Summary
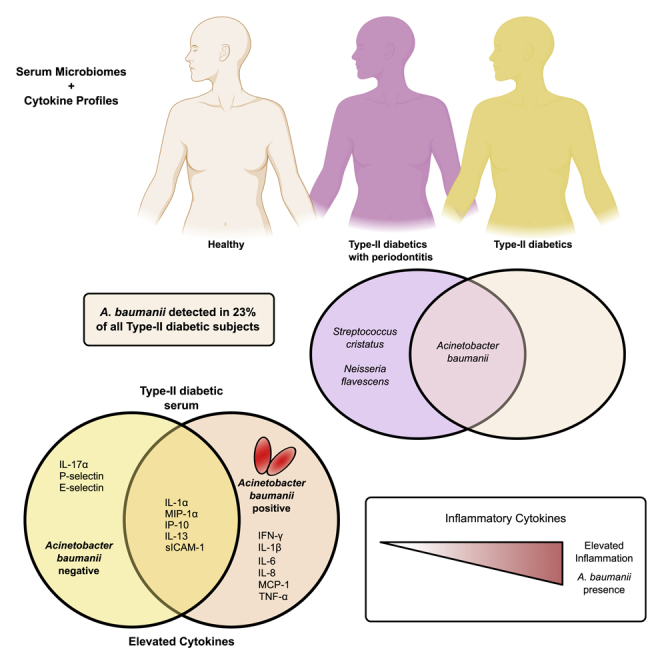
Type II diabetes (T2D) affects over 10% of the US population and is a growing disease worldwide that manifests with numerous comorbidities and defects in inflammation. This dysbiotic host response allows for infection of the host by numerous microorganisms. In the course of T2D disease, individuals can develop chronic infections including foot ulcers and periodontitis, which lead to further complications and opportunistic infections in multiple body sites. In this study, we investigated the serum of healthy subjects and patients with T2D with (T2DP) or without periodontitis for both microbiome signatures in addition to cytokine profiles. Surprisingly, we detected the presence of Acinetobacter baumanii in the serum of 23% individuals with T2D/T2DP tested. In T2DP, IL-1β, TNF-α, MCP-1, IL-6, IL-8, and IFN-γ were significantly elevated in ABC-positive subjects. As an emerging pathogen, A. baumanii infection represents a risk for impaired inflammation and the development of comorbidities in subjects with T2D.
Subject areas: Human Metabolism, Microbiology
Graphical abstract
Highlights
-
•
Acinetobacter baumanii was found in the serum of 23% type-II diabetic patients
-
•
Minor but significant serum microbiome changes between healthy vs type-II diabetic patients
-
•
Proinflammatory cytokines were elevated in A. baumanii-positive serum samples
-
•
A. baumanii + serum cytokine profiles were consistent with chronic inflammation
Human Metabolism; Microbiology
Introduction
Type-II diabetes (T2D) is a chronic inflammatory disease affecting 10.5% of the US population, with rising prevalence both in the United States and worldwide (Whiting et al., 2011; National Diabetes Statistics Report | Data & Statistics | Diabetes | CDC, 2020). Individuals with T2D typically have numerous comorbidities and are at a greater risk of both opportunistic and nosocomial infections, which can result in significant morbidity, including amputations, and lost quality of life (QOL) measures (Vardakas et al., 2007; Erben et al., 2013; McDonald et al., 2014; Ferlita et al., 2019; Kim et al., 2019). Thus, care must be taken to prevent and monitor the infection status of these individuals, as the development of diabetic mucosal and skin lesions across the body can impact the host immune and diabetic responses. Key infection prevention includes minimizing portals of entry and colonization by numerous pathogens. One example of such a portal of entry is the emergence of diabetic foot ulcers, which can develop diabetic foot infections (DFIs). Roughly 15% of adults with T2D develop foot ulcers and ∼14% of these subjects undergo amputation, with the remainder needing extensive debridement, negative pressure treatments, and other therapies including antibiotics, which result in prolonged hospitalization time, lengthy healing duration, and increased treatment costs (Reiber et al., 1998; Driver et al., 2010).
In addition to DFIs, subjects with T2D are ∼3 times more likely to develop periodontitis (Emrich et al., 1991; Wu et al., 2020). Periodontitis is an inflammatory oral disease accompanied by a polymicrobial biofilm infection that can lead to tissue damage, chronic lesions, and tooth/bone loss in the oral cavity (Irfan et al., 2001; Genco et al., 2020; Mann et al., 2020). During periodontal infection, the healthy oral microbiome, especially in the subgingival space (along teeth below the gumline, including periodontal ligament, epithelium, alveolar bone, and connective tissues), transitions to one enriched with several opportunistic pathogens that exacerbate inflammation and host tissue damage both locally and systemically (Socransky et al., 1998; Griffen et al., 2012; Hajishengallis and Lamont, 2012; Roberts and Darveau, 2015; Nowicki et al., 2018; Curtis et al., 2020). Although protective, acute inflammation is a helpful immune response, it is characterized by rapid activation of inflammation and subsequent return to homeostasis (resolution). Unresolved inflammation that fails to control the trigger leads to chronic lesions and is a hallmark of both periodontitis and T2D.
More acute bacteremia can manifest in diseases such as endocarditis or other distal infections by oral microbes (Lockhart and Durack, 1999; Parahitiyawa et al., 2009; Carrizales-Sepúlveda et al., 2018). Various systemic diseases are in fact influenced by microbial metabolism and host interactions. “How” and “why” immune system imbalance fails to control microbial pathogenic transition remains an active area of investigation. Increasing evidence implicates periodontal diseases, especially periodontitis in adults, as a potential risk factor for increased morbidity and mortality from systemic conditions including diabetes, cardiovascular diseases, adverse pregnancy outcomes, and others (Genco et al., 2020; Kleinstein et al., 2020b). This is due to two plausible mechanisms: a direct pathway leading to migration of the bacteria to distal organs and an indirect pathway leading to production of microbial metabolites and/or activating inflammatory mediators that activate the immune system locally and systemically. This is likely made possible as periodontitis presents as a chronic inflammatory condition of the tooth-supporting structures occurring with oral microbiome dysbiosis and continuous inflammatory burden. Notably, oral microbes have also been detected (Dowd et al., 2008; Gardner et al., 2013) and isolated from DFIs (Bowler and Davies, 1999; Citron et al., 2007). Thus, we hypothesized that patients with T2D might be at a greater risk of extraoral infection and may exhibit changes in microbiota that transiently occupy their bloodstream. We further hypothesized that these changes would be even more apparent in patients with T2D with periodontitis (T2DP) and that these patients may carry potential DFI-colonizing oral species in their bloodstream. Our initial investigations toward these hypotheses utilized samples derived from previous work on resolution of inflammation in T2D (Freire et al., 2017) and are reported here, with unexpected results.
To investigate the possibility of oral species presence in the bloodstream, we compared the serum microbiomes of subjects with T2D and T2DP versus a healthy cohort. Unexpectedly, we detected Acinetobacter baumanii in 23% of the subjects with T2D/P in our study. A. baumanii is an emerging infectious pathogen (Villar et al., 2014) notorious for its evolving antibiotic resistances (Gootz and Marra, 2008; Vázquez-López et al., 2020) and nosocomial (Dijkshoorn et al., 2007; Ayoub Moubareck and Hammoudi Halat, 2020) and bloodstream infections (Peleg et al., 2008) and is part of a cluster of Acinetobacter species with similar clinical manifestations known as the AB or ABC complex (Gerner-Smidt and Tjernberg, 1993), which are highly similar via ribotyping. ABC complex organisms present several particular risks to subjects with T2D, specifically higher mortality rates for those with bacteremia (Leão et al., 2016) and for those with higher blood glucose concentrations (Leung and Liu, 2019). Patients infected with A. baumanii after burns were 9.8 times more likely to develop glucose intolerance, and ∼3 times more patients with T2D with burns developed A. baumanii infections (27%) than patients without T2D (8.5%) (Furniss et al., 2005). Additionally, A. baumanii has also been observed in DFIs, particularly alongside other multidrug-resistant pathogens, presenting a grave concern for individuals with T2D (Castellanos et al., 2019; Henig et al., 2020). In addition to DFIs, ABC complex organisms can also spread through the body and form infections in numerous organs (Peleg et al., 2008; Al-Anazi and Al-Jasser, 2014). A. baumanii in a mouse pneumonia model led to increased proinflammatory cytokines, reduced neutrophil infiltration into the lung, and increased extrapulmonary dissemination (Qiu et al., 2009). In T2D, neutrophils present failure in early chemotaxis, but a hyper-inflammatory feedback loop emerges to compensate the initial myeloid cell failure (Kleinstein et al., 2020a).
In this work, we first investigated whether or not we could detect signatures of oral bacteria; our findings indicated minor, yet significant, microbiome compositional changes between healthy cohort and patients with T2D and T2DP. We discovered that nearly one-fourth of the subjects with T2DP in our study contained sequence reads identical to that of A. baumanii. The specific presence of A. baumanii was confirmed by further clone library and 16S rDNA Sanger sequencing. In addition, we assayed the host response by cytokine profiles of sera. When stratifying A. baumanii-positive T2D versus A. baumanii-negative T2D samples we observed unique cytokine profiles, suggesting a specific microbial impact on systemic inflammation. Although our methodology cannot indicate if A. baumanii was transiently present or a subclinical colonizer of our T2DP cohort, we propose that these data reveal a much greater risk of currently “uninfected” individuals with T2D for A. baumanii exposure and infection than previously understood and should justify more intensive and thorough investigation into AB complex colonization of patients with T2D.
Results
Patient demographics
Serum samples were generated from the population described in Table 1. A total of 81 subjects were investigated in this study, 57 with T2D, of which 29 had periodontitis (T2DP) as defined by the American Association of Diabetes (American Diabetes Association, 2015) and American Association of Periodontology (AAP) (Armitage, 1999) criteria, respectively. All T2D donors were poorly controlled diabetic subjects who had not taken antimicrobials, non-steroidal anti-inflammatory drugs, or insulin sensitizers within three months. Diagnostic serum glucose measures were taken for all subjects, and HbA1C was measured for diabetic patients to stage disease status. As expected, poorly controlled diabetic subjects (HbA1c% >6.5%) showed significantly elevated glucose levels (p < 0.0001; Table 1). There were no significant differences in gender or ethnicity between healthy volunteers and diabetic patients. Diabetic patients showed elevated body mass index and tended to be older than healthy controls (p < 0.05), consistent with known disease biology. Periodontal condition was further stratified among the subjects according to the severity of irreversible tissue loss (mild, moderate, severe) and reversible lesions called gingivitis according to AAP (Armitage, 1999) criteria. Periodontal condition was significantly worse in diabetic compared with healthy subjects (p < 0.0001). All healthy subjects had healthy periodontal condition compared with only 11% diabetic patients, with 15% diabetic subjects suffering from severe periodontitis. Current and former smokers were near uniformly part of the T2D and T2DP groups with only one current smoker in the healthy group. The entire study population had a median age range of 53 ± 11 years, and all subjects were outpatient and thus not part of an extended hospital stay cohort.
Table 1.
Study subject demographics
| Healthy (N = 24) | Type II diabetes (N = 57) | Total (N = 81) | p value | |
|---|---|---|---|---|
| Age (mean in years ±SD) | 46 ± 10.72 | 55 ± 10.78 | 52.78 ± 11.40 | <0.001 |
| Gender (no., %) | ||||
| Male | 13 (54%) | 29 (51%) | 42 (51.85%) | |
| Female | 11 (46%) | 28 (49%) | 39 (48.15) | 0.45 |
| Ethnicity | ||||
| Caucasian | 15 (63%) | 25 (43.86%) | 40 (49.38%) | |
| Hispanic | 2 (8%) | 7 (12.28%) | 9 (11.11%) | |
| African American | 6 (25%) | 23 (40.35%) | 29 (35.80%) | |
| Asian | 0 | 2 (3.51%) | 2 (2.47%) | |
| Other | 1 (4%) | 0 | 1 (1.23%) | 0.16 |
| Smoking status (no., %) | ||||
| Smokers | 1 (4%) | 5 (8.77%) | 6 (7.41%) | |
| Former smokers | 0 | 21 (36.84%) | 21 (25.93%) | |
| Never smokers | 23 (96%) | 31 (54.39%) | 54 (66.67%) | 0.02 |
| BMI (kg/m2; mean ± SD) | 29.34 ± 2.60 | 32.93 ± 6.02 | 31.87 ± 5.48 | <0.01 |
| Blood cholesterol (mg/dL; mean ± SD) | 148.89 ± 36.71 | 211.73 ± 78.69 | 194.01 ± 74.84 | <0.001 |
| HbA1c (%; mean ± SD) | – | 7.48 ± 1.61 | – | – |
| Blood Glucose (mg/dL; mean ± SD) | 99.25 ± 19.29 | 190.84 ± 60.58 | 163.70 ± 66.69 | <0.0001 |
| Periodontal condition (no., %) | ||||
| Healthy | 24 (100%) | 7 (12.28%) | 31 (38.27%) | |
| Mild | 0 | 15 (26.32%) | 15 (18.52%) | |
| Moderate | 0 | 11 (19.30%) | 11 (13.58%) | |
| Severe | 0 | 12 (21.05%) | 12 (14.81%) | |
| Gingivitis | 0 | 10 (17.54%) | 10 (12.35%) | |
| Stable periodontitis | 0 | 2 (3.51%) | 2 (2.47%) | <0.0001 |
| ABC positive (no., %) | ||||
| Positive | 0 | 13 (22.81%) | 13 (16.05%) | |
| Negative | 19 (79%) | 29 (50.88%) | 48 (59.26%) | |
| Not tested | 5 (21%) | 15 (26.32%) | 20 (24.69%) | 0.02 |
| Neutrophil count (million cells, mean ± SD) | 74.82 ± 58.56 | 158.22 ± 90.08 | 131.90 ± 94.51 | <0.0001 |
| Monocyte count (million cells, mean ± SD) | 63.79 ± 50.35 | 113.91 ± 60.09 | 99.22 ± 63.28 | <0.01 |
p values calculated by unpaired t tests or χ2 (two-sided p values; italicized p < 0.05 significant).
BMI, body-mass index; ABC, A. baumanii; %, percentage; mg/dL, milligrams per deciliter; kg/m2, kilograms per square meter.
Smoking classification according to CDC National Health Interview Survey (NHIS).
Microbiome analysis controls for low template sequencing
Data generated here was part of a post hoc study of serum samples collected for a previous study (Freire et al., 2017) and thus not available in the larger volumes ideal for 16S microbiome dataset generation. Low template concentrations can produce microbiome signatures not derived from that in the biological samples due to amplification of contaminating genomic DNA (gDNA) in sterile buffers and reagents (Kennedy et al., 2014; Salter et al., 2014; Kim et al., 2017), which often manifests as aquatic species signatures, many of which are not compatible with growth in the human host (i.e., many do not even grow at 37°C) (Kim et al., 2017). A description of no-template/reagent-only control samples is provided in the Methods. Data within this study are provided as either minimally filtered (mitochondrial, human, and chloroplast aligned reads removed) or strict filtered (reads removed based on homology with reagent-only controls) and indicated as such throughout the study. All sequence data are made available at NIH SRA Bioproject PRJNA664044, and all scripts used for data generation, filtering, and analyses are provided in the Supplemental Information.
We were able to generate abundant data from these samples with careful consideration paid to controls for amplification bias and background bacterial gDNA contamination of commercial sterile reagents. First, we utilized amplification of a mock standard library of 8 bacterial and 2 fungal species (Figures S1A and S1B) at 10 and 1 ng of total template, which indicated that our library preparation protocol was suitable for species-level analysis where possible by V1-V3 sequence. Second, we utilized 13 separate no-template/reagent-only controls, which were subjected to our sequence analysis pipeline, revealing frequently encountered contaminating taxa. These taxa were filtered from each human-derived sample in the strict-filtered datasets only.
V1-V3 16S microbiome diversity analyses reveal minor but significant differences between diabetic and nondiabetic groups
Serum and control samples were subject to bead beater lysis and gDNA purification. Purified gDNA was amplified using the V1-V3-targeted 27F-519R primer pair (Stackebrandt and Goodfellow, 1991; Turner et al., 1999). Quality trimmed reads were subjected to DADA2 analysis (Callahan et al., 2016) and aligned to the SILVA database (Quast et al., 2013) to determine species-level identity where possible. Rarefaction curve analysis was performed to ensure adequate sequencing depth (Figure S2), and resequencing of each Illumina index was performed to generate greater sequencing depth without further template amplification. Run-to-run variability between next-generation sequencing was found to be insignificant (Figure S3). Data analysis and comparisons were performed via QIIME2 (Bolyen et al., 2019) as detailed in the Methods.
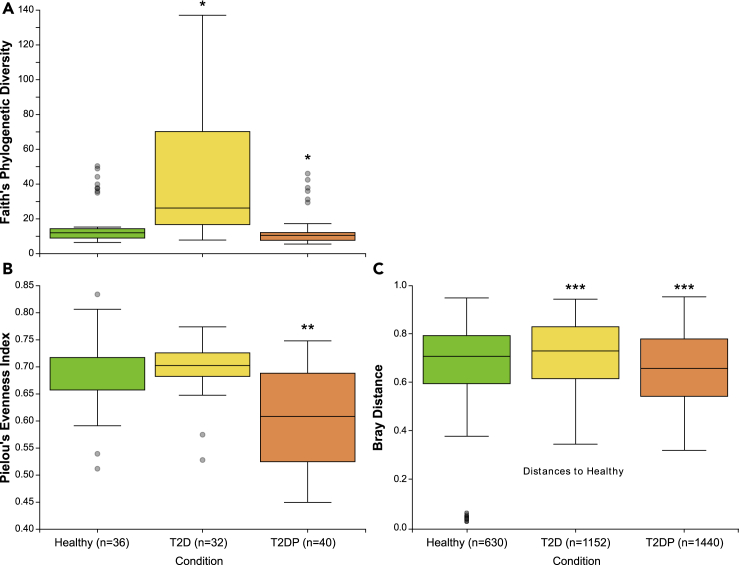
We first quantified alpha and beta diversity between the healthy, T2D, and T2DP subject groups for our strict filtered (Figures 1and S7) and minimally filtered data (Figure S4). For both datasets, species richness for T2D and T2DP groups were significantly different than the healthy cohort, which was also significantly different when compared with T2DP in each dataset for species evenness (T2D more rich, T2DP less rich, and less even). Beta diversity was significantly less for healthy subjects versus T2D and greater when compared with T2DP for either minimal or strict filtered datasets. Thus, we observed minor but significant shifts in microbiome composition between subject groups.
Figure 1.
16S alpha and beta diversity of serum microbiomes changes with subject status
(A–C) Species richness is displayed based on Faith's phylogenetic diversity (Faith, 1992) (A), and species evenness is based on Pielou's evenness index (Pielou, 1966) (B) for healthy, type-II diabetic patients (T2D), and type-II diabetic patients with periodontitis (T2DP) samples. Significance was determined by Kruskal-Wallis analysis of variance (Kruskal and Wallis, 1952) for each comparison indicated, and Benjamini-Hochberg correction (Benjamini and Hochberg, 1995) was applied to generate adjusted q-values. All data used were strictly filtered based on no-template control indexes. ∗q-value < 0.002 compared with healthy, ∗∗q-value < 0.0004 compared with healthy and with T2D. (C) Beta diversity Bray-Curtis distances. Pairwise PERMANOVA of each category versus each (group size of 3, n = 112) was performed in 999 permutations. ∗∗∗q-value differs from “healthy” < 0.001. See also Figure S4.
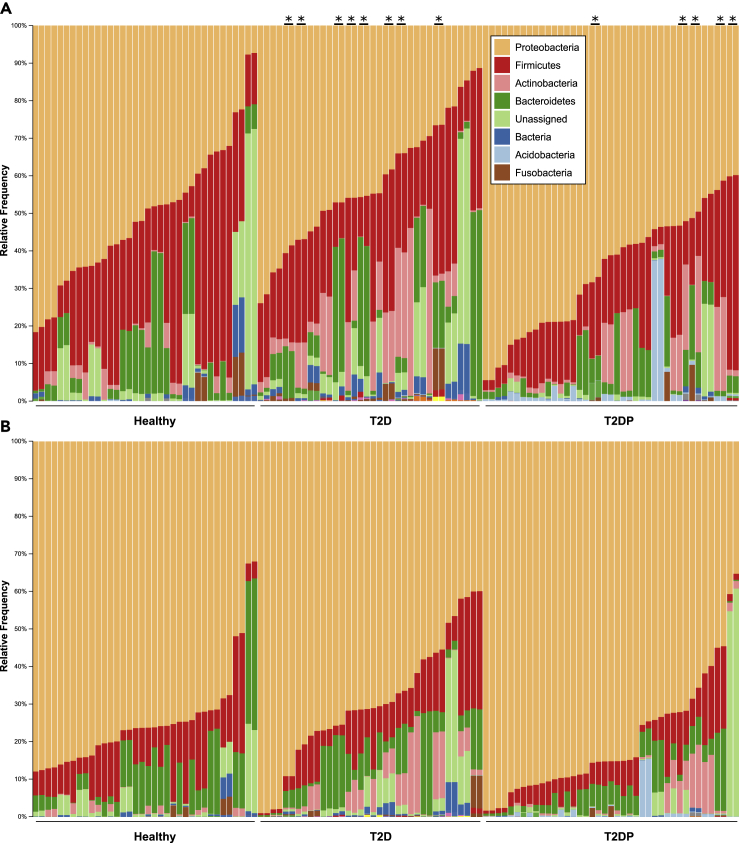
To begin investigating which taxa contributed to this composition difference we first looked at a phylum-level display of our taxonomically assigned data (Figure 2). Figures 2A and 2B, respectively, indicate phylum-level data for strict versus minimal filtered datasets. Similar trends in phyla distribution were evident for either dataset and reveal minor phylum-level changes between subject groups.
Figure 2.
Phylum-level diversity changes between subject status
(A and B) DADA2-analyzed data were aligned to the SILVA database for taxonomical assignment via QIIME2 as described in Methods. Data from both sequencing runs were collapsed to phylum-level taxa and displayed for (A) strict filtered and (B) minimally filtered conditions. Asterisks on the top indicate run-run sample pairs that were positive for A. baumanii.
MED and DADA2 analysis reveal the presence of Acinetobacter baumanii
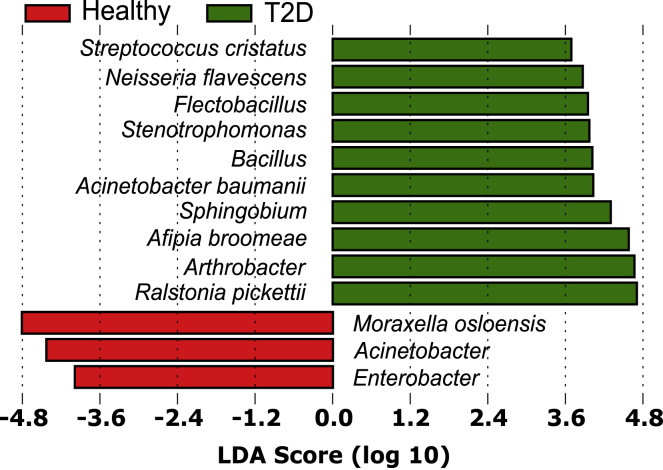
Given our initial investigation into whether or not there were differences between T2D and T2DP microbiota and a preliminary hypothesis that oral taxa would be enriched in T2DP serum, we first used a minimal entropy decomposition (MED) analysis identical to the one used previously to characterize oral microbiomes (Eren et al., 2014). Oligotyped “nodes” or representative sequences were aligned to both the Human Oral Microbiome (HOMD) (Escapa et al., 2018) and Ribosomal Database Project (RDP) (Cole et al., 2014) databases, and species were assigned based on 98% or higher sequence identity when comparing nodes to reference databases. We next compared species-assigned data between subject groups by linear discriminate analysis effect size measurements (LEfSe) (Segata et al., 2011). Using MED-analyzed data for healthy versus all subjects with T2D (Figure 3) we detected significant enrichments of some oral species (Streptococcus cristatus, Neisseria flavescens) and also some known reagent contaminants (Ralstonia picketti, Afipia broomeae) alongside other soil and freshwater microbes that we suspect are also contaminants (Flectobacillus, Stenotrophomonas, Sphingobium, Arthrobacter). Surprisingly, we observed A. baumanii significantly enriched in the T2D population as a whole. For MED analysis, reads assigned as A. baumanii were present from 0.01% to 7.3% of total reads in 8 and 5 subjects with T2D and T2DP, respectively. No reads assigned to A. baumanii were detected in any healthy subject. DADA2 amplicon sequence variants (ASVs) are similar to MED nodes, and we observed that one ASV node was a perfect match to the singular MED node assigned to A. baumanii based on 100% RDP alignment across its full sequence.
Figure 3.
Healthy subjects versus all subjects with T2D reveal a potential enrichment of A. baumanii
Initial minimal entropy decomposition (MED) analysis of 16S data with taxonomy assigned based on RDP database alignment to generate species-level assignments was performed followed by linear discriminate analysis effect size quantification (LEfSe). This revealed enrichment of several species/genera in subjects with T2D relative to healthy. DADA2 analysis of the same dataset was aligned to the SILVA database and shows similar patterns of enrichment (see also Figure S5).
In our DADA2/QIIME2-based analysis we performed taxonomical assignment for each DADA2 ASV node aligned to either the Greenegenes (DeSantis et al., 2006) (not shown) or SILVA database version 132 (Quast et al., 2013). Phylum-level taxonomic assignments of DADA2 data aligned to SILVA were used in Figure 2. ASVs are similar to MED nodes, and we observed that one ASV node was a perfect match to the singular MED node assigned to A. baumanii based on 100% RDP alignment across its full sequence. This ASV was assigned only to the Acinetobacter genus despite a 100% full-length match to A. baumanii sequence at the RDP database and the NCBI non-redundant database (not shown). Based on this, we manually assigned this ASV to A. baumanii and found that reads now assigned to A. baumanii were present from 0.01% to 9.1% of total reads in 8 and 5 subjects with T2D and T2DP, respectively, with no reads assigned to A. baumanii detected in any healthy subjects. This demonstrated agreement between both MED and DADA2-based 16S data analysis. LEfSe analysis was again performed on healthy subjects versus all with T2D, as well as between all three subject groups, and demonstrated again that A. baumanii was significantly elevated among all T2D samples (Figure S5).
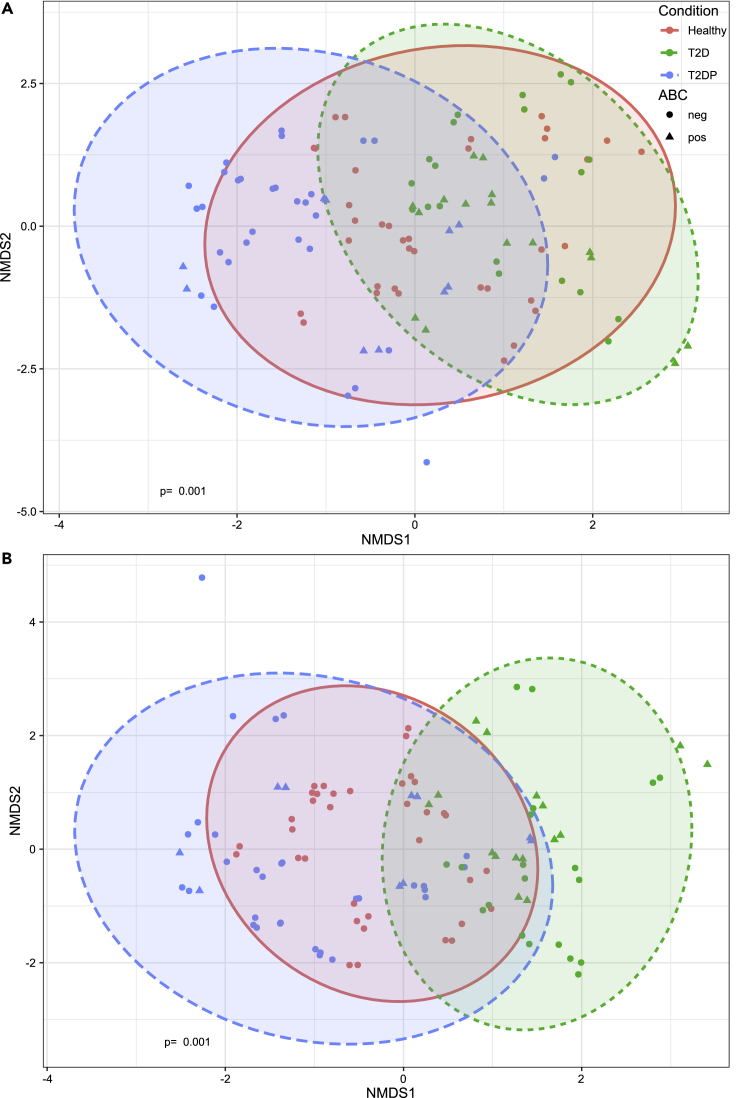
We next looked at differences in microbiome compositions across subjects by plotting Bray-Curtis distances via non-metric multidimensional scaling (NMDS) while distinguishing subject groups as well as presence or absence of ABC bacteria (Figure 4). Broadly, T2D and T2DP samples had less overlap in composition, whereas healthy samples intersected either group. ABC status did not seem to form a distinct microbiome cluster, suggesting that there was no unique microbiota composition for ABC-positive subjects apart from A. baumanii sequence detection.
Figure 4.
Beta diversity quantification between subject groups
(A and B) SILVA-assigned taxonomy of DADA2-analyzed 16S data was used to determine Bray-Curtis dissimilarity between samples for strictly filtered (A) and minimally filtered (B) data. Non-metric multidimensional scaling and statistical analysis for each subject group was performed in R using the vegan package as described in Methods. p-value of 0.001 determined by PERMANOVA test via the adonis2 function.
Validating the presence of A. baumanii in subjects with T2D
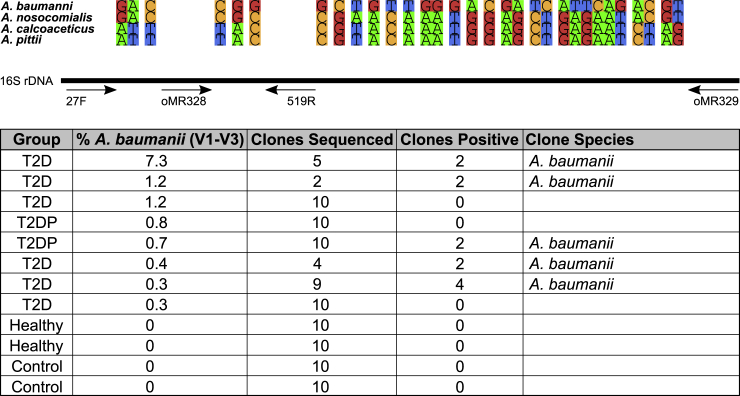
The finding of ABC sequences was unexpected, as study participants were not hospitalized or under treatment for any infection(s), and we wished to confirm this result by other techniques. This was especially needed as the V1-V3 portion of the 16S rDNA sequence only differs between A. baumanii and Acinetobacter nosocomialis by one nucleotide substitution. Using PCR primers for the 16S rDNA region that encompasses nearly all differentiating 16S nucleotides between Acinetobacter species we generated amplicons from a portion of our ABC-positive samples alongside negative healthy and no-template control samples (Figure 5). PCR products were ligated into a TA vector, and individual colonies were propagated and subjected to Sanger sequencing of plasmid inserts. The 1242-bp sequences were then aligned to the NCBI reference RNA sequence database (refseq_rna). Each sequence (12 clones across 5 samples) was identical and matched the A. baumanii 16S sequence across its length. This result validated our initial 16S detection of putative A. baumanii.
Figure 5.
Clone library design to verify ABC complex member identity
Amplification with oMR328-329 primers produced an amplicon between 120 and 1306 bp of the A. baumanii 16S rDNA sequence. This allowed for Sanger sequencing of information-rich nucleotides indicated above allowing for species differentiation beyond initial V1-V3 sequencing with the 27F-519R primers. The table beneath indicated the total number of individual clones sequenced per sample and which species they aligned to with 100% identity in the NCBI non-redundant database.
Host cytokine responses to T2D and T2DP versus health
In addition to serum microbiota, we also quantified 20 different human cytokines in serum samples across all 3 patient groups (Figure 6). Based on relative abundance profiles, we observed that proinflammatory cytokines were significantly elevated in A. baumannii-positive samples highlighted in the box (ABC+). MCP-1 and IL-1β, classic proinflammatory cytokines, were increased in diabetic patients. Intriguingly, T2D ABC-negative subjects did not show increase of these markers. ABC+ subjects showed significantly higher expression levels of cytokines IL-1β, IL-6, IL-8, MCP-1, and IFN- γ when compared with ABC-negative groups (I and V, p < 0.01).
Figure 6.
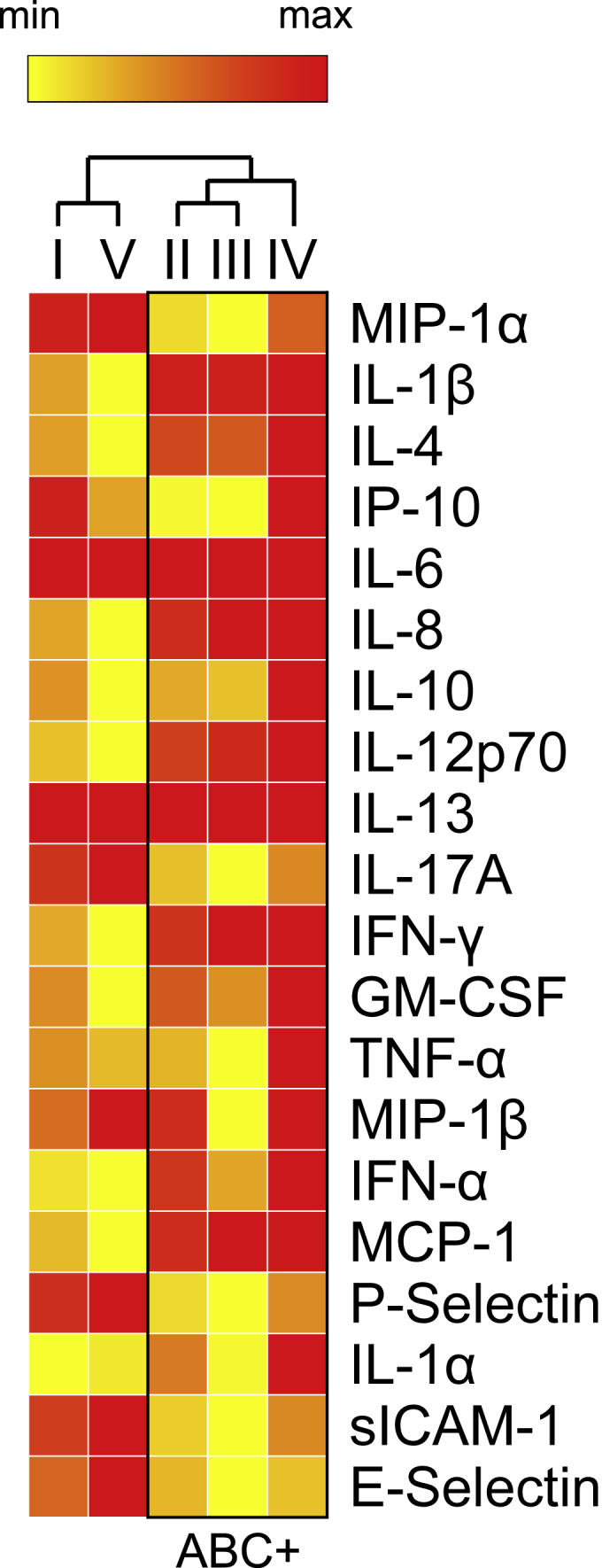
Cytokine profile quantification reveals distinct differences between subject groupings and A. baumanii (ABC) presence
Heatmap showing immune mediator concentrations derived from serum of diabetic and healthy samples were compared to understand the role of ABC in each sample group. (I) Diabetic/Healthy, (II) All ABC+/All ABC-, (III) ABC+ Diabetic/ABC- Diabetic, (IV) ABC+ Diabetic/Healthy, (V) ABC- Diabetic/Healthy. Data are clustered according to comparison groups (red, highest expression; yellow, lowest expression). Hierarchical clustering was employed by Morpheus from the Broad Institute (Morpheus, https://software.broadinstitute.org/morpheus). Observations from hierarchical clustering are shown in a tree, with ABC groups highlighted in the box. ABC+ groups (II, III, and IV) showed significantly different (p < 0.01) levels of IL-1β, IL-6, IL-8, MCP-1, IFN- γ. ∗p value <0.01 between groups via unpaired Student's t test (red, highest expression; yellow, lowest expression).
Host cytokine responses to specific microbiota
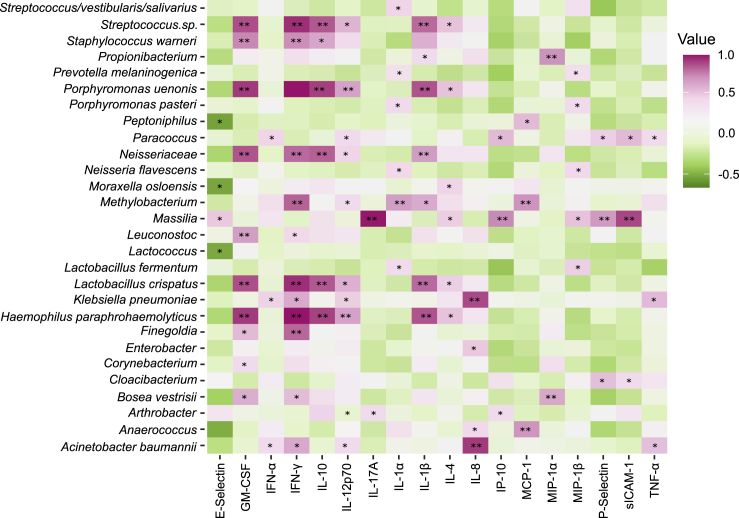
Based on the cytokine abundance data and earlier microbiome sequencing, we were able to determine if any specific taxa were significantly correlated with cytokine presence or absence in subjects with T2D (Figure 7). A. baumanii was significantly correlated with elevated amounts of IFN-α, IFN-γ, IL-12p70, IL-8, and TNF-α. ABC+ subjects presented cytokine correlations consistent with a profile of chronic inflammation. IL-8 is a chemokine that impacts neutrophil phenotype and function, and excessive amounts of this cytokine lead to chronic activation of phagocytes (Moore and Kunkel, 2019). Excessive production of TNF-α increases failure of resolution of inflammation on innate immune cells by controlling a resolution receptor (Freire and Van Dyke, 2013). Here, TNF-α showed a significant increase in ABC+ subjects, suggesting co-existence with ABC in the serum of patients with T2D. We also performed an identical analysis for healthy subjects and saw several genera or species with significant correlations (Figure S6). In healthy subjects unique profiles were found, correlated with lower levels of proinflammatory cytokines and co-clustered with ABC-negative samples.
Figure 7.
Host-microbial correlation-resolved type-II diabetic associations with inflammatory cytokines
HOMD/RDP assigned taxonomy of MED-analyzed 16S data for both T2D and T2DP samples and cytokine concentrations were analyzed via Pearson correlation coefficient in R using the rcorr function. Significance was determined using the asymptotic p values generated by rcorr with ∗p value <0.05, ∗∗p value <0.01. Data are strictly filtered with taxa present in no-template controls subtracted. See also Figures S6, S8, and S9.
Although low template concentration microbiome profiling presents many challenges, we were able to utilize numerous control steps to minimize the impact of contaminating taxa. We were also able to demonstrate that there were significant differences between subject groups that include taxa of likely biological origins, and also that specific cytokines significantly correlated with the presence of distinct taxa. Most surprisingly, we were able to detect A. baumanii and confirm its presence in serum samples of patients with T2D not known or currently diagnosed with any underlying infection. These data do not per se indicate active infection but could also indicate colonization or transient exposure to A. baumanii for these subjects compared with healthy subjects.
Discussion
Issues with amplification-based 16S studies from low template concentrations are well known (Sinha et al., 2015; Kim et al., 2017; Pollock et al., 2018). We began this study as a post hoc analysis of existing serum samples from a previous bank of anonymized specimens (Freire et al., 2017). As sample volumes of serum were mostly limited, we relied on amplification of 16S rDNA, using V1-V3 region primers to improve the likelihood of identifying oral taxa (Eren et al., 2014) that we hypothesized to exist in the diabetic with periodontitis part of our cohort. To minimize effects of amplification, we performed multiple high-throughput sequencing runs of amplified libraries to increase sequence depth as opposed to further amplification and using a higher capacity sequencing platform. Additionally, we also included numerous no-template controls throughout sample preparation and purification to identify as many outside contaminants as we could (Kennedy et al., 2014; Salter et al., 2014; Kim et al., 2017). As a further control, we carried out amplification of mock community libraries (Figure S1) to determine if amplification bias or infidelity affected taxonomic assignment. We also used two separate methods for correction of Illumina-sequenced amplicon errors, DADA2 (Callahan et al., 2016) and minimal entropy decomposition/oligotyping (MED) (Eren et al., 2015), as well as comparing our data from multiple ribosomal databases for taxonomic assignment (DeSantis et al., 2006; Quast et al., 2013; Cole et al., 2014). In addition to Illumina sequencing, we performed a separate amplification of 16S rDNA followed by Sanger sequencing to independently confirm the identity of putative A. baumanii sequence presence in a subset of our diabetic samples (Figure 5). Thus, we have made great efforts to validate our findings as far as is feasible and have taken care not to overinterpret data presented here.
We initially hypothesized that oral taxa would be enriched in the bloodstream of subjects with T2DP, and potentially all subjects with T2D, compared with healthy subjects. Our microbiome data revealed changes in composition between our three subject groups (Figures 1, 2, 3, 4, S4, and S5). However, there was only enrichment of few oral taxa when healthy subjects were compared with all subjects with T2D and not with subjects with T2DP alone, specifically S. cristatus and N. flavescens (Figure 3), which are not associated with periodontitis. Their presence here could indicate further access to the bloodstream by oral microbes in the T2D cohort, although this requires further verification. Given the limited template available for each sample, we cannot rule out that other oral taxa are not elevated in subjects with T2D/P, but more rigorous testing of that hypothesis would require larger serum volumes and using a longitudinal approach. By far the most surprising element of this dataset was the detection of A. baumanii- (Figures 3 and S5) assigned reads unique to 13 subjects with T2DP and not present in any control or healthy subject samples.
A. baumanii represents a considerable risk to subjects with T2D including higher mortality rates (Leão et al., 2016) and a higher risk of infection in burn wounds, as well as DFIs (Furniss et al., 2005; Castellanos et al., 2019; Henig et al., 2020). To confirm our V1-V3 amplicon data and to determine whether or not these were A. baumanii or another ABC species, we used targeted near-full-length 16S rDNA primers that allowed us to sequence the majority of differentiating nucleotides necessary for ribotyping. Sequencing individual clones of these amplicons revealed 100% matches to A. baumanii, and no clones (n = 50) were A. baumanii positive from indicated control samples (Figure 5). This result was surprising, as none of the subjects in our T2D/P cohort were currently under inpatient treatment or had any indication of underlying infection (apart from periodontitis). Given the methodology here, neither can we speculate on the true infection status of these individuals nor can we be certain if the A. baumanii present were circulating in the bloodstream or were on the skin and mixed with the sample during a blood draw. No matter the route of entry, we were able to determine significant cytokine profile changes in T2DP A. baumanii-positive subjects, as well as broader inflammatory changes in cytokine profiles between subjects with T2DP and T2D and healthy subjects (Figure 6).
Cytokine dysregulation can impact the host locally and systemically, making the subject more prone to severe infections and increased tissue damage (Tisoncik et al., 2012). Individuals who have T2D present an increased metabolic burden, while often comorbid periodontal disease promotes microbial dysbiosis, with both diseases resulting in a chronic state of inflammation. In the present study, A. baumanii was only found in the serum of diabetic subjects, where an increase in proinflammatory cytokines was also observed (Figures 6 and 7). We utilized an unbiased panel of 20 human cytokines to survey specific immune responses to T2D and T2DP compared with those of healthy individuals. Although there are complications presented by low template sizes in our samples, cytokine profiling from these same volumes were well within desired assay volumes. As mediators of inflammation, cytokine production feeds forward a cascade of signals that can impact tissue, organ, and overall host immunometabolism. In T2D, IL-1β, TNF-α, MCP-1, IL-6, IL-8, and IFN-γ were significantly elevated in ABC+ subjects and not in ABC-negative subjects. Of these proinflammatory cytokines, IL-1β, TNF-α, and IL-8 suppression has been investigated as therapeutic targets for clinical management of diabetes and associated chronic lesions (Peiró et al., 2017; Feng et al., 2018). We previously observed increased TNF-α levels in cell cultured neutrophils from subjects with T2D relative to cells derived from healthy subjects (Kleinstein et al., 2020a). Co-occurrence of bacteria in the serum and cytokines also differed among controls. In the current study, co-occurrence among cytokines was more evident in diabetic subjects (100 significant co-occurrences) versus healthy individuals (30 significant co-occurrences) (Figures 7 and S6). A baumannii especially correlated with neutrophil inflammatory signaling cytokine IL-8, which was not observed in healthy subjects. We have previously shown differences in gene expression in neutrophils of individuals with T2D, which are primed to mount an aberrant inflammatory response when compared with neutrophils of healthy individuals (Kleinstein et al., 2020a), consistent with production of inflammatory cytokines observed here. In contrast, IL-17α, P-selectin, and E-selectin levels were increased in diabetic subjects who were ABC-negative compared with ABC+ diabetic subjects. IL-4 and IL-10 did not co-occur with A. baumanii in any of the healthy versus diabetic groups. The cytokine results provide an initial glimpse into the host response to chronic inflammatory diseases, T2DP with and without the presence of A. baumannii, which may warrant further investigation in larger studies.
In conclusion, this study has revealed modest serum microbiome compositional changes and significant cytokine profile changes between subjects with T2D with or without periodontitis versus non-T2D individuals, revealing an impaired host response when A. baumanii was present. We also present here the potential for lurking A. baumanii colonization in asymptomatic subjects with T2D, possibly to a much larger extent than previously reported. In our opinion, these data present a strong justification for further monitoring of individuals with T2D for A. baumanii colonization and support the need for a more robust study to characterize the potentially greater spread of A. baumanii among individuals with T2D.
Limitations of the study
Limitations of this study primarily include small template volumes available for serum samples in this post hoc study. Blood and sera microbiome studies will always be more complex due to low bacterial template concentrations inherent to these types of sample. Although we have made utmost efforts to validate our main findings regarding A. baumanii presence, there are likely more interesting aspects to our study cohort microbiota that have been missed. These and other minor limitations have been indicated in the article.
Resource availability
Lead contact
Further information and requests for resources, data, code, and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Matthew Ramsey (mramsey@uri.edu).
Materials availability
This study did not generate new unique reagents or genetic constructs.
Data and code availability
All data is available at NIH – SRA Bioproject: PRJNA664044. All other code used for data analysis has been provided in the Transparent Methods section (see Scripts) in the Supplemental Information.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Thanks to the M. Ramsey and M. Freire lab members for their valuable input and assistance with both experimental work and writing. Thanks to Dr. Janet Atoyan at the Rhode Island Genomics and Sequencing Center for assistance with sequence library preparation and suggestions on sequence data analysis. Thanks to Dr. Thomas E. Van Dyke for helpful discussion and to the Forsyth Center for Clinical and Translational Research Center staff for recruiting patient samples and providing clinical data used in this study. Funding Sources: This work was supported by U.S. Public Health Service Grants from the National Institutes of Health/National Institute of Dental and Craniofacial Research, R00 DE0234804 (Freire), K23 DE018917 (Hasturk) and R01 DE027958 (Ramsey) NIGMS/RI-INBRE early career development award (P20GM103430, Ramsey), and the Rhode Island Foundation Medical Research Fund (20164348, Ramsey).
Author contributions
D.P., B.H., and S.E.K. performed the work. D.P., S.E.K., M.F., and M.R. wrote and edited the manuscript. M.F., H.H., and M.R. provided reagents and samples. D.P., R.E., S.E.K., M.F., and M.R. designed the experiments. M.F. and M.R. secured funding.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101941.
Contributor Information
Marcelo Freire, Email: mfreire@jcvi.org.
Matthew Ramsey, Email: mramsey@uri.edu.
Supplemental information
References
- Al-Anazi K.A., Al-Jasser A.M. Infections caused by acinetobacter baumannii in recipients of hematopoietic stem cell transplantation. Front. Oncol. 2014;4:186. doi: 10.3389/fonc.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- National diabetes Statistics Report | data & Statistics | diabetes | CDC (2020). https://www.cdc.gov/diabetes/data/statistics/statistics-report.html
- Ayoub Moubareck C., Hammoudi Halat D. Insights into acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics (Basal) 2020;9:119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 1995;57:289–300. [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler P.G., Davies B.J. The microbiology of infected and noninfected leg ulcers. Int. J. Dermatol. 1999;38:573–578. doi: 10.1046/j.1365-4362.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizales-Sepúlveda E.F., Ordaz-Farías A., Vera-Pineda R., Flores-Ramírez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27:1327–1334. doi: 10.1016/j.hlc.2018.05.102. [DOI] [PubMed] [Google Scholar]
- Castellanos N., Nakanouchi J., Yüzen D.I., Fung S., Fernandez J.S., Barberis C., Tuchscherr L., Ramirez M.S. A study on acinetobacter baumannii and Staphylococcus aureus strains recovered from the same infection site of a diabetic patient. Curr. Microbiol. 2019;76:842–847. doi: 10.1007/s00284-019-01696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron D.M., Goldstein E.J., Merriam C.V., Lipsky B.A., Abramson M.A. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J. Clin. Microbiol. 2007;45:2819–2828. doi: 10.1128/JCM.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.R., Wang Q., Fish J.A., Chai B., McGarrell D.M., Sun Y., Brown C.T., Porras-Alfaro A., Kuske C.R., Tiedje J.M. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.A., Diaz P.I., Van Dyke T.E. The role of the microbiota in periodontal disease. Periodontology. 2020;2000:14–25. doi: 10.1111/prd.12296. [DOI] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- Dowd S.E., Wolcott R.D., Sun Y., McKeehan T., Smith E., Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) PLoS One. 2008;3:e3326. doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver V.R., Fabbi M., Lavery L.A., Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J. Am. Podiatr. Med. Assoc. 2010;100:335–341. doi: 10.7547/1000335. [DOI] [PubMed] [Google Scholar]
- Emrich L.J., Shlossman M., Genco R.J. Periodontal disease in non-insulin-dependent diabetes mellitus. J. Periodontol. 1991;62:123–131. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- Erben N., Ozgunes I., Aksit F., Doyuk Kartal E., Colak E., Usluer G. Healthcare-associated infections and the distribution of causative pathogens in patients with diabetes mellitus. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:821–825. doi: 10.1007/s10096-013-1816-x. [DOI] [PubMed] [Google Scholar]
- Eren A.M., Borisy G.G., Huse S.M., Mark Welch J.L. Oligotyping analysis of the human oral microbiome. Proc. Natl. Acad. Sci. U S A. 2014;111:E2875–E2884. doi: 10.1073/pnas.1409644111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren A.M., Morrison H.G., Lescault P.J., Reveillaud J., Vineis J.H., Sogin M.L. Minimum entropy decomposition: unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J. 2015;9:968–979. doi: 10.1038/ismej.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escapa I.F., Morrison H.G., Lescault P.J., Reveillaud J., Vineis J.H., Sogin M.L. ‘New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): a resource for the microbiome of the human aerodigestive tract. Xu J., editor. mSystems. 2018;3 doi: 10.1128/mSystems.00187-18. http://msystems/3/6/msys.00187-18.atom e00187–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992;61:1–10. [Google Scholar]
- Feng S., Yu H., Yu Y., Geng Y., Li D., Yang C., Lv Q., Lu L., Liu T., Li G., Yuan L. Levels of inflammatory cytokines IL-1 β , IL-6, IL-8, IL-17a, and TNF- α in aqueous humour of patients with diabetic retinopathy. J. Diabetes Res. 2018;2018:1–6. doi: 10.1155/2018/8546423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlita S., Yegiazaryan A., Noori N., Lal G., Nguyen T., To K., Venketaraman V. Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially Mycobacterium tuberculosis. J. Clin. Med. 2019;8:2219. doi: 10.3390/jcm8122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire M.O., Van Dyke T.E. Natural resolution of inflammation. Periodontology. 2013;2000:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire M.O., Dalli J., Serhan C.N., Van Dyke T.E. Neutrophil resolvin E1 receptor expression and function in type 2 diabetes. J. Immunol. 2017;198:718–728. doi: 10.4049/jimmunol.1601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furniss D., Gore S., Azadian B., Myers S.R. Acinetobacter infection is associated with acquired glucose intolerance in burn patients. J. Burn Care Rehabil. 2005;26:405–408. doi: 10.1097/01.bcr.0000176882.69354.7e. [DOI] [PubMed] [Google Scholar]
- Gardner S.E., Hillis S.L., Heilmann K., Segre J.A., Grice E.A. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62:923–930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R.J., Graziani F., Hasturk H. Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontology. 2020;83:59–65. doi: 10.1111/prd.12271. [DOI] [PubMed] [Google Scholar]
- Gerner-Smidt P., Tjernberg I. Acinetobacter in Denmark: II. Molecular studies of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. APMIS. 1993;101:826–832. [PubMed] [Google Scholar]
- Gootz T.D., Marra A. Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev. Anti Infect. Ther. 2008;6:309–325. doi: 10.1586/14787210.6.3.309. [DOI] [PubMed] [Google Scholar]
- Griffen A.L., Beall C.J., Campbell J.H., Firestone N.D., Kumar P.S., Yang Z.K., Podar M., Leys E.J. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G., Lamont R.J. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henig O., Pogue J.M., Martin E., Hayat U., Ja'ara M., Kilgore P.E., Cha R., Dhar S., Kaye K.S. The impact of multidrug-resistant organisms on outcomes in patients with diabetic foot infections. Open Forum Infect. Dis. 2020;7:ofaa161. doi: 10.1093/ofid/ofaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfan U.M., Dawson D.V., Bissada N.F. Epidemiology of periodontal disease: a review and clinical perspectives. J. Int. Acad. Periodontol. 2001;3:14–21. [PubMed] [Google Scholar]
- Kennedy K., Hall M.W., Lynch M.D., Moreno-Hagelsieb G., Neufeld J.D. Evaluating bias of illumina-based bacterial 16S rRNA gene profiles. Wommack K.E., editor. Appl. Environ. Microbiol. 2014;80:5717–5722. doi: 10.1128/AEM.01451-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Hofstaedter C.E., Zhao C., Mattei L., Tanes C., Clarke E., Lauder A., Sherrill-Mix S., Chehoud C., Kelsen J. Optimizing methods and dodging pitfalls in microbiome research. Microbiome. 2017;5:52. doi: 10.1186/s40168-017-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Ha K.H., Kim D.J., Choi Y.H. Diabetes and the risk of infection: a national cohort study. Diabetes Metab. J. 2019;43:804–814. doi: 10.4093/dmj.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstein S.E., McCorrison J., Ahmed A., Hasturk H., Van Dyke T.E., Freire M. Transcriptomics of type 2 Diabetic and healthy human neutrophils. 2020. [DOI] [PMC free article] [PubMed]
- Kleinstein S.E., Nelson K.E., Freire M. Inflammatory networks linking oral microbiome with systemic health and disease. J. Dental Res. 2020;99:1131–1139. doi: 10.1177/0022034520926126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal W.H., Wallis W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952;47:583–621. [Google Scholar]
- Leão A.C.Q., Menezes P.R., Oliveira M.S., Levin A.S. Acinetobacter spp. are associated with a higher mortality in intensive care patients with bacteremia: a survival analysis. BMC Infect. Dis. 2016;16:386. doi: 10.1186/s12879-016-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.-H., Liu C.-P. Diabetic status and the relationship of blood glucose to mortality in adults with carbapenem-resistant Acinetobacter baumannii complex bacteremia. J. Microbiol. Immunol. Infect. 2019;52:654–662. doi: 10.1016/j.jmii.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Lockhart P.B., Durack D.T. Oral microflora as a cause of endocarditis and other distant site infections. Infect. Dis. Clin. North Am. 1999;13:833–850. doi: 10.1016/s0891-5520(05)70111-2. [DOI] [PubMed] [Google Scholar]
- Mann J., Bernstein Y., Findler M. Periodontal disease and its prevention, by traditional and new avenues. Exp. Ther. Med. 2020;19:1504–1506. doi: 10.3892/etm.2019.8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H.I., Nitsch D., Millett E.R.C., Sinclair A., Thomas S.L. New estimates of the burden of acute community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using linked electronic health records. Diabetic Med. 2014;31:606–614. doi: 10.1111/dme.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.B., Kunkel S.L. Attracting attention: discovery of IL-8/CXCL8 and the birth of the chemokine field. J. Immunol. 2019;202:3–4. doi: 10.4049/jimmunol.1801485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki E.M., Shroff R., Singleton J.A., Renaud D.E., Wallace D., Drury J., Zirnheld J., Colleti B., Ellington A.D., Lamont R.J. Microbiota and metatranscriptome changes accompanying the onset of gingivitis. mBio. 2018;9:e00575-18. doi: 10.1128/mBio.00575-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parahitiyawa N.B., Jin L.J., Leung W.K., Yam W.C., Samaranayake L.P. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin. Microbiol. Rev. 2009;22:46–64. doi: 10.1128/CMR.00028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró C., Lorenzo Ó., Carraro R., Sánchez-Ferrer C.F. IL-1β inhibition in cardiovascular complications associated to diabetes mellitus. Front. Pharmacol. 2017;8:363. doi: 10.3389/fphar.2017.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielou E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966;13:131–144. [Google Scholar]
- Pollock J., Glendinning L., Wisedchanwet T., Watson M. The madness of microbiome: attempting to find consensus “best practice” for 16S microbiome studies. Liu S.-J., editor. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.02627-17. e02627-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., KuoLee R., Harris G., Chen W. High susceptibility to respiratory Acinetobacter baumannii infection in A/J mice is associated with a delay in early pulmonary recruitment of neutrophils. Microbes Infect. 2009;11:946–955. doi: 10.1016/j.micinf.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber G.E., Lipsky B.A., Gibbons G.W. The burden of diabetic foot ulcers. Am. J. Surg. 1998;176:5S–10S. doi: 10.1016/s0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- Roberts F.A., Darveau R.P. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: symbiosis and dysbiosis. Periodontology. 2015;69:18–27. doi: 10.1111/prd.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter S.J., Cox M.J., Turek E.M., Calus S.T., Cookson W.O., Moffatt M.F., Turner P., Parkhill J., Loman N.J., Walker A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Abnet C.C., White O., Knight R., Huttenhower C. The microbiome quality control project: baseline study design and future directions. Genome Biol. 2015;16:276. doi: 10.1186/s13059-015-0841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Goodfellow M., editors. Nucleic Acid Techniques in Bacterial Systematics. Wiley; 1991. pp. 225–236. [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S., Pryer K.M., Miao V.P., Palmer J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 1999;46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- Vardakas K.Z., Siempos I.I., Falagas M.E. ‘Diabetes mellitus as a risk factor for nosocomial pneumonia and associated mortality’, Diabetic Medicine. A J. Br. Diabetic Assoc. 2007;24:1168–1171. doi: 10.1111/j.1464-5491.2007.02234.x. [DOI] [PubMed] [Google Scholar]
- Vázquez-López R., Solano-Gálvez S.G., Juárez Vignon-Whaley J.J., Abello Vaamonde J.A., Padró Alonzo L.A., Rivera Reséndiz A., Muleiro Álvarez M., Vega López E.N., Franyuti-Kelly G., Álvarez-Hernández D.A. Acinetobacter baumannii resistance: a real challenge for clinicians. Antibiotics (Basel) 2020;9:205. doi: 10.3390/antibiotics9040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar M., Cano M.E., Gato E., Garnacho-Montero J., Miguel Cisneros J., Ruíz de Alegría C., Fernández-Cuenca F., Martínez-Martínez L., Vila J., Pascual A. Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: a reappraisal. Medicine. 2014;93:202–210. doi: 10.1097/MD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Wu C.-Z., Yuan Y.H., Liu H.H., Li S.S., Zhang B.W., Chen W., An Z.J., Chen S.Y., Wu Y.Z., Han B. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20:204. doi: 10.1186/s12903-020-01180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available at NIH – SRA Bioproject: PRJNA664044. All other code used for data analysis has been provided in the Transparent Methods section (see Scripts) in the Supplemental Information.