Abstract
Background
The management of bone defect due to trauma or surgical debridement is a current problem in orthopedic trauma surgery, often complicated by infection and bone nonunion. The graft is one of the most challenging variables in surgical treatment. Bioactive Glass (BAG) as a biocompatible and osteogenic product is a promising bone substitute showing good results in maxillo-facial-, spine surgery and treatment of osteomyelitis. Surprisingly, there is very little data on BAG use in trauma surgery.
Case presentation
A 51-year-old male patient, involved in a motorcycle accident, suffered an open proximal tibia fracture, type IIIC, of the left leg. Patient was admitted in January of 2013 to a general orthopedic department for surgical treatment. After several surgical revisions due to infection, vascular damage, and bone nonunion, the patient was successfully treated with Masquelet therapy followed by GlassBONE™ grafting (GlassBONE™ 45S5; Norarker). The patient demonstrated excellent results over the course of a two-year follow-up.
Conclusions
In our experience, GlassBONE™ 45S5 has proven to be an effective bone substitute even in difficult grafting conditions, including multiple surgical revisions for bone nonunion and infection. In our case, at the end of 2 years and 3 months of follow-up, the patient reported no pain, and had no signs of infection. Bone union and full weight bearing was achieved.
This case report is oriented by the CARE guidelines for clinical case reports; the patient gave consent for publication.
Keywords: BAG, Bone nonunion, Tibia fracture
Highlights
-
•
GlassBONE™ 45S5 has proven to be an effective bone substitute even in difficult grafting conditions.
-
•
Bone nonunion was successfully treated with Masquelet therapy followed by GlassBONE™ grafting.
-
•
Bone loss due to fracture or surgical debridement is a current problem in orthopedic trauma surgery.
-
•
Bone fractures are the most widespread trauma in humans.
Background
Bone fractures account for the most widespread trauma in humans [1]. The management of bone defect and bone loss due to fracture or surgical debridement is a current problem in orthopedic trauma surgery. Bone loss usually requires grafting and implantation of stabilizing material, elevating the risk of infection and subsequently the risk of bone nonunion [2]. Bone nonunion leads to diminution of quality of life and even disability posing a vast impact on health systems and economies. A recent review of Schlund et al. found that 10–15% of patients experienced impaired fracture healing or even bone nonunion after bone injury [3]. In open fractures, the risk of nonunion is reported to be more than 30% [4]. In a study on 104 tibial shaft fractures, Karladani showed a relative risk of 8.2% of open fractures to develop nonunion [5].
The surgical management of bone defect has been addressed in a variety of ways, including bone-transfer, free soft-tissue flaps or antibiotic loaded polymethylmethacrylate (PMMA) [6,7]. Autologous bone grafting is limited by the amount of graft material accessible for harvest and large bone defects may not be able to be sufficiently filled. For example, some patients who have already undergone harvesting from both iliac crests and/or harvesting via reamer-irrigator- aspirator (RIA), may not be candidates for bone grafting due to anatomical reasons. In addition, autologous bone grafting requires a second surgical intervention, posing postoperative risks including infection, pain and serious cosmetic anomalies [8,9]. The main disadvantages of PMMA application include its multiple resistances and the requirement of additional intervention resulting in potential risk of associated complications and morbidities [10,11].
There is a need of a bone substitute that does not require harvesting, which is biocompatible, carries no risk of viral or bacterial disease transmission, has unlimited availability, and provides stable restitution of the bone. BAG, a biomaterial of the ceramic family, seems to meet these requirements in mechanical strength, biodegradability, and its osteoinductive and osteointegrative properties [12,13]. BAG induces the formation of hydroxyapatite by releasing calcium ions, responsible for its osteostimulative effect. In addition, it has an osteoconductive effect allowing bone to grow on its surface [[14], [15], [16]]. In our clinical case, we used the original form of BAG, GlassBONE™ (NORARKER) for grafting. GlassBONE™, belonging to the SiO2-Na2O-CaO-P2O5 system (Bioglass) was invented by Dr. Larry Hench in the late 1960s and is a degradable, bioactive glass (glass that activates specific responses of cells) with the groundbreaking characteristic to bond to bone [[17], [18], [19]]. 45S5 is the base of many variants of bioactive ceramics including S53P4 BAG, which is a promising BAG. This BAG is currently involved in a clinical trial comparing the outcome of two different treatment strategies involving nonunion of the tibia and femur. One group is treated with S53P4 BAG grafting after Masquelet therapy, the other group is treated in the regular way with autologous bone- and tricalcium phosphate grafting [20]. Open fractures with large skin lesions (greater than 5 cm), as we are presenting in our case report, are known to have a 5.7 times greater risk of delayed healing or nonunion than fractures with no skin injury [2]. In trauma surgery, the risk of infection is also elevated by the implantation of foreign material (fixation material) [21]. BAG could be an attractive biomaterial in trauma surgery due to its osteoconductive and osteostimulative effects as well as its antimicrobial properties. Bacterial adhesion and proliferation are inhibited with BAG due to increase of the local pH and elevation of osmotic pressure by release of sodium and calcium ions and phosphorus salts [22]. The great advantage of this local bactericidal action, without the addition of synthetical antibiotic, is that no bacterial resistance will be created, and no adverse reactions should be seen.
Case presentation
We report the case of a 51-year-old male who presented with an open proximal tibia fracture type IIIC of the left leg due to a motorcycle accident in January 2013. For three years, (between the initial surgery in January 2013 until March 2016) the patient underwent several surgical revisions to correct and combat vascular damage, bone nonunion and infection. Even after numerous attempts to achieve healing, patient's result was unsatisfying, as once again, nonunion and infection of the fracture site was noted. Successful treatment was finally initiated in September 2016 by Masquelet therapy followed by GlassBONE™ grafting in a second procedure showing excellent results upon 2-year follow-up.
A 51-year-old male was addressed to the orthopedic department (France) to receive medical treatment of an open proximal tibia fracture type IIIC of the left leg. Medical history: smoker, no diseases, no regular medication. The immediate surgery in January 2013 consisted of open reduction and internal plate fixation (lateral LCP, antero-lateral approach) and vascular bypass of popliteal artery. The postoperative phase was complicated by severe wound healing disorder, leading to septic osteitis with skin necrosis and bone exposition in March 2013. Treatment consisted of medial gastrocnemius muscle flap and skin graft at University Hospital. The tissue samples taken during surgery were positive for staphylococcus multi-r, Enterobacter cloacae multi-r and enterococcus species multi-r. Antibiotic therapy with Vancomycin and Co-trimoxazole (Trimethoprim/Sulfamethoxazole) was initiated. In December 2013 fracture of fixation material occurred leading to the necessity of several surgical revisions between December 2013 and October 2014, including material changement, decortication and partial resection of the tibia. In the two surgical revisions (January and October 2014) the same initial surgical approach (antero-lateral) was used. Internal fixation was performed as initially with lateral LCP. Allograft (half femoral head, Tissue Bank of France and OSTEOpure™, European Cell and Tissue Bank), and autograft (iliac crest) with Bone Morphogenetic Proteins (BMP) substitution were performed.
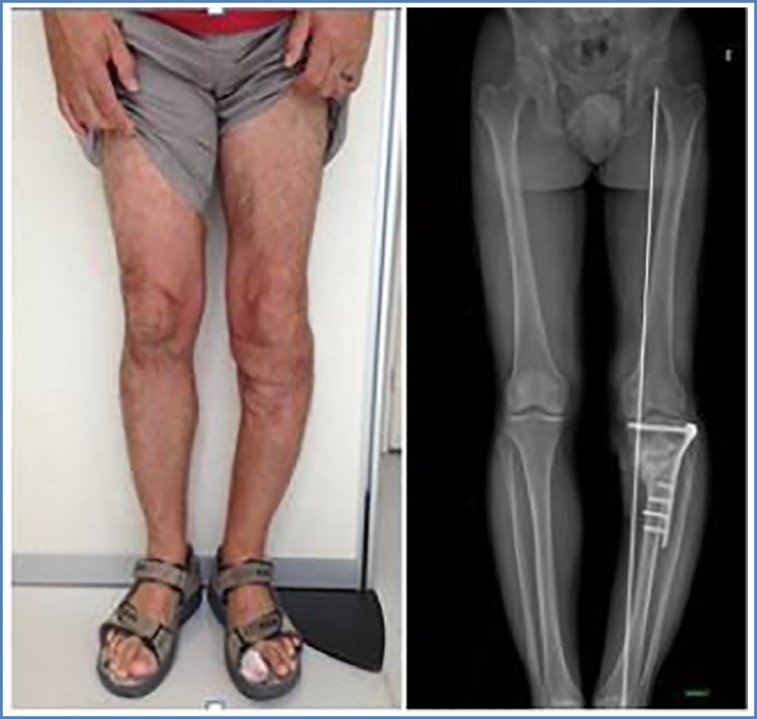
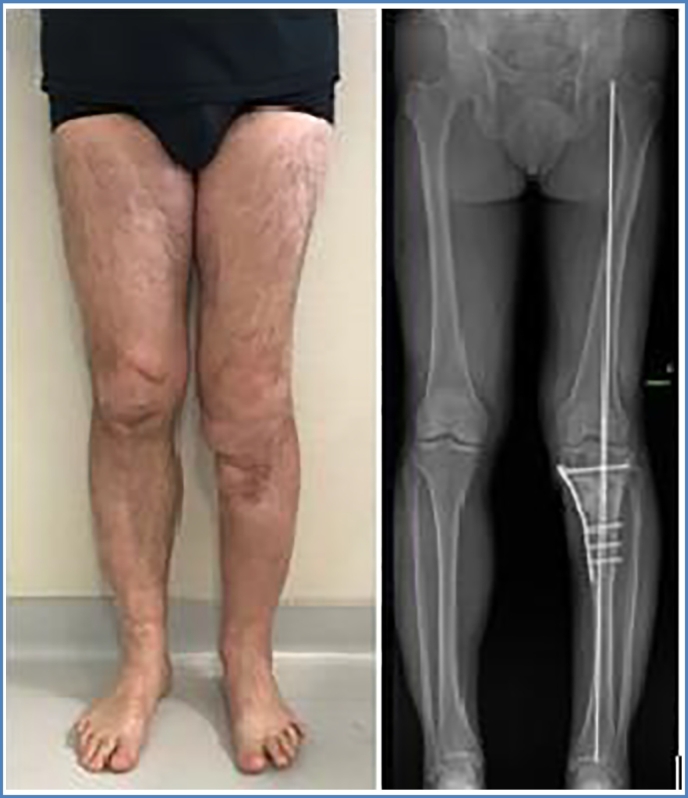
One year later, despite repeated surgical revisions and antibiotic treatment, the wound was inflammatory with subcutaneous collection without fistulation. X-ray and CT-scan performed in September 2015 showed that bone union was still not achieved. In addition, severe tibial varus deformation (10°) at the fracture site was noted (Fig. 1, Fig. 2). The suspected underlying infectious process was confirmed by bone scintigraphy in December 2015 with fixation at the site of fracture and surrounding soft tissues.
Fig. 1.
Clinical aspect and anterior-posterior view (ap view) of leg axis showing varus deformity before Masquelet therapy.
Fig. 2.
ap- and lateral view of left leg before Masquelet therapy.
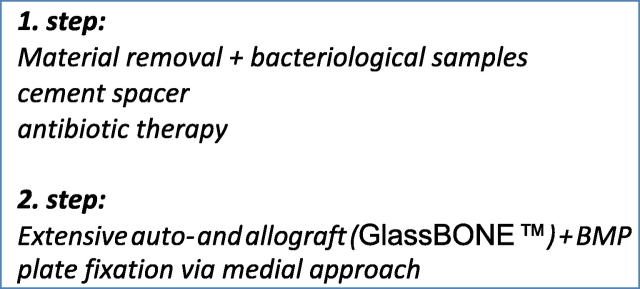
The final treatment we are presenting in this case was preoperatively confirmed by a pluridisciplinary meeting in April 2016. Treatment strategy consisted of a two-step therapy (Masquelet-therapy followed by auto- and GlassBONE™-grafting and plate fixation via different surgical approach (medial approach to proximal tibia)) (Fig. 3).
Fig. 3.
Therapy plan.
Preoperative exams were performed: Scintigraphy with polynuclear cells marked with 99mTc- HMPAO confirmed an osteo-infection at the lateral side of left tibia. As an additional complication, (in March 2016) preoperative C.T. angio and vascular Doppler ultrasound revealed a lower limb arteriopathy with stenosis (>70%) of the left inferior popliteal artery. This required preoperative vascular surgery in order to prime the vascular conditions for Masquelet and grafting surgery. Angioplasty of the left inferior popliteal artery was performed in June 2016 with good results. No complications, no stenosis was found during the follow-up.
Step one: material removal and Masquelet therapy
In September 2016, the first step of treatment was performed (Centre Hospitalier Saint Joseph Saint Luc, Lyon, France). Material associated to partial resection of the tibia was removed and a cement spacer was put in place. Provisory osteosynthesis via clamp was performed (Fig. 4). Multiple bacteriological samples were taken including PCR analysis. During surgery, due to correction of the varus, damage of popliteal artery occurred necessitating a venous bypass by allograft. Intraoperative thrombosis of the venous bypass was successfully managed by thrombectomy in the immediate postoperative suites. Subsequent postoperative evolution was positive (C.T. angio confirming good collateral flow despite repeated thrombosis of the venous bypass. There was no indication for revascularization, but medical treatment prescribed with Acide acétylsalicylique 75 mg per day). Antibiotic therapy with Tazocilline + Vancomycin was initiated until reception of negative bacteriological samples. The patient left hospital on day 20 in good physical condition. Bacteriological samples were negative; no weight bearing was allowed.
Fig. 4.
ap- and lateral view of left knee after cement spacer implantation.
Step two
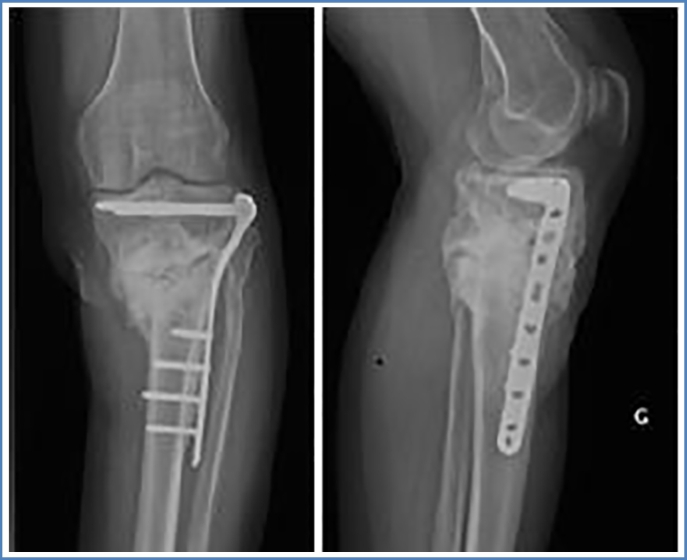
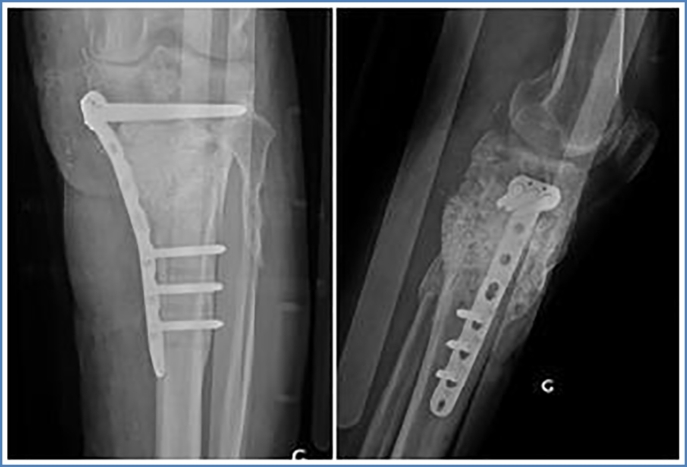
Three months later, in January 2017, second surgical procedure (Centre Hospitalier Saint Joseph Saint Luc, Lyon, France) was performed without complications. Via medial approach, the cement spacer was removed, respecting the induced membrane. Again, multiple bacteriological samples were taken including PCR analysis. The bone defect was filled with extensive auto- and allograft (Iliac crest, GlassBONE™) within the borders of induced membrane and fixed via medial LCP (Fig. 5). Postoperatively, the patient received antibiotic therapy for 5 days (Vancomycin) until the reception of the bacteriological analysis, showing negative results in all samples. The patient left hospital on day 7 in good condition without weight bearing. Clinical and radiological follow-up was performed on months 1, 3, 4, 6, 10 and 27 post operatively.
Fig. 5.
ap- and lateral view of left knee after GlassBONE grafting and plate fixation.
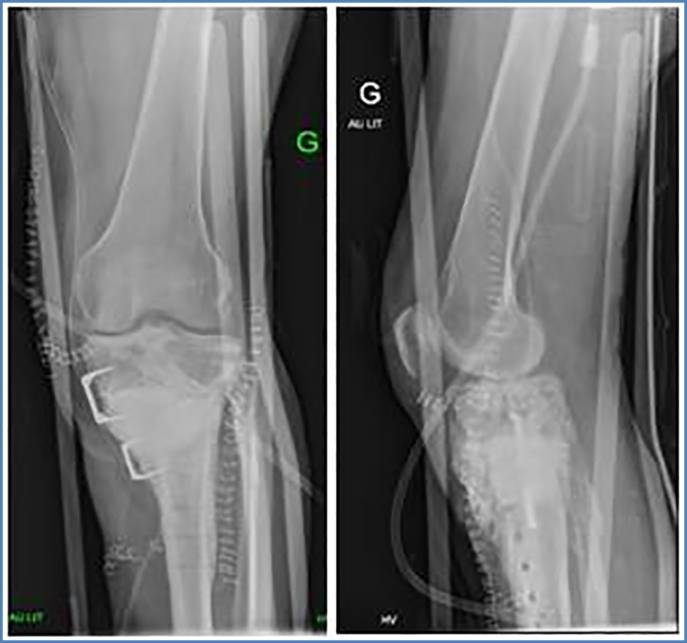
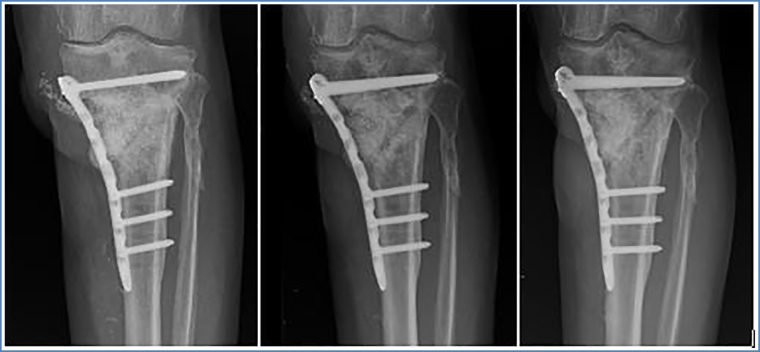
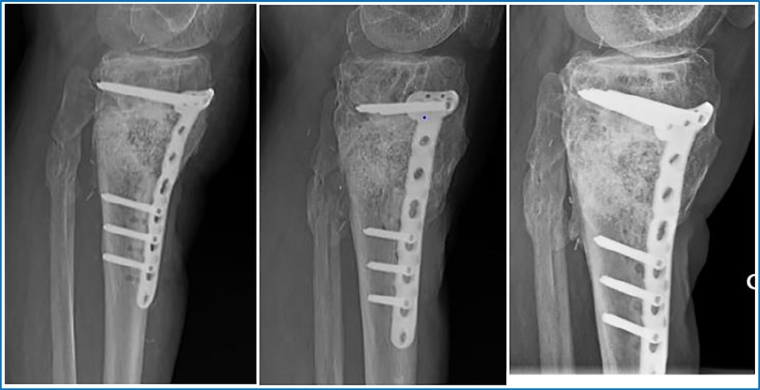
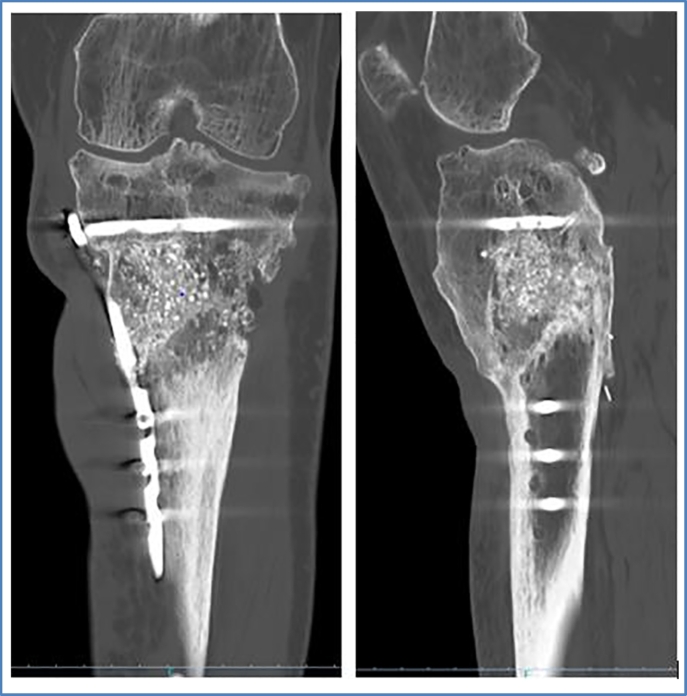
Control 1-month post operatively showed good results. Radiographically there was no secondary displacement of material, consolidation was beginning. Bacteriological samples and PCR were negative. There was no pain or sign of infection. Progressive weight bearing (15 kg) was started, adding 10 kg each week. During the following clinical controls, (3, 4, 6, and 10 months post operatively) the patient showed an excellent clinical evolution. Radiographically progressive homogenization of the graft and bone consolidation was noted with no material loosening. At 6 months postoperatively, the patient was able to regain half-time work activity. Pain at the antero-lateral side of the left tibia of ischemic character, due to the poor vascular status, was reported only after extensive walking or standing (>2 h). At 10-month postoperative evaluation, clinical status was still very satisfying. The pain symptomatology did not restrict the patient's daily life activities. Radiographically, bone consolidation was found and there was no deformity of axe nor signs of material loosening. 2 years postoperatively, radiographs and CT-scan showed transformation of the GlassBONE™ into bone and perfect bone consolidation with no material loosening (Fig. 6, Fig. 7, Fig. 8, Fig. 9). Clinical exam was very satisfying: no limping and walking was possible without crutches. The walking distance however was limited (pausing each 300 m) due to vascular claudication on peripheral obliterative arteriopathy.
Fig. 6.
ap view of left leg at 1 month, 6 months and 24 months postoperatively.
Fig. 7.
Lateral view of leg at 1 month, 6 months and 24 months postoperatively.
Fig. 8.
CT-scan: ap and lateral view of left leg 18 months postoperatively.
Fig. 9.
Clinical aspect and ap view of leg axis 24 months postoperatively.
Discussion and conclusions
In our case, a patient presenting with multiple complications after initial surgery of an open fracture of the proximal tibia was finally successfully treated with BAG grafting. This in a terrain of high risk as open fractures with large skin lesions are known to have a 5.7 times greater risk of delayed healing or result in nonunion than fractures with no skin injury [2]. There is little long-term data on BAG in long bones. Good results were shown in a case report of remodeling of the tibia after grafting a large cavity of the proximal tibia in treatment of fibrous dysplasia with BAG-hydroxyapatite (70% and 30% iliac crest). In the 13-year follow-up, the Swedish study group showed excellent clinical and radiographical results and histological degradation of BAG (no more BAG material in bone biopsy 13 years after grafting) [6]. BAG also shows good results in repairing bone defects of benign neoplasm. In a 2-year follow-up, 34 patients with larger bone defects (ranging from 3 × 2 × 1 cm to 11 × 3.5 × 3 cm) were grafted with a mixture of BAG and autogenous red bone marrow with rare complications. Bone remodeling was achieved 6 to 10 months postoperatively, and radiographically the majority of the implanted BAG was absorbed [23].
Another interesting point to take into consideration concerning our case is the area of fracture. Bone defects in metaphyseal area differ from the diaphyseal area concerning the mechanical environment and challenges. In contrast to a metaphyseal bone defect, where filling of greater cavities, reestablishing a support for the joint surface, and regaining bone stock are the primary objectives, in diaphyseal bone defect, repairing the cortical continuity is the main goal. There is clinical evidence that the filled defects in metaphysis heal faster when filled than when left open [24,25].
GlassBONE™ provides the quality of giving initial support and filling. It is available in granules and is therefore suitable for any size and form of cavities. Furthermore, it is subsequently resorbed and provides osteoconduction for new, in-growing bone [26]. In addition to variations concerning the mechanical environment and its challenges, there are several studies indicating a difference in the process of fracture healing in metaphyseal bone compared to the diaphysis. Inoue et Al. compared the bone repair mechanism of the metaphysis and the diaphysis of the mouse tibia. This study showed that in the metaphysis, the fracture was filled with newly formed bone produced from the bone marrow without detection of a cartilage formation on the periosteal side. This was contrary to the diaphysis, where cartilage was formed at the fracture site and then subsequently replaced by bone on the periosteal side. Furthermore, the study indicated that after injury, osteogenic markers in the bone marrow and medullary callus appeared earlier in metaphysis than in the diaphysis [27]. In addition, the metaphyseal region is rich in cancellous bone and contains more mesenchymal stem cells with high osteogenic potential [28]. This fact might suggest that the metaphyseal region is more efficient in providing mesenchymal stem cells to the injured bone marrow than the diaphyseal region. This condition might also have played a beneficial role for the successful bone-union in our case since the site of pseudarthrosis was mainly situated in the metaphyseal region. The Glassbone-autograft mixture was consequently placed in a favorable area regarding mesenchymal stem cell availability. In addition, based on the well-studied experiences with Masquelet-therapy in long bone defects, we suppose that the graft effectively consolidated since it was surrounded by the induced pseudo membrane, known to express osteoinductive growth factor molecules, comprised of osteoprogenitor cells, which stimulate osteogenesis [[29], [30], [31]].
A critical point of our case report is that we mostly utilized x-ray for measurement of union and bone healing while SPECT could be a more precise but also noninvasive method for showing an increase in the rate of mineral metabolism and remodeling of the cortex.
In our case, we grafted with a mixture of BAG and autograft relying on established trauma surgery experience. However, there should be more investigation regarding whether the use of BAG alone or in combination with autograft is superior (being interesting in many cases where autograft harvesting is complicated). A promising clinical trial that started in 2018 compares the treatment of nonunion of the tibia and femur with S53P4 BAG grafting alone in Masquelet therapy to regular combined grafting of autologous bone and tricalcium phosphate [20].
Contributor Information
L. Tetzel, Email: laura.tetzel@berlin.de.
M. Guyard, Email: mguyard@ch-stjoseph-stluc-lyon.fr.
References
- 1.Einhorn T.A., Gerstenfeld L.C., Fracture healing: mechaniEinhorn, T. A, Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. (http://doi.org/10.1038/nrrheum.2014.164sms and interventions. Nat Rev Rheumatol (2015)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audigé L., Griffin D., Bhandari M., Kellam J., Rüedi T.P. Path analysis of factors for delayed healing and nonunion in 416 operatively treated tibial shaft fractures. Clin. Orthop. Relat. Res. 2005;(438):221–232. doi: 10.1097/01.blo.0000163836.66906.74. [DOI] [PubMed] [Google Scholar]
- 3.Schlundt C. Clinical and research approaches to treat non-union fracture. Curr. Osteoporos. Rep. 2018;16:155–168. doi: 10.1007/s11914-018-0432-1. [DOI] [PubMed] [Google Scholar]
- 4.P, G., E, W., RA, E., MM, M. & CM, C.-B Fractures of the tibia. Can their outcome be predicted. J. Bone Joint Surg. Br. 1999:81. doi: 10.1302/0301-620x.81b1.8958. [DOI] [PubMed] [Google Scholar]
- 5.Karladani A.H., Granhed H., Kärrholm J., Styf J. The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch. Orthop. Trauma Surg. 2001 doi: 10.1007/s004020000252. [DOI] [PubMed] [Google Scholar]
- 6.Aho A. Case report: remodeling of the tibia after grafting of a large cavity with particulate bioactive glass-hydroxylapatiteon treatment of fibrous dysplasia with 13 years’ follow-up. Acta Orthop. Scand. 2003;74:766–770. doi: 10.1080/00016470310018342. [DOI] [PubMed] [Google Scholar]
- 7.Webb J.C.J., Spencer R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Joint Surg. Br. 2007;89:851–857. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- 8.Gazdag G., Lane L., Glaser G., Forster F. Alternatives to autogenous bone graft: efficacy and indications. J. Am. Acad. Orthop. Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ebraheim N.A., Elgafy H., Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J. Am. Acad. Orthop. Surg. 2001;9:210–218. doi: 10.5435/00124635-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bridgens J., Davies S., Tilley L., Norman P., Stockley I. Orthopaedic bone cement: do we know what we are using? J. Bone Joint Surg. Br. 2008;90:643–647. doi: 10.1302/0301-620X.90B5.19803. [DOI] [PubMed] [Google Scholar]
- 11.Slane J., Gietman B., Squire M. Antibiotic elution from acrylic bone cement loaded with high doses of tobramycin and vancomycin. J. Orthop. Res. 2018;36:1078–1085. doi: 10.1002/jor.23722. [DOI] [PubMed] [Google Scholar]
- 12.Baino F., Vitale-Brovarone C. Three-dimensional glass-derived scaffolds for bone tissue engineering: current trends and forecasts for the future. J. Biomed. Mater. Res. A. 2011;97:514–535. doi: 10.1002/jbm.a.33072. [DOI] [PubMed] [Google Scholar]
- 13.Maçon A.L.B. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015 doi: 10.1007/s10856-015-5403-9. [DOI] [PubMed] [Google Scholar]
- 14.Souza L.P.L. Evaluation of effectiveness of 45S5 bioglass doped with niobium for repairing critical-sized bone defect in in vitro and in vivo models. J. Biomed. Mater. Res. Part A. 2019 doi: 10.1002/jbm.a.36826. [DOI] [PubMed] [Google Scholar]
- 15.Williams D.F. Vol. 4. 1986. Definitions in biomaterials: proceedings of a consensus conference of the European Society for Biomaterials European cells & materials. (Elsevier, New York) [Google Scholar]
- 16.Westhauser F. Three-dimensional polymer coated 45S5-type bioactive glass scaffolds seeded with human mesenchymal stem cells show bone formation in vivo. J. Mater. Sci. Mater. Med. 2016 doi: 10.1007/s10856-016-5732-3. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes H.R. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials (Basel) 2018;11:2530. doi: 10.3390/ma11122530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hench L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 19.Paderni S., Terzi S., Amendola L. Major bone defect treatment with an osteoconductive bone substitute. Chir. Organi Mov. 2009;93:89–96. doi: 10.1007/s12306-009-0028-0. [DOI] [PubMed] [Google Scholar]
- 20.Tanner M.C. Evaluation of the clinical effectiveness of bioactive glass (S53P4) in the treatment of non-unions of the tibia and femur: study protocol of a randomized controlled non-inferiority trial. Trials. 2018;19 doi: 10.1186/s13063-018-2681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellantone M., Coleman N.J., Hench L.L. Bacteriostatic action of a novel four-component bioactive glass. J. Biomed. Mater. Res. 2000 doi: 10.1002/1097-4636(20000905)51:3<484::AID-JBM24>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Bortolin M. Antibiofilm agents against mdr bacterial strains: is bioactive glass bag-s53p4 also effective? J. Antimicrob. Chemother. 2016 doi: 10.1093/jac/dkv327. [DOI] [PubMed] [Google Scholar]
- 23.Liu H., Sun J., Wang Y., Yang X., Zhu E. Repairing bone defects of benign bone neoplasm by grafting of bioactive glass combined with autologous bone marrow. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22:1349–1353. [PubMed] [Google Scholar]
- 24.De Jong J.J.A. Fracture repair in the distal radius in postmenopausal women: a follow-up 2 years postfracture using HRpQCT. J. Bone Miner. Res. 2016 doi: 10.1002/jbmr.2766. [DOI] [PubMed] [Google Scholar]
- 25.Russell T.A., Leighton R.K. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J. Bone Jt. Surg. - Ser. A. 2008 doi: 10.2106/JBJS.G.01191. [DOI] [PubMed] [Google Scholar]
- 26.Blokhuis T.J. Management of traumatic bone defects: metaphyseal versus diaphyseal defects. Injury. 2017 doi: 10.1016/j.injury.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Inoue S., Otsuka H., Takito J., Nakamura M. Decisive differences in the bone repair processes of the metaphysis and diaphysis in young mice. Bone Reports. 2018 doi: 10.1016/j.bonr.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yildiz B.O., Suchard M.A., Wong M.L., McCann S.M., Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannoudis P.V., Harwood P.J., Tosounidis T., Kanakaris N.K. Restoration of long bone defects treated with the induced membrane technique: protocol and outcomes. Injury. 2016 doi: 10.1016/S0020-1383(16)30840-3. [DOI] [PubMed] [Google Scholar]
- 30.Masquelet A.C., Fitoussi F., Begue T., Muller G.P. Annales de Chirurgie Plastique Esthetique. 2000. Reconstruction of the long bones by the induced membrane and spongy autograft. [PubMed] [Google Scholar]
- 31.Pelissier P., Masquelet A.C., Bareille R., Mathoulin Pelissier S., Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004;22:73–79. doi: 10.1016/S0736-0266(03)00165-7. [DOI] [PubMed] [Google Scholar]