect effects on peripheral immune cell response to pathogens [29], we observed neither direct nor indirect effects on microglia in this study, despite the fact that the exposure paradigm activated AhR target genes in the brain. This suggests a disparate susceptibility to TCDD insult of the central vs. peripheral immune system. The lack of an acute, adult TCDD exposure effect on microglia characteristics and function could be a result of the immune-privileged status of the brain. Although adult microglia express AhR [17] and TCDD crosses the BBB [30], the efficiency of TCDD penetration into the brain is low [30] and as a result microglia in the adult brain may be buffered from exposure to the levels of TCDD seen by peripheral cells or may be intrinsically less sensitive to such exposure.
While our results suggest a contrast between peripheral and central immune cell susceptibility to outside insult once established as a mature immune system, these two systems may be similar in that the level of susceptibility is dependent on age of exposure. While there was no overt defect in microglia as a result of exposure in adulthood, a time point when microglia are established within the confines of the BBB, they may be more susceptible earlier on before this protected environment has been established [31–34]. There is increasing evidence that microglia undergo a critical period of development during and shortly after gestation, prior to microglial migration into the brain and BBB formation [35,36]. The alignment of this critical developmental period and the absence of the protective environment of the BBB renders them especially vulnerable to outside insult. This concept, combined with the demonstrated absence of TCDD-induced microglial defect in adulthood, is consistent with peripheral immune literature suggesting that gestational TCDD exposure is more toxic to peripheral immune cells than adult TCDD exposure [22]. Disruption of the standard microglial developmental program during early life could lead to long term alterations in function later in life. This is supported by recent research demonstrating that microglial development is in fact susceptible to environmental perturbations during gestation, resulting in altered microglial transcriptomes postnatally [36].
4.2. Sex-dependent effects of TCDD exposure on microglial behavior
While sex-dependent effects in microglia are only beginning to be revealed, recent research suggests that microglia respond differently to environmental insults in male vs. female animals [37]. Our results confirm this, as we found a sexually dimorphic microglial response to moderate-dose LPS treatment in adult mice which normalized with higher dose LPS exposure, although the power of this analysis is limited by sample size. However, the male-specific sensitivity to lower levels of LPS aligns with recent research demonstrating that microglia from male adult mice have a more inflammatory phenotype in response to LPS treatment while those from female mice have a more protective phenotype [38]. While sex-dependent effects on the toxicity phenotype of TCDD have also been demonstrated [39], we did not find evidence of sex-specific effects of TCDD exposure on microglia in regards to the baseline characteristics and inflammatory insults assayed here. This is not surprising as sex-dependent differences in the effects of TCDD exposure vary based on many factors such as organ or brain region, model organism, age, and time. These factors also influence the heterogeneity of microglia phenotypes, leading to similar variability in sex-dependent differences in their susceptibility to toxicants such as TCDD [36,40]. Our findings underscore the importance of additional research to elucidate critical periods of microglial developmental and sexual differentiation that would clarify windows of susceptibility to environmental insults and identify candidate periods of therapeutic intervention.
4.3. Conclusions and future outlook
Our findings are encouraging as they suggest that several critical baseline characteristics of microglial pathological and physiological functions are not drastically altered by peripheral exposure to TCDD. However, while we did not see overt microglial responses or priming after acute exposure in adulthood, subtler or potentially region-specific TCDD-induced microglial deficits and their impacts on cognition need to be examined as do more prolonged exposures to TCDD. More so, while microglia appear protected from the negative impact of peripheral exposure to environmental toxins once established within the confines of the BBB, it will be critical to examine how microglia may be harmed by exposure to such insults during gestation and postnatal periods as they are navigating the critical period of microglial development.
Acknowledgements
We thank the Arnivan Ghosh Laboratory for the Sholl analysis ImageJ plug-in, Paige Lawrence for critical experimental guidance, and Evelyn Matei and MaKenna Cealie for experimental assistance.
Funding
This work was supported by the National Institutes of Health (NIH) grant EY019277, NS114480, NS099973, AA027111 (AKM) and NRSA institutional training grantT32 ES007026 (RLL), the National Science Foundation grant NSF 1557971 (AKM), as well as a pilot project from the core center grant P30ES001247 (AKM) from the National Institute of Environmental Health Sciences. The work also used the technical core facilities supported by P30EY001319. These sponsors had no involvement beyond providing study funding.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- [1].World Health Organization, World Health Organization, vol. 2018, 2016. [Google Scholar]
- [2].Institute of Medicine, Dioxins and Dioxin-like Compounds in the Food Supply: Strategies to Decrease Exposure (Washington (DC), 2003. [PubMed] [Google Scholar]
- [3].Poland A, Knutson JC, 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity, Annu. Rev. Pharmacol. Toxicol. 22 (1982) 517–554. [DOI] [PubMed] [Google Scholar]
- [4].Fallarino F, Romani L, Puccetti P, AhR: far more than an environmental sensor, Cell Cycle 13 (2014) 2645–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gu YZ, Hogenesch JB, Bradfield CA, The PAS superfamily: sensors of environmental and developmental signals, Annu. Rev. Pharmacol. Toxicol. 40 (2000) 519–561. [DOI] [PubMed] [Google Scholar]
- [6].Lai ZW, Pineau T, Esser C, Identification of dioxin-responsive elements (DREs) in the 5’ regions of putative dioxin-inducible genes, Chem. Biol. Interact. 100 (1996) 97–112. [DOI] [PubMed] [Google Scholar]
- [7].Tan Z, Chang X, Puga A, Xia Y, Activation of mitogen-activated protein kinases (MAPKs) by aromatic hydrocarbons: role in the regulation of aryl hydrocarbon receptor (AHR) function, Biochem. Pharmacol. 64 (2002) 771–780. [DOI] [PubMed] [Google Scholar]
- [8].Mandal PK, Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology, J. Comp. Physiol. B 175 (2005) 221–230. [DOI] [PubMed] [Google Scholar]
- [9].Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N, Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age, J. Pediatr. 140 (2002) 48–56. [DOI] [PubMed] [Google Scholar]
- [10].Mitchell KA, Lawrence BP, Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) renders influenza virus-specific CD8+ T cells hyporesponsive to antigen, Toxicol. Sci. 74 (2003) 74–84. [DOI] [PubMed] [Google Scholar]
- [11].Warren TK, Mitchell KA, Lawrence BP, Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung, Toxicol. Sci. 56 (2000) 114–123. [DOI] [PubMed] [Google Scholar]
- [12].Gutierrez-Vazquez C, Quintana FJ, Regulation of the immune response by the aryl hydrocarbon receptor, Immunity 48 (2018) 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marshall NB, Kerkvliet NI, Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells, Ann. N. Y. Acad. Sci. 1183 (2010) 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vorderstrasse BA, Kerkvliet NI, 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects the number and function of murine splenic dendritic cells and their expression of accessory molecules, Toxicol. Appl. Pharmacol. 171 (2001) 117–125. [DOI] [PubMed] [Google Scholar]
- [15].Vorderstrasse BA, Steppan LB, Silverstone AE, Kerkvliet NI, Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression, Toxicol. Appl. Pharmacol. 171 (2001) 157–164. [DOI] [PubMed] [Google Scholar]
- [16].Cheon H, et al. , Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced TNF-alpha production in differentiated THP-1 human macrophages, Exp. Mol. Med. 39 (2007) 524–534. [DOI] [PubMed] [Google Scholar]
- [17].Lee YH, et al. , Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia, Glia 63 (2015) 1138–1154. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, et al. , 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces microglial nitric oxide production and subsequent rat primary cortical neuron apoptosis through p38/JNK MAPK pathway, Toxicology 312 (2013) 132–141. [DOI] [PubMed] [Google Scholar]
- [19].Xu G, et al. , 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced inflammatory activation is mediated by intracellular free calcium in microglial cells, Toxicology 308 (2013) 158–167. [DOI] [PubMed] [Google Scholar]
- [20].Kettenmann H, Hanisch UK, Noda M, Verkhratsky A, Physiology of microglia, Physiol. Rev. 91 (2011) 461–553. [DOI] [PubMed] [Google Scholar]
- [21].Pegram RA, Diliberto JJ, Moore TC, Gao P, Birnbaum LS, 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) distribution and cytochrome P4501A induction in young adult and senescent male mice, Toxicol. Lett. 76 (1995) 119–126. [DOI] [PubMed] [Google Scholar]
- [22].Kerkvliet NI, Immunological effects of chlorinated dibenzo-p-dioxins, Environ. Health Perspect. 103 (Suppl 9) (1995) 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gasiewicz TA, Geiger LE, Rucci G, Neal RA, Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice, Drug Metab. Dispos. 11 (1983) 397–403. [PubMed] [Google Scholar]
- [24].Huang P, et al. , TCDD-induced expression of Ah receptor responsive genes in the pituitary and brain of cellular retinol-binding protein (CRBP-I) knockout mice, Toxicol. Appl. Pharmacol. 192 (2003) 262–274. [DOI] [PubMed] [Google Scholar]
- [25].Gyoneva S, et al. , Cx3cr1-deficient microglia exhibit a premature aging transcriptome, Life Sci. Alliance 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Corona AW, et al. , Fractalkine receptor (CX(3)CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide, J. Neuroinflamm. 7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hoogland ICM, et al. , Microglial activation after systemic stimulation with lipopolysaccharide and Escherichia coli, Front. Cell. Neurosci. 12 (2018) 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li Q, Barres BA, Microglia and macrophages in brain homeostasis and disease, Nat. Rev. Immunol. 18 (2018) 225–242. [DOI] [PubMed] [Google Scholar]
- [29].Boule LA, Burke CG, Jin GB, Lawrence BP, Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome, Sci. Rep. 8 (1826) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pohjanvirta R, Vartiainen T, Uusi-Rauva A, Monkkonen J, Tuomisto J, Tissue distribution, metabolism, and excretion of 14C-TCDD in a TCDD-susceptible and a TCDD-resistant rat strain, Pharmacol. Toxicol. 66 (1990) 93–100. [DOI] [PubMed] [Google Scholar]
- [31].Alliot F, Godin I, Pessac B, Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain, Brain Res. Dev. Brain Res. 117 (1999) 145–152. [DOI] [PubMed] [Google Scholar]
- [32].Ben-Zvi A, et al. , Mfsd2a is critical for the formation and function of the blood-brain barrier, Nature 509 (2014) 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ginhoux F, et al. , Fate mapping analysis reveals that adult microglia derive from primitive macrophages, Science 330 (2010) 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schulz C, et al. , A lineage of myeloid cells independent of Myb and hematopoietic stem cells, Science 336 (2012) 86–90. [DOI] [PubMed] [Google Scholar]
- [35].Matcovitch-Natan O, et al. , Microglia development follows a stepwise program to regulate brain homeostasis, Science 353 (2016) aad8670. [DOI] [PubMed] [Google Scholar]
- [36].Thion MS, et al. , Microbiome influences prenatal and adult microglia in a sex-specific manner, Cell 172 (2018), 500–516 e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bolton JL, et al. , Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner, Front. Synaptic Neurosci. 9 (2017) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Villa A, et al. , Sex-specific features of microglia from adult mice, Cell Rep. 23 (2018) 3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pohjanvirta R, Miettinen H, Sankari S, Hegde N, Linden J, Unexpected gender difference in sensitivity to the acute toxicity of dioxin in mice, Toxicol. Appl. Pharmacol. 262 (2012) 167–176. [DOI] [PubMed] [Google Scholar]
- [40].Prokopec SD, Watson JD, Lee J, Pohjanvirta R, Boutros PC, Sex-related differences in murine hepatic transcriptional and proteomic responses to TCDD, Toxicol. Appl. Pharmacol. 284 (2015) 188–196. [DOI] [PubMed] [Google Scholar]