Summary
Human cancer tissue-derived organoids maintain the mutational spectrum and histological characteristics of their parental tumors and thus provide a platform for predicting patients’ responses to anticancer drugs. Here, we provide a fully detailed, step-by-step protocol to derive lung adenocarcinoma organoids from primary tumor tissues. Organoid lines can be generated with a success rate of 80% using our protocol.
For complete details on the use and execution of this protocol, please refer to Li et al. (2020).
Subject areas: Cancer, Cell Biology, Organoids
Graphical Abstract
Highlights
-
•
Protocol for generating organoids from human lung adenocarcinoma samples
-
•
Passage, cryopreservation, and revival of lung adenocarcinoma organoids
-
•
Tissue processing of lung adenocarcinoma organoids for histological characterization
Human cancer tissue-derived organoids maintain the mutational spectrum and histological characteristics of their parental tumors and thus provide a platform for predicting patients’ responses to anticancer drugs. Here, we provide a fully detailed, step-by-step protocol to derive lung adenocarcinoma organoids from primary tumor tissues. Organoid lines can be generated with a success rate of 80% using our protocol.
Before you begin
CRITICAL: All procedures should be performed in a Class II Biological Safety Cabinet to maintain sterility.
Preparation of reagents and solutions
Timing: 1 day
-
1.250 mg/mL Collagenase type II (50×)
-
a.Dissolve 1 g of collagenase type II in 4 mL of adDMEM/F12+++ (advanced DMEM/12 containing 1× antibiotic/antimycotic, 1× GlutaMAX supplement, and 10 mM HEPES).
-
b.Vortex to ensure complete dissolution.
-
c.Filter the solution with a 0.22-μm filter.
-
d.Aliquot 100 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 1 year.
-
a.
-
2.10 mg/mL DNase I (100×)
-
a.Dissolve 50 mg in 5 mL of 0.15 M NaCl.
-
b.Filter the solution with a 0.22-μm filter.
-
c.Aliquot 50 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 6 months.
-
a.
-
3.100 mM Y-27632 dihydrochloride (10,000 ×)
-
a.Dissolve 50 mg in 1.56 mL of ultra-pure water.
-
b.Filter the solution with a 0.22-μm filter.
-
c.Aliquot 5 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 6 months.
-
a.
-
4.100 μg/mL R-spondin 1 (200×)
-
a.Dissolve 250 μg in 2.5 mL of sterile PBS + 0.1% (wt/vol) bovine serum albumin.
-
b.Aliquot 250 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 1 month.
-
a.
-
5.100 μg/mL Noggin (1,000×)
-
a.Dissolve 100 μg in 1 mL of sterile PBS + 0.1% (wt/vol) bovine serum albumin.
-
b.Aliquot 50 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 2 months.
-
a.
-
6.200 μg/mL FGF-10 (10,000×)
-
a.Dissolve 100 μg in 0.5 mL of sterile PBS + 0.1% (wt/vol) bovine serum albumin.
-
b.Aliquot 5 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 3 months.
-
a.
-
7.250 μg/mL FGF-7 (12,500×)
-
a.Dissolve 100 μg in 0.4 mL of sterile PBS + 0.1% (wt/vol) bovine serum albumin.
-
b.Aliquot 4 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 3 months.
-
a.
-
8.500 mM N-acetylcysteine (400×)
-
a.Dissolve 408 mg in ultra-pure water to a total volume of 5 mL.
-
b.Filter the solution with a 0.22-μm filter.
-
c.Aliquot 125 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 3 months.
-
a.
-
9.2 M nicotinamide (200×)
-
a.Dissolve 6.1 g in PBS to a total volume of 25 mL.
-
b.Filter the solution with a 0.22-μm filter.
-
c.Aliquot 250 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 3 months.
-
a.
-
10.30 mM SB202190 monohydrochloride hydrate (3,000×)
-
a.Dissolve 5 mg in 0.453 mL of dimethyl sulfoxide (DMSO).
-
b.Aliquot 16.67 μL into a 1.5 mL black microcentrifuge tube and store at −20°C for up to 3 months.
-
a.
CRITICAL: Do not filter solutions in DMSO through syringe filters with polyvinylidene difluoride membranes because DMSO will dissolve these filters.
CRITICAL: Use black microcentrifuge tubes to protect this solution from light.
Note: DMSO-safe filters, such as Nylon membrane filters are recommended for filtering solutions in DMSO.
-
11.25 mM A83-01 (50,000×)
-
a.Dissolve 5 mg in 0.474 mL of DMSO.
-
b.Aliquot 2 μL into a 1.5 mL microcentrifuge tube and store at −20°C for up to 3 months.
-
a.
CRITICAL: Do not filter solutions in DMSO through syringe filters with polyvinylidene difluoride membranes because DMSO will dissolve these filters.
-
12.Aliquot Matrigel
-
a.Thaw a new bottle of Matrigel in a 4°C refrigerator for ∼8 h.
-
b.Invert the bottle several times to mix well the Matrigel.
-
c.Put 1.5 microcentrifuge tubes on ice to cool down the tubes.
-
d.Put Matrigel and 1.5 mL microcentrifuge tubes on ice, aliquot required volume of Matrigel into a 1.5 mL microcentrifuge tube, and store the aliquots at −20°C until the expiration date.
-
a.
CRITICAL: P1000 tips should be pre-wetted and pre-cold by aspirating and expelling cold sterile PBS + 0.1% BSA for several times before aspirating Matrigel to prevent Matrigel from sticking to the tips.
Note: Matrigel is a reconstituted basement membrane preparation enriched in extracellular proteins, freeze-thaw cycles should be avoided.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| p40 (1:100) | Maixin Biotech (http://www.maxim.com.cn/) | Cat#RAB-0666 |
| TTF1 (1:100) | Maixin Biotech | Cat#MAB-0677; Clone MX011 |
| Napsin A (1:100) | Maixin Biotech | Cat#MAB-0704; Clone MX015 |
| CK5 (1:100) | Thermo Fisher | Cat#RM-2106-S0; Clone EP1601Y |
| Donkey anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 488 (1:1,000) | Thermo Fisher | Cat#A21206; RRID: AB_2535792 |
| Goat anti-mouse IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor 555 (1:1,000) | Thermo Fisher | Cat#A21422; RRID: AB_141822 |
| Biological samples | ||
| Human lung adenocarcinoma tissue from a male patient | Shenzhen Second People's Hospital, China | n/a |
| Chemicals, peptides, and recombinant proteins | ||
| Advanced DMEM/F-12 | Gibco | Cat#12634-010 |
| Antibiotic-antimycotic (100×) contains 10,000 units/mL of penicillin, 10,000 μg/mL of streptomycin, and 25 μg/mL of Gibco amphotericin B | Gibco | Cat#15240-062 |
| GlutaMAX supplement | Gibco | Cat#35050-061 |
| HEPES | Gibco | Cat#15630-080 |
| B-27 supplement (50×), serum free | Gibco | Cat#17504-044 |
| N-Acetylcysteine | Sigma | Cat#A9165; CAS: 616-91-1 |
| Nicotinamide | Sigma | Cat#N0636; CAS: 98-92-0 |
| SB202190 | Sigma | Cat#S7076; CAS: 350228-36-3 (anhydrous) |
| A83-01 | Sigma | Cat#SML0788; CAS: 909910-43-6 |
| Recombinant human R-spondin 1 protein | R&D Systems | Cat#4645-RS-250 |
| Human Noggin | Peprotech | Cat#120-10C |
| Y-27632 dihydrochloride | Abmole Bioscience | Cat#M1817; CAS: 129830-38-2 |
| Recombinant human FGF-10 | Peprotech | Cat#100-26 |
| Recombinant human KGF (FGF-7) | Peprotech | Cat#100-19 |
| Recovery cell culture freezing medium | Gibco | Cat#12648010 |
| TrypLE Express | Gibco | Cat#12605010 |
| Collagenase, type II | Gibco | Cat#17101015 |
| DNase I | Roche | Cat#11284932001 |
| Fetal bovine serum | Gibco | Cat#10091148 |
| PBS | Gibco | Cat#10010023 |
| Matrigel growth factor reduced (GFR) basement membrane matrix | Corning | Cat#356231 |
| Other | ||
| 70 μm cell strainer | Corning | Cat#352350 |
| Falcon 10 mL serological pipet | Corning | Cat#357551 |
| Costar 6-well clear TC-treated multiple well plates | Corning | Cat#3516 |
| 0.22 μm filter | Millipore | Cat#SLGV033RB |
| 50 mL centrifuge tube | Corning | Cat#CLS430829 |
| 15 mL centrifuge tube | Corning | Cat#CLS430791 |
| 1.5 mL centrifuge tube | Beyotime | Cat#FCT116 |
| Cryogenic vials | Corning | Cat#38053 |
| Agarose | Sigma-Aldrich | Cat#A9414 |
| Biopsy processing/embedding cassettes | Citotest Scientific | Cat#0103-1101-16 |
| Hematoxylin solution | Baso Diagnostics | Cat#BA-4041 |
| Eosin solution | Baso Diagnostics | Cat#BA-4099 |
| Antigen retrieval buffer (50× citrate-EDTA buffer) | Maixin Biotech | Cat#MVS-0098 |
Materials and equipment
Prepare the following media
Digestion medium I
| Reagent | Final concentration | Amount |
|---|---|---|
| AdDMEM/F12+++ | 1× | 4.85 mL |
| Collagenase type II (250 mg/mL) | 5 mg/mL | 100 μL |
| DNase I (10 mg/mL) | 100 μg/mL | 50 μL |
| Y-27632 dihydrochloride (100 mM) | 10 μM | 0.5 μL |
| Total | n/a | 5 mL |
Note: This medium should be made freshly and be prewarmed in a 37°C water bath before use.
Organoid culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| AdDMEM/F12 | n/a | 46.79 mL |
| Antibiotic-antimycotic (100×) | 1× | 0.5 mL |
| GlutaMAX supplement (100×) | 1× | 0.5 mL |
| HEPES (100×) | 1× | 0.5 mL |
| B-27 supplement (50×) | 1× | 1 mL |
| R-Spondin 1 (100 μg/mL) | 500 ng/mL | 250 μL |
| Noggin (100 μg/mL) | 100 ng/mL | 50 μL |
| 0.2 mg/mL FGF-10 (0.2 mg/mL) | 20 ng/mL | 5 μL |
| FGF-7 (0.25 mg/mL) | 20 ng/mL | 4 μL |
| N-Acetylcysteine (500 mM) | 1.25 mM | 125 μL |
| Nicotinamide (2 M) | 10 mM | 250 μL |
| SB202190 monohydrochloride hydrate (30 mM) | 10 μM | 16.7 μL |
| A83-01 (25 mM) | 500 nM | 1 μL |
| Y-27632 dihydrochloride (100 mM) | 10 μM | 5 μL |
| Total | n/a | 50 mL |
CRITICAL: The medium should be stored at 4°C for no longer than 1 week.
Step-by-step method details
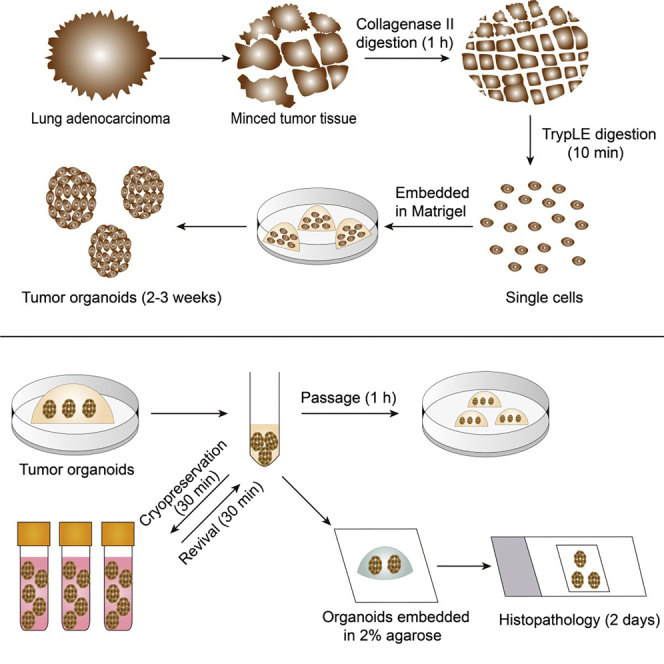
Establishment of LADC organoids from clinical tissues
Timing: 2 h
This step describes the digestion of human lung adenocarcinoma (LADC) tissues and the derivation of LADC organoids.
CRITICAL: Some critical preparations should be made before digesting clinical tissues and passing organoids. (1) Cell culture plates should be prewarmed by being incubated in the incubator for at least 2 h before plating cells; (2) Thaw aliquoted Matrigel at 4°C.
-
1.
Collect the tissue sample from clinical site, put the sample on ice in 40 mL of ice cold adDMEM/F12+++ containing antibiotic-antimycotic (100 units/mL of penicillin, 100 μg/mL of streptomycin, and 250 ng/mL of Amphotericin B) in a thermocol ice box, and transfer the sample immediately to the laboratory. The patient provided informed consent and this study was approved by the Ethics Committee of the First Affiliated Hospital of Shenzhen University.
Pause point: Tissue samples can be kept in adDMEM/F12+++ at 4°C for up to 12 h without significant loss of organoid outgrowth.
-
2.
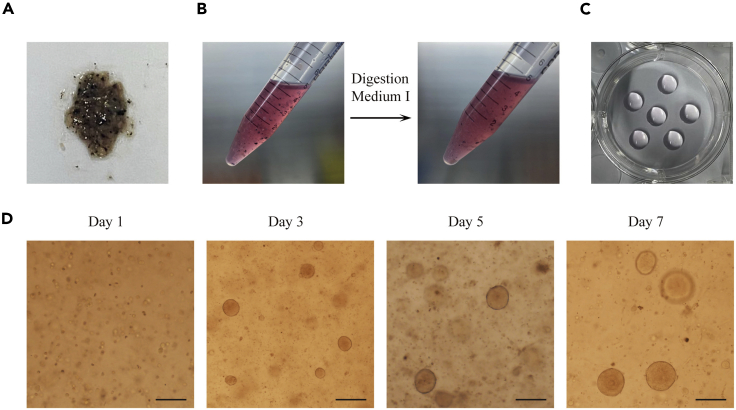
Wash the tumor sample twice with cold PBS and mince it into <2 mm pieces in a 10 cm tissue culture plate using scissors (Figure 1A). Incubate the minced tissue with Digestion Medium I for 1 h at 37°C on a shaking platform (120 rpm). Centrifuge the digested tissues at 200 × g for 5 min at 22°C–25°C, discard the supernatant. (see Troubleshooting 1)
Note: Every 200 mg of minced tissue was placed in 5 mL of Digestion Medium I in a 15-mL falcon tube. The sample should look cloudy to the eye after digestion with Digestion Medium I (Figure 1B).
-
3.
Wash the pellet with 10 mL of adDMEM/F12+++, and centrifuge the tube again at 200 × g for 5 min at 22°C–25°C. Discard the supernatant.
CRITICAL: Cool down the centrifuge to 4°C after this step.
-
4.
Incubate the tissue with 5 mL of TrypLE Express + Y-27632 dihydrochloride (10 μM) for ∼10 min at 37°C on a shaking platform (120 rpm).
-
5.
Add 10 mL of cold adDMEM/F12+++ supplemented with 20% FBS to stop the digestion. Centrifuge the tube at 200 × g for 5 min at 4°C. Discard the supernatant.
-
6.
Add 2 mL of cold adDMEM/F12+++ and dissociate the cells by pipetting up and down for 5–10 times. Centrifuge at 200 × g for 5 min at 4°C. Discard the supernatant.
Note: The dissociation status can be checked using a light microscope. This solution mainly consist of single cells after this step.
-
7.
Resuspend the cell pellet in 10 mL of cold adDMEM/F12+++ and filter the cells through a 70-μM cell strainer to remove large undigested tissue clusters.
-
8.
Count the cells using a hemocytometer, collect 100,000 cells, and centrifuge at 200 × g for 5 min at 4°C. Discard the supernatant. (see Troubleshooting 1)
Note: Every 100,000 cells were collected for being embedded in 300 μL of Matrigel and plated into one well of a 6-well culture plate.
-
9.
Resuspend the cells in 30 μL of cold organoid culture medium first before mixing the cell suspension with Matrigel. Matrigel is viscous and it is difficult to thoroughly resuspend cells directly in Matrigel.
-
10.
Add 300 μL of Matrigel to the cell suspensions and mix well by gently pipetting up and down for several times.
CRITICAL: P1000 tips should be pre-wetted and pre-cold by aspirating and expelling cold sterile PBS + 0.1% BSA for several times before aspirating Matrigel to prevent the Matrigel from sticking to the tips.
CRITICAL: Mix well the cell suspensions with Matrigel slowly on ice, and do not introduce bubbles.
Alternatives: Matrigel can be substituted with other extracellular matrix substrates such as Cultrex basement membrane extract BME (Trevigen 3432-001-01), Cultrex basement membrane extract BME type II (Trevigen 3532-001-02), or Geltrex basement membrane matrix (Gibco A1413202).
-
11.
Dispense Matrigel-cell mixture as 50-μL droplets (∼15, 000 cells/drop) into one well of a 6-well plate. Usually, 6 droplets were plated into each well. For a visual guide, refer to Methods Video S1 (Plating the Matrigel-cell mixture). The drops were solidified for 1 min right side up and for another 9 min upside down at 37°C and 5% CO2. If Matrigel-cell mixture are plated as smaller droplets, such as 30-μL droplets, the cell culture plate can be put upside down immediately after cell plating, and be placed in incubator for 10 min. Matrigel should form domes (Figure 1C).
Figure 1.
Establishment of human lung adenocarcinoma organoid cultures
(A) Photo of minced tumor tissue.
(B) Images of tumor tissue before and after digestion with Digestion Medium I.
(C) Photo of Matrigel domes plated in a well of a 6-well culture plate.
(D) Representative bright-field images of LADC organoids after seeding. Scale bar, 100 μm.
CRITICAL: P100 tips should be pre-wetted and pre-cold by aspirating and expelling cold sterile PBS + 0.1% BSA for several times before aspirating Matrigel-cell mixture.
CRITICAL: Cell culture plates should be prewarmed by incubating in the incubator for at least 2 h before cell plating.
CRITICAL: Plate the cells as quickly as possible to prevent the Matrigel from solidifying.
Note: To make good use of the expensive culture medium, it is better to plate as more droplets as possible. However, plating more droplets takes more time, the Matrigel may solidify, and plated droplets may flatten out in the well.
-
12.
After the drops solidified, 2.5 mL of warm LADC organoid medium was added to each well. Fill each unused well with 2 mL of sterile PBS to prevent the evaporation.
CRITICAL: Do not pipette the culture medium directly onto the Matrigel domes, because this may break up the dome structure. The culture medium should be added gently down the sidewall of each well.
CRITICAL: The remaining unused wells should be filled with 2 mL of sterile PBS to reduce the evaporation of the medium.
Maintenance of LADC organoid cultures
Timing: 2–3 weeks
This step describes the culture of LADC organoids until the initial passaging.
-
13.
The medium was replaced every 3 days and fresh medium was constituted every week. Examples of morphology of LADC organoids after several days of culture are shown (Figure 1D). (see Troubleshooting 2)
CRITICAL: Y-27632 dihydrochloride was added in the digestion mediums and culture medium to prevent dissociation-induced anoikis, but should be withdrawn from the organoid medium after the first week of culture.
Passaging of LADC organoids
Timing: 1 h
This step describes the passaging of LADC organoids using the mechanical dissociation method.
-
14.
Scrap the Matrigel domes containing organoids (2–3 weeks after the culture) from one well of a 6-well cell culture plate using a P1000 pipette tip and transfer them into a 15 mL tube. Centrifuge at 200 × g for 5 min at 4°C.
CRITICAL: P1000 pipette should be pre-wetted by aspirating and expelling sterile PBS + 0.1% BSA for several times to prevent the cells from sticking to the pipette before use.
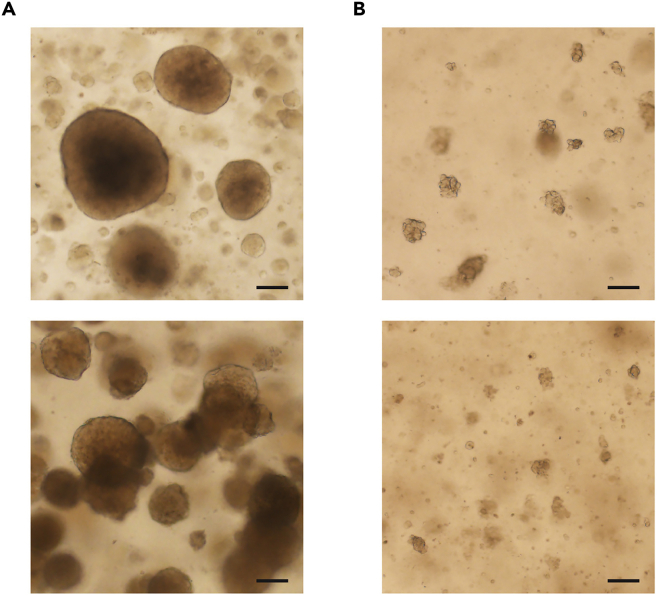
Note: LADC organoids were usually passaged after the initial 2–3 weeks of culture and then passaged every 2–3 weeks at a 1:2–1:3 ratio in future maintenance. The exact timing for the passage of a specific LADC organoids culture is variable and is determined largely by the growth rate of organoids. Organoid cultures in which more than 30% of the organoids are larger than 200 μm in diameter or many organoids are about to connected to each other should be passaged (Figure 2).
Figure 2.
Passaging of LADC organoids
(A) Representative bright-field images of LADC organoids need passaging.
(B) Representative bright-field images of organoids after dissociation using the mechanical dissociation method. Scale bar, 100 μm.
-
15.
Discard the supernatant and digest the Matrigel with 2 mL of TrypLE Express + Y-27632 dihydrochloride (10 μM) for ∼5 min at 37°C on a shaking platform (60 rpm).
-
16.
Stop the trypsinization by the addition of 4 mL of cold adDMEM/F12+++ supplemented with 20% FBS. Centrifuge at 200 × g for 5 min at 4°C.
-
17.
Discard the supernatant and wash the cell pellet with 10 mL of cold adDMEM/F12.
-
18.
Centrifuge at 200 × g for 5 min at 4°C and discard the supernatant.
-
19.
Use 10 mL Stripette Serological Pipets to dissociate the organoids into smaller clusters of cells through trituration in 10 mL of cold adDMEM/F12+++. Photos of organoids before and after pipette trituration were provided in the Figure 2.
CRITICAL: Serological Pipets should be pre-wetted by aspirating and expelling sterile PBS + 0.1% BSA for several times to prevent the cells from sticking to the pipette before being used to dissociate the organoids.
-
20.
Add 2 mL of cold adDMEM/F12 to rinse the 10 mL pipette to wash down cells stick to the pipette. Gently pipette the cells up and down for several times to further dissociate organoids.
-
21.
Centrifuge the tube at 200 × g for 5 min at 4°C and discard the supernatant.
-
22.
Add 30 μL of cold organoid culture medium to the cell pellet and resuspend the cells. Place the tube on ice to cool down the solution.
-
23.
Add 600–900 μL of Matrigel to the tube and performed the cell plating process as above mentioned (step 11).
-
24.
For potential contamination, see Troubleshooting 3 and 4.
Cryopreservation of LADC organoids
Timing: 30 min
This step describes the cryopreservation of LADC organoids
-
25.
Collect LADC organoids from one well of a 6-well cell culture plate, dissolve the Matrigel surrounding organoids using TrypLE Express, and dissociate the LADC organoids into smaller cell clusters as described in steps 14–19.
-
26.
Centrifuge the tube at 200 × g for 5 min at 4°C and discard the supernatant.
-
27.
Add 2–3 mL of Recovery Cell Culture Freezing Medium, and resuspend the cell pellet. Transfer 1 mL into a 2 mL cryogenic vial, place the vial into a freezing container, and put the freezing container at −80°C.
CRITICAL: Freezing container should be at room temperature (22°C–25°C) before use.
Alternatives: Freezing medium contains 90% FBS and 10% DMSO can also be used for the cryopreservation of LADC organoids.
-
28.
The following day, transfer the frozen cryogenic vials to a liquid nitrogen tank for long-term storage.
Pause point: Cryopreserved organoids can be stored in liquid nitrogen for >2 years.
Revival of LADC organoids
Timing: 30 min
This step describes the cryopreservation of LADC organoids.
-
29.
Transfer frozen cryogenic vials from liquid nitrogen storage to a 37°C water bath. Gently swirling the cryogenic vial.
-
30.
Transfer 5 mL of prewarmed adDMEM/F12+++ to a 15 mL centrifuge tube, and add thawed cells dropwise to the medium.
-
31.
Centrifuge the tube at 200 × g for 5 min and discard the supernatant.
-
32.
Resuspend the cell pellet in organoid culture medium and performed the organoid culture as described in steps 9–12.
Tissue processing of LADC organoids for histology
Timing: 2 days
Organoids are small cell clusters, miniature versions of tissues. Due to their small sizes, organoids cannot be treated in the conventional tissue processing method. This step describes the way we use to perform the tissue processing of organoids for histology. Briefly, the organoids are embedded in 2% agarose and subjected to dehydration, clearing, wax infiltration, and embedding.
-
33.
Collect LADC organoids from 6-well cell culture plates by scraping the Matrigel domes from the cell culture plate using a P1000 pipette tip.
-
34.
Centrifuge the tube at 200 × g for 5 min at 4°C and discard the supernatant.
-
35.
Add 5 mL of cold adDMEM/F12+++ to the tube, put the tube on a shaking platform at 4°C for 10 min.
-
36.
Centrifuge the tube at 200 × g for 5 min at 4°C and discard the supernatant. Repeat this step for 3 times to dissolve the Matrigel surrounding organoids.
CRITICAL: Do not digest the Matrigel surrounding the organoids using the TrypLE digestion method, as this will destroy the organoids’ histological structure. It is acceptable to leave a little of Matrigel with the organoids.
-
37.
Add 10 mL of 10% neutral-buffered formalin to fix the organoids for over 2 h at 22°C–25°C.
Pause point: Organoids can be kept up to 1 week in 10% neutral-buffered formalin without affecting the staining results.
-
38.
Centrifuge the tube at 200 × g for 5 min and discard the supernatant.
-
39.
Resuspend the organoids in 20–50 μL of PBS.
CRITICAL: Resuspend organoids in PBS first and mix the solution with 2% agarose, as 2% agarose is viscous and it is difficult to thoroughly resuspend organoids directly 2% agarose solution.
-
40.
Mix 0.2 g of agarose with 10 mL of water in a 15-mL Falcon tube, and microwave for 30 s to melt the agarose.
-
41.
Let the agarose solution cool down to about 50°C. Gently mix the organoids with 50–100 μL of 2% agarose solution.
CRITICAL: Cut off the end of a P100 tip and use it to pipette the 2% agarose solution, as the agarose solution is viscous and difficult to pipette.
Alternatives: Histogel can be used instead of the 2% agarose solution. Other alternatives could include 2% agar: 2.5% gelatin solution, 2% agarose: 2.5% gelatin or pregelatinized starch as embedding medium for small tissues or cell clusters.
-
42.
Slowly pipette the mixture of organoids and agarose onto a piece of parafilm. Put the parafilm upside down to let the agarose solution form a dome structure. For a visual guide, refer to Methods Video S2 (Embedding the organoids in 2% agarose).
Alternatives: Pipette the mixture of organoids and agarose onto a piece of parafilm on ice. Agarose will solidify rapidly and it is not necessary to put the parafilm upside down.
-
43.
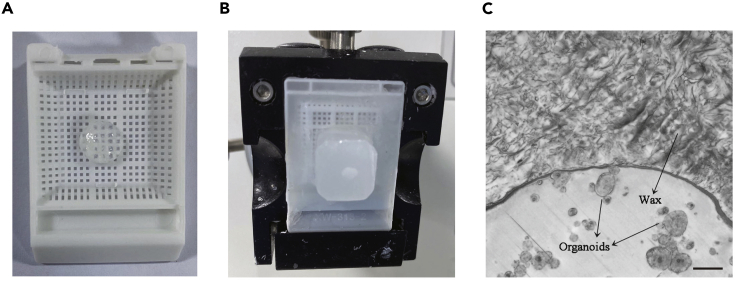
Let the agarose containing the organoids to solidify for 10 min. The agarose dome can be enclosed in a cassette and subjected to the following process in a similar procedure to that of tissue samples (Figure 3A).
| Step | Reagent | Time |
|---|---|---|
| Dehydration | 70% ethanol | 1 h |
| 80% ethanol | 1 h | |
| 95% ethanol | 1 h | |
| 100 % ethanol | 45 min | |
| Clearing | 50% ethanol + 50% xylene | 30 min |
| 100% xylene | 15 min | |
| 100% xylene | 15 min | |
| Wax infiltration | 50% xylene + 50% wax | 1 h |
| 100% wax | 1 h | |
| 100% wax | 1 h |
CRITICAL: Xylene is a hazardous solvent and evaporates easily. Perform the steps in a fume hood while handling xylene. Xylene substitutes with reduced health and environment risks can also be used but have not been tested directly by the authors.
-
44.
Embed the organoid sample in wax and cut sections at a thickness of 4–6 μm. Organoids-agarose mixture embed in wax looks like a small tissue (Figure 3B).
CRITICAL: Organoids embed in paraffin are hardly be noticed by the naked eye, so you may need to pick the sections out of the water bath using a microscope slide and confirm the presence of organoids by observing the slides under light microscopy (Figure 3C).
-
45.
Perform hematoxylin and eosin (H&E) staining and immunofluorescence staining of organoid sections to characterize LADC organoids.
Figure 3.
Processing of LADC organoids
(A) Photo of organoids embedded in 2% agarose in an embedding cassette.
(B) Photo of organoids embedded in wax.
(C) Bright-field images of microscope slides containing lung adenocarcinoma organoids. Scale bar, 100 μm.
H&E staining of LADC organoids
Timing: 1 h
This step describes the protocol we use to perform H&E staining of paraffin sections of the organoids.
-
46.
Perform H&E staining of paraffin sections in the following procedures.
| Step | Reagent | Time |
|---|---|---|
| Deparaffinization | 100% xylene | 5 min |
| 100% xylene | 5 min | |
| Rehydration | 100% ethanol | 3 min |
| 100% ethanol | 3 min | |
| 95% ethanol | 3 min | |
| 80% ethanol | 1 min | |
| water | 5 min | |
| Hematoxylin staining | hematoxylin | 1 min |
| water | 3 min | |
| Differentiation | 0.5% hydrochloric acid in 70% ethanol | 10 s |
| water | 5 min | |
| Eosin staining | 95% ethanol | 10 s |
| eosin | 45 s | |
| Dehydration | 95% ethanol | 10 s |
| 95% ethanol | 10 s | |
| 100% ethanol | 1 min | |
| 100% ethanol | 1 min | |
| Clearing | 100% xylene | 1 min |
| 100% xylene | 1 min |
Note: The slides should be rinsed with 95% ethanol for 10 s before staining in alcoholic eosin. For aqueous eosin, this step should be eliminated.
-
47.
Mount with neutral resin and glass coverslips.
CRITICAL: The slides should not be allowed to dry during the process. Drying out will cause high background staining.
-
48.
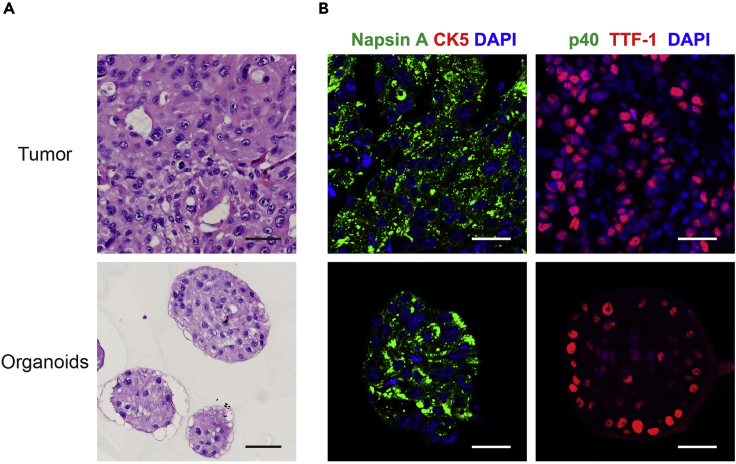
Capture images under a light microscopy. LADC organoids should retain the histological characteristics of the original tumor (Figure 4A).
Figure 4.
LADC organoids preserve the histopathological features of the original tumor tissues
Histological H&E (A) and immunofluorescence images (B) of LADC organoids and the corresponding parental tumor. Paired LADC organoids and tumor tissue were stained for TTF-1, Napsin A, p40, and CK5. Nuclei were stained with DAPI (blue). Scale bar, 50 μm.
Immunofluorescence staining of LADC organoids
Timing: 2 days
This step describes the protocol we use to perform immunofluorescence staining of paraffin sections of the organoids.
-
49.
Perform the Deparaffinization and Rehydration step as described in step 46.
-
50.
Antigen retrieval: incubate slides in boiled 1× citrate-EDTA buffer for 20 min. Let the slides in citrate-EDTA buffer cool down to 22°C–25°C. Wash the slides with water for 5 min.
CRITICAL: This antigen retrieval method works well for the antibodies used in this study. The protocol used for antigen retrieval in other circumstances should be optimized depending on the target antigen, the antibody used, the type of tissue, and the fixation method.
-
51.
Encircle the section with a PAP pen. Make sure that the PAP pen dry completely and wash the slides 3 × 5 min in 100 mL PBS with gentle shaking (50 rpm).
-
52.
Blocking endogenous peroxidase: add 100 μL of endogenous peroxidase blocker (3% H2O2) to each encircled section, and incubate for 10 min at 22°C–25°C. Wash the slides 3 × 5 min in 100 mL of PBS with gentle shaking (50 rpm).
-
53.
Blocking: incubate the encircled sections with 5% BSA in PBS for 1 h at 22°C–25°C with gentle shaking (50 rpm).
-
54.
Incubation with primary antibodies: discard blocking buffer, incubate the sections in 100 μL of diluted primary antibodies in 3% BSA in PBS in a humidified chamber for ∼12 h at 4°C with gentle shaking (50 rpm).
-
55.
Incubation with secondary antibodies: wash the slides 3 × 5 min in 100 mL of PBS with gentle shaking (50 rpm). Incubate the sections in fluorescent-dye conjugated secondary antibodies diluted in 100 mL of PBS for 1 h at 22°C–25°C in the dark with gentle shaking (50 rpm). Wash the slides 3 × 5 min in PBS with gentle shaking (50 rpm).
Note: Propidium iodide (PI) can be added in this step to stain nucleus.
-
56.
Mount coverslip with a drop of mounting medium and seal coverslip with nail polish.
-
57.
Capture images under a confocal microscopy. LADC organoids should retain the expression profiles of specific markers expressed in the corresponding tumor (Figure 4B).
Expected outcomes
Using this step-by-step protocol, organoid cultures from human lung adenocarcinoma samples can be established with a success rate of 80%. Organoids are usually formed within 1 week of culture (Figure 1C). Usually, LADC organoids will be passaged after the first 2–3 weeks of culture at a 1:2–1:3 ratio.
The morphology of LADC organoids can vary greatly between patients with different LADC subtypes; organoids from acinar subtype and enteric subtype are usually cystic, whereas solid adenocarcinoma and papillary adenocarcinoma-derived organoids usually display solid structures. Of note, lung adenocarcinomas often are composed of complex histologic patterns (Travis et al., 2015), the corresponding organoids generated may contain heterogeneous mixtures of structures.
To characterize the LADC organoids, mutational analysis by whole-exome sequencing and histopathological analysis by immunohistochemical staining can be performed. Most of LADC organoids and the corresponding parental tumors exhibited positive staining of thyroid transcription factor-1 (TTF-1) and Napsin A (Travis et al., 2010), and are negative for squamous marker p40 (Pelosi et al., 2012) and basal marker cytokeratin 5 (CK5).
Limitations
Using this protocol, we failed in establishing organoids from some clinical lung adenocarcinoma samples, we think that this success rate varies greatly between organoid cultures from different patients.
Also, lung adenocarcinoma organoid cultures can be easily contaminated by normal airway organoids (Dijkstra et al., 2020; Sachs et al., 2019). There are several studies reporting the use of different ways to purify cancer organoids, including the ways described in the following troubleshooting section. However, the establishment rate of pure lung cancer organoids may remain low, more effort should be made to solve this problem before applying this model for personalized medicine.
Troubleshooting
Problem 1
Low yield of cells after tissue digestion.
Potential solution
-
1.
If large tissue clusters remain after digestion, incubate the tissue with collagenase II for a prolonged time period. Pipette up and down 5–10 times using 10 mL Stripette Serological Pipets to promote the tissue disruption process.
-
2.
If cell suspension remains viscous after collagenase II digestion, add more DNase I to the solution.
Problem 2
Dying organoids.
Potential solution
-
1.
Add Rho kinase inhibitor Y27632 during dissociation step to avoid detach-induced anoikis.
-
2.
Splitting organoids without mechanical disruption. After harvesting organoids, add 1 mL of adDMEM/F12+++ and incubate for 15 min. Organoids will sink down while debris and dead cells will not. Gently take off 200 μL of the medium including dead cells and debris, spin down, and reseed organoids. In this way, we get rid of dead cells which might be the reason for anoikis and the death of organoids.
Problem 3
Contamination with normal lung organoids.
Potential solution
-
1.
Add TP53 activator nutlin-3a (5–10 μM) to the culture medium to enrich for tumor organoids carrying TP53 loss-of-function mutations, as described elsewhere (Broutier et al., 2017; Sachs et al., 2019; Yan et al., 2018).
-
2.
Dissolve the Matrigel surrounding organoids by incubating Matrigel containing organoids in cold adDMEM/F12+++ on ice for 20 min and then pipetting up and down for 5–10 times. Centrifuge the tube at 200 × g for 5 min at 4°C, resuspend the cells in cold adDMEM/F12+++ and pick up cancer organoids manually based on the morphology differences between normal lung organoids and cancer organoids (Figure 5) under a light microscope (Broutier et al., 2017; Sachs et al., 2019; Yan et al., 2018).
Figure 5.
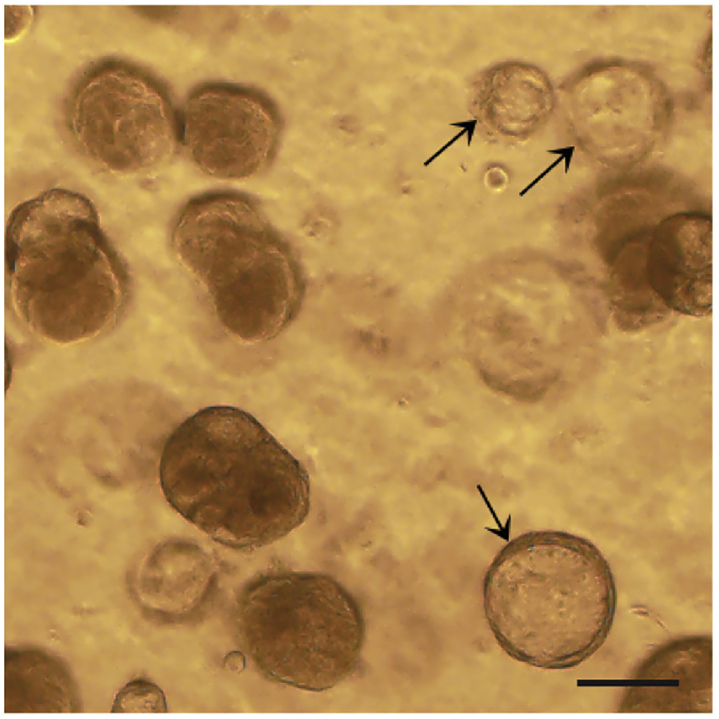
LADC organoid culture contaminated with normal lung organoids
Arrows indicate normal lung organoids in a LADC organoid culture. Scale bar, 100 μm.
Problem 4
Contamination with fibroblasts.
Potential solution
-
1.
Pipette cold adDMEM/F12 directly to the Matrigel domes to break down the domes and collect organoids for passage, instead of scraping the domes from the cell culture plate. Fibroblasts tend to adhere to the surface of cell culture plates and will be left behind.
-
2.
Dissolve the Matrigel surrounding organoids and pick up the cancer organoids manually under a light microscope.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Weiren Huang (pony8980@163.com).
Materials availability
This study did not generate any new unique reagents.
Data and code availability
This study did not generate any datasets or code.
Acknowledgments
This work was supported by the National Key R&D Program of China (2019YFA0906000), the National Natural Science Foundation of China (81772737, 81772736, 81972867), the National Science Foundation Projects of Guangdong Province, China (2017B030301015, 2020A1515010235), the Shenzhen Municipal Government of China (GJHZ20180926165202081, JCYJ20200109120016553), the Sanming Project of Shenzhen Health and Family Planning Commission, China (SZSM201512037, SZSM202011017), and the China Postdoctoral Science Foundation grant (2019M653215).
Author contributions
Conceptualization, Z.L., W.C., and W.H.; Methodology, Z.L., L.Y., D.C., and Z.M.; Investigation, Z.L.; Writing – Original Draft, Z.L.; Writing – Review & Editing, W.C. and W.H.; Resources, W.H.; Supervision, W.C. and W.H.; Funding Acquisition, W.H., W.C., and Z.L.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100239.
Contributor Information
Wei Chen, Email: jessie_chenwei@163.com.
Weiren Huang, Email: pony8980@163.com.
References
- Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarro L.M., Bradshaw C.R., Allen G.E., Arnes-Benito R., Sidorova O., Gaspersz M.P. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra K.K., Monkhorst K., Schipper L.J., Hartemink K.J., Smit E.F., Kaing S., de Groot R., Wolkers M.C., Clevers H., Cuppen E. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep. 2020;31:107588. doi: 10.1016/j.celrep.2020.107588. [DOI] [PubMed] [Google Scholar]
- Li Z., Qian Y., Li W., Liu L., Yu L., Liu X., Wu G., Wang Y., Luo W., Fang F. Human lung adenocarcinoma-derived organoid models for drug screening. iScience. 2020;23:101411. doi: 10.1016/j.isci.2020.101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi G., Fabbri A., Bianchi F., Maisonneuve P., Rossi G., Barbareschi M., Graziano P., Cavazza A., Rekhtman N., Pastorino U. ΔNp63 (p40) and thyroid transcription factor-1 immunoreactivity on small biopsies or cellblocks for typing non-small cell lung cancer: a novel two-hit, sparing-material approach. J. Thorac. Oncol. 2012;7:281–290. doi: 10.1097/JTO.0b013e31823815d3. [DOI] [PubMed] [Google Scholar]
- Sachs N., Papaspyropoulos A., Zomer-van Ommen D.D., Heo I., Böttinger L., Klay D., Weeber F., Huelsz-Prince G., Iakobachvili N., Amatngalim G.D. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38:e100300. doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- Travis W.D., Rekhtman N., Riley G.J., Geisinger K.R., Asamura H., Brambilla E., Garg K., Hirsch F.R., Noguchi M., Powell C.A. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: a paradigm shift. J. Thorac. Oncol. 2010;5:411–414. doi: 10.1097/JTO.0b013e3181d57f6e. [DOI] [PubMed] [Google Scholar]
- Yan H.H.N., Siu H.C., Law S., Ho S.L., Yue S.S.K., Tsui W.Y., Chan D., Chan A.S., Ma S., Lam K.O. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–897.e11. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any datasets or code.