Abstract
Background and Objectives
The relationship between metabolic stress, inflammation, and cardiovascular disease is being studied steadily. The aim of this study was to evaluate the effect of palmitate (PA) and minimally modified low-density lipoprotein (mmLDL) on macrophages and to identify the associated pathways.
Methods
J774 macrophages were incubated with PA or mmLDL and lipopolysaccharide (LPS). Secretion of inflammatory chemokines and the expression of corresponding genes were determined. The phosphorylation of extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase was also assessed. RNA sequencing of macrophages was performed to identify the genes regulated by PA or mmLDL. Some of the genes regulated by the 2 agents were validated by knocking down the cells using small interfering RNA.
Results
PA or mmLDL promoted the secretion of interleukin (IL)-6 and IL-1β in LPS-stimulated macrophages, and this was accompanied by higher phosphorylation of ERK. RNA sequencing revealed dozens of genes that were regulated in this process, such as Csf3 and Edn1, which were affected by PA and mmLDL, respectively. These agents also increased Nlrp3 expression. The effect of Csf3 or Edn1 silencing on inflammation was modest, whereas toll-like receptor (TLR) 4 inhibition reduced a large proportion of macrophage activation.
Conclusions
We demonstrated that the proinflammatory milieu with high levels of PA or mmLDL promoted macrophage activation and the expression of associated genes such as Nlrp3, Csf3, and Edn1. Although the TLR4 pathway appeared to be most relevant, additional role of other genes in this process provided insights regarding the potential targets for intervention.
Keywords: Atherosclerosis, Metabolic syndrome, Immunity, Obesity
INTRODUCTION
Oxidative stress, inflammation, and immune system activation are known to be closely related to metabolic disorders such as diabetes, obesity, and associated cardiovascular complications.1) When atherosclerosis develops, metabolic stress may significantly affect the inflammatory response of endothelial cells, monocytes, and macrophages.2) Although the mechanism is not completely understood, diverse types of oxidized low-density lipoproteins (LDLs) are have also been shown to contribute to steatohepatitis. Thus, intervention or inhibition of certain metabolic pathways is being considered as a therapeutic option against inflammatory disorders.3)
Saturated fatty acids and oxidized LDL commonly coexist and contribute to vascular inflammation. Saturated fatty acids can induce inflammatory response via the toll-like receptor (TLR) 4 pathways, and can affect lipopolysaccharide (LPS) production via modification of gut microbiota. Subsequent endotoxemia may trigger oxidative stress and result in the production of oxidized LDL, a major form of atherogenic lipids.4) We chose palmitate (PA) in this study, as it is a major form of saturated fatty acid in human circulation, and it is a player in metabolically pathologic conditions like insulin resistance.5) Several studies have reported metabolic inflammation caused by PA.5),6)
The purpose of this study was to evaluate proinflammatory effects and to identify genes regulated by metabolic stimuli, saturated fatty acids or modified LDL. Specifically, we aimed to examine the effects of PA and minimally modified LDL (mmLDL) on LPS-primed macrophages with pivotal roles in chronic inflammatory conditions, such as atherosclerosis. We also investigated the genes regulated in this process and validated the expression of some of them. Our targets included NLRP3, an essential mediator of the interleukin (IL)-1 pathway, which is currently being actively investigated. The IL-1 pathway upregulated in vascular inflammation, is one of key pathways suggested as a target for anti-inflammatory therapy. For example, the CANTOS clinical trial showed that an IL-1β antagonist reduced cardiovascular risk.7) Building on our previous research on the combined effects of PA and mmLDL,8) we sought to further investigate the individual effects of these 2 agents and genes associated with the observed effects in the current study.
METHODS
Macrophages and other reagents
Dulbecco's modified Eagle's medium (DMEM), penicillin-streptomycin, fetal bovine serum (FBS) and Dulbecco's phosphate-buffered saline were purchased from Gibco (Grand Island, NY, USA). The murine macrophage-like cell line J774A.1 (abbreviated as J774) was provided by Yury I Miller in University of California, San Diego, USA. LPS and PA were purchased from Sigma-Aldrich (St. Louis, MO, USA) for macrophage stimulation. U0126, an extracellular signal-regulated kinase (ERK) inhibitor, was purchased from Cell Signaling Technology (Danvers, MA, USA). It was dissolved in dimethyl sulfoxide (DMSO), and a stock solution of 10 mM was prepared before experiments. TAK-242, a TLR4 inhibitor, was purchased from InvivoGen (San Diego, CA, USA). PA was dissolved in 0.1 M 70% ethanol and a control solution containing ethanol was also prepared. TAK-242 was dissolved in DMSO. Stock solutions of 30 mM PA and 10 mM TAK-242 were prepared before the experiment. LDL (density, 1.019−1.063 g/mL) was isolated from the plasma of healthy donors using sequential ultracentrifugation.9) To produce mmLDL, 50 µg/mL LDL was incubated in serum-free DMEM for 18 hours with murine fibroblast cells overexpressing human 15-lipoxygenase.8) mmLDL has been characterized in detail previously.10) Preparations of PA and mmLDL were assessed for LPS contamination using the Limulus amebocyte lysate assay (Lonza, Basel, Switzerland). Endotoxin levels were < 0.05 EU/mL in all experiments. We chose 100 µM PA8),11),12) and 50 µM/mL mmLDL8),13) for the experiment as these doses have frequently been used to test effects of PA or mmLDL on macrophages in previous studies.
Cell culture and treatment
J774 cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin- streptomycin (Gibco, Carlsbad, CA, USA) at 37°C in a 5% CO2 environment. Prior to stimulation, the cells were maintained in serum-poor (0.5% FBS) medium for 6 hours. For protein expression analysis, J774 cells were plated (5 × 105 cells/well) on 60-mm 6-well plates and incubated overnight. The cells were then either not treated or treated with 100 µM PA for 18 hours before being stimulated with 3 ng/mL LPS. Otherwise, the cells were treated with 3 ng/mL LPS with or without 50 µg/mL mmLDL for 6 hours. After 30 minutes, the cells were harvested for immunoblotting. The supernatant was collected 6 hours post-treatment with LPS with or without mmLDL, and then analyzed using enzyme-linked immunosorbent assay (ELISA). For quantitative polymerase chain reaction (qPCR) analysis, cells were harvested 6 hours post-treatment with LPS with or without mmLDL. All experiments were conducted in biological triplicates.
Enzyme-linked immunosorbent assay
The cell culture supernatant was collected and the amounts of secreted IL-6, IL-1β, or tumor necrosis factor (TNF)-α were quantified using ELISA kits (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's protocols. Technical duplicates of all samples were used.
Immunoblot analysis
Cells were harvested and solubilized in radioimmunoprecipitation assay buffer (Biosesang, Seongnam, Korea) containing a protease inhibitor cocktail (Roche, Inc., Indianapolis, IN, USA). We determined the protein concentration using a bicinchoninic acid protein assay (Pierce Biotechnology, Inc., Rockford, IL, USA). Cell lysates were resolved using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was then incubated at room temperature for 1 hour with 5% bovine serum albumin in Tris-buffered saline (TBS) solution with Tween-20 and with a primary antibody overnight at 4°C. On the following day, the membranes were washed with TBS and incubated with a horse radish peroxidase-conjugated secondary antibody at room temperature. The immunoreactive bands were visualized using the enhanced chemiluminescence kit (EMD Millipore).
RNA extraction and real-time polymerase chain reaction
RNA was extracted from cells using a Ribospin RNA extraction kit (GeneAll, Seoul, Korea) according to the manufacturer's protocol. RNA concentration and purity were assessed using a NanoDrop spectrophotometer. One microgram of RNA was then used for complementary DNA (cDNA) synthesis with a QuantiTect reverse transcription kit (Qiagen, Venlo, Netherlands). Real-time polymerase chain reaction (PCR) was performed using the SYBR dye system and Step One Plus real-time PCR machine (Applied Biosystems, Foster City, CA, USA). Gene expression analysis was performed using the LightCycler software based on cycle threshold values and is shown as fold change of expression. Messenger RNA (mRNA) levels were normalized to glyceraldehyde 3-phosphate dehydrogenase mRNA. All results were derived from technical duplicates.
RNA sequencing and analysis
RNA purity was determined using a NanoDrop ND1000 spectrophotometer. Total RNA sequencing libraries were prepared according to the manufacturer's instructions using a Truseq stranded total RNA sample prep kit (Illumina, San Diego, CA, USA). One microgram of total RNA was further treated for ribosomal RNA (rRNA) depletion using Ribo-zero rRNA removal kit (human/mouse/rat) (Illumina). Then, the rRNA-depleted total RNA was fragmented into small pieces at 94°C for 8 minutes. The cleaved RNA fragments were copied to first strand cDNA using reverse transcriptase and random primers. This was followed by second strand cDNA synthesis using DNA polymerase I and RNase H. Finally, one 'A' base was added, and the adapter was subsequently ligated. The products were purified and enriched using PCR to create the final cDNA library. After real-time PCR, index-tagged libraries were combined in equimolar ratio. RNA sequencing was performed using the Illumina NextSeq 500 platform according to the manufacturer's recommended protocol.
The reads were aligned with the gene expression values using Cufflinks (fragments per kilobase of transcript per million mapped reads). The differentially expressed genes of the vehicle treatment group were compared to that of the PA or mmLDL treatment group using Cuffdiff. Upregulated or downregulated genes with q-value <0.05 and 2-fold change were identified.
Small interfering RNA transfection
Small interfering RNAs (siRNAs) against Csf3r and Edn1 and the scrambled siRNA were purchased from Mbiotech (Hanam, Korea). For gene silencing, J774 cells were treated with 100 nM siRNA using Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA, USA). After 24 hours, the media with siRNA were removed and stimulants were treated for each experiment.
Statistical analysis
All data are presented as the mean±standard error of the mean. We performed t-test for comparison between groups. p<0.05 (2-sided) was considered statistically significant. We used the software package Prism 5.0 for all data analyses (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Palmitate or minimally modified low-density lipoprotein promoted chemokine secretion in macrophages
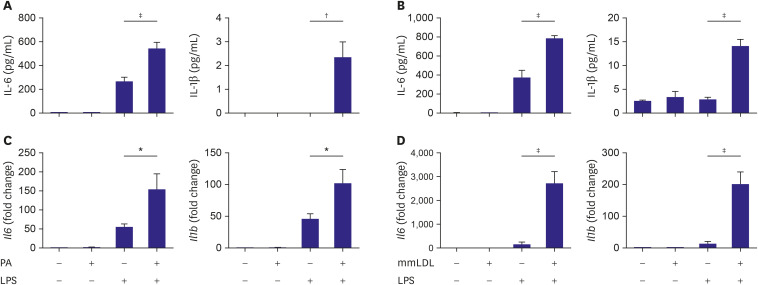
To evaluate the effect of PA on chemokine secretion in LPS-stimulated macrophages, macrophages were incubated with PA and treated with or without LPS. ELISA showed that the cells treated with PA and LPS secreted significantly more IL-6 and IL-1β in the culture media than the cells treated with LPS alone (Figure 1A). To determine the effect of mmLDL, macrophages were treated with LPS with or without mmLDL. IL-6 and IL-1β secretion in the culture media was higher in cells treated with mmLDL and LPS than in cells treated with LPS alone (Figure 1B).
Figure 1. Chemokine secretion and the corresponding mRNA levels increased additively after treatment with PA or mmLDL in LPS-stimulated J774 macrophages. Macrophages were incubated with PA, followed by treatment with or without LPS. The culture media was collected and the levels of IL-6 and IL-1β were determined using ELISA (A). Macrophages were treated with LPS with or without mmLDL. The culture media were collected and the levels of IL-6 and IL-1β were determined (B). J774 cells were incubated with PA, followed by treatment with or without LPS. The cells were harvested and the relative expression levels of Il6 and Il1b were determined using qPCR and normalized to that of Gapdh (C). J774 cells were treated with LPS with or without mmLDL. mRNA expression was determined as mentioned above (D). Experiments were conducted with technical duplicates, and data presented are based on 3 independent replicates.
ELISA = enzyme-linked immunosorbent assay; IL = interleukin; LPS = lipopolysaccharide; mmLDL = minimally modified low-density lipoprotein; mRNA = messenger RNA; PA = palmitate.
*p<0.05, †p<0.01, ‡p<0.001.
J774 cells were incubated with PA and then treated with or without LPS. The relative expression of Il6 and Il1b were determined using qPCR and normalized to that of Gapdh. Compared to that in LPS alone, both genes were upregulated by co-treatment with PA and LPS (Figure 1C). Next, the cells were treated with LPS with or without mmLDL. The mRNA levels of Il6 and Il1b increased significantly in cells treated with mmLDL and LPS compared to those treated with LPS alone (Figure 1D).
Palmitate or minimally modified low-density lipoprotein promoted extracellular signal-regulated kinase activation in macrophages
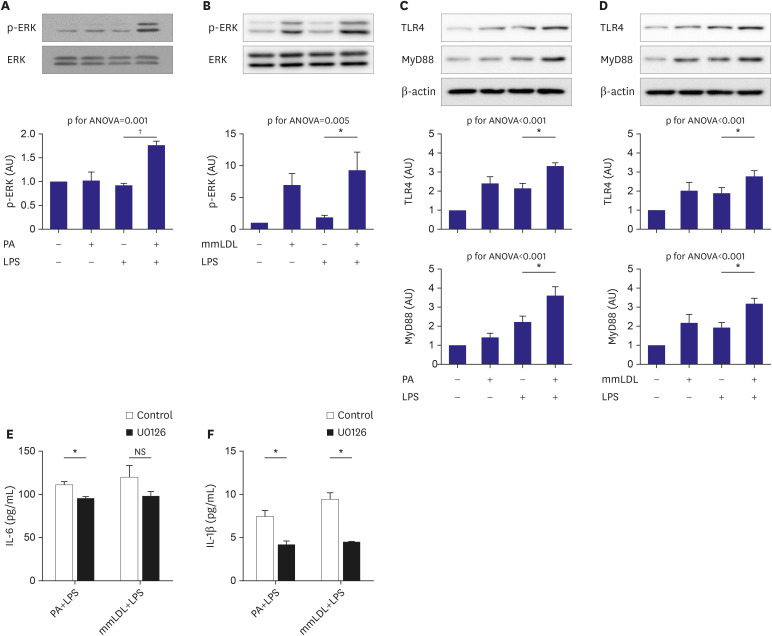
The J774 cells were incubated with PA and then treated with or without LPS. Immunoblotting using cell extracts showed higher ERK phosphorylation in cells treated with PA and LPS than in those treated with LPS alone (Figure 2A). The macrophages were treated with LPS with or without mmLDL. The cells treated with mmLDL and LPS showed higher ERK phosphorylation than those treated with LPS alone (Figure 2B). TLR4 and MyD88, upstream of ERK mitogen-activated protein kinase (MAPK), were analyzed after treatment of LPS with or without PA or mmLDL in the same manner described above. We found that PA increased TLR4 expression (Figure 2C), whereas mmLDL upregulated TLR4 and MyD88 (Figure 2D). To validate the role of ERK in the action of PA or mmLDL, J774 cells were incubated with U0126, an ERK inhibitor, or DMSO for 2 hours and then treated with LPS with or without PA or mmLDL. PA-induced elevation of IL-6 and IL-1β secretion was significantly reduced by ERK inhibition with U0126 (Figure 2E and F). Conversely, U0126 significantly only attenuated mmLDL-induced elevation of IL-1β secretion (Figure 2F).
Figure 2. Phosphorylation of ERK MAPK and its upstream targets were additively promoted by PA or mmLDL in LPS-stimulated macrophages. J774 cells were incubated with PA and then treated with or without LPS. Cell extracts were collected and phosphorylation of ERK was analyzed using immunoblotting (A). Macrophages were treated with LPS with or without mmLDL. Phosphorylation of ERK was analyzed as mentioned above (B). TLR4 and MyD88, the upstream molecules of ERK MAPK, were analyzed after treatment LPS with or without PA (C) or mmLDL (D). J774 cells were incubated with U0126, an ERK inhibitor, or DMSO and then treated with or without PA or mmLDL. The effect of U0126 on PA or mmLDL-induced elevation of IL-6 (E) and IL-1β (F) secretion was analyzed. Experiments were conducted with technical duplicates, and data presented are based on 3 independent replicates.
ANOVA = analysis of variance; DMSO = dimethyl sulfoxide; ERK = extracellular signal-regulated kinase; IL = interleukin; LPS = lipopolysaccharide; MAPK = mitogen-activated protein kinase; mmLDL = minimally modified low-density lipoprotein; NS = not significant; PA = palmitate; TLR = toll-like receptor.
*p<0.05, †p<0.01.
Genes regulated by palmitate or minimally modified low-density lipoprotein
To identify the genes regulated by PA or mmLDL, the mRNA profiles of the cells were analyzed using RNA sequencing. Compared to the cells stimulated with LPS alone, those stimulated with PA and LPS showed nine differentially expressed and upregulated genes. The cells stimulated with mmLDL and LPS showed 30 differentially expressed upregulated genes (Table 1).
Table 1. Highly-ranked genes that are differentially upregulated after treatment with PA or mmLDL in LPS-stimulated macrophages.
| Genes | PA | Genes | mmLDL |
|---|---|---|---|
| Fold change | Fold change | ||
| Pus2f2 | 3.21 | Thbs1 | 8.10 |
| Tgfbi | 2.68 | Il1a | 6.78 |
| Il1f9 | 2.59 | Slc39a10 | 5.35 |
| Edil3 | 2.33 | Nr4a1 | 5.14 |
| C1pc | 2.21 | Tnfsf9 | 4.52 |
| Csf3 | 2.21 | Osbp2 | 4.42 |
| Cd3001b | 2.18 | Dusp5 | 4.25 |
| Cd93 | 2.15 | Cd93 | 4.07 |
| Osbp2 | 2.01 | Il1b | 3.78 |
| Pxdc1 | 3.64 | ||
| Pitpnc1 | 3.35 | ||
| Edil3 | 2.91 | ||
| Pid1 | 2.91 | ||
| Fosl2 | 2.82 | ||
| Peli2 | 2.74 | ||
| Plaur | 2.68 | ||
| Ppp1r3b | 2.45 | ||
| Cxcl3 | 2.45 | ||
| Epha2 | 2.43 | ||
| Il1r2 | 2.38 | ||
| Mmp10 | 2.34 | ||
| Cd300lf | 2.33 | ||
| Bhlhe40 | 2.28 | ||
| Bnip3 | 2.27 | ||
| Ski | 2.26 | ||
| Il23a | 2.26 | ||
| Edn1 | 2.22 | ||
| Fgr | 2.18 | ||
| Kctd12 | 2.16 | ||
| Kdm5b | 2.16 |
LPS = lipopolysaccharide; mmLDL = minimally modified low-density lipoprotein; PA = palmitate.
Among the target genes regulated by PA, we selected Csf3 for validation. Csf3 is expressed in diverse immune cells and is involved in the maturation of granulocytes and modulation of immune response.14) Among the target genes regulated by mmLDL, we selected Edn1 for validation. Although its role in macrophages is not clear, it is known to be involved in inflammation in endothelial cells and affect the transcription of inflammatory chemokines.15),16)
Validation of the role of Csf3 in macrophage activation by palmitate
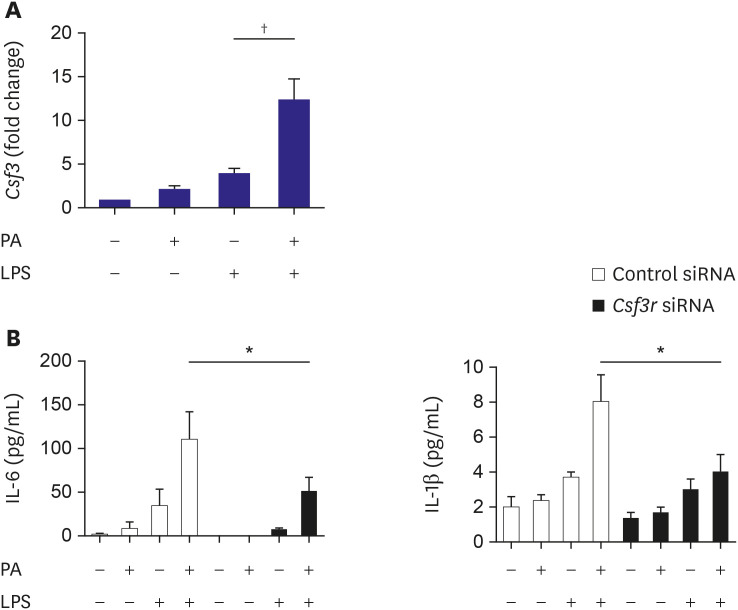
To validate the RNA sequencing results, the mRNA level of Csf3 in macrophages was determined after PA treatment. J774 cells were incubated with PA and then treated with or without LPS. The relative expression of Csf3 in cells treated with PA and LPS was more than that of cells treated with LPS alone (Figure 3A). CSF3 is the primary ligand for CSF3R, a member of the cytokine receptor superfamily. To analyze the role of CSF3R in PA-associated macrophage activation, we determined the changes in chemokine secretion after silencing of Csf3r. J774 cells were transfected with siRNA against Csf3r prior to stimulation with PA with or without LPS. ELISA of the culture media showed significant attenuation of the elevated IL-6 and IL-1β secretion (Figure 3B).
Figure 3. Role of Csf3 in macrophage activation after treatment with PA. To validate the results of RNA sequencing, the Csf3 mRNA level in macrophages was determined after PA treatment. J774 cells were incubated with PA and then treated with or without LPS. The cells were harvested and the relative expression of Csf3 was measured using qPCR and normalized to Gapdh expression (A). Silencing of Csf3r attenuated the PA-induced increased secretion of chemokines in LPS-stimulated macrophages. J774 cells were transfected with siRNA against Csf3r prior to stimulation of the cells with PA with or without LPS. In the culture media, the levels of chemokines were determined using ELISA. Experiments were conducted with technical duplicates, and data presented are based on 3 independent replicates.
ELISA = enzyme-linked immunosorbent assay; IL = interleukin; LPS = lipopolysaccharide; mmLDL = minimally modified low-density lipoprotein; mRNA = messenger RNA; PA = palmitate; qPCR = quantitative polymerase chain reaction; siRNA = small interfering RNA.
*p<0.05, †p<0.01.
Validation of the role of Edn1 in macrophage activation by minimally modified low-density lipoprotein
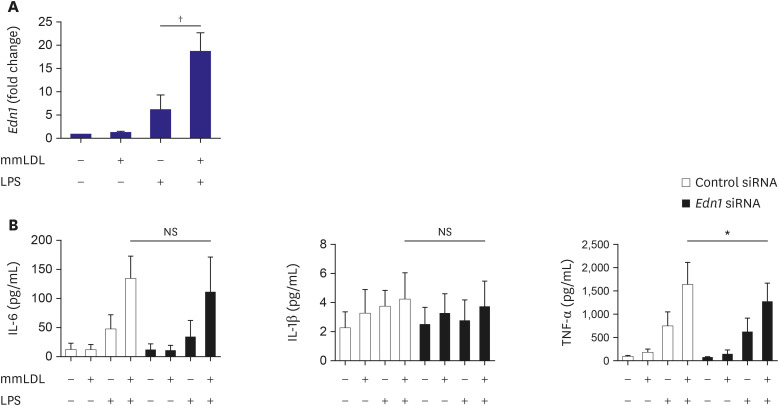
To validate the results of RNA sequencing, the Edn1 mRNA level in macrophages was determined after mmLDL treatment. Macrophages were treated with LPS with or without mmLDL. The relative expression of Edn1 in cells treated with mmLDL and LPS was higher than that in cells treated with LPS alone (Figure 4A). Silencing of Edn1 did not attenuate the increased IL-6 and IL-1β secretion, whereas it reduced the secretion of TNF-α (Figure 4B).
Figure 4. Role of Edn1 in macrophage activation after treatment with mmLDL. To validate the results of RNA sequencing, Edn1 mRNA expression in macrophages was determined after treatment with mmLDL. J774 cells were treated with LPS with or without mmLDL. The relative expression of Edn1 was determined using qPCR and normalized to Gapdh expression (A). J774 cells were transfected with siRNA against Edn1 prior to stimulation of the cells with mmLDL with or without LPS. The chemokine levels in the culture media of J774 cells was determined using ELISA. Edn1 silencing did not attenuate the increased secretion of IL-6 and IL-1β, whereas it reduced that of TNF-α. Experiments were conducted with technical duplicates, and data presented are based on 3 independent replicates.
ELISA = enzyme-linked immunosorbent assay; IL = interleukin; LPS = lipopolysaccharide; mmLDL = minimally modified low-density lipoprotein; mRNA = messenger RNA; NS = not significant; qPCR = quantitative polymerase chain reaction; siRNA = small interfering RNA; TNF = tumor necrosis factor.
*p<0.05, †p<0.01.
Role of the NLRP3 and TLR4 pathways in macrophage activation
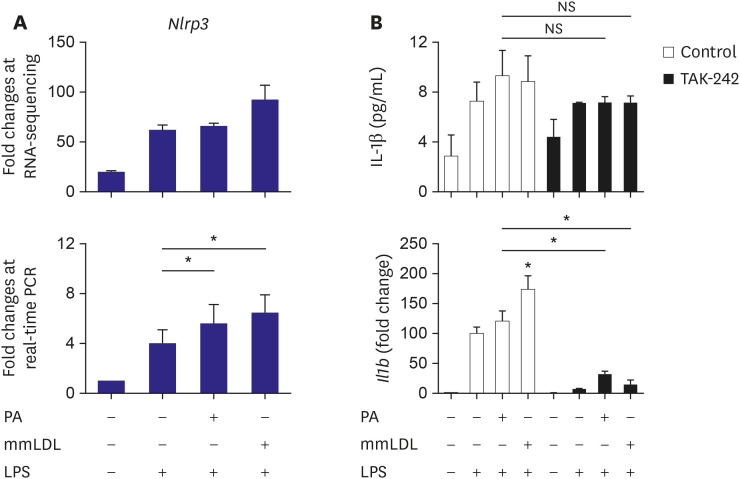
We have observed upregulation of Il1b, a well-known inflammation-related gene, after PA or mmLDL treatment (Figure 1). Hence, we sought to evaluate whether these agents acted via the NLRP3-dependent pathway. The results of real-time PCR showed that Nlrp3, the expression of which tended to increase after PA or mmLDL treatment in RNA sequencing, was significantly upregulated (Figure 5A). Finally, we investigated whether the increased secretion and expression of IL-1β was dependent on the TLR4 pathway. TAK-242, a TLR4 inhibitor, appeared to reduce, albeit not significantly, the elevated secretion of IL-1β with PA or mmLDL (top of Figure 5B). However, after TAK-242 treatment, we observed that the induction of Il1b with PA or mmLDL was remarkably attenuated (bottom of Figure 5B).
Figure 5. Involvement of the NLRP3 and the TLR4-mediated pathway in macrophage activation with PA or mmLDL. J774 cells were treated with PA or mmLDL in addition to LPS. RNA-sequencing showed a tendency of upregulation of Nlrp3 (top of A). Real-time PCR demonstrated significant increase in Nlrp3 expression after PA or mmLDL treatment (bottom of A). To investigate the role of the TLR4 pathway in mediating the effects of PA or mmLDL on IL-1β, its expression and secretion were determined with or without a TLR4 inhibitor, TAK-242. TAK-242 inhibited, albeit not significantly, the effect of PA or mmLDL-induced IL-1β secretion (top of B). However, the effect of PA or mmLDL on Il1b upregulation was remarkably reduced after TLR4 inhibition (bottom of B).
IL = interleukin; LPS = lipopolysaccharide; mmLDL = minimally modified low-density lipoprotein; NS = not significant; PA = palmitate; PCR = polymerase chain reaction; TLR = toll-like receptor.
*p<0.05.
DISCUSSION
The findings of our study include: 1) PA or mmLDL promoted inflammatory chemokine secretion in LPS-stimulated macrophages. 2) This was accompanied by the activation of the ERK MAP kinase pathway. 3) Dozens of PA and mmLDL-regulated genes were identified, which included Csf3 and End1, respectively. 4) Nlrp3 was also found to be activated by these 2 agents. 5) The effect of silencing either Csf3 or Edn1 appeared to be modest, whereas TLR4 inhibition reduced a large proportion of macrophage activation by PA or mmLDL. In this study, we demonstrated the effect of the proinflammatory metabolic milieu on macrophages and the associated molecular pathways.
We demonstrated, for the first time, that mmLDL can upregulate Nlrp3. Although the TLR4 pathway appeared to contribute the most to this process, evidence on the role of other genes was also provided. The strength of the current study also relies on the identification of genes regulated by PA or mmLDL. These can be suggested as novel therapeutic targets against poor metabolic conditions. It has been reported that PA could promote insulin resistance and inflammation via TLR4-dependent and some other pathways.17) However, we are the first to provide data demonstrating that Csf3 is upregulated by PA. Conversely, the pathways on which oxidized LDL works have been substantially investigated and understood, particularly, oxidized LDL's interactions with CD36 and its influence on inflammasomes.18) Remarkably, however, we revealed that mmLDL not containing advanced lipid oxidation products upregulates Nlrp3.
The NLRP3 inflammasome is a well-known signaling complex involved in innate immunity and is a key mediator of the production of IL-1 family of cytokines. It is activated by various endogenous signals during the progression of atherosclerosis, which include pathogen-associated molecular patterns, danger-associated molecular patterns, and oxidized LDL.19) Free fatty acids such as PA have been reported to promote NLRP3 production via the pattern recognition receptor.19) Oxidized LDL is known to be involved in the formation of the NLRP3 inflammosome.19),20) This effect of oxidized LDL can also result in disorders not only in cardiovascular organs. Uptake of oxidized LDL by Kupffer cells has been reported to induce inflammasome activation, impairment of autophagy and hepatic inflammation and non-alcoholic steatoheaptitis. In addition, activated inflammasome and promoted apoptosis of hepatocytes are known to induce inflammation. Furthermore, oxidized LDL can activate hepatic stellate cells and leads to pro-fibrotic response.21) However, for the first time, we showed that mmLDL upregulated Nlrp3 expression.
Studies have suggested that PA promotes inflammation via multiple pathways, and TLR2 and TLR4 are considered the major receptors mediating this effect. However, alternative mediators such as diacylglycerol, ceramide, and lysophosphatidylcholine also contribute to intracellular inflammation by PA. Hence, controlling the response to PA by inhibiting a single pathway may be challenging.22) Reports have shown that mmLDL promotes inflammation via TLR4.23) Choi et al.24) showed that oxidized cholesteryl ester of mmLDL induces secretion of inflammatory cytokines, which is mediated by TLR4 and spleen tyrosine kinase. In our study, a TLR4 inhibitor attenuated a large proportion of IL-1β secretion. Thus, we confirmed that this pathway can be a target for efficiently controlling macrophage activation with PA or mmLDL. We confirmed that PA or mmLDL activated ERK MAPK and upregulated TLR4 in LPS-stimulated macrophages. This finding was validated by using ERK inhibitor. However, because the activation of ERK by these agents was previously shown and was not the main purpose of the current study, we did not further explore ERK-related issues.
In the present study, we observed that silencing of 2 genes, Csf3 and Edn1, modestly but significantly reduced the cellular effect of PA and mmLDL, respectively. Csf3 function has been reported to be dependent on TLR4.8) This gene is associated with inflammation and is upregulated in the epicardial adipose tissue of patients with diabetes and coronary artery disease. This suggests that Csf3 expression may be related to metabolism and atherosclerosis.25) Furthermore, Csf3 was reported to be one of the differentially expressed genes during the dedifferentiation of human smooth muscle cell phenotype, indicating its possible relationship with atherogenesis.26) Upregulation of Csf3 by PA in our study implied that Csf3-related pathways may contribute to the proinflammatory and proatherogenic activities of PA.
We observed that Edn1 was upregulated by mmLDL. A previous human genetic study has revealed the association of Edn1 with fibromuscular dysplasia or coronary artery disease.27) Edn1 is one of the transcriptionally regulated genes in macrophage response.28) In Cd73-deficient mice, Edn1 was found to be upregulated and atherosclerosis was promoted.29) The extent of mmLDL-mediated upregulation of Edn1 that can contribute to vascular pathology remains to be investigated. The other genes affected by PA or mmLDL include, but are not limited to, the following: Il1f9, C1qc, and Cd93 were upregulated by PA, whereas Thbs1, Il1a, and Slc39a10 were upregulated by mmLDL. However, till date, the role of these genes in cardiovascular health remains uncertain.
In our experiments, LPS frequently showed a greater impact than PA or mmLDL on chemokine secretion or expression of related genes. However, our focus was on PA or mmLDL in LPS-primed macrophages, not to compare any of them. Although an environment with elevated endotoxin, represented by LPS, is of importance,30) our study sought to investigate the effect of PA and mmLDL, on top of endotoxemia, which are known mediators in poor metabolic conditions.
Our study has a few limitations. The doses of PA or mmLDL used in our study may differ from the concentration found in human tissues or in circulation. However, we referred to doses used in previous studies, and our dosages were within the published ranges. We validated the roles of Csf3 and Edn1, as well as that of TLR4, and observed that the effect of TLR4 inhibition was the most obvious. As TLR4, an upstream receptor, plays a major role in these processes, the feasibility of using other genes that play minor roles in these processes as intervention targets could be uncertain. However, we identified several PA- and mmLDL-affected genes, and multiple relevant genes may possibly act cooperatively. In particular, risk factors other than saturated fatty acids and modified LDL may coexist in metabolic conditions that are prone to vascular diseases. Therefore, an understanding of the diverse risk factors and associated targets will be valuable for translating the results of this study into practice.
In conclusion, we demonstrated that proinflammatory metabolic conditions involving elevation in PA or mmLDL levels promoted macrophage activation via regulation of the expression of various genes such as Nlrp3, Csf3, and Edn1. Although the TLR4 pathway appeared to be the most relevant, validation of the role of other genes provided insights regarding the potential targets for intervention in this environment.
Footnotes
Funding: This study was supported by the National Research Foundation of Korea, funded by the Korean government (No. 20181D1A1B07043855 and 2019R1F1A1057952).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Lee SH.
- Data curation: Youk H, Kim M, Ann SJ.
- Formal analysis: Youk H, Kim M.
- Funding acquisition: Lee SH, Ann SJ.
- Investigation: Youk H, Kim M, Ann SJ, Lee SH.
- Methodology: Youk H, Kim M, Kim JH, Ann SJ, Lee SH.
- Project administration: Ann SJ, Lee SH.
- Resources: Kim JH, Lee SH.
- Supervision: Lee SH, Ann SJ.
- Validation: Lee CJ, Oh J, Park S, Kang SM.
- Visualization: Youk H, Kim M.
- Writing - original draft: Youk H, Kim M, Ann SJ, Lee SH.
- Writing - review & editing: Lee CJ, Oh J, Park S, Kang SM.
References
- 1.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali L, Schnitzler JG, Kroon J. Metabolism: the road to inflammation and atherosclerosis. Curr Opin Lipidol. 2018;29:474–480. doi: 10.1097/MOL.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 3.Bettencourt IA, Powell JD. Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation, and transplantation. J Immunol. 2017;198:999–1005. doi: 10.4049/jimmunol.1601318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016;244:211–215. doi: 10.1016/j.atherosclerosis.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Chung JH, Jeon HJ, Hong SY, et al. Palmitate promotes the paracrine effects of macrophages on vascular smooth muscle cells: the role of bone morphogenetic proteins. PLoS One. 2012;7:e29100. doi: 10.1371/journal.pone.0029100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 8.Ann SJ, Kim KK, Cheon EJ, et al. Palmitate and minimally-modified low-density lipoprotein cooperatively promote inflammatory responses in macrophages. PLoS One. 2018;13:e0193649. doi: 10.1371/journal.pone.0193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 10.Choi SH, Yin H, Ravandi A, et al. Polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS One. 2013;8:e83145. doi: 10.1371/journal.pone.0083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz EA, Zhang WY, Karnik SK, et al. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M, Boytard L, Hadi T, et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci Rep. 2020;10:5555. doi: 10.1038/s41598-020-62272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiesner P, Choi SH, Almazan F, et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deotare U, Al-Dawsari G, Couban S, Lipton JH. G-CSF-primed bone marrow as a source of stem cells for allografting: revisiting the concept. Bone Marrow Transplant. 2015;50:1150–1156. doi: 10.1038/bmt.2015.80. [DOI] [PubMed] [Google Scholar]
- 15.Hathaway CK, Grant R, Hagaman JR, et al. Endothelin-1 critically influences cardiac function via superoxide-MMP9 cascade. Proc Natl Acad Sci U S A. 2015;112:5141–5146. doi: 10.1073/pnas.1504557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolar P, Wack A. Monocytes work harder under pressure. Nat Immunol. 2019;20:1422–1424. doi: 10.1038/s41590-019-0523-x. [DOI] [PubMed] [Google Scholar]
- 17.Palomer X, Pizarro-Delgado J, Barroso E, Vázquez-Carrera M. Palmitic and oleic acid: the yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol Metab. 2018;29:178–190. doi: 10.1016/j.tem.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Miller YI, Shyy JY. Context-dependent role of oxidized lipids and lipoproteins in inflammation. Trends Endocrinol Metab. 2017;28:143–152. doi: 10.1016/j.tem.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- 20.Rhoads JP, Lukens JR, Wilhelm AJ, et al. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcγR cooperation and is dependent on CARD9. J Immunol. 2017;198:2105–2114. doi: 10.4049/jimmunol.1601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houben T, Brandsma E, Walenbergh SM, Hofker MH, Shiri-Sverdlov R. Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:416–429. doi: 10.1016/j.bbalip.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68:915–932. doi: 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller YI, Choi SH, Wiesner P, Bae YS. The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. Br J Pharmacol. 2012;167:990–999. doi: 10.1111/j.1476-5381.2012.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SH, Sviridov D, Miller YI. Oxidized cholesteryl esters and inflammation. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:393–397. doi: 10.1016/j.bbalip.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camarena V, Sant D, Mohseni M, et al. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr Metab Cardiovasc Dis. 2017;27:739–750. doi: 10.1016/j.numecd.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagiannis GS, Weile J, Bader GD, Minta J. Integrative pathway dissection of molecular mechanisms of moxLDL-induced vascular smooth muscle phenotype transformation. BMC Cardiovasc Disord. 2013;13:4. doi: 10.1186/1471-2261-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adlam D, Olson TM, Combaret N, et al. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol. 2019;73:58–66. doi: 10.1016/j.jacc.2018.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denisenko E, Guler R, Mhlanga M, Suzuki H, Brombacher F, Schmeier S. Transcriptionally induced enhancers in the macrophage immune response to Mycobacterium tuberculosis infection. BMC Genomics. 2019;20:71. doi: 10.1186/s12864-019-5450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchheiser A, Ebner A, Burghoff S, et al. Inactivation of CD73 promotes atherogenesis in apolipoprotein E-deficient mice. Cardiovasc Res. 2011;92:338–347. doi: 10.1093/cvr/cvr218. [DOI] [PubMed] [Google Scholar]
- 30.Bowman JD, Surani S, Horseman MA. Endotoxin, toll-like receptor-4, and atherosclerotic heart disease. Curr Cardiol Rev. 2017;13:86–93. doi: 10.2174/1573403X12666160901145313. [DOI] [PMC free article] [PubMed] [Google Scholar]