Abstract
The lower airways (larynx to alveoli) are protected by a complex array of neural networks that regulate respiration and airway function. Harmful stimuli trigger defensive responses such as apnea, cough and bronchospasm by activating a subpopulation of sensory afferent nerves (termed nociceptors) which are found throughout the airways. Airway nociceptive fibers are projected from the nodose vagal ganglia, the jugular vagal ganglia and the dorsal root ganglia, which are derived from distinct embryological sources: the former from the epibranchial placodes, the latter two from the neural crest. Embryological source determines nociceptive gene expression of receptors and neurotransmitters and recent evidence suggests that placode- and neural crest-derived nociceptors have distinct stimuli sensitivity, innervation patterns and functions. Improved understanding of the function of each subset in specific reflexes has substantial implications for therapeutic targeting of the neuronal components of airway disease such as asthma, viral infections and chronic obstructive pulmonary disease.
Keywords: Afferent nerves, vagal, nociception, embryological source, anatomy, function
Introduction
Visceral organ function is modulated by efferent nerve pathways arising from neural networks within the peripheral and central nervous systems. Sensory afferents innervating visceral organs report local conditions to these neural networks, thus facilitating feedback regulation. The larynx, trachea, bronchi and intrapulmonary airways are innervated by sensory afferents provided by the vagus ganglia and, to a much lesser extent, the thoracic dorsal root ganglia (DRG) [1–9]. Vagal sensory nerves are heterogeneous with respect to stimuli sensitivity, protein expression, conduction velocity, anatomical connection and embryological origin, and their activation triggers distinct changes in respiratory and cardiovascular function [10].
Sensory neuron soma within the vagus nerve (cranial nerve X) are located in two discrete ganglia: the inferior vagal ganglion or nodose ganglion, and the superior vagal ganglion or jugular ganglion [11]. The nodose ganglion is larger than the jugular and contains ~5–10 times the number of neurons. In larger mammals such as humans, monkey, cats, rabbits and guinea pigs, the nodose and jugular ganglia are anatomically separated [2, 5, 12–16], whereas in rats and mice the ganglia are partially fused [17–20] (Figure 1). Studies of the airway afferent innervation in guinea pig suggest that the recurrent laryngeal nerve carries mainly nodose afferent fibers, whereas the superior laryngeal nerve mainly carries jugular afferent fibers [21].
Figure 1:
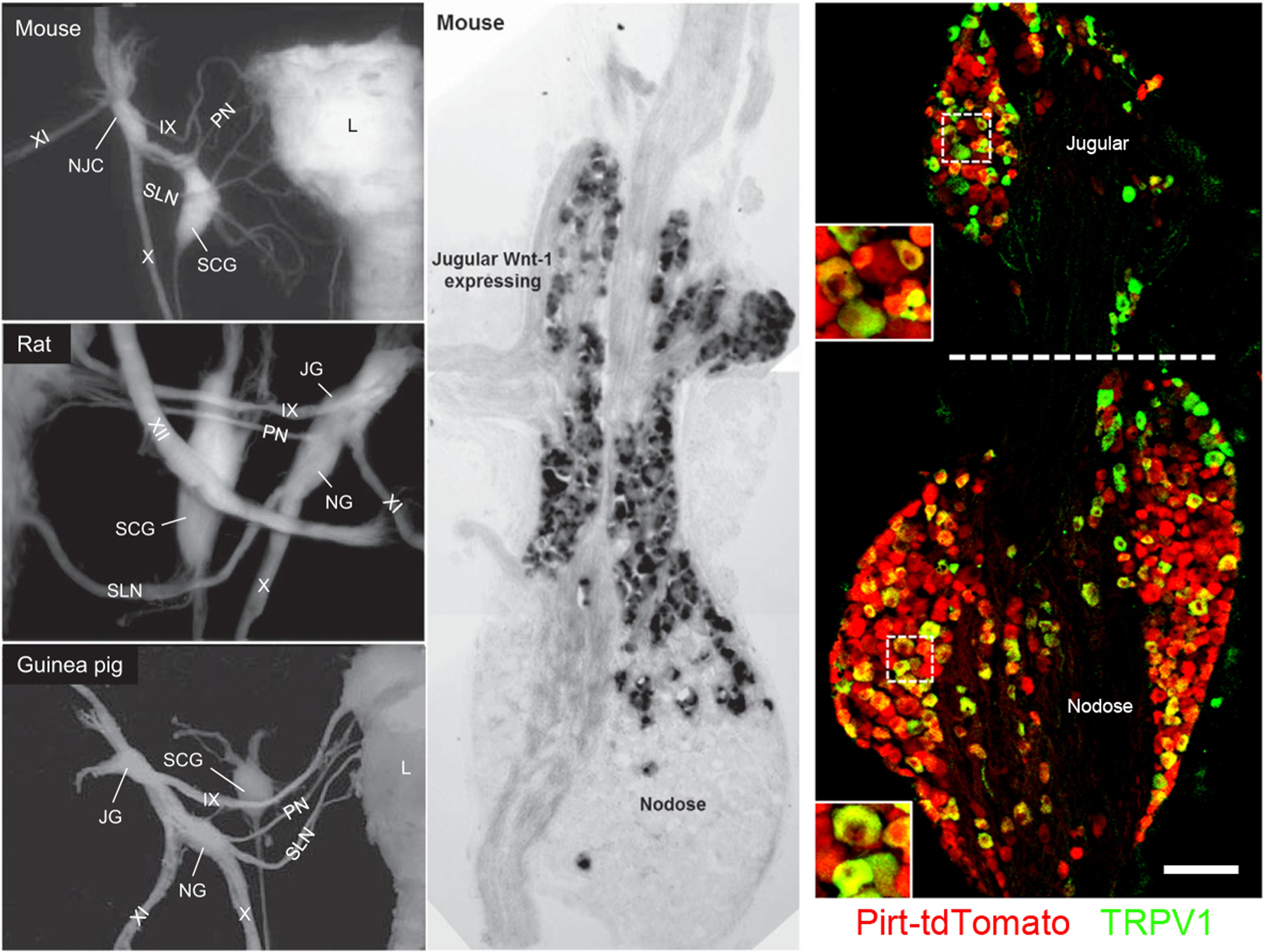
Vagal sensory ganglia. Left, dissections of mouse, rat, guinea pig vagal ganglia (NG, JG and NJC) and vagus nerve (Cranial nerve X), with other cranial nerves (IX, XI), superior cervical ganglion (SCG), superior laryngeal nerve (SLN), paryngeal nerve (PN) and larynx (L). From [10]. Middle, X-Gal staining of nodose-jugular complex from Wnt1Cre/R26R mice identifies neural crest-derived neurons. From [18]. Right, nodose-jugular complex from Pirt-tdTomato mouse, stained for TRPV1 expression (green). Pirt (red) is expressed in almost all sensory neurons. Data shows that TRPV1+ is expressed in many nodose and jugular neurons. Scale denotes 100μm, with inserts showing enlarged views of jugular and nodose neurons. From [20].
Studies in both mammals and birds indicate that the nodose and jugular ganglia are derived from distinct embryological sources [11, 22, 23]: Nodose neurons are derived from the third epibranchial placode, whereas the jugular neurons arise from the neural crest. The initial development of neural crest-derived sensory structures, which also includes the DRG, is dependent on transient β-catenin-dependent Wnt1 signaling [24], followed by the expression of the transcription factors Brn3a (and its homologs) and Prdm12 [25, 26]. Prdm12 continues to be selectively expressed in adult neural crest-derived sensory neurons [27–30]. Nodose neurons are characterized by the expression of the transcription factors Phox2a and Phox2b and a lack of Brn3a and Prdm12 [11, 25]. Phox2a is transiently expressed during development, and its deletion causes severe ganglionic atrophy [31, 32]. Phox2b is switched on slightly after Phox2a and its deletion causes epibranchial sensory neurons to revert into a neural crest-like sensory phenotype [11, 25]. Phox2b continues to be selectively expressed in adult nodose neurons [28–30]. Using the Wnt1Cre/R26R mouse, which expresses β-galactosidase in Wnt1 lineage (i.e. neural crest-derived) neurons, jugular and nodose neurons were distinguished in the fused jugular-nodose complex [18]. X-gal staining of the jugular neurons indicates that there is significant anatomical mixing of placodal and neural crest neurons in some mouse vagal ganglia (Figure 1). Any designation of “nodose” or “jugular” based upon the location within the mouse or rat vagal ganglia should therefore be used with caution. Populationally, jugular neurons are significantly smaller than nodose neurons in the guinea pig and mouse [2, 20]. Nevertheless, size is not a good predictor of embryological origin [20].
Nociception
Electrophysiological recordings of individual vagal afferents innervating the airways show that the majority are unmyelinated C-fibers [1, 3, 5, 33]. These fibers are mechanically-sensitive, but have a high threshold for mechanical stimulation and are typically quiescent during eupneic breathing. Both nodose and jugular neurons project C-fibers, with jugular C-fibers preferentially innervating the larger airways and nodose C-fibers preferentially innervating the intrapulmonary airways [2, 5]. The vast majority of vagal airway C-fibers in guinea pigs and rats are directly activated by capsaicin, the selective agonist for Transient Receptor Potential (TRP) Vanilloid 1 (V1) (Figure 1)[3, 5, 33]. In the somatosensory system, activation of TRPV1-expressing afferents causes pain and nocifensive reflexes [34, 35]. In the airways, activation of TRPV1-expressing vagal afferents evokes defensive respiratory and cardiovascular reflexes such as bradypnea, tachypnea, bronchospasm, cough and bradycardia [21, 33, 36–40]. These responses mimic neural responses to inflammation, infection and inhalation of smoke and other irritants, which can contribute to the morbidity of asthma, chronic obstructive pulmonary disease (COPD) and viral infections [10]. As such, nodose and jugular C-fibers may be considered, consistent with Sherrington’s definition [41], to be nociceptive sensory nerves: afferents that detect noxious or potentially noxious stimuli. In mice, TRPV1 is only expressed on the subset of airway C-fibers that conduct in the slower 0.3 to 0.7m/s range [42]. Consistent with other mammals, capsaicin inhalation evokes bradypnea in mice [43, 44]. The function of the faster mouse C-fibers that lack TRPV1 expression is not known. Other irritants such as bradykinin, allyl isothiocyanate and cinnamaldehyde activate nociceptive afferent subsets that almost completely overlap with TRPV1 expression, although they are mediated by different receptors [45–47].
The airways are also innervated by myelinated vagal A-fibers, most of which are highly sensitive to mechanical stimulation. These include pulmonary slowly adapting receptors (SARs) and rapidly adapting receptors (RARs), whose activation by stretch during eupneic breathing regulates central respiratory networks [6, 48–52]. These TRPV1-negative pulmonary fibers are derived from nodose neurons [3, 8, 21, 33], and evidence suggests that these fibers are not directly activated by noxious stimuli (although they may respond indirectly to agents that cause bronchoconstriction, edema or mucus secretion) and thus are considered here as ‘non-nociceptive’ [10]. Another well-characterized afferent subset is the population of nodose Aδ-fibers that specifically innervate the trachea and large bronchi [2, 53]. These nerves do not express TRPV1, but are exquisitely sensitive to acid and punctate mechanical stimulation. Activation of these nodose Aδ-fibers evokes cough in guinea pigs, and thus can also be considered to be “nociceptive” [21, 54]. Finally, there is a population of Aδ-fibers in extrapulmonary airways with high-threshold mechanosensitivity. These fibers represent about 50% of the jugular fibers and are activated by capsaicin [2, 21]. As such these Aδ-fibers are similar to the nociceptive jugular C-fibers mentioned above. Vagal soma that project myelinated A-fibers are characterized by a large diameter and robust neurofilament expression [2, 55, 56].
Markers for neural crest and placodal vagal neurons
Differences in anatomy and gene expression between nodose and jugular nociceptors innervating the airways have been determined using functional studies (single fiber recordings or patch clamp/Ca2+ imaging studies of dissociated neurons), immunohistochemistry or in situ hybridization of sectioned ganglia, or single neuron RT-PCR of dissociated neurons. More recently, unbiased screens of low-input or single neuron RNAseq transcriptomics [28–30] have dramatically increased our understanding of nodose vs. jugular function, expanding the number of putative subsets. The following is a non-exhaustive list of selective expression patterns (Table 1).
Table 1:
Embryological origin (placodal vs. neural crest) of TRPV1+ nociceptive sensory nerves innervating the lower airways determines gene expression and anatomy.
| Placodal | Neural crest | |
|---|---|---|
| Ganglia | Nodose | Jugular, DRG |
| Autacoid receptors | P2X2/3, 5HT3 | P2X3 |
| Neuropeptides (substance P, CGRP) | Few neurons | Many neurons |
| Neurotrophin receptors | TRKB | TRKA |
| Central terminals | nTS | nTS, Pa5 |
| Peripheral terminals | Mainly lung | Mainly large airways (trachea, bronchi) |
Purinergic receptors
The ionotropic purinergic receptor P2X3 is expressed on most, if not all, nodose and jugular neurons [29, 30, 57]. Whereas P2X2 is expressed on more than 80% of nodose neurons but less than 2% of jugular neurons according to a P2X2-cre reporter mouse (Figure 2A)[58]. Single neuron RT-PCR of airway-specific TRPV1+ in guinea pig and mouse studies confirm this stark difference in P2X2 expression between nodose and jugular neurons [18, 57]. The ATP analog α,β-methylene ATP (αβmATP) activates both P2X3 and P2X2/3, but the P2X3 homomeric response is inactivated so quickly as to largely negate its effect [59]. Consistent with the expression pattern of P2X2 and P2X3, αβmATP selectively activates nodose airway C-fibers (but not jugular C-fibers), and nodose A-fibers innervating the intrapulmonary airways of rats, guinea pigs and mice (Figure 2A)[5, 18, 21, 33]. Surprisingly, nodose tracheal Aδ-fibers are insensitive to αβmATP [21]. The expression of purinergic receptors in this afferent subset has yet to be reported. Nodose C-fibers innervating the guinea pig lungs are also robustly activated by adenosine, whereas jugular C-fibers are relatively insensitive [60]. Nodose TRPV1+ neurons express both the adenosine A1 and A2a receptors.
Figure 2:
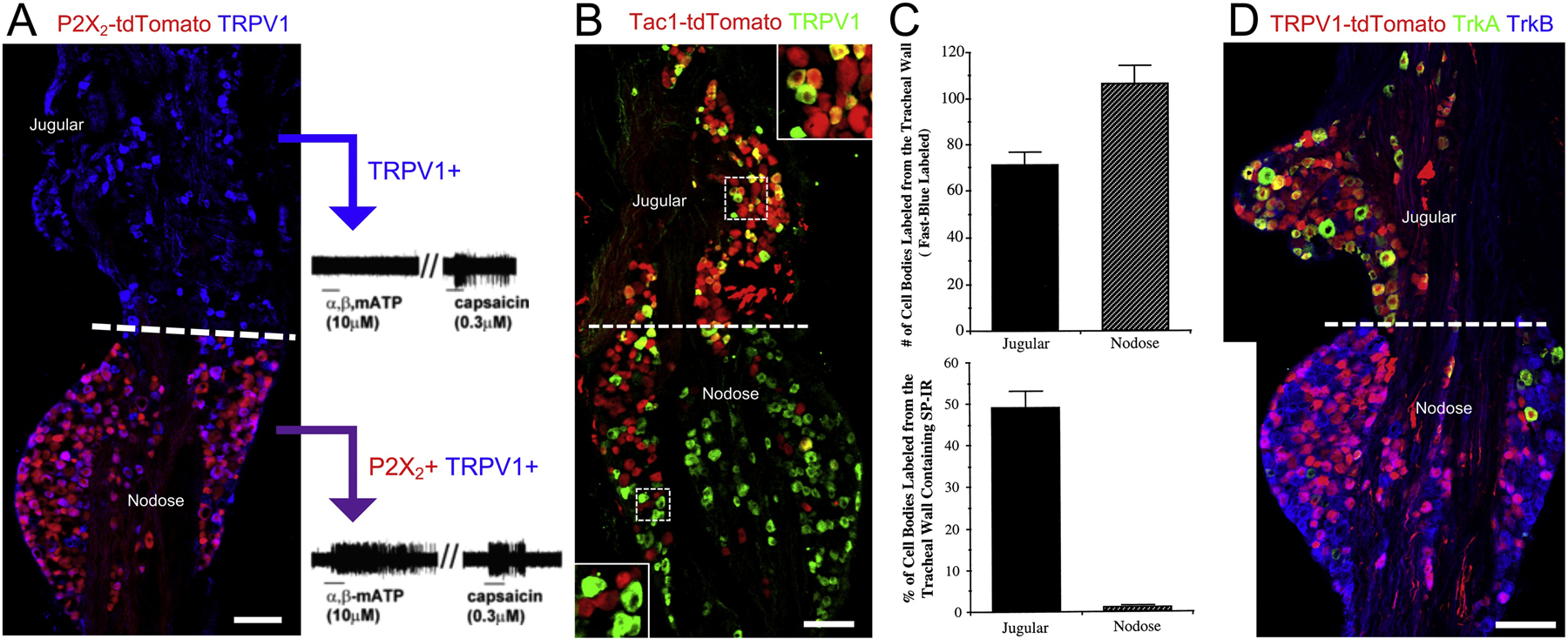
Preferential markers of nodose and jugular neurons. A, nodose-jugular complex from P2X2-tdTomato (red) mouse, stained for TRPV1 expression (blue). From [58]. Single fiber recordings of mouse lung C-fiber afferents indicate that some (likely nodose) respond to both αβmATP (P2X2/3 agonist) and capsaicin (TRPV1 agonist), whereas others (likely jugular) are only activated by capsaicin. From [18]. B, nodose-jugular complex from Tac1-tdTomato (red) mouse, stained for TRPV1 expression (green). From [20]. C, the relative proportion of nodose and jugular neurons labeled retrogradely from the guinea pig trachea (top) and the percentage of retrogradely-labeled neurons that express substance P (SP-IR). From [66]. D, nodose-jugular complex from TRPV1-tdTomato (red) mouse, stained for TrkA (green) and TrkB (blue) expression. From [20]. Scale denotes 100μm (A, B, D).
Serotonergic receptors
Serotonin robustly activates nodose C-fibers innervating the guinea pig lung, but has little effect on jugular C-fibers [61]. Nodose C-fibers were also activated by a selective 5HT3 agonist, and serotonin-evoked responses were largely blocked by a selective 5HT3 antagonist. Similar 5HT3-dependent nodose responses are observed in mice, but in this species airway jugular C-fibers are also activated by serotonin, albeit in a non-5HT3-mediated manner [62]. A reporter mouse has shown that 5HT3 expression is overwhelmingly found in nodose neurons but in very few jugular neurons [20]. Jugular TRPV1+ neurons innervating the mouse lung frequently express 5HT1 and 5HT4 mRNA, which are rarely expressed in nodose neurons [62].
Neuropeptides
Substance P is a neuropeptide mediator that contributes to neurogenic inflammation in the airways, characterized by bronchospasm and increased vascular permeability/edema [63, 64]. Substance P causes its effects through neurokinin receptors (NK1, NK2 and NK3). Substance P immunoreactivity in the vagal ganglia varies somewhat between species, although in general it is found in small-diameter neurofilament-negative neurons. In rats, substance P immunoreactivity is reported to be rarely expressed in nodose neurons (especially in the distal areas of the ganglion) but frequently expressed in jugular neurons [17, 65]. A reporter mouse for the gene Tac1, which encodes preprotachykinin A (PPT-A), the precursor for substance P and other tachykinins, shows that ~75% of Tac1+ vagal neurons are located in the jugular ganglion (Figure 2B)[20]. Interestingly, the jugular Tac1+ neurons are more likely to co-express TRPV1, compared to the nodose Tac1+ neurons which are largely TRPV1-negative [20, 29]. In a study of lung-specific vagal afferents in the guinea pig, substance P was found in ~55% and ~90% of neurofilament-negative neurons in the nodose and jugular ganglia, respectively [5]. Whereas, virtually all nodose and jugular neurofilament-negative neurons innervating the guinea pig trachea express substance P [2, 5, 66], although it should be emphasized that neurofilament-negative neurons are only 1% of the nodose tracheal innervation (Figure 2C). Interestingly, jugular neurofilament+ fibers innervating the trachea (presumed Aδ-fibers) do not express substance P, despite their similar chemical sensitivity to jugular C-fibers (which do express substance P). Calcitonin-gene related peptide (CGRP), another pro-inflammatory neurogenic mediator, is expressed in almost all jugular neurons in the mouse and rat [17, 28, 29, 65]. Again, expression of this neuropeptide is limited in the nodose ganglia, but CGRP may be expressed in as much as 40% of nodose neurons. Vasointestinal peptide (VIP) expression is more typically found in nodose neurons compared to jugular neurons in the mouse [28] and rat [17].
Growth factor receptors
Studies in rat, guinea pig and mice have shown that tropomyosin receptor kinases (Trk) are selectively expressed in the vagal sensory system (Figure 2D). TrkA, which is activated by nerve growth factor (NGF), is expressed on >85% of jugular neurons but <5–20% of nodose neurons [20, 67–69]. Whereas TrkB, which is activated by brain-derived neurotrophic factor (BDNF), is expressed on >80–95% of nodose neurons [20, 67–69]. TrkB expression has been reported to be in 5 to 30% of mouse jugular neurons [20, 29], whereas TrkB expression was noted in ~40–50% guinea pig jugular neurons innervating the airways [69]. TrkC is expressed on a limited number of nodose and jugular neurons [67–69]. Similar Trk expression patterns are found in the jugular and nodose TRPV1+ (nociceptive) populations. Other growth factor receptors are heterogeneously expressed in subpopulations of vagal neurons, although no others have been shown to correlate with embryological origin. One possible exception is GFRα3, which has been shown to be preferentially expressed in jugular neurons compared to nodose neurons in mouse RNAseq studies [28, 29]. Nevertheless, another study showed that a substantial proportion of nodose airway afferents also express GFRα3 [69].
Neurotrophic factor signaling is critical for the development of sensory ganglia and neuronal maturation [70], but there is also substantial evidence showing that NGF and BDNF are able to cause phenotypic changes in adult vagal neurons. Overexpression of NGF causes an increase in subepithelial innervation within the mouse airways [71]. NGF injections into the tracheal wall of guinea pigs evoked de novo expression of substance P in ~10% of large diameter neurofilament+ nodose neurons (presumed to project Aδ-fibers) [72]. Similar data was observed in subsequent murine studies [73]. On the other hand, BDNF injections evoked an increase in the number of tracheal-labeled nodose neurons (presumed to be mostly Aδ-fiber projecting neurons) expressing TRPV1 from 20% to 60% [53]. The relative proportion of tracheal-labeled nodose neurons modulated by BDNF and NGF is consistent with the selective expression of Trk receptors in these neurons. Neurotrophic factors have been implicated in phenotypic changes in airway afferents caused by allergic and viral inflammation [55, 74–78].
Dorsal Root Ganglia
Sensory neurons within the DRG are derived from the neural crest [24, 25]. While DRG transcriptomic signatures are more similar to the neural crest jugular neurons than placode-derived nodose neurons, the existence of both peptidergic and non-peptidergic nociceptive populations have been well characterized in DRG afferents [27, 29]. Nevertheless, it should be noted that the vast majority of DRG neurons project to somatic tissues, so whole ganglionic analyses are unlikely to represent the DRG population that innervates specific visceral organs. Retrograde tracing from the lung and trachea indicate that both cervical and thoracic DRG project afferent fibers to these organs, with the majority derived from C8-T5 [4, 79, 80]. TRPV1 expression in lung DRG afferents seems to vary across species: 30% of rat [80],12–34% of mouse [4, 18] and 61% of guinea pig lung DRG afferents express TRPV1. Most lung DRG TRPV1+ afferents express P2X3, but very few express P2X2 [18, 57]. Similar to jugular afferents, there is widespread expression of neuropeptides (e.g. Tac1, substance P and CGRP) in mouse and guinea pig lung DRG [18, 79, 81], although another study found only limited substance P expression [4]. In general, neurofilament staining in lung DRG afferents occurs only in a subset of TRPV1-negative/substance P-negative neurons [4, 80, 81], suggesting that TRPV1+/substance P+ afferents project C-fiber terminals to the lungs. Lastly, TrkA expression is also widespread in lung DRG afferents [4, 18].
Mapping neural crest- and placode-derived nociceptors
Central terminations in the brainstem medulla
Intraganglionic injection of a GFP-expressing AAV vector into the vagal ganglia causes GFP expression throughout the central terminals of the transfected nodose and jugular neurons. Following unilateral intraganglionic injections in both mice and guinea pigs, GFP-expressing axons were found within the ipsilateral tractus solitarius and GFP-expressing terminals were observed throughout the entire rostral-caudal axis of the ipsilateral nucleus tractus solitarius (NTS), as well in the contralateral nTS from the area of the obex and more caudally (Figure 3)[20, 82]. Such terminations are consistent with electrophysiological recordings of vagal afferents in larger mammals [6]. Some GFP-expressing terminations were also noted in the neighboring dorsal motor nucleus of the vagus (DMX) and the area postrema [20]. In addition, some GFP-expressing fibers coursed from their entrance into the medulla up through the lateral aspect to the paratrigeminal complex (Pa5) [20, 82]. Using intraganglionic injections of a cre-sensitive AAV reporter into the vagal ganglia of TRPV1-cre mice, the central terminations of vagal nociceptors were also studied [20]. TRPV1+ terminations made up the majority of vagal terminations within the medial and caudal subnuclei of the nTS (e.g. SolC, SolG, SolDL) (Figure 3F), consistent with electrophysiological recordings of bronchopulmonary C-fiber afferents [83]. There were few TRPV1+ terminations within the rostral or lateral subnuclei of the nTS. Much of the vagal innervation of the Pa5 was TRPV1+ (Figure 3G).
Figure 3:
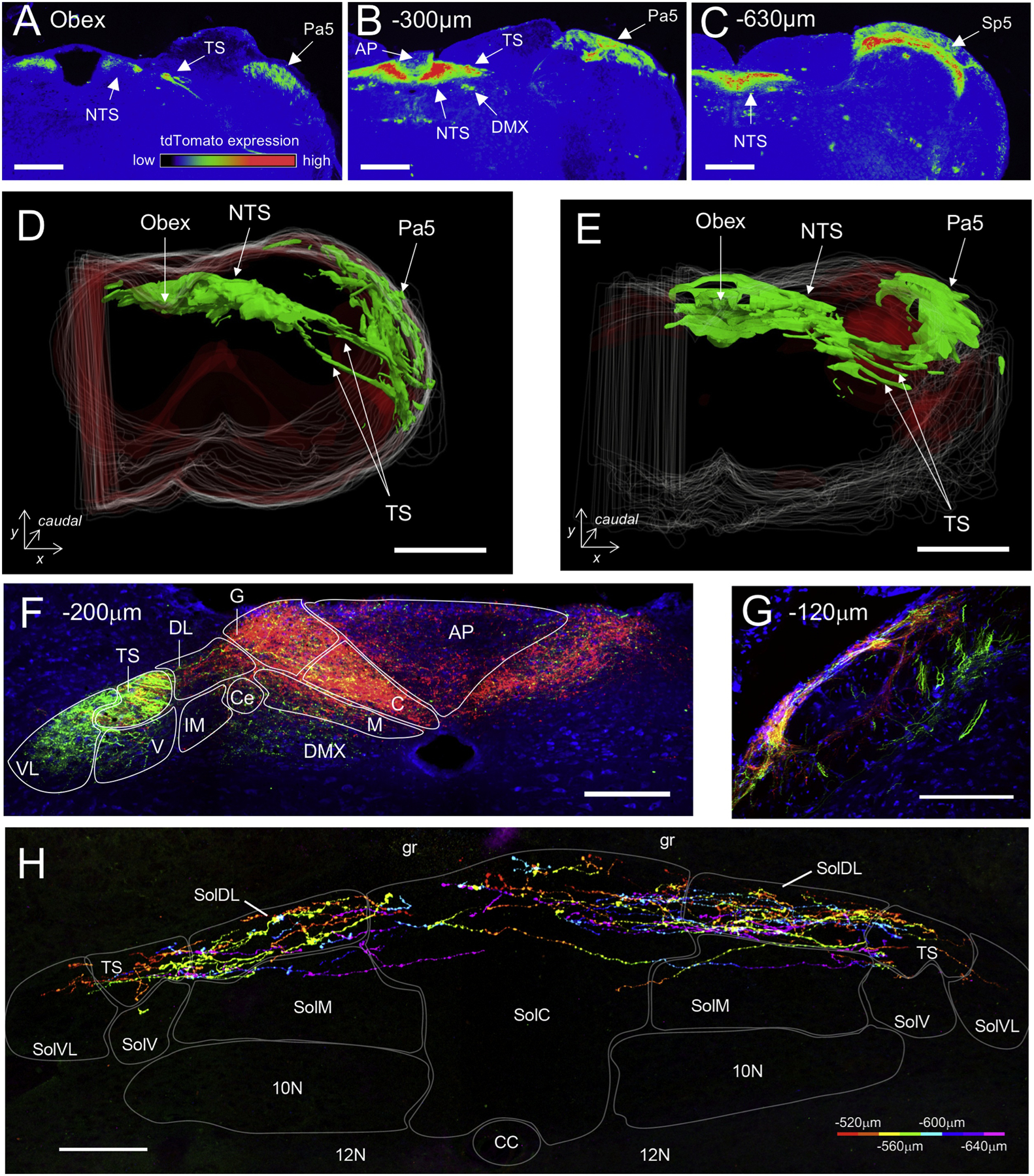
Sensory terminations within the murine medulla. A–C, tdTomato-expressing TRPV1+ fibers in the nTS, tractus solitarius (TS), Pa5, trigeminal complex (Sp5) and area postrema (A) of the TRPV1-tdTomato mouse. tdTomato is also expressed in some dorsal moter nucelus of the vagus (DMX) neurons. D and E, Green fluorescent protein (GFP) expression in brainstem projections of Tac1+ (D) and TRPV1+ (E) vagal afferents following unilateral injection of AAV9-flex-GFP into the vagal ganglion of Tac1-tdTomato (D) and TRPV1-tdTomato (E) mice. Data in the 3D reconstruction of medulla along entire rostral-caudal axis shows that vagal subsets project to both nTS and the Pa5. F, reporter expression in nTS projections of TRPV1+ (red, or yellow) and TRPV1-negative vagal afferents (green only) following unilateral injection of AAV9-GFP and AAV9-flex-tdTomato into the vagal ganglion of TRPV1-Cre mice. The following structures are identified: area postrema (AP), dorsal motor nucleus of the vagus (DMX), SolC (C), SolCe (Ce), SolDL (DL), SolG (G), SolIM (IM), SolM (M), SolV (V), SolVL (VL) and tractus solitarius (TS). G, reporter expression in Pa5 projections of TRPV1+ (red, or yellow) and TRPV1-negative vagal afferents (green only), from the same studies as (F). H, composite image of serial coronal sections showing nTS terminations of lung-specific TRPV1+ afferents. Pseudo-rainbow encoded by rostral-caudal position. The following structures are identified: dorsal motor nucleus of the vagus (10N), gracile fasciculus (gr), hypoglossal motor nucleus (12N). In all panels the rostral (+) or caudal (−) distance from obex is labeled in μm. Scale denotes 1mm (D, E), 400μm (A, B, C), 200μm (F, G) or 100μm (H). From [20].
Almost all the published evidence indicates that placode-derived nodose afferents terminate within the nTS and do not innervate the Pa5. P2X2 expression is confined to nodose sensory neurons and is virtually absent in jugular and trigeminal neurons (see above). Reporter expression in P2X2-cre mice was shown to be restricted to fibers terminating in nTS subnuclei, and no P2X2-expressing afferents were noted innervating the Pa5 [58]. Furthermore, in tracing using either AAV or horseradish peroxidase in which only the nodose neurons (and not the jugular neurons) were labeled, central terminations were restricted to only nTS subnuclei in rats and guinea pigs [82, 84]. Lastly, injection of the rat Pa5 with fluorescently-conjugated (488) cholera toxin subunit B (CT-B) caused retrograde labeling of virtually no nodose neurons (Figure 4A–C)[19]. Instead, the Pa5 tracing labeled a large number of jugular neurons, indicating that at least some populations of jugular afferents project to the Pa5.
Figure 4:
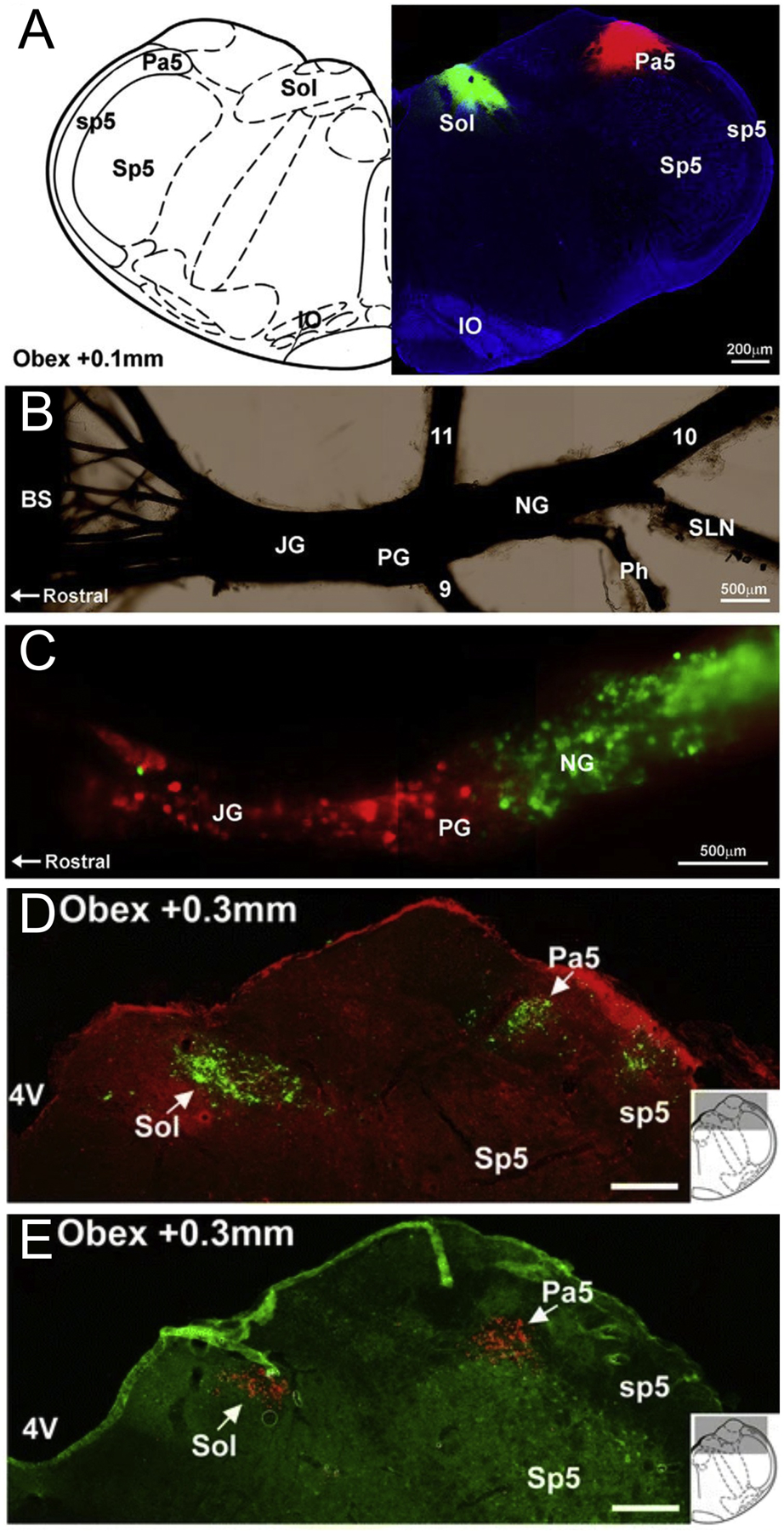
Brainstem projections of rat vagal afferents. A, caudal brainstem section showing the nTS (Sol) and Pa5 locations for microinjection of CT-B tracers conjugated with 488 (green) or 594 (red) fluorophores, respectively. B, whole-mount preparation of rat vagal ganglia demonstrating the location of the jugular (JG) and nodose ganglia (NG). C, neurons in rat vagal ganglia retrogradely labeled with CT-B from the nTS (green) and Pa5 (red). Also labeled: 9, Glossopharyngeal nerve; 10, vagus nerve; 11, accessory nerve; BS, brainstem; IO, inferior olives; PG, petrosal ganglia; Ph, pharyngeal branch; SLN, superior laryngeal nerve; Sp5, spinal trigeminal nucleus; sp5, spinal trigeminal tract. D and E, reporter expression in medulla neurons following trachea injection of HSV-1-tdTomato (red, D) or lung injection of HSV-1-GFP (green, E). Scale denotes 200μm (D, E). From [19, 97].
However, there is some conflicting data on the exclusivity of the jugular-Pa5 connection. In the same rat study, McGovern et al [19] simultaneously injected another fluorescently-conjugated (594) CT-B into the nTS immediately rostral to obex, and only observed retrograde tracing into nodose neurons (Figure 4A–C). Thus suggesting that jugular neurons do not project to the nTS at all. Similar results were observed in identical dual CT-B studies in guinea pigs [85]. However, in a different guinea pig retrograde tracing study, fast blue injections into the SolC labeled small diameter neurons within both the nodose and jugular ganglia [38]. It is possible that jugular afferents terminate in only the caudal subnuclei of the nTS, which were possibly not labeled in the studies by McGovern et al [19, 85]. As mentioned above, substance P/Tac1 expression is a preferential marker of jugular neurons. Intraganglionic injection of a cre-sensitive AAV reporter into the vagal ganglia of Tac1-cre mice, showed that, in addition to vagal Tac1+ terminations in the Pa5 (consistent with jugular connectivity), vagal Tac1+ fibers also terminated throughout the rostral-caudal axis of the nTS, in both medial and lateral subnuclei (Figure 3D)[20]. Most of the Tac1+ neurons labeled in this study were located in the jugular ganglia and many also expressed TRPV1+, thus it is likely that some of the nTS terminations were of Tac1+ jugular nociceptive fibers. These were likely the terminations within the caudal, medial subnuclei. Other immunohistochemical studies have shown that substance P expression is found throughout the nTS and there is evidence that this is partially reduced by neonatal capsaicin toxicity or vagotomy [86–88], suggesting a vagal nociceptor contribution.
Functionally, tachykinins have been implicated in nociceptive neurotransmission within the nTS. In guinea pigs, intracerebroventricular NK receptor antagonism inhibited both laryngeal capsaicin-evoked reflex increase in parasympathetic drive to the airways [38], and capsaicin-evoked sensitization of cough reflex [37, 89]. Microinjection of NK antagonists into SolC blocked both bradypnea evoked by SolC microinjection of capsaicin in rats [90] and cough elicited by cigarette smoke inhalation in conscious GP [91], but failed to inhibit capsaicin-evoked sensitization of the guinea pig cough reflex [89]. Such observations question whether or not tachykinins contribute to reflex modulation directly at the level of the primary afferent synapse or further along the connectome within the dorsal medulla, thus invalidating their use as evidence for peptidergic/jugular afferent synapses within the nTS. Indeed, a large number of intrinsic neurons in the nTS express Tac1 [20] and microinjection of substance P into the SolC produces qualitatively different respiratory effects to capsaicin in rats and guinea pigs [89, 92]. There is some evidence in electrophysiological recordings of nTS neurons receiving direct input from vagal C-fibers that NK1 antagonism reduces neurotransmission [93], but other studies have shown that such neurotransmission is exclusively glutamatergic [94, 95].
The development of a herpes simplex virus 1 (HSV-1) strain (H129) with anterograde transsynaptic capabilities has been instrumental in determining the central projections of nerves specifically innervating the airways [7]. In a series of rat studies, instillation of HSV-1 into the tracheal lumen [7, 19, 96], or injected into the tracheal wall [97] or into the lung [97] caused uptake of HSV-1 into the peripheral terminals of terminals innervating the airways, resulting in HSV-1 protein and reporter expression within the cell bodies of placode-derived (e.g. P2X2+, TrkB+) and neural crest-derived (e.g. preprotachykinin A+, TrkA+) vagal neurons, most of which were nociceptive (i.e. TRPV1+). HSV-1-derived protein expression was then noted in the downstream connectome of the airway afferents, starting at the earliest time frame within the nTS, Pa5 and trigeminal complex (although no trigeminal neurons were labeled)(Figure 4D, E). At later timepoints, neurons within the pons (e.g. parabrachial nuclei), thalamus, hypothalamus, amygdala and the forebrain cortex were labeled. The connectome labeling of nTS neurons by airway HSV-1 inoculation is consistent with electrophysiological antidromic mapping of airway afferents [6], and the inhibition of multiple airway reflexes by nTS microinjection of glutamatergic antagonists [98–101]. The connectome labeling of Pa5 neurons by airway HSV-1 inoculation is less well characterized. Recent studies in anesthetized guinea pigs have provided evidence that the Pa5 may play a role in reflexes evoked from the larynx [82, 85]. Stimulation of the larynx electrically or chemically with capsaicin evoked bradycardia, which was moderately inhibited by Pa5 microinjections of NK antagonists but was abolished by Pa5 microinjections of either ionotropic glutamate receptor antagonists (CNQX and AP-5) or the GABAA agonist muscimol [82, 85]. There seems to be significant complexity within the Pa5-associated respiratory circuits, however, as Pa5 microinjections of the glutamate analog D,L-Homocysteic acid evoked bradycardia, but Pa5 microinjections of capsaicin evoked tachypnea (which was inhibited by NK antagonists but not by glutamatergic antagonists) [82]. Ablation of NK1-expressing neurons within the Pa5 inhibited, but did not abolish, cough evoked by inhalation of the irritant bradykinin in conscious guinea pigs [102]. In mouse studies, however, there is no evidence of an airway afferent innervation of the Pa5: instillation of a retrograde cre-sensitive AAV reporter vector into the distal trachea of TRPV1-Cre and Tac1-Cre mice only labeled fibers terminating in the nTS (exclusively in the caudal and medial subnuclei) and failed to label fibers terminating within the Pa5 (Figure 3H)[20]. It is not clear if the differences between these anatomical and functional studies are due to species differences, differences in the tropism of the viral vectors, differences in the targeted tissue (i.e. proximal vs. distal airway) or some other technical issue. Following airway administration, AAV vectors label only the central fibers of airway afferents in the medulla, thus the terminations can be easily determined. The HSV-1-mediated tracing is transsynaptic and expresses reporters in downstream neurons. The network organization of the labeled neurons is potentially open to interpretation and often the somal fluorescent signal of intrinsic neurons swamps the afferent fiber labeling. More work is needed to map the central terminations of nodose and jugular airway afferents within the nTS and Pa5, and determine their specific contribution to reflex modulation from the airways.
Peripheral terminals within the airways
The characterization of nerve fibers in the airways has been largely achieved with immunohistochemistry and dye labeling. The airways from the larynx through to the distal lung parenchyma are densely innervated by nerves labeled by the pan-neuronal marker pgp9.5 [103, 104]. In addition to free nerve endings of heterogeneous structure and complexity throughout the airways, nerve terminations occur in close proximity to clusters of pulmonary neuroendocrine cells, termed neuroepithelial bodies (NEBs) [105–111], and laryngeal neuroendocrine cells analogous to taste buds [9]. The majority of nerves innervating the airways are thought to be afferent, but autonomic fibers are also present: tyrosine hydroxylase-expressing sympathetic fibers have been described innervating blood vessels and smooth muscle in the tracheal and bronchial subepithelium [79, 112]; and studies show choline acetyltransferase (ChAT)-expressing parasympathetic ganglia in the subepithelial layer of larger airways and ChAT-expressing fibers innervating the smooth muscle layer throughout the airways [113, 114].
Given that TRPV1 expression is a well characterized functional marker of nociceptive C-fibers projected from neural crest- and placode-derived afferents, there is surprisingly little data on the distribution of TRPV1+ afferents in the airways. Studies of TRPV1 immunoreactivity in the guinea pig and rat airways showed that TRPV1+ fibers densely innervated the tracheal subepithelial layer and to a lesser extent the trachealis smooth muscle (Figure 5A, B)[115–117]. Dual labeling with pgp9.5 indicated that many other TRPV1-negative fibers were present in the subepithelium (Figure 5C)[115]. The number of TRPV1+ fibers intercalated into the tracheal epithelial layer was variable, although many pgp9.5+ fibers were consistently observed. The structure of the TRPV1+ fibers within the airways was relatively simple: fine varicose fibers with some branching but no structured arborization.
Figure 5:
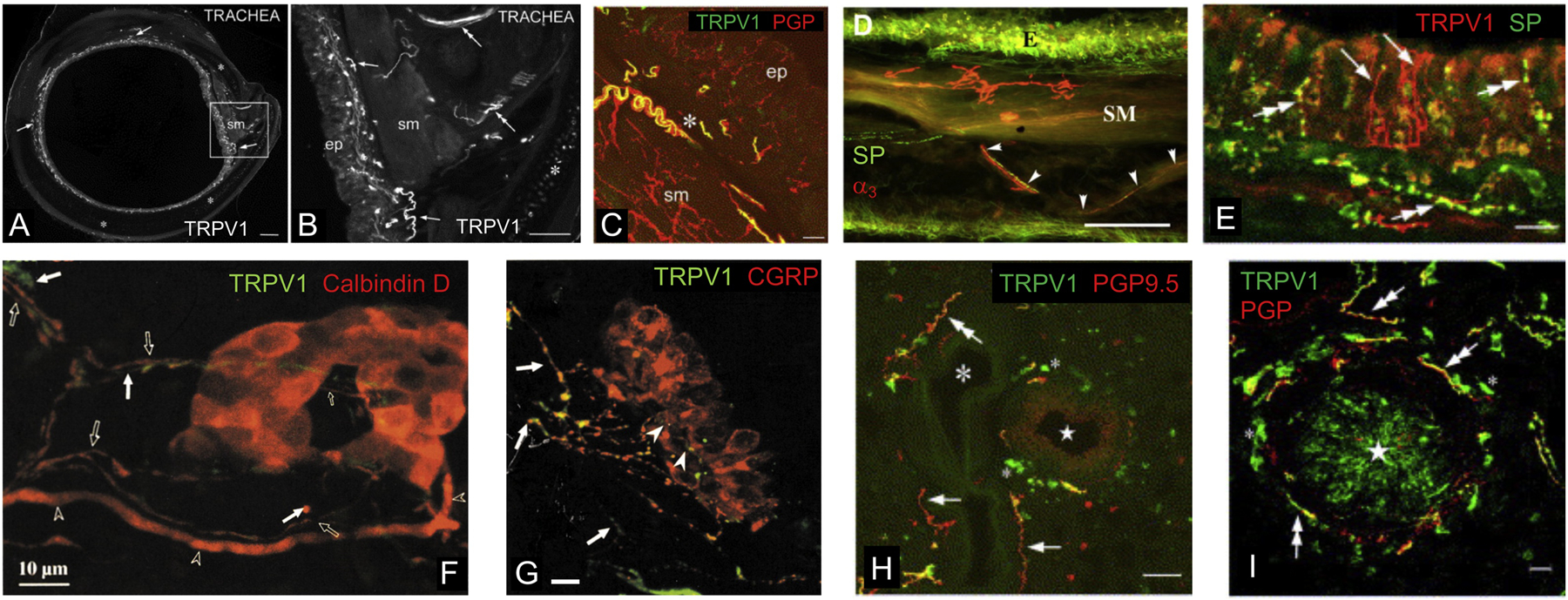
Nociceptive afferent fibers innervating the airways. A and B, TRPV1+ structures in the guinea pig trachea. TRPV1+ fibers are noted (arrows) between the smooth muscle (sm) of the trachealis muscle and the epithelium (ep) and also (double arrows) in and outside the smooth muscle layer. Asterisk indicates cartilage. From [115]. C, guinea pig trachea stained for TRPV1 (green) and pan-neuronal pgp9.5 (red). TRPV1+ fibers mainly noted in subepithelial plexus (asterisk), whereas pgp9.5+ fibers noted in plexus, epithelial (ep) and smooth muscle (sm). From [115]. D, guinea pig trachea stained for substance P (SP, green) and α3 Na+/K+ ATPase (red). Substance P+ fibers noted within the epithelium (E) and beneath the tracheal smooth muscle (SM). α3+ terminals have a complex arbor, do not express substance P and are found exclusively within the smooth muscle. Arrowheads denote putative parental axons of α3+ fibers and substance P+ fibers projecting to the smooth muscle layer together. Taken from [54]. E, guinea pig tracheal epithelium stained for TRPV1 (red) and substance P (SP, green). Most TRPV1+ fibers also express substance P (double arrows), but a minor subset does not express substance P (arrows). From [115]. F, neuroepithelial bodies from rat lungs stained for TRPV1 (green) and calbindin D (red). Calbindin D is expressed in both the neuroendocrine cells of the NEB and in a subset of thin fibers (open arrows) and thick fibers (open arrowheads) innervating the NEB. TRPV1+ fibers (arrows) do not express calbindin D. From [109]. G, neuroepithelial bodies from rat lungs stained for TRPV1 (green) and CGRP (red). CGRP is expressed in both the neuroendocrine cells of the NEB and in thin fibers innervating the base of the NEB (arrowheads). TRPV1+ fibers (arrows) innervating the NEB also express CGRP. From [109]. H and I, guinea pig lung stained for TRPV1 (green) and pan-neuronal pgp9.5 (red). Fibers expressing pgp9.5 and TRPV1 (double arrows) innervate bronchiole (star), blood vessel (large asterisk) and alveolar tissue. Some pgp9.5+ fibers do not express TRPV1 (arrows). Small asterisks indicate non-specifically labeled white blood cells. From [115]. Scale denotes 200μm (A), 100μm (B), 50μm (D, H), 20μm (C, E, I) or 10μm (F, G).
Most studies investigating nociceptive peripheral terminals within the airways have instead used immunohistochemistry of either substance P or CGRP [54, 79, 105, 108, 109, 115, 118–126], which are preferential markers of neural crest-derived neurofilament-negative nociceptive neurons. Note that neuropeptide immunoreactivity alone will not distinguish between jugular and DRG neurons. In the trachea, a dense network of substance P+ and CGRP+ fibers are found in the epithelial and subepithelial layers of most species (Figure 5D)[54, 79, 115, 116, 118, 120, 121, 124, 126], although a study in sheep indicated few peptidergic fibers within the epithelium [122]. Some peptidergic fibers were also noted in the trachealis smooth muscle of all species studied. Counterstaining for pgp9.5 suggests that >90% of epithelial fibers are substance P+ [121, 124]. Capsaicin toxicity eliminated 96% of substance P/CGRP labeling in the rat trachea [116, 121], and almost all the substance P+ tracheal fibers in the guinea pig were TRPV1+ (Figure 5E)[115]. In pig and guinea pig trachea, some peptidergic fibers intercalate into the epithelial layer [79, 115, 116, 124], but this was not seen in the rat larynx [126]. Peptidergic fibers innervating the trachea are not carried by the recurrent laryngeal nerve [120]. Overall, the data indicates that peptidergic TRPV1+ fibers (likely neural crest-derived nociceptors) are the dominant fiber subtype within the tracheal epithelium. This is consistent with the labeling of substance P-expressing jugular neurons following retrograde tracing from the trachea [2, 5, 66]. There is also evidence in the guinea pig and rat trachea of non-peptidergic TRPV1+ fibers within the subepithelium and intercalated into the epithelium [115, 117] (although these were not observed in a follow up guinea pig study [116]), which are projected by either nodose C-fibers or jugular Aδ-fibers. To date, the structures of tracheal terminations of peptidergic and non-peptidergic TRPV1+ fibers have not been rigorously compared.
Only a small percentage of fibers within the trachealis smooth muscle express substance P [54, 79, 121]. Some non-peptidergic fibers innervating the smooth muscle may express TRPV1 [115, 116], but many are thought to be TRPV1-negative mechanoreceptors projected from the nodose ganglia [54, 120], which in the guinea pig trachea are labeled by the styryl dye FM2–10 and the α3 subunit of the Na+/K+ ATPase pump (Figure 5D). FM2–10 labeled terminals have complex dendritic arbors that are more common near the tracheal carina and the main bronchi [54]. These structures were not observed in the trachea of a P2X2 reporter mouse [58], possibly due to species differences. Instead, the P2X2+ fibers (presumed nodose) which densely innervated the muscle layers of the membranous portion of the trachea, were structurally simple - following the transversal axis of the muscle in parallel arrays. P2X2+ terminations were also noted intercalated into the epithelium of the membranous portion of the mouse trachea [58]. These terminals were highly arborized in the epithelial plane and so did not resemble the non-peptidergic TRPV1+ terminations within the guinea pig tracheal epithelium [115], perhaps suggesting that the latter are projected by jugular Aδ-fibers instead of nodose C-fibers.
Counterstaining for pgp9.5 in the intrapulmonary airways demonstrated that 50–75% of fibers surrounding bronchioles were TRPV1+ and almost all alveolar fibers were TRPV1+ (Figure 5H, I)[115]. Substance P+ and CGRP+ fibers have been routinely observed in the subepithelial layer of bronchioles in cats, rats, mice and guinea pig [79, 105, 109, 111, 116, 119], and these are likely projected from jugular and DRG neurons. A series of elegant studies have focused on the peptidergic innervation of NEBs, whose neuroendocrine cells in the epithelial layer are themselves immunoreactive for pgp9.5 and CGRP (Figure 5 F, G) [105–111]. NEBs are directly innervated by multiple fiber subtypes, including myelinated fibers that intercalate into the NEB, and thin CGRP+ fibers that only contact the basal pole of the NEB [105, 109, 111]. All the NEB-associated CGRP+ fibers expressed TRPV1 and were depleted by capsaicin toxicity, suggesting that they are neural crest-derived nociceptors. This is consistent with the observation that Wnt1-lineage fibers innervate the basal pole of NEBs [127]. Brouns et al [109] concluded that NEB-associated CGRP+ fibers were derived from DRG, but evidence discounting their potential jugular origin was lacking. Myelinated fibers innervating the NEB do not express TRPV1, and are likely low threshold mechanoreceptors, although electrophysiological evidence suggests that they are not SAR fibers [128]. There is little CGRP+ innervation of the smooth muscle layer of bronchioles [111]. There is substantial variation in reports of peptidergic fibers within the alveolar compartment. One study found that peptidergic fibers rarely innervated alveoli in guinea pig lung [79], suggesting that the majority of TRPV1+ fibers in this region [115] are projected by nodose C-fibers. This is consistent with presence of Phox2b-lineage fibers innervating the alveolar compartment [127]. Nevertheless, another study in the guinea pig found that all TRPV1+ fibers innervating the alveoli expressed substance P and CGRP [116]. Indeed, this study found little evidence of non-peptidergic TRPV1+ fibers within the entire away, which is inconsistent with retrograde tracing data and electrophysiological data in this species [2, 5, 57, 66]. As such, there is significant uncertainty regarding the structure of peptidergic and non-peptidergic TRPV1+ terminals within the lower airways. Similar to the trachea, there is some peptidergic innervation of pulmonary blood vessels [111, 121, 124]. Finally, peptidergic fibers are found innervating parasympathetic ganglia in the larger airways [123, 125]. These fibers are likely nociceptive, due to their sensitivity to capsaicin toxicity, and can regulate parasympathetic cholinergic neuron activity via tachykinin-dependent neurotransmission.
Function of airway nociceptors: Reflexes
Activation of nociceptive airway afferents evokes neurogenic inflammation, respiratory and cardiovascular reflexes, and aversive sensations such as dyspnea. There is evidence that the application of nociceptive stimuli at different concentrations or the application of the same nociceptive stimuli to distinct regions/tissues within the airways can activate distinct reflex mechanisms [1, 21, 39, 89, 129]. But in many cases the contribution of neural crest- and placode-derived nociceptors has yet to be elucidated.
Electrical or punctate mechanical stimulation of the trachea evokes cough in anesthetized guinea pigs, due to the activation of placode-derived nodose Aδ-fibers innervating the trachea [21, 54, 130, 131]. Citric acid, but not capsaicin, activates these fibers and accordingly also evokes cough in anesthetized guinea pigs (Figure 6C, D). Acid-induced stimulation of nodose tracheal Aδ-fibers has little effect on respiratory rate [21, 39]. Capsaicin inhalation has disparate effects on the cough reflex, likely due to different subsets being activated in different experimental setups. Inhalation of capsaicin causes cough in conscious guinea pigs and humans via the activation of TRPV1-expressing vagal afferents (Figure 6A)[36, 37, 132–134]. Other irritants that similarly activate both nodose and jugular C-fibers also evoke cough [21, 40, 131, 135–137]. Whereas selective stimulants of nodose C-fibers (via P2X2/3 or 5HT3 receptors) fail to evoke cough [131], and selective silencing of nodose afferents has no effect on capsaicin-evoked cough (Figure 6A, B)[131]. Thus, it is likely that capsaicin-evoked cough is mediated by the activation of neural crest-derived TRPV1+ fibers projected from the jugular ganglion, although this has yet to be definitely proven. In anesthetized guinea pigs, capsaicin and bradykinin fails to evoke cough (Figure 6C)[21, 89]. Nevertheless, activation of tracheal TRPV1+ afferents causes a synergistic increase in the sensitivity of cough evoked by nodose tracheal Aδ-fiber activation (Figure 6E)[89]. Interestingly, selective activation of pulmonary nodose C-fibers (via adenosine or 5HT3 receptors) completely abolished cough evoked by nodose tracheal Aδ-fiber activation (Figure 6F)[138]. It was therefore concluded that nodose and jugular TRPV1+ afferents have opposing effects on cough sensitivity, with jugular nociceptors (preferentially innervating the larger airways) mediating capsaicin-evoked increases in cough sensitivity, potentially through neuropeptide signaling in the medulla (Figure 6E).
Figure 6:
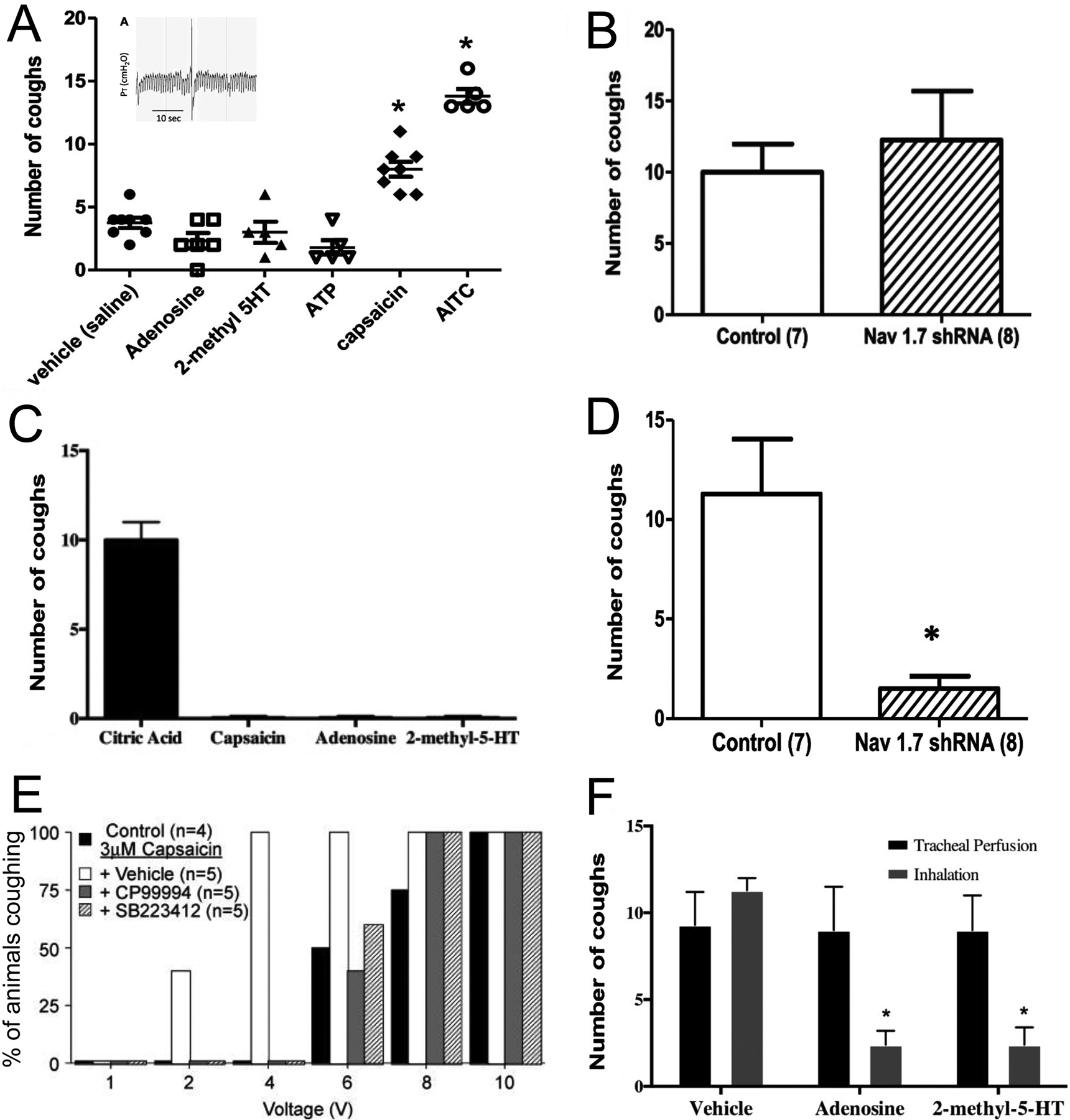
The contribution of vagal afferent nociceptive subsets to the cough reflex in guinea pigs. A, coughs evoked in conscious animals by nebulized vehicle, adenosine (10mM), 2-methyl 5HT (5mM), ATP (10μM), capsaicin (10μM) and allyl isothiocyanate (AITC, 10mM)(n = 5 to 8). Insert, representative air pressure trace of a capsaicin-evoked cough. From [131]. B, mean coughs (+ SE) evoked by capsaicin (1–10μM) in conscious animals who had previously been administered intranodose injection of either control shRNA or shRNA targeting NaV1.7 (n = 7 to 8). From [131]. C, mean coughs (+ SE) evoked in anesthetized animals by tracheal application of citric acid (0.001–2M), capsaicin (10μM), adenosine (0.1μM) and 2-methyl 5HT (10μM) (n = 3 to 40). From [138]. D, mean coughs (+ SE) evoked by mechanical punctate stimuli to the trachea of anesthetized animals who had previously been administered intranodose injection of either control shRNA or shRNA targeting NaV1.7 (n = 7 to 8). * denotes significant difference (p<0.05). From [131]. E, the percentage of anesthetized animals that cough in response to electrical stimulation of the trachea (16Hz, 10s, 1–10V) under control conditions (black columns). Combined tracheal application of capsaicin (3μM) with intracerebroventricular administration of vehicle (white columns) increases cough sensitivity. Intracerebroventricular administration of either CP99994 (NK1 antagonist, grey columns) or SB223412 (NK3 antagonist, hatched columns) blunts the increased cough sensitivity caused by tracheal capsaicin. From [89]. F, mean coughs (+ SE) evoked by application of citric acid (0.001–2M) to the trachea of anesthetized animals with either tracheal perfusion of vehicle (saline), adenosine (0.1μM) or 2-methyl 5HT (10μM), or pulmonary inhalation of nebulized vehicle (saline), adenosine (2mg/ml) or 2-methyl 5HT (1mg/ml). * denotes significant difference to vehicle (p<0.05). From [138].
In studies of anesthetized guinea pigs, capsaicin applied to the trachea evokes bradypnea (likely via activation of jugular TRPV1+ fibers), whereas inhalation or intravenous application evokes multiphasic tachypnea and bradypnea (possibly due to the activation of both nodose and jugular TRPV1+ fibers) [39, 138]. Similar responses to intravenous/inhalation capsaicin have been noted in anesthetized rats and dogs and conscious dogs [139, 140], but multiphasic reflex responses were also noted following tracheal administration. In guinea pigs, selective nodose C-fiber stimulants applied to the pulmonary system cause tachypnea but have little effect on respiration rate when applied to the trachea (where there are few nodose C-fibers) [39, 138]. This is consistent with the tachypnea evoked by optogenetic-based activation of a lower airway vagal subset that has significant overlap with TRPV1 expression in the anesthetized mouse [8]. Such data would also suggest that vagally-dependent tachypnea caused by embolism [1, 141, 142] is triggered by nodose afferent activation. Nevertheless, intravenous administration of 5HT3 agonists (selectively activates nodose afferents) caused significant bradypnea (followed by tachypnea) in anesthetized cats [1], rats [143, 144] and mice [145], and selective activation of a subset of airway jugular afferents caused tachypnea in mice [146]. As such there is a lack of consistency in the data regarding the specific role of nodose and jugular nociceptors in the reflex control of respiratory rate - suggesting further complexity, perhaps based upon anatomical innervation patterns [1]. Nevertheless, almost all of the respiratory reflex is mediated by vagal afferents [39, 139, 140].
Airway smooth muscle is controlled by both the parasympathetic and sympathetic nervous systems, whose net effect at eupnea is a baseline cholinergic-mediated tone [147–150]. In multiple species under anesthesia, capsaicin or bradykinin applied intravenously or to the larynx or trachea causes a reflex increase in tracheal tone and lower airway resistance (bronchospasm) [1, 38, 149–151]. This response is abolished by atropine, ganglionic blockade and vagotomy, indicating that airway vagal TRPV1+ fibers trigger a reflex increase in parasympathetic drive to the airways. Central administration of neurokinin antagonists abolished the reflex evoked by tracheal bradykinin treatment, consistent with the hypothesis that peptidergic jugular afferents were involved [38, 146, 151]. The extent to which nodose C-fibers can also evoke this reflex is unclear, although phenylbiguanide (5HT3 agonist) reportedly increases airway resistance in rabbits [152] and cats [153], while having no direct effect on smooth muscle [154]. Capsaicin also increased sympathetic drive to the guinea pig airways via the activation of tracheal TRPV1+ DRG afferents [81]. This reflex was blocked by intravenous tachykinin antagonists, which were interpreted to be acting centrally in the spinal cord, but the specific anatomic pathways have not been elucidated.
In guinea pigs and rats, but not humans and other large mammals, activation of peptidergic TRPV1+ afferents in the airways causes tachykinin-dependent bronchospasm [63, 155–159]. This ‘neurogenic inflammation’ is due to capsaicin-induced depolarization of the afferent peripheral terminals which causes local release of tachykinins, independently of action potential conduction. Capsaicin also causes airway smooth muscle dilation in atropinized guinea pig airways via tachykinin-dependent activation of parasympathetic non-adrenergic non-cholinergic (NANC) nerves [159]. This appears to be a peripheral reflex (sensitive to voltage-gated sodium channel block) mediated by contralateral terminals of peptidergic vagal (putative jugular) TRPV1+ afferents innervating parasympathetic ganglia.
Similar to the neural control of airway smooth muscle, mucus secretion from epithelial goblet and serous cells and submucosal glands is under reflex control [1]. Capsaicin, either topically or injected via the bronchial artery or the right atria, evoked an increase in mucus secretion [160–165]. In most cases this response was due to a central reflex activation of atropine-sensitive parasympathetic signaling, although local actions of afferent-derived tachykinins may also contribute [166]. This suggests a role for peptidergic (likely neural crest-derived) nociceptors. There are no reports confirming nodose C-fiber activation can trigger reflex hypersecretion [10].
Inhalation of capsaicin or allyl isothiocyanate evokes cardiovascular reflexes, although these are highly sensitive to anesthesia compared to their evoked respiratory reflexes [1, 33, 129]. Under normal conditions, the prevailing response is an increase in parasympathetic drive to the heart causing atropine-sensitive bradycardia (and accompanying hypotension). This central reflex is activated by vagal TRPV1+ afferents innervating the airways, but the contribution of nodose and jugular afferents is presently unclear - although ATP (nodose stimulant) failed to evoke substantial bradycardia when inhaled [33]. In vagotomized rats, there is also evidence that topical application of capsaicin, AITC or bradykinin to the pulmonary surface evokes an increase in sympathetic drive via the activation of DRG (neural crest) afferents innervating the lung [167, 168].
Conclusions and implications
Current evidence suggests that placode- and neural crest-derived nociceptors innervating the respiratory tract have distinct gene expression, neuroanatomy and function. However, it is important to note that there are significant caveats to the use of common markers as definitive identifiers of placode- and neural crest-derived nociceptors. For example, tachykinin expression in afferents is often assumed to indicate neural crest but substance P/Tac1 expression is not an exclusive marker of neural crest nociceptors. In addition, there is a lack of ligands that provide selective activation of specific afferent subsets (e.g. jugular C-fibers), and caution should be taken when interpreting immunofluorescence in thin afferent fibers. Thus, there is still much to be learnt of afferent subset function and neuroanatomy (both peripheral and central). In addition, most of the nodose vs. jugular studies have focused on small mammals, and it remains to be seen how the placode- and neural crest-derived afferent delineation matches to the wealth of respiratory afferent data in larger mammals (cf. pulmonary vs. bronchial C-fibers in the dog [1]) or translates to human physiology. The recent spate of single neuron transcriptomics suggests even greater subset complexity than is described here, and little is known of the global transcriptomic response to viral or allergic inflammation. Knowledge of the role of each subset in specific reflexes, and the sensitivity of these subsets to activation and remodeling by inflammation has broad implications for therapeutic targeting of the neuronal components of airway disease. In addition to their established role in cough and reflex bronchospasm and secretion, which have particular relevance to viral infection, asthma and COPD, vagal afferent nerves have also been implicated in airway hyperreactivity (increased response to bronchospastic agent) in asthma [10, 149, 169, 170] and in debilitating cardiopulmonary events in response to pollution [129, 171–173]. As such, deciphering the specific function of vagal afferent subsets may offer novel therapeutic strategies.
Highlights.
Airway afferents are projected by nodose (placode) and jugular (neural crest) neurons
Most airway afferents are activated by noxious stimuli, provoking defensive reflexes
Embryological source of afferents defines gene expression, anatomy and function
Acknowledgements
This work was supported by the National Institutes of Health’s Office of Director SPARC Commonfund program (OT2OD023854) and the National Institute for Neurological Disorders and Stroke (U01NS113868).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coleridge JC and Coleridge HM, Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol, 1984. 99: p. 1–110. [DOI] [PubMed] [Google Scholar]
- 2.Ricco MM, Kummer W, Biglari B, Myers AC, and Undem BJ, Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol, 1996. 496 (Pt 2): p. 521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho CY, Gu Q, Lin YS, and Lee LY, Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol, 2001. 127(2–3): p. 113–24. [DOI] [PubMed] [Google Scholar]
- 4.Dinh QT, Groneberg DA, Peiser C, Mingomataj E, Joachim RA, Witt C, Arck PC, Klapp BF, and Fischer A, Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir Physiol Neurobiol, 2004. 144(1): p. 15–24. [DOI] [PubMed] [Google Scholar]
- 5.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, and Kollarik M, Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol, 2004. 556(Pt 3): p. 905–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubin L, Alheid GF, Zuperku EJ, and McCrimmon DR, Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol, 2006. 101(2): p. 618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGovern AE, Davis-Poynter N, Farrell MJ, and Mazzone SB, Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience, 2012. 207: p. 148–66. [DOI] [PubMed] [Google Scholar]
- 8.Chang RB, Strochlic DE, Williams EK, Umans BD, and Liberles SD, Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell, 2015. 161(3): p. 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott SL, Umans BD, Williams EK, Brust RD, and Liberles SD, An Airway Protection Program Revealed by Sweeping Genetic Control of Vagal Afferents. Cell, 2020. 181(3): p. 574–589.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzone SB and Undem BJ, Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev, 2016. 96(3): p. 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker CV and Bronner-Fraser M, Vertebrate cranial placodes I. Embryonic induction. Dev Biol, 2001. 232(1): p. 1–61. [DOI] [PubMed] [Google Scholar]
- 12.Katz DM and Karten HJ, Substance P in the vagal sensory ganglia: localization in cell bodies and pericellular arborizations. J Comp Neurol, 1980. 193(2): p. 549–64. [DOI] [PubMed] [Google Scholar]
- 13.Keller JT, Saunders MC, Beduk A, and Jollis JG, Innervation of the posterior fossa dura of the cat. Brain Res Bull, 1985. 14(1): p. 97–102. [DOI] [PubMed] [Google Scholar]
- 14.Sato D, Sato T, Urata Y, Okajima T, Kawamura S, Kurita M, Takahashi K, Nanno M, Watahiki A, Kokubun S, Shimizu Y, Kasahara E, Shoji N, Sasano T, and Ichikawa H, Distribution of TRPVs, P2X3, and parvalbumin in the human nodose ganglion. Cell Mol Neurobiol, 2014. 34(6): p. 851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollarik M, Ru F, and Undem BJ, Phenotypic distinctions between the nodose and jugular TRPV1-positive vagal sensory neurons in the cynomolgus monkey. Neuroreport, 2019. 30(8): p. 533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atsumi K, Yajima T, Tachiya D, Kokubun S, Shoji N, Sasano T, Ichikawa H, and Sato T, Sensory neurons in the human jugular ganglion. Tissue Cell, 2020. 64: p. 101344. [DOI] [PubMed] [Google Scholar]
- 17.Helke CJ and Hill KM, Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience, 1988. 26(2): p. 539–51. [DOI] [PubMed] [Google Scholar]
- 18.Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, and Undem BJ, Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol, 2010. 588(Pt 23): p. 4769–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGovern AE, Driessen AK, Simmons DG, Powell J, Davis-Poynter N, Farrell MJ, and Mazzone SB, Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci, 2015. 35(18): p. 7041–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Hadley SH, Maddison M, Patil M, Cha BJ, Kollarik M, and Taylor-Clark TE, Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for Pirt, TRPV1, 5HT3 and Tac1 expression. eNeuro, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, and Undem BJ, Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol, 2004. 557(Pt 2): p. 543–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narayanan CH and Narayanan Y, Neural crest and placodal contributions in the development of the glossopharyngeal-vagal complex in the chick. Anat Rec, 1980. 196(1): p. 71–82. [DOI] [PubMed] [Google Scholar]
- 23.Ayer-Le Lievre CS and Le Douarin NM, The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Dev Biol, 1982. 94(2): p. 291–310. [DOI] [PubMed] [Google Scholar]
- 24.Lee HY, Kléber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, and Sommer L, Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science, 2004. 303(5660): p. 1020–3. [DOI] [PubMed] [Google Scholar]
- 25.D’Autréaux F, Coppola E, Hirsch MR, Birchmeier C, and Brunet JF, Homeoprotein Phox2b commands a somatic-to-visceral switch in cranial sensory pathways. Proc Natl Acad Sci U S A, 2011. 108(50): p. 20018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YC, Auer-Grumbach M, Matsukawa S, Zitzelsberger M, Themistocleous AC, Strom TM, Samara C, Moore AW, Cho LT, Young GT, Weiss C, Schabhüttl M, Stucka R, Schmid AB, Parman Y, Graul-Neumann L, Heinritz W, Passarge E, Watson RM, Hertz JM, Moog U, Baumgartner M, Valente EM, Pereira D, Restrepo CM, Katona I, Dusl M, Stendel C, Wieland T, Stafford F, Reimann F, von Au K, Finke C, Willems PJ, Nahorski MS, Shaikh SS, Carvalho OP, Nicholas AK, Karbani G, McAleer MA, Cilio MR, McHugh JC, Murphy SM, Irvine AD, Jensen UB, Windhager R, Weis J, Bergmann C, Rautenstrauss B, Baets J, De Jonghe P, Reilly MM, Kropatsch R, Kurth I, Chrast R, Michiue T, Bennett DL, Woods CG, and Senderek J, Transcriptional regulator PRDM12 is essential for human pain perception. Nat Genet, 2015. 47(7): p. 803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, and Ernfors P, Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci, 2015. 18(1): p. 145–53. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Kollarik M, Ru F, Sun H, McNeil B, Dong X, Stephens G, Korolevich S, Brohawn P, Kolbeck R, and Undem B, Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One, 2017. 12(10): p. e0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kupari J, Haring M, Agirre E, Castelo-Branco G, and Ernfors P, An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep, 2019. 27(8): p. 2508–2523.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzone SB, Tian L, Moe AAK, Trewella MW, Ritchie ME, and McGovern AE, Transcriptional Profiling of Individual Airway Projecting Vagal Sensory Neurons. Mol Neurobiol, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Pattyn A, Morin X, Cremer H, Goridis C, and Brunet JF, Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development, 1997. 124(20): p. 4065–75. [DOI] [PubMed] [Google Scholar]
- 32.Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, and Brunet JF, Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron, 1997. 18(3): p. 411–23. [DOI] [PubMed] [Google Scholar]
- 33.Hooper JS, Hadley SH, Morris KF, Breslin JW, Dean JB, and Taylor-Clark TE, Characterization of cardiovascular reflexes evoked by airway stimulation with allylisothiocyanate, capsaicin, and ATP in Sprague-Dawley rats. J Appl Physiol (1985), 2016. 120(6): p. 580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, and Julius D, The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature, 1997. 389(6653): p. 816–24. [DOI] [PubMed] [Google Scholar]
- 35.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, and Julius D, Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science, 2000. 288(5464): p. 306–13. [DOI] [PubMed] [Google Scholar]
- 36.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, and Barnes PJ, Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol (1985), 1995. 79(4): p. 1082–7. [DOI] [PubMed] [Google Scholar]
- 37.Bolser DC, DeGennaro FC, O’Reilly S, McLeod RL, and Hey JA, Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP-99,994 and SR 48968, in the guinea-pig and cat. Br J Pharmacol, 1997. 121(2): p. 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzone SB and Canning BJ, Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol, 2002. 283(1): p. R86–98. [DOI] [PubMed] [Google Scholar]
- 39.Chou YL, Scarupa MD, Mori N, and Canning BJ, Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol, 2008. 295(5): p. R1572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brozmanova M, Mazurova L, Ru F, Tatar M, and Kollarik M, Comparison of TRPA1-versus TRPV1-mediated cough in guinea pigs. Eur J Pharmacol, 2012. 689(1–3): p. 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherrington C, The integrative action of the nervous system. 1906: New Haven. [Google Scholar]
- 42.Kollarik M, Dinh QT, Fischer A, and Undem BJ, Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol, 2003. 551(Pt 3): p. 869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nassenstein C, Kammertoens T, Veres TZ, Uckert W, Spies E, Fuchs B, Krug N, and Braun A, Neuroimmune crosstalk in asthma: dual role of the neurotrophin receptor p75NTR. J Allergy Clin Immunol, 2007. 120(5): p. 1089–96. [DOI] [PubMed] [Google Scholar]
- 44.Ren J, Ding X, and Greer JJ, Mechanistic Studies of Capsaicin-Induced Apnea in Rodents. Am J Respir Cell Mol Biol, 2017. 56(2): p. 252–260. [DOI] [PubMed] [Google Scholar]
- 45.Kajekar R, Proud D, Myers AC, Meeker SN, and Undem BJ, Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther, 1999. 289(2): p. 682–7. [PubMed] [Google Scholar]
- 46.Lee MG, Macglashan DW Jr., and Undem BJ, Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol, 2005. 566(Pt 1): p. 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, and Undem BJ, Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol, 2008. 586(6): p. 1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donoghue S, Garcia M, Jordan D, and Spyer KM, The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol, 1982. 322: p. 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufman MP, Iwamoto GA, Ashton JH, and Cassidy SS, Responses to inflation of vagal afferents with endings in the lung of dogs. Circ Res, 1982. 51(4): p. 525–31. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, Wang YF, and Zhang JW, Structure of slowly adapting pulmonary stretch receptors in the lung periphery. J Appl Physiol, 2003. 95(1): p. 385–93. [DOI] [PubMed] [Google Scholar]
- 51.Davies RO and Kubin L, Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol, 1986. 373: p. 63–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies RO, Kubin L, and Pack AI, Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J Physiol, 1987. 383: p. 571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lieu TM, Myers AC, Meeker S, and Undem BJ, TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol, 2012. 302(9): p. L941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazzone SB, Reynolds SM, Mori N, Kollarik M, Farmer DG, Myers AC, and Canning BJ, Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci, 2009. 29(43): p. 13662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chuaychoo B, Hunter DD, Myers AC, Kollarik M, and Undem BJ, Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol, 2005. 116(2): p. 325–31. [DOI] [PubMed] [Google Scholar]
- 56.Mazzone SB and McGovern AE, Immunohistochemical characterization of nodose cough receptor neurons projecting to the trachea of guinea pigs. Cough, 2008. 4: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwong K, Kollarik M, Nassenstein C, Ru F, and Undem BJ, P2X2 Receptors Differentiate Placodal vs Neural Crest C-fiber Phenotypes Innervating Guinea Pig Lungs and Esophagus. Am J Physiol Lung Cell Mol Physiol, 2008. 295(5): p. L858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SH, Bahia PK, Patil M, Sutton S, Sowells I, Hadley SH, Kollarik M, and Taylor-Clark TE, Development of a mouse reporter strain for the purinergic P2X(2) receptor. eNeuro, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, and Humphrey PP, International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev, 2001. 53(1): p. 107–18. [PubMed] [Google Scholar]
- 60.Chuaychoo B, Lee MG, Kollarik M, Pullmann R Jr., and Undem BJ, Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol, 2006. 575(Pt 2): p. 481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chuaychoo B, Lee MG, Kollarik M, and Undem BJ, Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther, 2005. 18(4): p. 269–76. [DOI] [PubMed] [Google Scholar]
- 62.Potenzieri C, Meeker S, and Undem BJ, Activation of Mouse Bronchopulmonary C-fibers by Serotonin and Allergen-Ovalbumin Challenge. J Physiol, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lundberg JM, Brodin E, Hua X, and Saria A, Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand, 1984. 120(2): p. 217–27. [DOI] [PubMed] [Google Scholar]
- 64.Ellis JL, Undem BJ, Kays JS, Ghanekar SV, Barthlow HG, and Buckner CK, Pharmacological examination of receptors mediating contractile responses to tachykinins in airways isolated from human, guinea pig and hamster. J Pharmacol Exp Ther, 1993. 267(1): p. 95–101. [PubMed] [Google Scholar]
- 65.Zhuo H, Ichikawa H, and Helke CJ, Neurochemistry of the nodose ganglion. Prog Neurobiol, 1997. 52(2): p. 79–107. [DOI] [PubMed] [Google Scholar]
- 66.Hunter DD and Undem BJ, Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med, 1999. 159(6): p. 1943–8. [DOI] [PubMed] [Google Scholar]
- 67.Michael GJ and Priestley JV, Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci, 1999. 19(5): p. 1844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kashiba H, Uchida Y, and Senba E, Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain Res Mol Brain Res, 2003. 110(1): p. 52–62. [DOI] [PubMed] [Google Scholar]
- 69.Lieu T, Kollarik M, Myers AC, and Undem BJ, Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol, 2011. 300(5): p. L790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindsay RM, Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci, 1996. 351(1338): p. 365–73. [DOI] [PubMed] [Google Scholar]
- 71.Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, and Friedman M, Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol, 1998. 18(2): p. 149–57. [DOI] [PubMed] [Google Scholar]
- 72.Hunter DD, Myers AC, and Undem BJ, Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med, 2000. 161(6): p. 1985–90. [DOI] [PubMed] [Google Scholar]
- 73.Dinh QT, Groneberg DA, Peiser C, Springer J, Joachim RA, Arck PC, Klapp BF, and Fischer A, Nerve growth factor-induced substance P in capsaicin-insensitive vagal neurons innervating the lower mouse airway. Clin Exp Allergy, 2004. 34(9): p. 1474–9. [DOI] [PubMed] [Google Scholar]
- 74.Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, and Renz H, Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol, 2004. 141(3): p. 431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang G, Lin RL, Wiggers M, Snow DM, and Lee LY, Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol, 2008. 586(Pt 23): p. 5771–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, and Fischer A, Airway hyper-responsiveness in allergic asthma in guinea-pigs is mediated by nerve growth factor via the induction of substance P: a potential role for trkA. Clin Exp Allergy, 2006. 36(9): p. 1192–200. [DOI] [PubMed] [Google Scholar]
- 77.Carr MJ, Hunter DD, Jacoby DB, and Undem BJ, Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med, 2002. 165(8): p. 1071–5. [DOI] [PubMed] [Google Scholar]
- 78.Braun A, Quarcoo D, Schulte-Herbruggen O, Lommatzsch M, Hoyle G, and Renz H, Nerve growth factor induces airway hyperresponsiveness in mice. Int Arch Allergy Immunol, 2001. 124(1–3): p. 205–7. [DOI] [PubMed] [Google Scholar]
- 79.Kummer W, Fischer A, Kurkowski R, and Heym C, The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience, 1992. 49(3): p. 715–37. [DOI] [PubMed] [Google Scholar]
- 80.Groth M, Helbig T, Grau V, Kummer W, and Haberberger RV, Spinal afferent neurons projecting to the rat lung and pleura express acid sensitive channels. Respir Res, 2006. 7: p. 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh EJ, Mazzone SB, Canning BJ, and Weinreich D, Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol, 2006. 573(Pt 2): p. 549–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Driessen AK, Farrell MJ, Dutschmann M, Stanic D, McGovern AE, and Mazzone SB, Reflex regulation of breathing by the paratrigeminal nucleus via multiple bulbar circuits. Brain Struct Funct, 2018. 223(9): p. 4005–4022. [DOI] [PubMed] [Google Scholar]
- 83.Kubin L, Kimura H, and Davies RO, The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. J Physiol, 1991. 435: p. 207–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalia M and Sullivan JM, Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol, 1982. 211(3): p. 248–65. [DOI] [PubMed] [Google Scholar]
- 85.Driessen AK, Farrell MJ, Mazzone SB, and McGovern AE, The Role of the Paratrigeminal Nucleus in Vagal Afferent Evoked Respiratory Reflexes: A Neuroanatomical and Functional Study in Guinea Pigs. Front Physiol, 2015. 6: p. 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lorez HP, Haeusler G, and Aeppli L, Substance P neurones in medullary baroreflex areas and baroreflex function of capsaicin-treated rats. Comparison with other primary afferent systems. Neuroscience, 1983. 8(3): p. 507–23. [DOI] [PubMed] [Google Scholar]
- 87.Helke CJ, Shults CW, Chase TN, and O’Donohue TL, Autoradiographic localization of substance P receptors in rat medulla: effect of vagotomy and nodose ganglionectomy. Neuroscience, 1984. 12(1): p. 215–23. [DOI] [PubMed] [Google Scholar]
- 88.Takano Y, Nagashima A, Kamiya H, Kurosawa M, and Sato A, Well-maintained reflex responses of sympathetic nerve activity to stimulation of baroreceptor, chemoreceptor and cutaneous mechanoreceptors in neonatal capsaicin-treated rats. Brain Res, 1988. 445(1): p. 188–92. [PubMed] [Google Scholar]
- 89.Mazzone SB, Mori N, and Canning BJ, Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol, 2005. 569(Pt 2): p. 559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazzone SB and Geraghty DP, Respiratory action of capsaicin microinjected into the nucleus of the solitary tract: involvement of vanilloid and tachykinin receptors. Br J Pharmacol, 1999. 127(2): p. 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, and Bonham AC, Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am J Respir Crit Care Med, 2004. 169(4): p. 499–504. [DOI] [PubMed] [Google Scholar]
- 92.Mazzone SB and Geraghty DP, Respiratory actions of tachykinins in the nucleus of the solitary tract: characterization of receptors using selective agonists and antagonists. Br J Pharmacol, 2000. 129(6): p. 1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paton JF, Importance of neurokinin-1 receptors in the nucleus tractus solitarii of mice for the integration of cardiac vagal inputs. Eur J Neurosci, 1998. 10(7): p. 2261–75. [DOI] [PubMed] [Google Scholar]
- 94.Jin YH, Bailey TW, Li BY, Schild JH, and Andresen MC, Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci, 2004. 24(20): p. 4709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doyle MW, Bailey TW, Jin YH, and Andresen MC, Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci, 2002. 22(18): p. 8222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McGovern AE, Davis-Poynter N, Rakoczy J, Phipps S, Simmons DG, and Mazzone SB, Anterograde neuronal circuit tracing using a genetically modified herpes simplex virus expressing EGFP. J Neurosci Methods, 2012. 209(1): p. 158–67. [DOI] [PubMed] [Google Scholar]
- 97.McGovern AE, Davis-Poynter N, Yang SK, Simmons DG, Farrell MJ, and Mazzone SB, Evidence for multiple sensory circuits in the brain arising from the respiratory system: an anterograde viral tract tracing study in rodents. Brain Struct Funct, 2015. 220(6): p. 3683–99. [DOI] [PubMed] [Google Scholar]
- 98.Canning BJ and Mori N, An essential component to brainstem cough gating identified in anesthetized guinea pigs. Faseb J, 2010. 24(10): p. 3916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Canning BJ and Mori N, Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol, 2011. 300(2): p. R369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonham AC, Coles SK, and McCrimmon DR, Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J Physiol, 1993. 464: p. 725–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mutolo D, Bongianni F, Cinelli E, and Pantaleo T, Role of excitatory amino acids in the mediation of tracheobronchial cough induced by citric acid inhalation in the rabbit. Brain Res Bull, 2009. 80(1–2): p. 22–9. [DOI] [PubMed] [Google Scholar]
- 102.Driessen AK, McGovern AE, Behrens R, Moe AAK, Farrell MJ, and Mazzone SB, A role for neurokinin 1 receptor expressing neurons in the paratrigeminal nucleus in bradykinin-evoked cough in guinea-pigs. J Physiol, 2020. 598(11): p. 2257–2275. [DOI] [PubMed] [Google Scholar]
- 103.Scott GD, Blum ED, Fryer AD, and Jacoby DB, Tissue optical clearing, three-dimensional imaging, and computer morphometry in whole mouse lungs and human airways. Am J Respir Cell Mol Biol, 2014. 51(1): p. 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.West PW, Canning BJ, Merlo-Pich E, Woodcock AA, and Smith JA, Morphologic Characterization of Nerves in Whole-Mount Airway Biopsies. Am J Respir Crit Care Med, 2015. 192(1): p. 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larson SD, Schelegle ES, Hyde DM, and Plopper CG, The three-dimensional distribution of nerves along the entire intrapulmonary airway tree of the adult rat and the anatomical relationship between nerves and neuroepithelial bodies. Am J Respir Cell Mol Biol, 2003. 28(5): p. 592–9. [DOI] [PubMed] [Google Scholar]
- 106.Van Genechten J, Brouns I, Burnstock G, Timmermans JP, and Adriaensen D, Quantification of neuroepithelial bodies and their innervation in fawn-hooded and Wistar rat lungs. Am J Respir Cell Mol Biol, 2004. 30(1): p. 20–30. [DOI] [PubMed] [Google Scholar]
- 107.Adriaensen D, Timmermans JP, Brouns I, Berthoud HR, Neuhuber WL, and Scheuermann DW, Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res, 1998. 293(3): p. 395–405. [DOI] [PubMed] [Google Scholar]
- 108.Brouns I, Oztay F, Pintelon I, De Proost I, Lembrechts R, Timmermans JP, and Adriaensen D, Neurochemical pattern of the complex innervation of neuroepithelial bodies in mouse lungs. Histochem Cell Biol, 2009. 131(1): p. 55–74. [DOI] [PubMed] [Google Scholar]
- 109.Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans JP, and Adriaensen D, Dual sensory innervation of pulmonary neuroepithelial bodies. Am J Respir Cell Mol Biol, 2003. 28(3): p. 275–85. [DOI] [PubMed] [Google Scholar]
- 110.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, and Sun X, Pulmonary neuroendocrine cells amplify allergic asthma responses. Science, 2018. 360(6393). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brouns I, Pintelon I, De Proost I, Alewaters R, Timmermans JP, and Adriaensen D, Neurochemical characterisation of sensory receptors in airway smooth muscle: comparison with pulmonary neuroepithelial bodies. Histochem Cell Biol, 2006. 125(4): p. 351–67. [DOI] [PubMed] [Google Scholar]
- 112.Mazzone SB, Lim LH, Wagner EM, Mori N, and Canning BJ, Sympathetic nerve-dependent regulation of mucosal vascular tone modifies airway smooth muscle reactivity. J Appl Physiol (1985), 2010. 109(5): p. 1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Canning BJ and Fischer A, Localization of cholinergic nerves in lower airways of guinea pigs using antisera to choline acetyltransferase. Am J Physiol, 1997. 272(4 Pt 1): p. L731–8. [DOI] [PubMed] [Google Scholar]
- 114.Myers AC, Anatomical characteristics of tonic and phasic postganglionic neurons in guinea pig bronchial parasympathetic ganglia. J Comp Neurol, 2000. 419(4): p. 439–50. [PubMed] [Google Scholar]
- 115.Watanabe N, Horie S, Michael GJ, Spina D, Page CP, and Priestley JV, Immunohistochemical localization of vanilloid receptor subtype 1 (TRPV1) in the guinea pig respiratory system. Pulm Pharmacol Ther, 2005. 18(3): p. 187–97. [DOI] [PubMed] [Google Scholar]
- 116.Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, and Priestley JV, Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience, 2006. 141(3): p. 1533–43. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto Y, Sato Y, and Taniguchi K, Distribution of TRPV1- and TRPV2-immunoreactive afferent nerve endings in rat trachea. J Anat, 2007. 211(6): p. 775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Inoue N, Magari S, and Sakanaka M, Distribution of peptidergic nerve fibers in rat bronchus-associated lymphoid tissue: light microscopic observations. Lymphology, 1990. 23(3): p. 155–60. [PubMed] [Google Scholar]
- 119.Dey RD, Altemus JB, Zervos I, and Hoffpauir J, Origin and colocalization of CGRP- and SP-reactive nerves in cat airway epithelium. J Appl Physiol (1985), 1990. 68(2): p. 770–8. [DOI] [PubMed] [Google Scholar]
- 120.Baluk P and Gabella G, Afferent nerve endings in the tracheal muscle of guinea-pigs and rats. Anat Embryol (Berl), 1991. 183(1): p. 81–7. [DOI] [PubMed] [Google Scholar]
- 121.Baluk P, Nadel JA, and McDonald DM, Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol, 1992. 319(4): p. 586–98. [DOI] [PubMed] [Google Scholar]
- 122.Corcoran BM, Distribution of calcitonin gene-related peptide, vasoactive intestinal polypeptide, neuropeptide Y, substance P and dopamine beta-hydroxylase immunoreactive nerve fibres in the trachea of sheep. Res Vet Sci, 1996. 60(1): p. 69–75. [DOI] [PubMed] [Google Scholar]
- 123.Canning BJ, Reynolds SM, Anukwu LU, Kajekar R, and Myers AC, Endogenous neurokinins facilitate synaptic transmission in guinea pig airway parasympathetic ganglia. Am J Physiol Regul Integr Comp Physiol, 2002. 283(2): p. R320–30. [DOI] [PubMed] [Google Scholar]
- 124.Lamb JP and Sparrow MP, Three-dimensional mapping of sensory innervation with substance p in porcine bronchial mucosa: comparison with human airways. Am J Respir Crit Care Med, 2002. 166(9): p. 1269–81. [DOI] [PubMed] [Google Scholar]
- 125.Kajekar R and Myers AC, Calcitonin gene-related peptide affects synaptic and membrane properties of bronchial parasympathetic neurons. Respir Physiol Neurobiol, 2008. 160(1): p. 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takahashi N, Nakamuta N, and Yamamoto Y, Morphology of P2X3-immunoreactive nerve endings in the rat laryngeal mucosa. Histochem Cell Biol, 2016. 145(2): p. 131–46. [DOI] [PubMed] [Google Scholar]
- 127.Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, and Patapoutian A, Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature, 2017. 541(7636): p. 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu J, Zhang J, Wang Y, Fan F, and Yu A, Neuroepithelial bodies not connected to pulmonary slowly adapting stretch receptors. Respir Physiol Neurobiol, 2004. 144(1): p. 1–14. [DOI] [PubMed] [Google Scholar]
- 129.Hooper JS, Stanford KR, Alencar PA, Alves NG, Breslin JW, Dean JB, Morris KF, and Taylor-Clark TE, Nociceptive pulmonary-cardiac reflexes are altered in the Spontaneously Hypertensive rat. J Physiol, 2019. 597(13): p. 3255–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Canning BJ, Farmer DG, and Mori N, Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol, 2006. 291(2): p. R454–63. [DOI] [PubMed] [Google Scholar]
- 131.Muroi Y, Ru F, Chou YL, Carr MJ, Undem BJ, and Canning BJ, Selective inhibition of vagal afferent nerve pathways regulating cough using Nav 1.7 shRNA silencing in guinea pig nodose ganglia. Am J Physiol Regul Integr Comp Physiol, 2013. 304(11): p. R1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bolser DC, Aziz SM, and Chapman RW, Ruthenium red decreases capsaicin and citric acid-induced cough in guinea pigs. Neurosci Lett, 1991. 126(2): p. 131–3. [DOI] [PubMed] [Google Scholar]
- 133.Laude EA, Higgins KS, and Morice AH, A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol, 1993. 6(3): p. 171–5. [DOI] [PubMed] [Google Scholar]
- 134.Tanaka M and Maruyama K, Mechanisms of capsaicin- and citric-acid-induced cough reflexes in guinea pigs. J Pharmacol Sci, 2005. 99(1): p. 77–82. [DOI] [PubMed] [Google Scholar]
- 135.Andre E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, and Geppetti P, Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol, 2009. 158(6): p. 1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, and Morice AH, TRPA1 Agonists Evoke Coughing in Guinea-pig and Human Volunteers. Am J Respir Crit Care Med, 2009. 180(11): p. 1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grace M, Birrell MA, Dubuis E, Maher SA, and Belvisi MG, Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax, 2012. 67(10): p. 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chou YL, Mori N, and Canning BJ, Opposing effects of bronchopulmonary C-fiber subtypes on cough in guinea pigs. Am J Physiol Regul Integr Comp Physiol, 2018. 314(3): p. R489–r498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Palecek F, Sant’Ambrogio G, Sant’Ambrogio FB, and Mathew OP, Reflex responses to capsaicin: intravenous, aerosol, and intratracheal administration. J Appl Physiol, 1989. 67(4): p. 1428–37. [DOI] [PubMed] [Google Scholar]
- 140.Clifford PS, Litzow JT, and Coon RL, Pulmonary depressor reflex elicited by capsaicin in conscious intact and lung-denervated dogs. Am J Physiol, 1987. 252(2 Pt 2): p. R394–7. [DOI] [PubMed] [Google Scholar]
- 141.Paintal AS, Vagal sensory receptors and their reflex effects. Physiol Rev, 1973. 53(1): p. 159–227. [DOI] [PubMed] [Google Scholar]
- 142.Roberts AM, Bhattacharya J, Schultz HD, Coleridge HM, and Coleridge JC, Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res, 1986. 58(4): p. 512–22. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Z, Zhuang J, Zhang C, and Xu F, Isoflurane inhibits bronchopulmonary C-fiber-mediated apneic response to phenylbiguanide by depressing 5-HT3 receptor function in anesthetized rats. Neurosci Lett, 2013. 552: p. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hsu CC, Ruan T, Lee LY, and Lin YS, Stimulatory Effect of 5-Hydroxytryptamine (5-HT) on Rat Capsaicin-Sensitive Lung Vagal Sensory Neurons via Activation of 5-HT(3) Receptors. Front Physiol, 2019. 10: p. 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang JW, Walker JF, Guardiola J, and Yu J, Pulmonary sensory and reflex responses in the mouse. J Appl Physiol (1985), 2006. 101(3): p. 986–92. [DOI] [PubMed] [Google Scholar]
- 146.Han L, Limjunyawong N, Ru F, Li Z, Hall OJ, Steele H, Zhu Y, Wilson J, Mitzner W, Kollarik M, Undem BJ, Canning BJ, and Dong X, Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness. Nat Neurosci, 2018. 21(3): p. 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Diamond L and O’Donnell M, A nonadrenergic vagal inhibitory pathway to feline airways. Science, 1980. 208(4440): p. 185–8. [DOI] [PubMed] [Google Scholar]
- 148.Canning BJ and Undem BJ, Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of guinea-pig trachealis. J Physiol, 1993. 471: p. 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coleridge HM, Coleridge JC, and Schultz HD, Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol Ther, 1989. 42(1): p. 1–63. [DOI] [PubMed] [Google Scholar]
- 150.Canning BJ, Reflex regulation of airway smooth muscle tone. J Appl Physiol, 2006. 101(3): p. 971–85. [DOI] [PubMed] [Google Scholar]
- 151.Canning BJ, Reynolds SM, and Mazzone SB, Multiple mechanisms of reflex bronchospasm in guinea pigs. J Appl Physiol (1985), 2001. 91(6): p. 2642–53. [DOI] [PubMed] [Google Scholar]
- 152.Karczewski W and Widdicombe JG, The role of the vagus nerves in the respiratory and circulatory responses to intravenous histamine and phenyl diguanide in rabbits. J Physiol, 1969. 201(2): p. 271–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boushey HA, Richardson PS, and Widdicombe JG, Reflex effects of laryngeal irritation on the pattern of breathing and total lung resistance. J Physiol, 1972. 224(2): p. 501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szarek JL, Zhang JZ, and Gruetter CA, Mechanisms of 5-hydroxytryptamine-induced contraction of isolated rat intrapulmonary bronchi. Pulm Pharmacol, 1995. 8(6): p. 273–81. [DOI] [PubMed] [Google Scholar]
- 155.Joos G, Kips J, Pauwels R, and van der Straeten M, The effect of tachykinins on the conducting airways of the rat. Arch Int Pharmacodyn Ther, 1986. 280(2 Suppl): p. 176–90. [PubMed] [Google Scholar]
- 156.Ellis JL, Sham JS, and Undem BJ, Tachykinin-independent effects of capsaicin on smooth muscle in human isolated bronchi. Am J Respir Crit Care Med, 1997. 155(2): p. 751–5. [DOI] [PubMed] [Google Scholar]
- 157.Ellis JL and Undem BJ, Non-adrenergic, non-cholinergic contractions in the electrically field stimulated guinea-pig trachea. Br J Pharmacol, 1990. 101(4): p. 875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ellis JL and Undem BJ, Inhibition by capsazepine of resiniferatoxin- and capsaicin-induced contractions of guinea pig trachea. J Pharmacol Exp Ther, 1994. 268(1): p. 85–9. [PubMed] [Google Scholar]
- 159.Canning BJ and Undem BJ, Evidence that antidromically stimulated vagal afferents activate inhibitory neurones innervating guinea-pig trachealis. J Physiol, 1994. 480 (Pt 3): p. 613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rogers DF and Barnes PJ, Opioid inhibition of neurally mediated mucus secretion in human bronchi. Lancet, 1989. 1(8644): p. 930–2. [DOI] [PubMed] [Google Scholar]
- 161.Davis B, Roberts AM, Coleridge HM, and Coleridge JC, Reflex tracheal gland secretion evoked by stimulation of bronchial C-fibers in dogs. J Appl Physiol Respir Environ Exerc Physiol, 1982. 53(4): p. 985–91. [DOI] [PubMed] [Google Scholar]
- 162.Schultz HD, Roberts AM, Bratcher C, Coleridge HM, Coleridge JC, and Davis B, Pulmonary C-fibers reflexly increase secretion by tracheal submucosal glands in dogs. J Appl Physiol (1985), 1985. 58(3): p. 907–10. [DOI] [PubMed] [Google Scholar]
- 163.Khansaheb M, Choi JY, Joo NS, Yang YM, Krouse M, and Wine JJ, Properties of substance P-stimulated mucus secretion from porcine tracheal submucosal glands. Am J Physiol Lung Cell Mol Physiol, 2011. 300(3): p. L370–9. [DOI] [PubMed] [Google Scholar]
- 164.Karmouty-Quintana H, Cannet C, Sugar R, Fozard JR, Page CP, and Beckmann N, Capsaicin-induced mucus secretion in rat airways assessed in vivo and non-invasively by magnetic resonance imaging. Br J Pharmacol, 2007. 150(8): p. 1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramnarine SI, Hirayama Y, Barnes PJ, and Rogers DF, ‘Sensory-efferent’ neural control of mucus secretion: characterization using tachykinin receptor antagonists in ferret trachea in vitro. Br J Pharmacol, 1994. 113(4): p. 1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rogers DF, Neurokinin receptors subserving airways secretion. Can J Physiol Pharmacol, 1995. 73(7): p. 932–9. [DOI] [PubMed] [Google Scholar]
- 167.Shanks J, Xia Z, Lisco SJ, Rozanski GJ, Schultz HD, Zucker IH, and Wang HJ, Sympatho-excitatory response to pulmonary chemosensitive spinal afferent activation in anesthetized, vagotomized rats. Physiol Rep, 2018. 6(12): p. e13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Adam RJ, Xia Z, Pravoverov K, Hong J, Case AJ, Schultz HD, Lisco SJ, Zucker IH, and Wang HJ, Sympathoexcitation in response to cardiac and pulmonary afferent stimulation of TRPA1 channels is attenuated in rats with chronic heart failure. Am J Physiol Heart Circ Physiol, 2019. 316(4): p. H862–h872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trankner D, Hahne N, Sugino K, Hoon MA, and Zuker C, Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A, 2014. 111(31): p. 11515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McAlexander MA, Gavett SH, Kollarik M, and Undem BJ, Vagotomy reverses established allergen-induced airway hyperreactivity to methacholine in the mouse. Respir Physiol Neurobiol, 2015. 212–214: p. 20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, and Kaufman JD, Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation, 2010. 121(21): p. 2331–78. [DOI] [PubMed] [Google Scholar]
- 172.Robinson RK, Birrell MA, Adcock JJ, Wortley MA, Dubuis ED, Chen S, McGilvery CM, Hu S, Shaffer MSP, Bonvini SJ, Maher SA, Mudway IS, Porter AE, Carlsten C, Tetley TD, and Belvisi MG, Mechanistic link between diesel exhaust particles and respiratory reflexes. J Allergy Clin Immunol, 2018. 141(3): p. 1074–1084.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Deering-Rice CE, Memon T, Lu Z, Romero EG, Cox J, Taylor-Clark T, Veranth JM, and Reilly CA, Differential Activation of TRPA1 by Diesel Exhaust Particles: Relationships between Chemical Composition, Potency, and Lung Toxicity. Chem Res Toxicol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


