Abstract
The current study examined the feasibility and acceptability of an integrated Cognitive-Behavioral Social Skills Training and Compensatory Cognitive Training (CBSST-CCT) intervention compared with Goal-Focused Supportive Contact (SC) in a pilot randomized controlled trial for people with schizophrenia with high negative symptom severity. The sample included 55 participants from five community settings; masters-level study clinicians delivered interventions on-site. Participants completed assessments of cognitive, functional, and psychiatric symptoms at baseline, mid-treatment, post-treatment (12.5 weeks), and 6-month follow-up. Enrollment goals were not initially met, necessitating the addition of a fifth site; however, all groups and assessments were completed on-site. Study procedures were acceptable, as evidenced by 100% enrollment and completion of baseline assessments following informed consent; however, over 1/3rd of participants dropped out. No modifications were necessary to the intervention procedures and CBSST-CCT fidelity ratings were acceptable. The intervention was deemed acceptable among participants who attended ≥1 session, as evidenced by similar attendance rates in CBSST-CCT compared to SC. Among CBSST-CCT participants, lower positive symptoms were significantly associated with better attendance. Overall, we found mixed evidence for the feasibility and acceptability of the CBSST-CCT protocol in people with schizophrenia with high negative symptoms. Challenges are highlighted and recommendations for future investigations are provided.
Keywords: psychosis, severe mental illness, cognitive rehabilitation, psychosocial intervention
1. Introduction
Schizophrenia affects more than 3 million Americans, costs more than $155 billion annually, and leads to profound deficits in everyday functioning (Cloutier et al., 2016). Negative symptoms such as amotivation, anhedonia, and blunted affect are highly prevalent (Bobes et al., 2010; Galderisi et al., 2013) and remain an unmet treatment need in many patients due to their link to poor functioning (Fenton and Mcglashan, 1991; Rabinowitz et al., 2012; Ventura et al., 2015, 2009) and quality of life (Meltzer et al., 1990; Narvaez et al., 2008; Norman et al., 2000; Wegener et al., 2005), combined with an unresponsiveness to pharmacological treatments (Buckley and Stahl, 2007; Mucci et al., 2017). As such, the NIMH-MATRICS Consensus Statement on Negative Symptoms (Kirkpatrick et al., 2006) emphasized the need to develop and test treatments for negative symptoms. Similarly, cognitive impairment is common in schizophrenia (Gonzalez-Pablos et al., 2019; Schaefer et al., 2013; Thompson et al., 2013) and is closely tied to negative symptoms and functional deficits (Green et al., 2004; Keefe and Harvey, 2012). Accordingly, the NIMH-MATRICS initiative highlights the need for treatments of cognitive impairment to improve daily functioning (Marder and Fenton, 2004).
The overall evidence regarding the efficacy of psychosocial treatments for negative symptoms of schizophrenia is limited as most trials have not specifically targeted negative symptoms (Jauhar et al., 2014; Velthorst et al., 2015). An emerging evidence-base focused on the target population (i.e., individuals with significant negative symptoms) supports the feasibility and acceptability of behavioral treatments, such as Cognitive Behavioral Therapy (CBT) and cognitive remediation, for negative symptoms (Choi et al., 2016; Farvod et al., 2019; Li et al., 2019; Mueller et al., 2017). A recent meta-analysis found small-to-moderate effects of these behavioral interventions on negative symptoms (Riehle et al., 2020), underscoring the need for further development of high-quality trials.
A potential method for boosting CBT effects on negative symptoms would be to pair CBT with a complementary intervention such as Social Skills Training (SST; Bellack, 2004), which may reduce negative symptoms (Turner et al., 2017), and it is possible that the bundling of interventions can have a stronger, synergistic impact. Cognitive-Behavioral Social Skills Training (CBSST) and Compensatory Cognitive Training (CCT) are two interventions that have shown promise for treating negative symptoms and cognitive functioning (Granholm et al., 2002, 2005, 2007, 2013, 2014, 2018; Mahmood et al., 2018, 2020; McQuaid et al., 2000; Mendella et al., 2015; Twamley et al., 2012, 2019). CBSST integrates two evidence-based treatments for schizophrenia (CBT and SST) and has demonstrated clinically significant effect sizes in reducing negative symptoms (diminished motivation; d=.22 at post-treatment, d=.72 at follow up; Granholm et al., 2014). Clinically significant effect size improvements in negative symptom severity have also been demonstrated with CCT (d=.92 at post-treatment, d=.43 at follow-up), which uses compensatory strategy training and habit learning to improve cognition and everyday functioning (Twamley et al., 2012). Notably, both CBSST and CCT are highly driven by participants’ recovery goals and some evidence suggests that both CBT and cognitive remediation improve negative symptoms to a comparable degree (Riehle et al., 2020). Another trial examined CBT paired with restorative cognitive training in comparison to a) pure CBT and b) an active control group (Kukla et al., 2018). Although the authors did not target negative symptoms specifically, they did report differential improvements in the CBT + cognitive training group in work and neuropsychological outcomes. Neither CBSST nor CCT has been used to target negative symptoms as the primary outcome; however, both approaches reduce negative symptoms, have demonstrated feasibility and acceptability (Granholm et al., 2005, 2013; Mendella et al., 2015; Twamley et al., 2012, 2017), and are likely to have different mechanisms of action, thereby allowing for additive or synergistic treatment effects.
Given the heterogeneity of symptom presentation in schizophrenia, it is unlikely that a single intervention will work equally well for all patients. The CBT components of CBSST are used to target defeatist attitudes (e.g., “Why try, I’ll just fail again”), which have been linked to negative symptoms (Avery et al., 2009; Campellone et al., 2016; Grant and Beck, 2009; Horan et al., 2010; Rector et al., 2005), and the SST components promote social engagement and behavioral activation. CCT may also reduce defeatist attitudes and promote self-efficacy as participants learn that they can acquire new skills and succeed at tasks. Additionally, CCT may help participants learn and remember the CBSST skills. Research also demonstrates stronger effects of cognitive remediation interventions within a psychosocial rehabilitation context (Wykes et al., 2011) such as CBSST provides. Therefore, a combined CBSST-CCT approach may lead to greater reductions in negative symptoms than have been reported in prior psychosocial intervention trials.
We conducted a pilot randomized controlled trial of an integrated CBSST-CCT intervention compared with Goal-Focused Supportive Contact (SC) to target negative symptoms within a high-negative-symptom sample. Two novel intervention features - i.e., treatment in community environments (e.g., board-and-care homes, clubhouses) and use of masters-level clinicians - were expected to (1) improve session attendance in participants with high negative symptoms (e.g., amotivation) by bringing the intervention to a highly convenient place, (2) improve the transfer of new skills to the natural environment, and (3) increase the likelihood of intervention uptake in typical community mental health settings, which often rely on group treatments and masters-level clinicians. As such, the purpose of the current study is to report on the feasibility and acceptability of the trial procedures and the CBSST-CCT intervention. Evidence for feasibility and acceptability is particularly important in interventions for people with schizophrenia and high negative symptom severity, as amotivation and defeatist attitudes have the potential to interfere with study participation. Feasibility markers for the current study were those indicating that procedures could be completed, whereas acceptability markers were those indicating that participants would complete them. We hypothesized that 64 participants at four community sites would be enrolled in the trial, and at least 80% would complete the trial. Because the current study represents a pilot RCT, examining initial feasibility/acceptability of the intervention in order to inform future studies, we sought a sample size of 64 in order to achieve statistical power of .80 to detect a Cohen’s d effect size of at least 0.5. Additionally, predictors of treatment session attendance were explored. Primary outcome results from the current study will be described in a separate paper.
2. Methods
2.1. Participant characteristics
Study procedures were approved by the University of California San Diego Institutional Review Board (Clinical Trial registration number NCT02170051); all participants provided written informed consent after being informed that all sessions would be conducted in a group format. Participants were paid $30 for participating in each assessment and they were given a $20 bonus payment for completing all four assessments ($140 maximum). They were not paid for attending treatment sessions.
The sample included 55 participants (see Table 1 for demographic and clinical characteristics). Inclusion criteria were: (1) ability to provide voluntary informed consent, (2) Age 18 to 65, (3) DSM-IV diagnosis of schizophrenia or schizoaffective disorder based on Structured Clinical Interview for DSM-IV (First et al., 2002), (4) Moderate-to-severe negative symptoms on the Clinical Assessment Interview for Negative Symptoms (CAINS; total score >19; (Kring et al., 2013), (5) ≥ 6th grade reading level on the WRAT-4 Reading subtest, and (6) Stable on psychiatric medications for the past three months. Exclusion criteria were: (1) Prior CBT, SST, or CCT in the past 5 years, (2) Severe depression on the Calgary Depression Scale for Schizophrenia (CDSS >8), (3) Ocular damage/disease/surgery/medications that affect pupil dilation, (4) DSM-IV alcohol or substance dependence diagnosis in past 6 months, and (5) Level of care required precluded outpatient therapy (e.g., hospitalized; severe medical illness). The CONSORT diagram is presented in Figure 1.2.1
Table 1.
Participant Characteristics
| Baseline characteristic | CBSST-CCT n=26 | SC n=29 | Full sample N=55 | |||
|---|---|---|---|---|---|---|
| M±SD | Range | M±SD | Range | M±SD | Range | |
| Age, years | 47.73±11.36 | 22–63 | 53.24±7.35 | 28–65 | 50.64±9.77 | 22–65 |
| Education, years | 12.04±1.69 | 8–16 | 11.72±2.63 | 2–18 | 11.87±2.22 | 2–18 |
| Duration of illness (years) | 28.61±14.44 | 3–54 | 31.59±10.39 | 11–60 | 30.27±12.31 | 3–60 |
| n | % | n | % | n | % | |
| Gender | ||||||
| Female | 9 | 34.6 | 13 | 44.8 | 22 | 40 |
| Male | 17 | 65.4 | 16 | 55.2 | 33 | 60 |
| Ethnicity | ||||||
| Hispanic | 8 | 30.8 | 10 | 34.5 | 18 | 32.7 |
| Non-Hispanic | 18 | 69.2 | 19 | 65.5 | 37 | 67.3 |
| Race | ||||||
| African American | 11 | 42.3 | 4 | 13.8 | 15 | 27.3 |
| American Indian/Alaskan | 0 | 0 | 1 | 3.4 | 1 | 1.8 |
| Asian | 1 | 3.8 | 0 | 0 | 1 | 1.8 |
| Native Hawaiian/Pacific | 2 | 7.7 | 0 | 0 | 2 | 3.6 |
| Islander | ||||||
| White | 12 | 46.2 | 24 | 82.8 | 36 | 65.5 |
| Never married | 16 | 61.5 | 17 | 58.6 | 33 | 60 |
| Living independently | 17 | 65.4 | 17 | 58.6 | 34 | 61.8 |
| Diagnosis | ||||||
| Schizoaffective | 10 | 38.5 | 9 | 31 | 19 | 34.5 |
| Schizophrenia | 16 | 61.5 | 20 | 69 | 36 | 65.5 |
| Medication class | ||||||
| No atypical antipsychotics | 4 | 15.4 | 3 | 10.3 | 7 | 12.7 |
| Atypical antipsychotics | 17 | 65.4 | 18 | 62.1 | 35 | 63.6 |
Figure 1.
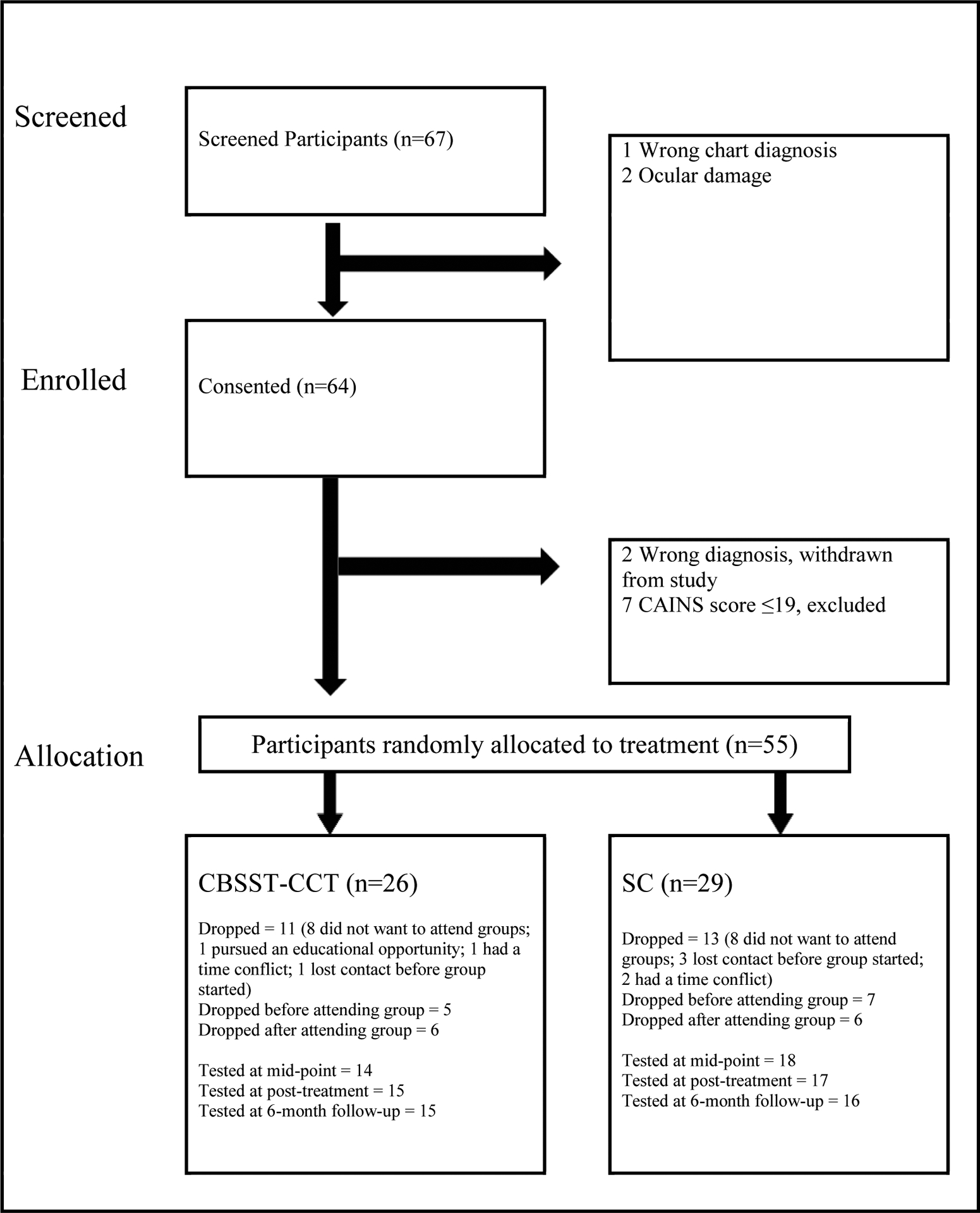
CONSORT Flow Diagram
2.2. CBSST-CCT
The CBSST-CCT intervention involved 25 twice-weekly 1-hour sessions (12.5 weeks total duration) of manualized group treatment. Table 2 details the integration of CBSST and CCT by content area. Briefly, CBSST (Granholm et al., 2016) was modified to strengthen its impact on negative symptoms as follows: (1) more extensive focus on challenging defeatist beliefs; (2) on-site therapy model and motivational interviewing techniques (e.g., using decisional balance sheets to facilitate discussion of the pros and cons of coming to group or working on goals); (3) more extensive targeting of affect expression and recognition in behavioral role plays in the SST module; and (4) inclusion of behavioral activation activities (e.g., scheduling pleasant activities; behavioral day scheduling) to the problem solving module. The three CBSST modules (i.e., cognitive skills, social skills, and problem-solving skills) were retained in their original order; however, an additional introductory module on cognitive training was added to the protocol. The purpose of beginning the intervention with this module was to introduce CCT strategies to bolster impaired functions in prospective memory, attention, and learning and memory, thereby enhancing attention to CBSST content, learning of content, and memory for content. CCT content was also integrated within the social skills module (conversational attention strategies) and problem-solving skills module (cognitive flexibility and planning strategies).
Table 2.
Integration of CBSST and CCT by Content Area
| CBSST | CCT | |
|---|---|---|
| Cognitive Training Module: 8 sessions | ||
| CCT domains and strategies: | ||
| Calendar use; to-do lists; prioritizing tasks; linking tasks by using planned cues; automatic places; using routines to automate tasks | ||
| Eye contact, paraphrasing, asking questions during conversations; taking breaks to refocus | ||
| Taking notes; paraphrasing and association; chunking; categorization; acronyms; visual imagery; overlearning | ||
| Cognitive Skills Module: 5 sessions | ||
| Introduce the general concepts of CBT, including the relationship between thoughts, actions and feelings, automatic thoughts, thought challenging by examining evidence for beliefs, and mistakes in thinking; Address symptoms and challenge defeatist beliefs that interfere with real-world skills execution; Help participants learn and remember to use their CBT skills | ||
| Social Skills Module: 6 sessions | ||
| Improve: (1) communication skills and psychosocial interactions (e.g., asking someone for support) via behavioral role plays; (2) expression of positive and negative feelings, making requests, comfort with sharing feelings, and communicating assertively; and (3) self-efficacy and defeatist performance beliefs | Teach and reinforce conversational attention skills (e.g., listening actively, eliminating distractions, asking questions, and paraphrasing [LEAP]) to improve cognitive aspects of communication and social communication | |
| Problem Solving Skills Module: 6 sessions | ||
| Improve basic problem-solving skills; Help participants develop plans to solve real-world problems and improve illness self-management; Behavioral activation to improve negative symptoms; Develop confidence in effective problem-solving | Teach cognitive flexibility and planning strategies in addition to problem-solving (CBSST and CCT use a very similar problem-solving strategy already); Using self-talk and self-monitoring while problem-solving; Hypothesis testing using pro and con evidence; Reinforce cognitive flexibility strategies to help participants realize when they should try a different strategy to achieve their goals; Use repetition and practice of executive skills to increase confidence | |
2.3. Goal-focused supportive contact (SC)
SC was used as a robust control condition, providing the same frequency and amount of contact with therapists and other group members as CBSST-CCT. The primary focus of the SC intervention was to help participants set and achieve recovery goals (e.g., living, learning, working, and socializing) to enhance motivation for treatment and reduce dropout in this population with high negative symptoms. Sessions were semi-structured and consisted of checkins about symptoms and potential crisis management, followed by a flexible discussion about setting and working toward recovery goals, with minimal therapist guidance. Sessions typically included therapist empathy and non-directive reinforcement of goal setting and planning to achieve goals. In order to ensure that SC did not overlap with CBSST-CCT, no specific training was provided in cognitive-behavioral coping strategies, social skills, problem solving, or cognitive strategies.
2.4. Therapists
The CBSST-CCT and SC interventions were delivered by two grant-funded masters-level clinicians in five community settings (a large board and care facility, a clubhouse, and three San Diego County-funded outpatient biopsychosocial rehabilitation mental health clinics). All CBSST and SC groups had two co-therapists and the same therapists delivered both interventions. Three therapists participated in the trial (two had master’s degrees in counseling and one had a master’s degree in psychology; all were unlicensed). One therapist co-facilitated groups at all five sites; another therapist co-facilitated the interventions at the first two locations, but had to leave the position due to family care responsibilities; and the third therapist co-facilitated at the last three locations. Following a one-day training workshop, the therapists received weekly supervision from authors EG and EWT.
2.5. Assessment protocol
Participants completed a 3-hour comprehensive assessment battery including diagnostic assessment, measures of neurocognitive and functional performance, psychiatric symptom severity, and pupillometry at baseline, mid-treatment, post-treatment, and 6-month follow-up (see Table 3 for a detailed list of measures and assessment timeline). Examiners administering the assessment battery were trained to a high degree of interrater reliability (.80 or higher) and were blinded to randomization status.
Table 3.
Assessment Protocol and Timeline
| Domain | Measure | Baseline | Midway | Post | 6-mo f/u |
|---|---|---|---|---|---|
| Diagnosis | Structured Clinical Interview for DSM-IV (First et al., 2002) | X | |||
| Premorbid IQ estimate | Wide Range Achievement Test-4 Reading (Wilkinson and Robertson, 2006) | X | |||
| Neurocognition | MATRICS Consensus Cognitive Battery (no MSCEIT) (Nuechterlein et al., 2008) | X | X | X | |
| Performance-based functional capacity | UCSD Performance-Based Skills Assessment - Brief (Mausbach et al., 2007) | X | X | X | |
| Performance-based social competence | Social Skills Performance Assessment (Patterson et al., 2001) | X | X | X | |
| Negative symptom severity | Clinical Assessment Interview for Negative Symptoms (Kring et al., 2013) | X | X | X | X |
| Scale for the Assessment of Negative Symptoms (Andreasen, 1984) | X | X | X | X | |
| Positive symptom severity | Brief Psychiatric Rating Scale - positive subscale (Overall and Gorham, 1962) | X | X | X | X |
| Depressive symptom severity | Calgary Depression Scale for Schizophrenia (Addington et al., 1990) | X | X | X | X |
| Insight | Birchwood Insight Scale (Birchwood et al., 1994) | X | X | X | X |
| Rehabilitation goals/milestones | Psychosocial Rehabilitation (PSR) Toolkit (Arns et al., 2001) | X | X | X | |
| Self-reported living skills | Specific Levels of Functioning Scale (Schneider and Struening, 1983) | X | X | X | X |
| Independent Living Skills Survey (Wallace et al., 2000) | X | X | X | X | |
| CBSST-CCT Skill Knowledge | Comprehensive Modules Test (Liberman et al., 1993) | X | X | X | X |
| Defeatist Attitudes | Defeatist Performance Attitudes Scale (15-item subscale of the Defeatist Attitude Scale; (Cane et al., 1986) | X | X | X | X |
| Asocial Belief Scale (Grant and Beck, 2010) | X | X | X | X | |
| Self-Efficacy Scale (19-item social factor) | X | X | X | X | |
| QLS (3 items on Intrinsic Motivation; (Fervaha et al., 2014) | X | X | X | X | |
| Pupil response, digit span recall | 6th-digit dilation amplitude | X | X | X | X |
| Pupil response | Light reflex task | X | X | X | X |
3. Results
3.1. Feasibility of study procedures
We operationalized feasibility of study procedures as the degree to which enrollment goals were met for each site and the ability to complete all assessments and groups on-site. Our initial target enrollment goal was 16 participants at each of four sites (a board and care facility, a clubhouse, and two outpatient biopsychosocial rehabilitation mental health clinics); we did not meet this target for any of the four sites, necessitating the addition of a fifth site (an additional San Diego County-funded outpatient mental health clinic). However, in support of feasibility, the providers who referred participants to the study understood the need for high negative symptom severity participants and most referred participants (86%) did indeed meet criteria for high negative symptom severity. All assessments and groups took place at the five sites, although study therapists reported space shortages and excessive noise at the board and care facility. Participants did not differ by site in terms of demographic characteristics. Overall, our findings provide mixed support for the feasibility of the study procedures.
3.2. Acceptability of study procedures
We defined acceptability of study procedures as a) the proportion of participants who elected to enroll in the study following consent procedures, b) the proportion of enrolled participants who completed baseline assessment procedures, and c) the number of participants who dropped out of the study across both intervention groups (defined as participants missing both post-treatment and follow-up assessments). All participants who met inclusion/exclusion criteria agreed to consent to the study (100% consent rate), and 100% of consented participants (55/55) completed baseline assessments. A similar proportion of participants dropped out of the study across both intervention groups (see Figure 1; dropout rate across both groups was 23/55; 41.8%). Overall, individuals who participated in the informed consent process enrolled in the study and completed their baseline assessments; however, a substantial subset of those participants did not complete the entire treatment protocol due to a variety of reasons, the most common of which was an unwillingness to participate in group treatment (see Figure 1). Out of the 220 possible assessment sessions, 148 (67.3%) were attended.
3.3. Feasibility and acceptability of the CBSST-CCT intervention
We defined intervention feasibility and acceptability as a) the degree to which the CBSST-CCT groups were delivered as planned at each of the five study locations, b) CBSST-CCT fidelity ratings, and c) treatment session attendance in CBSST-CCT compared to SC. No modifications to the CBSST-CCT manual, session length, or group duration were necessary, study costs met a priori expectations, and study therapists delivered 100% of sessions at all five sites. With regard to fidelity ratings, all CBSST-CCT and SC sessions were audio recorded, and a random 20% of the sessions were selected for fidelity rating by author DP, who was not involved with therapist supervision or assessment procedures. Fidelity was assessed with a measure adapted from the Cognitive Therapy Scale for Psychosis (CTS-Psy) (Haddock, Gillian, 2001) and the Social Skills Training Fidelity Scale (Bellack, 2004), and modified to include items specifically related to CCT and CBSST content, including maintaining focus on participants’ goals. Fidelity ratings for CBSST-CCT ranged from 73% to 82% across the five settings (M=77%). A score of 50% or more on the CTS-Psy has been viewed as indicating fidelity to cognitive behavior therapy for psychosis (Turkington et al., 2002). Fidelity ratings for SC ranged from 7% to 10% (M=9%), indicating that the treatments delivered in the two conditions were sufficiently distinct, as intended. Overall, CBSST-CCT was feasible to deliver as planned, with good fidelity.
Attendance did not differ by group assignment; CBSST-CCT participants attended 42.9% (SD=31.0%) of sessions, whereas SC participants attended 54.9% (SD=32.9%) of sessions, t(41)=1.23, p=.22. There were also no significant differences in attendance rates based on participant characteristics such as sex t(41)=1.23, p=.23, race (white vs non-white; t(41)=1.50, p=.14), and ethnicity (Hispanic vs non-Hispanic; t(41)=0.16, p=.88). Among study completers (those who completed ≥3 assessments), attendance rates were 56.5% in CBSST-CCT and 68% in SC, t(30)=1.29, p=.21. Duration of illness did not differ in study completers compared to non-completers, t(50)=0.35, p=.73.
Within each treatment group, Pearson and point biserial correlations were conducted to explore predictors of session attendance for participants who attended at least one treatment session (n=43). Less severe positive symptoms at baseline were associated with greater session attendance within the CBSST-CCT group (r=−.48, p=.029), whereas less severe depressive symptoms were associated with greater session attendance within the SC group (r=−.43, p=.048). No other significant associations were found (see Table 4 for correlation coefficients).
Table 4.
Pearson/Point Biserial Correlations Examining Predictors of Treatment Attendance (n=43)
| Session Attendance | ||||
|---|---|---|---|---|
| SC | ||||
| p | p | |||
| Age | .05 | .837 | .24 | .278 |
| Sex | −.40 | .072 | −.04 | .857 |
| Race/ethnicity | .08 | .737 | −.11 | .625 |
| Education (years) | .02 | .946 | .06 | .798 |
| WRAT reading | −.27 | .237 | −.29 | .191 |
| CAINS | −.22 | .333 | −.16 | .489 |
| SANS | −.28 | .219 | .17 | .440 |
| BPRS positive subscale | −.48 | .029 | .01 | .958 |
| CDSS | −.15 | .517 | −.43 | .048 |
| BIS | .27 | .235 | −.15 | .497 |
| UPSA-B | .02 | .926 | −.06 | .787 |
| SSPA | .29 | .206 | .13 | .557 |
| SLOF | .17 | .454 | .19 | .392 |
| ILSS | .10 | .652 | −.09 | .707 |
| MCCB global | .05 | .836 | −.07 | .749 |
| Intrinsic motivation | .29 | .202 | −.15 | .504 |
| DPAS | −.09 | .701 | .15 | .499 |
Note. Bold font denotes p<.05. BIS=Birchwood Insight Scale; BPRS=Brief Psychiatric Rating Scale; CAINS=Clinical Assessment Interview for Negative Symptoms; CDSS=Calgary Depression Scale for Schizophrenia; DPAS=Defeatist Performance Attitude Scale; ILSS=Independent Living Skills Survey; MCCB=MATRICS Consensus Cognitive Battery; SANS=Scale for the Assessment of Negative Symptoms; SLOF=Specific Level of Functioning; SSPA=Social Skills Performance Assessment; UPSA-B=UCSD Performance-based Skills Assessment; WRAT=Wide Range Achievement Test -Fourth Edition;.
4. Discussion
The goals of the integrated CBSST-CCT intervention are to facilitate recovery goal attainment by improving cognition and skill learning, decreasing severity of defeatist beliefs, and strengthening the impact of CBSST and CCT on negative symptoms (particularly amotivation and asociality). The current study specifically aimed to examine the feasibility and acceptability of this novel CBSST-CCT intervention in comparison to a Goal-Focused SC control condition in the context of a pilot randomized controlled trial conducted in individuals with schizophrenia and high negative symptom severity. Due to the difficulty in facilitating engagement and participation in the current study population, findings may inform planning (e.g., target sample size) and design (e.g., choice of real-world environments) of future RCTs in negative symptom-enriched samples.
Overall, our results provide mixed evidence regarding the feasibility and acceptability of study procedures and the CBSST-CCT intervention and highlight some of the challenges associated with conducting research in people with schizophrenia with high levels of negative symptoms. For example, we added a fifth community site due to failure to meet initial enrollment goals; however, 100% of those who participated in the informed consent process elected to enroll. Noise and space challenges occurred during interventions in the board and care setting, underscoring the need to strategize logistics and board and care operator buy-in prior to study procedures. These issues did not occur at the mental health clinics and clubhouse.
Across groups, 41.8% of participants dropped out of the study; the most common reason provided for dropout was a lack of desire to participate in group treatment. A systematic review evaluating studies that assessed the impact of psychosocial interventions on negative symptoms of schizophrenia reported that over three-quarters of the papers reviewed had dropout rates less than 20% (Elis et al., 2013). However, dropout was inconsistently defined across studies. Moreover, the interventions were not specifically designed to treat negative symptoms, with one exception (Klingberg et al., 2011), and only two required the presence of moderate negative symptom severity or higher for study enrollment (e.g., Grant et al., 2012; Klingberg et al., 2011). Consequently, the high severity of negative symptoms in the current study sample is unique, and this may partially explain our dropout rate. Importantly, several other randomized controlled trials in schizophrenia have reported similar dropout rates to the current study (46% in Granholm et al., 2014; 35% in Grant et al., 2012; 32% in Rector et al., 2003; 32% in Valmaggia et al., 2005), although treatments and follow-up periods were longer in these studies compared to the current study. With regard to community-based mental health settings specifically, Carrion and colleagues (1993) found that forgetting appointments, needing to work, and transportation-related difficulties most impacted retention rates in a study of outpatients with schizophrenia. It is possible that suboptimal public transportation also contributed to our dropout rate, given evidence for better session attendance in prior CBSST trials when transportation was provided (Granholm et al., 2013), compared to when it was not (Granholm et al., 2014). Overall, our rates of dropout and attendance align with high rates of nonadherence (17–64%) found in general community mental health clinic populations, including patients with psychosis (Cullen 2018; Dworkin et al., 1986; Üçok et al., 2007).
Session attendance did not differ statistically between CBSST-CCT (42.9% [31.0%]) and SC (54.9% [32.9%]), but this could have been due to a lack of power, as participants in the SC group attended an average of three more sessions (13.7/25) than did participants in the CBSST-CCT group (10.7/25). To the extent that this difference is clinically meaningful, it is not clear why attendance would be lower in CBSST-CCT participants. It is possible that greater cognitive demand in the CBSST-CCT group relative to the SC group influenced attendance rates.
Future studies of interventions for individuals with schizophrenia and elevated negative symptoms may benefit from including tasks measuring reward processing deficits as they relate to motivational impairments, intervention adherence, and study retention (Strauss et al., 2014). In addition to linking intervention strategies to personal goals, it is possible that motivational interviewing techniques could be used to boost treatment adherence (Rüsch & Corrigan, 2002; Steinberg et al., 2004). Future community-based intervention studies in people with schizophrenia may also enhance retention by taking financial and transportation barriers into consideration (Carrion et al., 1993).
We were able to deliver all sessions of CBSST-CCT as planned with high fidelity, lending support for the use of masters-level clinicians in such treatments, which could result in more accessible care in this population. Exploratory analyses determined that less severe positive symptoms and depressive symptoms at baseline were associated with higher treatment session attendance in CBSST-CCT and SC, respectively, with moderate to large effect sizes (r=−.48 and −.43, respectively). These results are consistent with those of prior studies (Nosé et al., 2003); but see also (Ruggeri et al., 2007). Concurrently targeting these psychiatric symptoms in future negative symptom trials may lead to direct health benefits, as well as treatment-associated improvements in outcomes via improved treatment adherence. Although it may appear surprising that negative symptoms did not correlate with session attendance, low variability in negative symptom severity in this sample (CAINS total score >19) may have artificially attenuated the relationship with session attendance.
In summary, our findings reflect the challenges of retaining participants and providing interventions to people with schizophrenia and severe negative symptoms in the community. Dropout rates may be minimized in future research by individualizing treatment and by using motivational interviewing techniques or other incentives. Overall, our preliminary results still provide some support for the feasibility and acceptability of the CBSST-CCT intervention itself, which may be integrated into existing services and delivered by masters-level providers. If the CBSST-CCT trial leads to positive outcomes, it could pave the way for a transformation in treatment of negative symptoms in schizophrenia. Due to the unmet need for negative symptom treatments and the potential for CBSST-CCT to reduce negative symptoms and improve everyday functioning in individuals with schizophrenia, future research implementing CBSST-CCT on a larger scale may be warranted.
Highlights.
New treatments are needed for the disabling negative symptoms of schizophrenia
We report on a pilot trial of CBSST-CCT, a new treatment for negative symptoms
CBSST-CCT was able to be delivered at community sites by masters-level clinicians
Study dropout rates were high, but CBSST-CCT adherence did not differ from controls
Lower positive symptom severity was associated with better CBSST-CCT attendance
Acknowledgement
The authors thank all the study participants for their contributions to this work.
Funding
This work was funded by the National Institute of Mental Health of the National Institutes of Health (R34 MH101250), which had no other role in this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Dr. Granholm has an equity interest in Granholm Consulting, Inc., a company that may potentially benefit from the research results as he receives income from the company for CBSST workshops and consulting. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
- Addington D, Addington J, Schissel B, 1990. A depression rating scale for schizophrenics. Schizophr. Res 3, 247–251. 10.1016/0920-9964(90)90005-R [DOI] [PubMed] [Google Scholar]
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, Knegtering H, 2017. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr. Res 186, 55–62. 10.1016/j.schres.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Arns P, Rogers ES, Cook J, Mowbray C, Committee, M. of the I.R., 2001. The IAPSRS Toolkit: Development, utility, and relation to other performance measurement systems. [DOI] [PubMed]
- Avery R, Startup M, Calabria K, 2009. The role of effort, cognitive expectancy appraisals and coping style in the maintenance of the negative symptoms of schizophrenia. Psychiatry Res. 167, 36–46. 10.1016/j.psychres.2008.04.016 [DOI] [PubMed] [Google Scholar]
- Bailer J, Takats I, Westermeier C, 2001. Efficacy of individualized cognitive-behavioral therapy for schizophrenic patients with negative symptoms and social disabilities: A controlled trial. Z. Klin. Psychol. Psychother 30, 268–278. [Google Scholar]
- Bellack A, 2004. Skills training for people with severe mental illness. Psychiatr. Rehabil. J 27, 375–391. 10.2975/27.2004.375.391 [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M, 1994. A self-report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr. Scand 89, 62–67. 10.1111/j.1600-0447.1994.tb01487.x [DOI] [PubMed] [Google Scholar]
- Bobes J, Arango C, Garcia-Garcia M, Rejas J, 2010. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: Findings from the CLAMORS study. J. Clin. Psychiatry 71, 280–286. 10.4088/JCP.08m04250yel [DOI] [PubMed] [Google Scholar]
- Buckley PF, & Stahl SM, 2007. Pharmacological treatment of negative symptoms of schizophrenia: Therapeutic opportunity or cul-de-sac? Acta Psychiatr. Scand 115, 93–100. 10.1111/j.1600-0447.2007.00992.x [DOI] [PubMed] [Google Scholar]
- Campellone TR, Sanchez AH, & Kring AM, 2016. Defeatist performance beliefs, negative symptoms, and functional outcome in Schizophrenia: A meta-analytic review. Schizophr. Bull 42, 1343–1352. 10.1093/schbul/sbw026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane DB, Olinger LJ, Gotlib IH, Kuiper NA, 1986. Factor structure of the dysfunctional attitude scale in a student population. J. Clin. Psychol 42, 307–309. [DOI] [Google Scholar]
- Carrion PG, Swann A, Kellert-Cecil H, Barber M, 1993. Compliance with clinical attendance by outpatients with schizophrenia. Psychiatr. Serv 44, 764–767. 10.1176/ps.44.8.764 [DOI] [PubMed] [Google Scholar]
- Cella M, Preti A, Edwards C, Dow T, Wykes T, 2017. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clin. Psychol. Rev 52, 43–51. 10.1016/j.cpr.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Choi K-H, Jaekal E, & Lee G-Y, 2016. Motivational and behavioral activation as an adjunct to psychiatric rehabilitation for mild to moderate negative symptoms in individuals with schizophrenia: A proof-of-concept pilot study. Front. Psychol 7 10.3389/fpsyg.2016.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier M, Aigbogun MS, Annie G, Nitulescu R, Ramanakumar AV, Kamat SA, DeLucia M, Duffy R, Legacy SN, Henderson C, Francois C, & Wu E, 2016. The economic burden of schizophrenia in the United States in 2013. J. Clin. Psychiatry 77, 764–771. 10.4088/JCP.15m10278 [DOI] [PubMed] [Google Scholar]
- Cullen BA, 2018. Altering the attendance rate successfully for new patients at an outpatient mental health clinic. Psychiatr. Serv 69, 1212–1214. 10.1176/appi.ps.201800161 [DOI] [PubMed] [Google Scholar]
- Daniels L, 1998. A group cognitive-behavioral and process-oriented approach to treating the social impairment and negative symptoms associated with chronic mental illness. J. Psychother. Pract. Res 7, 167–176. [PMC free article] [PubMed] [Google Scholar]
- Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, Lehman A, Tenhula WN, Calmes C, Pasillas RM, Peer J, Kreyenbuhl J, 2010. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr. Bull 36, 48–70. 10.1093/schbul/sbp115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RJ, Adams GL, Telschow RL, 1986. Cues of disability and treatment continuation of chronic schizophrenics. Soc. Sci. Med 22, 521–526. 10.1016/0277-9536(86)90018-3 [DOI] [PubMed] [Google Scholar]
- Elis O, Caponigro JM, Kring AM, 2013. Psychosocial treatments for negative symptoms in schizophrenia: Current practices and future directions. Clin. Psychol. Rev 33, 914–928. 10.1016/j.cpr.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton WS, & Mcglashan TH, 1991. Natural history of schizophrenia subtypes: II. Positive and negative symptoms and long-term course. Arch. Gen. Psychiatry 48, 978–986. 10.1001/archpsyc.1991.01810350018003 [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G, 2014. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr. Scand 130, 290–299. 10.1111/acps.12289 [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. SCID-I/P. [Google Scholar]
- Galderisi Silvana, Mucci A, Bitter I, Libiger Jan, Bucci P, Wolfgang Fleischhacker W, Kahn René S., Kahn RS, Fleischhacker WW, Boter H, Keet IPM, Brugman C, Davidson M, Dollfus S, Gaebel W, Galderisi S, Gheorghe M, Gonen I, Grobbee DE, Hranov LG, Hummer M, Libiger J, Lindefors N, López-Ibor JJ, Nijssen K, Peuskens J, Prelipceanu D, Riecher-Rössler A, Rybakowski JK, Sedvall G, Von Wilmsdorff M, 2013. Persistent negative symptoms in first episode patients with schizophrenia: Results from the European First Episode Schizophrenia Trial. Eur. Neuropsychopharmacol 23, 196–204. 10.1016/j.euroneuro.2012.04.019 [DOI] [PubMed] [Google Scholar]
- Gaynor K, Dooley B, Lawlor E, Lawoyin R, O’Callaghan E, 2011. Group cognitive behavioural therapy as a treatment for negative symptoms in first-episode psychosis: Group CBT in first-episode and stable psychosis. Early Interv. Psychiatry 5, 168–173. 10.1111/j.1751-7893.2011.00270.x [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pablos E, Sanguino-Andres R, Lopez-Villalobos JA, Polonia Iglesias-Santa F, Gonzalez-Sanguino C, 2019. Cognitive deterioration in schizophrenia: aging and cerebrovascular disease. Int. Psychogeriatr 31, 303–304. 10.1038/mp.2017.170 [DOI] [PubMed] [Google Scholar]
- Granholm E, Holden J, Link PC, McQuaid JR, Jeste DV, 2013. Randomized controlled trial of cognitive behavioral social skills training for older consumers with schizophrenia: defeatist performance attitudes and functional outcome. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 21, 251–62. 10.1097/JGP.0b013e31823e2f70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Holden J, Link PC, & McQuaidm JR, 2014. Randomized clinical trial of cognitive behavioral social skills training for schizophrenia: Improvement in functioning and experiential negative symptoms. J. Consult. Clin. Psychol 82, 1173–1185. 10.1037/a0037098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Holden J, & Worley M, 2018. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: Mediation by defeatist performance attitudes and asocial beliefs. Schizophr. Bull 44, 653–661. 10.1093/schbul/sbx099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Mcquaid JR, Mcclure FS, Auslander LA, Perivoliotis D, Pedrelli P, Patterson T, & Jeste DV, 2005. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am. J. Psychiatry, 520–529. 10.1176/appi.ajp.162.3.520 [DOI] [PubMed] [Google Scholar]
- Granholm E, Mcquaid JR, Mcclure FS, Link PC, Perivoliotis D, Gottlieb JD, Patterson TL, & Jeste DV, 2007. Randomized controlled trial of cognitive behavioral social skills training for older people with schizophrenia: 12-month follow-up. Journal of Clinical Psychiatry, 730–737. 10.4088/jcp.v68n0510 [DOI] [PubMed] [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Pedrelli P, Jeste DV, 2002. A randomized controlled pilot study of cognitive behavioral social skills training for older patients with schizophrenia. Schizophr. Res 53, 167–169. 10.1176/appi.ajp.162.3.520 [DOI] [PubMed] [Google Scholar]
- Granholm EL, McQuaid JR, Holden JL, 2016. Cognitive-Behavioral Social Skills Training for Schizophrenia: A Practical Treatment Guide. Guilford Publications. [Google Scholar]
- Grant PM, Beck AT, 2010. Asocial beliefs as predictors of asocial behavior in schizophrenia. Psychiatry Res. 177, 65–70. 10.1016/j.psychres.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Grant PM, Beck AT, 2009. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr. Bull 35, 798–806. 10.1093/schbul/sbn008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT, 2012. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch. Gen. Psychiatry 69, 121–127. 10.1001/archgenpsychiatry.2011.129 [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK, 2004. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophr. Res 72, 41–51. 10.1016/j.schres.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Haddock Gillian, 2001. An investigation into the psychometric properties of the cognitive therapy scale for psychosis (CTS-Psy). Behav. Cogn. Psychother 29, 221–223. 10.1017/S1352465801002089 [DOI] [Google Scholar]
- Horan WP, Rassovsky Y, Kern RS, Lee J, Wynn JK, & Green MF, 2010. Further support for the role of dysfunctional attitudes in models of real-world functioning in schizophrenia. J. Psychiatr. Res 44, 449–505. 10.1016/j.jpsychires.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR, 2014. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br. J. Psychiatry 204, 20–29. 10.1192/bjp.bp.112.116285 [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Harvey PD, 2012. Cognitive Impairment in Schizophrenia, in: Geyer MA, Gross G (Eds.), Novel Antischizophrenia Treatments. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 11–37. 10.1007/978-3-642-25758-2_2 [DOI] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR, 2006. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull 32, 214–219. 10.1093/schbul/sbj053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg S, Wölwer W, Engel C, Wittorf A, Herrlich J, Meisner C, Buchkremer G, Wiedemann G, 2011. Negative symptoms of schizophrenia as primary target of cognitive behavioral therapy: Results of the randomized clinical TONES study. Schizophr. Bull 37, S98–S110. 10.1093/schbul/sbr073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP, 2013. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. Schizophr. Res 1700, 165–172. 10.1176/appi.ajp.2012.12010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukla M, Bell MD, Lysaker PH, 2018. A randomized controlled trial examining a cognitive behavioral therapy intervention enhanced with cognitive remediation to improve work and neurocognition outcomes among persons with schizophrenia spectrum disorders. Schizophr. Res 197, 400–406. 10.1016/j.schres.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Liberman RP, Wallace CJ, Blackwell G, Wckman TA, Vaccaro JV, Kuehnel TG, 1993. Innovations in skills training for the seriously mentally ill: The UCLA social and independent living skills survey modules 2, 43–59. [Google Scholar]
- Lutgens D, Gariepy G, Malla A, 2017. Psychological and psychosocial interventions for negative symptoms in psychosis: Systematic review and meta-analysis. Br. J. Psychiatry 210, 324–332. 10.1192/bjp.bp.116.197103 [DOI] [PubMed] [Google Scholar]
- Mahmood Z, Clark JMR, & Twamley EW, 2018. Compensatory cognitive training for psychosis: Effects on negative symptom subdomains. Schizophr. Res 204, 397–400. 10.1016/j.schres.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood Z, Kelsven S, Cadenhead K, Wyckoff J, Reyes-Madrigal F, de la Fuente-Sandoval C, & Twamley EW, 2020. Compensatory cognitive training for Latino youth at clinical high risk for psychosis: Study protocol for a randomized controlled trial. Front. Psychiatry 10, 951 10.3389/fpsyt.2019.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, & Fenton W, 2004. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr. Res 72, 5–9. 10.1016/j.schres.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL, 2007. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr. Bull 33, 1364–1372. 10.1093/schbul/sbm014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcquaid JR, Granholm E, Mcclure FS, Roepke S, Pedrelli P, Patterson TL, & Jeste DV, 2000. Development of an integrated cognitive-behavioral and social skills training intervention for older patients with schizophrenia. J. Psychother. Pract. Res 9, 149–156. [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Burnett S, Bastani B, Ramirez LF, 1990. Effects of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hosp. Community Psychiatry 41, 892–7. 10.1176/ps.41.8.892 [DOI] [PubMed] [Google Scholar]
- Mendella PD, Burton CZ, Tasca GA, Roy P, St Louis L, Twamley EW, 2015. Compensatory cognitive training for people with first-episode schizophrenia: results from a pilot randomized controlled trial. Schizophr. Res 162, 108–111. 10.1016/j.schres.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Mucci A, Merlotti E, Üçok A, Aleman A, Galderisi S, 2017. Primary and persistent negative symptoms: Concepts, assessments and neurobiological bases. Schizophr. Res 186, 19–28. 10.1016/j.schres.2016.05.014 [DOI] [PubMed] [Google Scholar]
- Narvaez JM, Twamley EW, McKibbin CL, Heaton RK, Patterson TL, 2008. Subjective and objective quality of life in schizophrenia. Schizophr. Res 98, 201–208. 10.1016/j.schres.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman R, Malla A, McLean T, Voruganti L, Cortese L, McIntosh E, Cheng S, & Rickwood A, 2000. The relationship of symptoms and level of functioning in schizophrenia to general wellbeing and the Quality of Life Scale. Acta Psychiatr. Scand 102, 303–309. 10.1034/j.1600-0447.2000.102004303.x [DOI] [PubMed] [Google Scholar]
- Nosé M, Barbui C, Tansella M, 2003. How often do patients with psychosis fail to adhere to treatment programmes? A systematic review. Psychol. Med 33, 1149–1160. 10.1017/S0033291703008328 [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry 165, 203–213. 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The Brief Psychiatric Rating Scale. Psychol. Rep 10, 799–812. 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV, 2001. Social skills performance assessment among older patients with schizophrenia. Schizophr. Res 48, 351–60. 10.1016/S0920-9964(00)00109-2 [DOI] [PubMed] [Google Scholar]
- Penn DL, Mueser KT, Tarrier N, Gloege A, Cather C, Serrano D, & Otto MW, 2004. Supportive therapy for schizophrenia: Possible mechanisms and implications for adjunctive psychosocial treatments. Schizophr. Bull 30, 101–112. 10.1093/oxfordjournals.schbul.a007055 [DOI] [PubMed] [Google Scholar]
- Perivoliotis D, Cather C, 2009. Cognitive behavioral therapy of negative symptoms. J. Clin. Psychol 65, 815–830. 10.1002/jclp.20614 [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S, 2012. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: Analysis of CATIE data. Schizophr. Res 137, 147–150. 10.1016/j.schres.2012.01.015 [DOI] [PubMed] [Google Scholar]
- Rector NA, Beck AT, & Stolar N, 2005. The negative symptoms of schizophrenia: A cognitive perspective. Can. J. Psychiatry 50, 247–257. 10.1177/070674370505000503 [DOI] [PubMed] [Google Scholar]
- Rector NA, Seeman MV, Segal ZV, 2003. Cognitive therapy for schizophrenia: a preliminary randomized controlled trial. Schizophr. Res 63, 1–11. 10.1016/S0920-9964(02)00308-0 [DOI] [PubMed] [Google Scholar]
- Riehle M, Böhl MC, Pillny M, & Lincoln TM, 2020. Efficacy of psychological treatments for patients with schizophrenia and relevant negative symptoms: A meta-analysis. Clin. Psychol. Eur 2, 1–23. 10.32872/cpe.v2i3.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri M, Salvi G, Bonetto C, Lasalvia A, Allevi L, Parabiaghi A, Bertani M, Tansella M, 2007. Outcome of patients dropping out from community-based mental health care: a 6-year multiwave follow-up study. Acta Psychiatr. Scand 116, 42–52. 10.1111/j.1600-0447.2007.01092.x [DOI] [PubMed] [Google Scholar]
- Rüsch N, Corrigan PW, 2002. Motivational interviewing to improve insight and treatment adherence in schizophrenia. Psychiatr. Rehabil. J 26, 23–32. 10.2975/26.2002.23.32 [DOI] [PubMed] [Google Scholar]
- Schaefer J, Giangrandea E, Weinberger DR, & Dickinson D, 2013. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophr. Res 150, 42–50. 10.1016/j.schres/2013/07/009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LC, & Struening EL, 1983. SLOF: A behavioral rating scale for assessing the mentally ill. Soc. Work Res. Abstr 19, 9–21. 10.1093/swra/19.3.9 [DOI] [PubMed] [Google Scholar]
- Staring ABP, ter Huurne M-AB, & van der Gaag M, 2013. Cognitive behavioral therapy for negative symptoms (CBT-n) in psychotic disorders: A pilot study. J. Behav. Ther. Exp. Psychiatry 44, 300–306. 10.1016/j.jbtep.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Ziedonis DM, Krejci JA, Brandon TH, 2004. Motivational interviewing with personalized feedback: A brief intervention for motivating smokers with schizophrenia to seek treatment for tobacco dependence. J. Consult. Clin. Psychol 72, 723–728. 10.1037/0022-006X.72.4.723 [DOI] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, & Gold JM, 2014. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull 40, S107–S116. 10.1093/schbul/sbt197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WK, Savla GN, Vahia IV, Depp CA, O’Hara R, Jeste DV, Palmer BW, 2013. Characterizing trajectories of cognitive functioning in older adults with schizophrenia: Does method matter? Schizophr. Res 143, 90–96. 10.1016/j.schres.2012.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson MF, & Wright JD, 2018. Identifying the “therapy targets” for treating the negative symptoms of psychosis using cognitive behavioral therapy. J. Cogn. Psychother 32, 203–220. 10.1891/0889-8391.32.3.203 [DOI] [PubMed] [Google Scholar]
- Turkington D, Kingdon D, Turner T, 2002. Effectiveness of a brief cognitive-behavioural therapy intervention in the treatment of schizophrenia. Br. J. Psychiatry 180, 523–527. 10.1192/bjp.180.6.523 [DOI] [PubMed] [Google Scholar]
- Turner DT, Mcglanaghy E, Cuijpers P, van der Gaag M, Karyotaki E, & Macbeth A, 2017. A meta-analysis of social skills training and related interventions for psychosis. Schizophr. Bull 44, 475–491. 10.1093/schbul/sbx146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Thomas KR, Burton CZ, Vella L, Jeste DV, Heaton RK, McGurk SR, 2019. Compensatory cognitive training for people with severe mental illnesses in supported employment: A randomized controlled trial. Schizophr. Res 203, 41–48. 10.1016/j.schres.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Heaton RK, & Jeste DV, 2012. Compensatory cognitive training for psychosis: Effects in a randomized controlled trial. J. Clin. Psychiatry 73, 1212–1219. 10.4088/JCP.12m07686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üçok A, Yazici M, Mete L, Kültür S, Göğüs A, Erkoç Ş, Alptekin K, 2007. Factors associated with dropout and noncompliance in patients with schizophrenia: Results of a one-year follow-up. Clin. Schizophr. Relat. Psychoses 1, 161–167. 10.3371/CSRP.1.2.5 [DOI] [Google Scholar]
- Valmaggia LR, van der Gaag M, Tarrier N, Pijnenborg M, Slooff CJ, 2005. Cognitive-behavioural therapy for refractory psychotic symptoms of schizophrenia resistant to atypical antipsychotic medication: Randomised controlled trial. Br. J. Psychiatry 186, 324–330. 10.1192/bjp.186.4.324 [DOI] [PubMed] [Google Scholar]
- Velthorst E, Koeter M, van der Gaag M, Nieman DH, Fett A-KJ, Smit F, Staring ABP, Meijer C, de Haan L, 2015. Adapted cognitive-behavioural therapy required for targeting negative symptoms in schizophrenia: meta-analysis and meta-regression. Psychol. Med 45, 453–465. 10.1017/S0033291714001147 [DOI] [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH, 2009. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: A meta-analysis. Schizophr. Res 113, 189–199. 10.1016/j.schres.2009.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Subotnik KL, Gitlin MJ, Gretchen-Doorly D, Ered A, Villa KF, Hellemann GS, & Nuechterlein KH, 2015. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophr. Res 161, 407–413. 10.1016/j.schres.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CJ, Liberman RP, Tauber R, Wallace J, 2000. The Independent Living Skills Survey: A comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophr. Bull 26, 631–658. 10.1093/oxfordjournals.schbul.a033483 [DOI] [PubMed] [Google Scholar]
- Wegener S, Redoblado-Hodge MA, Wegener S, Redoblado-Hodge MA, Lucas S, Fitzgerald D, Harris A, Brennan J, 2005. Relative contributions of psychiatric symptoms and neuropsychological functioning to quality of life in first-episode psychosis. Aust. N. Z. J. Psychiatry 39, 487–492. 10.1080/j.1440-1614.2005.01608.x [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ, 2006. Wide range achievement test (WRAT4). Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, & Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am. J. Psychiatry 168, 472–485. 10.1176/appi.ajp.2010.10060855 [DOI] [PubMed] [Google Scholar]


