Abstract
Background
Breast cancer (BC) patients who are treated with mastectomy are frequently offered immediate breast reconstruction. This study aimed to assess decisional conflict in patients considering immediate breast reconstruction, and to identify factors associated with clinically significant decisional conflict (CSDC).
Methods
Baseline data of a multicenter randomized controlled trial evaluating the impact of an online decision aid for BC patients considering immediate breast reconstruction after mastectomy were analyzed. Participants completed questionnaires assessing sociodemographic and clinical characteristics, decisional conflict and other patient-reported outcomes related to decision-making such as breast reconstruction preference, knowledge, information resources used, preferred involvement in decision-making, information coping style, and anxiety. Multivariable logistic regression analysis was performed to identify factors associated with CSDC (score > 37.5 on decisional conflict).
Results
Of the 250 participants, 68% experienced CSDC. Patients with a slight preference for breast reconstruction (odds ratio (OR) = 6.19, p < .01), with no preference for or against breast reconstruction (OR = 11.84, p < .01), and with a strong preference for no breast reconstruction (OR = 5.20, p < .05) were more likely to experience CSDC than patients with a strong preference for breast reconstruction. Furthermore, patients with more anxiety were more likely to experience CSDC (OR = 1.03, p = .01).
Conclusion
A majority of BC patients who consider immediate breast reconstruction after mastectomy experience clinically significant decisional conflict. The findings emphasize the need for decision support, especially for patients who do not have a strong preference for breast reconstruction.
Keywords: Decisional conflict, Immediate breast reconstruction, Surgical preferences
Highlights
-
•
A majority of patients considering immediate breast reconstruction experience decisional conflict.
-
•
Patients without a strong preference for breast reconstruction are more likely to experience decisional conflict.
-
•
Patients with more anxiety are more likely to experience decisional conflict.
1. Introduction
Immediate breast reconstruction (BR) after mastectomy is increasingly performed [[1], [2], [3]]. The choice for immediate BR after mastectomy largely depends on the values and preferences of the patient [4]. A breast cancer (BC) patient treated with mastectomy has to decide whether or not to have immediate BR, and, if immediate BR is chosen, make a decision among the types of BR (i.e. with an implant, autologous tissue or a combination) that are available to her.
Decision-making on immediate BR is complex as outcomes are uncertain and there are multiple reconstructive options with numerous advantages and disadvantages associated with each option [5]. For example, BR may have a positive impact on patients’ body image, self-esteem and quality of life after mastectomy, but it also increases the risk of surgical complications compared to mastectomy without BR [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. BR with autologous tissue often leads to a more natural looking and feeling breast, but it also entails additional scarring to the donor site [5]. Patients undergoing mastectomy have to weigh these pros and cons to make a personal choice about which option is best for them [4,16]. Decision-making is further complicated by the fact that patients often need to make this decision in a short period of time, between diagnosis and mastectomy. During this period it is common for patients to feel distressed and anxious [[17], [18], [19]], which may limit their cognitive functioning and decision making skills [20,21].
The complexity of this decision might increase feelings of decisional conflict in patients considering immediate BR. Decisional conflict is defined as a state of uncertainty about the course of action to take [22]. Behavioral manifestations of decisional conflict include feeling unsure about what to choose, wanting to delay the decision, questioning what is important, feeling distressed, wavering between the options, and constantly thinking about the options [23]. Although a certain level of decisional conflict might be inherent when deliberately making a complex decision, high levels of decisional conflict are associated with delayed decision-making, indecisiveness, and feelings of depression and regret [[24], [25], [26]]. These outcomes should be prevented, especially in the context of BR, as the primary goal of BR is to improve psychosocial outcomes and patient satisfaction.
Literature is sparse on decisional conflict about immediate BR, and the factors associated with it. Therefore, in this study we aimed to assess the levels of decisional conflict in BC patients who consider immediate BR after mastectomy, and to identify factors associated with clinically significant decisional conflict (CSDC).
2. Methods
2.1. Study sample & procedure
For this study we used baseline data from a multicenter randomized controlled trial evaluating the impact of an online patient decision aid (pDA) for BC patients who are considering immediate BR after mastectomy. For a detailed description of the trial see ter Stege et al. [27,28]. In short, patients were invited for trial participation by their surgical oncologist, nurse specialist or BC nurse during a routine treatment consultation in which the possibility of immediate BR was discussed. After written approval to share contact information with the researchers, patients were provided more study details and screened for eligibility via a telephone call by a member of the research team. Patients were eligible for participation if they were ≥18 years of age, diagnosed with BC or ductal carcinoma in situ, undergoing mastectomy, eligible for immediate BR and referred to a plastic surgeon. The consultation with a plastic surgeon had to be scheduled at least three working days after the study invitation to allow patients to have sufficient time to complete the informed consent form and baseline questionnaire, and to use the pDA before consultation. Participants were required to have internet access, basic computer skills and sufficient command of the Dutch language. Eligible patients who were interested in participating completed the informed consent form and baseline questionnaire via an online platform [29]. They were subsequently randomized to either the intervention group, in which they received access to the pDA, or to the usual care group, in which they received a standard BR information leaflet from the Dutch Cancer Society. Participants were invited to complete follow-up questionnaires one week after consultation with their plastic surgeon, and three and 12 months after mastectomy.
2.2. Measures
2.2.1. Sociodemographic and clinical characteristics
We collected data on patient’s age, country of birth, educational level, marital status, parity, body mass index (BMI), current smoking status, comorbidities, diagnosis, date of diagnosis, laterality, history of BC, prior BC treatment, hereditary or familial increased risk for BC, neoadjuvant therapy, and indication for adjuvant radiotherapy.
2.2.2. Decisional conflict
Decisional conflict was measured by the 16 item Decisional Conflict Scale (DCS) for which there is demonstrated reliability and validity [24,25,30,31]. Items are rated on a 5-point response scale (0 = strongly disagree, 4 = strongly agree), with positive statements having reversed scoring such that a higher score indicates higher decisional conflict. A total score is calculated, as well as five subscale scores (uncertainty (3 items), feeling informed (3 items), feeling clear about values (3 items), feeling supported (3 items) and effective decision making (4 items). Since consultation with a plastic surgeon had not yet taken place at the time of administration, the effective decision making subscale was omitted from baseline assessment. The total score was based on 12 items (DCS Total–12). According to the published scoring algorithm, total and subscale scores were calculated by averaging the sum of the individual item scores, multiplied by 25 [25]. Scores ranged from 0 (no decisional conflict) to 100 (extremely high decisional conflict). According to the published manual [25], scores of 25 or below are associated with implementing decisions, and scores exceeding 37.5 are associated with delaying decision making and feeling unsure about implementation. These cut-offs are derived from data in women considering preventive hormone therapy, whereby those who delayed their decision had average scores above 37.5 (unpublished data referred to in O’Connor et al. (1998) [32]. The cut-off (>37.5) served as a gold standard by which a checklist to screen on CSDC in clinical practice was validated [33].
2.2.3. Patient-reported outcomes related to decision-making
Breast reconstruction preference (BR preference) was assessed by a study-specific item asking patients to indicate which of the following five statements suits them best: (a) ‘I have a strong preference for BR’, (b) ‘I have a slight preference for BR’, (c) ‘I do not (yet) have a preference for or against BR’, (d) ‘I have a slight preference for no BR’, or (e) ‘I have a strong preference for no BR’.
Knowledge was measured with ten statements about BR that participants indicated as being “true/false/I don’t know”. These statements were translated and adapted from statements used in prior research evaluating knowledge in women with increased risk for BC deciding about risk-reducing mastectomy and BR [34]. Statements concerned topics such as risk factors, recovery time, and the impact of BR on sensation in the breast (See Appendix A for full instrument). The total score is the number of correctly answered items, ranging from 0 to 10.
Information resources used were assessed with an item, asking participants to select all types of BR information resources they used from a set of predefined answers (i.e. surgical oncologist, plastic surgeon, nurse/nurse specialist, information leaflet(s), book(s), website(s), relative(s), scientific article(s), article(s) from magazines or newspapers, other).
Preferred involvement in decision-making was measured by the Control Preferences Scale (CPS) [35]. Patients were asked to select one of five statements that best reflected their preferred role in BR decision-making: I prefer (a) to make the decision alone, (b) to make the decision alone, after considering the clinician’s opinion, (c) to make the decision together with the clinician, (d) the clinician to make the decision after considering my opinion, (e) the clinician to make the decision alone.
Information coping style was assessed with the Threatening Medical Situations Inventory (TMSI) [36]. This 24-item questionnaire measures cognitive confrontation [i.e., the tendency to actively search for information in case of a medical threat (‘monitoring’)], and cognitive avoidance [i.e., the tendency to avoid information/look for distraction in case of a medical threat (‘blunting’)] within the domain of a medical threat. It consists of four scenarios of threatening medical situations followed by three monitoring and three blunting alternatives [e.g., ‘I plan to ask the specialist as many questions as possible’ (monitoring) and ‘I think things will turn out to be alright’ (blunting)]. Participants were asked to rate the extent to which the alternative is applicable to them on a 5-point scale (1 = not at all applicable to me, to 5 = strongly applicable to me). Total monitoring and blunting scores are obtained by summing the relevant items. Scores on both scales range between 12 and 60 [36].
Anxiety was measured by the 6-item state scale of the Spielberger State-Trait Anxiety Inventory (STAI-6) [37,38]. Participants were asked to indicate on a 4-point scale to what extent a state applies to them at that moment (1 = not at all, 4 = very much). A total score is calculated by taking the mean of the items multiplied by 20 and ranges from 20 to 80, with higher scores indicating more anxiety.
2.3. Statistical analyses
Descriptive statistics were used to characterize the sample and to evaluate the levels of decisional conflict. Decisional conflict was dichotomized into CSDC (score >37.5 on DCS) and no CSDC (score ≤ 37.5 on DCS) [25,32]. Based on literature [32,[39], [40], [41], [42]], and expert opinions, we evaluated possible explanatory variables for CSDC using logistic regression analysis. The following variables were considered for selection in the regression model: (1) Sociodemographic characteristics (age, country of birth, educational level [low (primary school, lower vocational education), intermediate (secondary school, intermediate vocational education), or high (higher vocational education, university)], marital status, BMI [underweight (BMI <18.5), normal (BMI 18.5-<25), overweight (BMI 25-<30), obese (BMI 30+)], current smoking status, number of comorbidities, type of hospital); (2) Clinical characteristics (time since diagnosis, laterality, prior BC diagnosis, diagnosis in irradiated breast(s), hereditary or familial increased risk for BC, indication for adjuvant radiotherapy); and, (3) Patient-reported outcomes (BR preference, knowledge, information resources used, preferred involvement in decision-making, information coping style, anxiety). Explanatory variables were included in the multivariable model if the association with the outcome was significant at p < .10. Furthermore, if potential explanatory variables were strongly correlated with each other (r > 0.80), we selected one of the predictors to represent the whole set. Results of the multivariable model were considered statistically significant at a P value < .05. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25 (Armonk, NY: IBM corp.).
3. Results
3.1. Participants
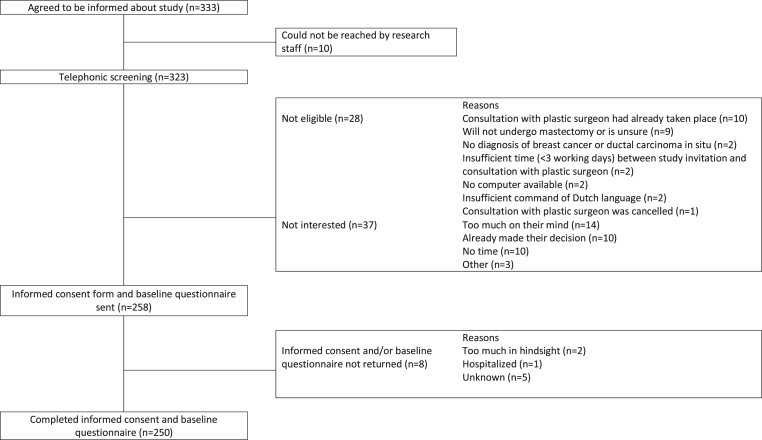
Fig. 1 shows the flowchart of the study. In total, 333 patients agreed to be informed about the study. Of these patients, 323 were reached by the research staff. Twenty-eight patients were ineligible. Of the remaining 295 patients, 37 were not interested in participating. The informed consent form and baseline questionnaire were sent to 258 patients. In total, 250 patients returned both and were included for analyses. This resulted in a participation rate of 85% (250/295).
Fig. 1.
Flowchart of study participants.
3.2. Background characteristics
Sociodemographic and clinical background characteristics are provided in Table 1. Patients had an average age of 50.12 years (SD = 11.03), and more than half (52%) were highly educated. The median time since diagnosis was three weeks (interquartile range (ICR) = 18), and 60% of the patients were recruited in a cancer-specific hospital.
Table 1.
Background characteristics (N = 250).
| No. | % | |
|---|---|---|
| Age (years), mean (SD) | 50.12 (11.03) | |
| Born in NL | 233 | 93 |
| Highest level of education | ||
| High | 129 | 52 |
| Intermediate | 109 | 44 |
| Low | 10 | 4 |
| Missing | 2 | 1 |
| Married or in a relationship | 214 | 86 |
| Having children | 199 | 80 |
| BMI | ||
| Underweight (<18.5) | 5 | 2 |
| Normal (18.5 - <25) | 139 | 56 |
| Overweight (25 - <30) | 75 | 30 |
| Obese (30+) | 31 | 12 |
| Current smoking status (yes) | 14 | 6 |
| Comorbidities | ||
| None | 128 | 51 |
| 1 | 79 | 32 |
| 2 or more | 42 | 17 |
| Missing | 1 | 0 |
| Diagnosis | ||
| Invasive BC | 151 | 60 |
| Invasive BC and DCIS | 37 | 15 |
| DCIS | 61 | 24 |
| Other | 1 | 0 |
| Time since diagnosis (weeks), median (ICR) | 3 (18) | |
| Bilateral diagnosis | 12 | 5 |
| Diagnosis in irradiated breast(s) | 27 | 11 |
| Prior diagnosis of BC and/or DCIS | 40 | 16 |
| Surgical treatment for BC and/or DCIS in the past | ||
| Breast conserving surgery | 32 | 13 |
| Mastectomy | 4 | 2 |
| Mastectomy with BR | 5 | 2 |
| Genetic predisposition or familial increased risk of BC | 40 | 16 |
| Neoadjuvant therapy | 91 | 36 |
| Chemotherapy | 86 | 34 |
| Hormone therapy | 9 | 4 |
| Immunotherapy | 23 | 9 |
| Indication for adjuvant radiotherapy | ||
| No | 71 | 28 |
| Yes | 61 | 24 |
| Maybe | 75 | 30 |
| I don’t know | 43 | 17 |
| Type of hospital | ||
| Cancer-specific center | 150 | 60 |
| Academic center | 27 | 11 |
| General hospital | 73 | 29 |
Abbreviations: SD, standard deviation; NL, Netherlands; BMI, body mass index; BC, breast cancer; DCIS, ductal carcinoma in situ; ICR, interquartile range; BR, breast reconstruction.
3.3. Decisional conflict
The mean total and subscale scores are summarized in Table 2. Sixty-eight percent of the patients experienced CSDC. Statements with which most patients agreed, reflecting more conflict, were: ‘I want more advice and information about the options’, ‘I am not sure what to decide’, and ‘this decision is difficult for me to make’ (82%, 40%, and 39% (strongly) agreed, respectively). Statements with which the least patients agreed (or if appropriate disagreed), reflecting less conflict, were: ‘I feel pressured by others in making this decision’, and, ‘I have enough support from others to make a choice’ (4% (strongly) agreed and 7% (strongly) disagreed, respectively) (See Appendix B for results on all items).
Table 2.
Decisional conflict in breast cancer patients considering immediate breast reconstruction.
| Mean (SD) | |
|---|---|
| DCS Total-12 | 46.18 (15.22) |
| CSDC, no. (%) | 169 (68%) |
| DCS subscales | |
| Uncertainty | 48.40 (27.60) |
| Feeling informed | 49.30 (22.26) |
| Feeling clear about values | 45.43 (19.37) |
| Feeling supported | 41.60 (14.46) |
Abbreviations: DCS, Decisional Conflict Scale; CSDC, clinically significant decisional conflict; SD, standard deviation.
3.4. Factors associated with clinically significant decisional conflict
Based on the univariable analyses (p < .10) and correlations among the potential explanatory variables (r > 0.80), the following variables were included in the multivariable logistic regression analysis: educational level, BR preference, being informed by scientific article(s), blunting coping style, and anxiety (see Appendix C for results of the univariable analyses).
The results of multivariable analyses are shown in Table 3. We found a significant effect for BR preference and anxiety. Specifically, patients with a slight preference for BR were 6.19 times more likely (95% confidence interval (CI) = 2.47–15.54), and patients with no preference for or against BR were 11.84 times more likely to experience CSDC (95% CI = 2.68–52.28) than patients with a strong preference for BR. Additionally, patients with a strong preference for no BR were 5.20 times more likely to experience CSDC (95% CI = 1.04–25.86) than patients with a strong preference for BR. Patients with more anxiety were 1.03 times more likely to experience CSDC (95% CI = 1.01–1.06). No significant effects were found for educational level, blunting information coping style, and being informed by scientific article(s).
Table 3.
Multivariable logistic regression predicting clinically significant decisional conflict (N = 248a).
| No. | CSDC | B (SE) | p | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Constant | −0.85 (1.24) | 0.49 | 0.43 | |||
| Educational level | ||||||
| Low | 10 | 40% | −1.37 (0.77) | 0.08 | 0.26 | 0.06–1.16 |
| Intermediate | 109 | 68% | −0.23 (0.32) | 0.48 | 0.80 | 0.42–1.50 |
| High | 129 | 69% | ref | |||
| BR preference | ||||||
| Strong preference for BR | 142 | 52% | ref | |||
| Slight preference for BR | 51 | 86% | 1.82 (0.47) | 0.00 | 6.19 | 2.47–15.54 |
| No preference for or against BR | 32 | 94% | 2.47 (0.76) | 0.00 | 11.84 | 2.68–52.28 |
| Slight preference for no BR | 9 | 89% | 1.63 (1.09) | 0.13 | 5.10 | 0.60–43.21 |
| Strong preference for no BR | 14 | 86% | 1.65 (0.82) | 0.04 | 5.20 | 1.04–25.86 |
| Informed by scientific article(s) | 21 | 48% | −1.08 (0.55) | 0.05 | 0.34 | 0.12–1.01 |
| Blunting coping style (TMSI)b | −0.01 (0.03) | 0.70 | 0.99 | 0.94–1.04 | ||
| Anxiety (STAI-6)c | 0.03 (0.01) | 0.01 | 1.03 | 1.01–1.06 | ||
Abbreviations: CSDC, clinically significant decisional conflict; B, beta; SE, standard error; OR, odds ratio; CI, confidence interval; BR, breast reconstruction; TMSI, Threatening Medical Situations Inventory; STAI-6, State scale of the State-Trait Anxiety Inventory.
Note. R2 = 0.30 (Nagelkerke). Model χ2(9) = 59.10, p < .001. Significant values (at p < .05) are shown in bold.
N = 248 due to 2 missings on variable educational level.
Mean = 34.02, Standard Deviation = 6.33.
Mean = 46.39, Standard Deviation = 12.91.
Based on the above results, we performed an explorative analysis into the association between anxiety and BR preference using analysis of variance. Group differences were accompanied by effect sizes (ES) (ES of 0.2 = small, 0.5 = moderate and clinically relevant, 0.8 = large) [43]. Patients with a strong preference for BR were significantly less anxious (M (SD) = 44.15 (12.65)) than patients with a slight preference for BR (M (SD) = 48.30 (12.53), p < .05, ES = 0.33), and patients with no preference for or against BR (M (SD) = 50.20 (12.36), p = .01, ES = 0.48). And, although not significant, effect sizes show (almost) clinically relevant differences in anxiety between patients with a strong preference for BR and patients with a slight preference or a strong preference for no BR (M (SD) = 51.85 (11.32), p = .08, ES = 0.61, and M (SD) = 49.76 (15.66), p = .12, ES = 0.43, respectively).
4. Discussion
Our results show that more than two thirds of BC patients considering immediate BR after mastectomy experienced clinically significant decisional conflict, and that this was associated with BR preference and anxiety. Patients with a slight preference for BR, patients with no preference for or against BR and patients with a strong preference for no BR were more likely to experience CSDC than patients with a strong preference for BR. In addition, patients with higher levels of anxiety were more likely to experience CSDC than patients with lower levels of anxiety.
To our knowledge, this is the first study in which decisional conflict regarding immediate BR was assessed in a large sample of BC patients. The levels of decisional conflict in our sample are comparable to the levels of decisional conflict in two prior studies in BC patients who considered delayed BR [44,45], and relatively high compared to levels in a sample of BC patients who considered immediate BR (mean (SD) = 33 (24)) [39], and to average decisional conflict regarding a variety of health-related decisions [31]. The specific population, complexity of the decision, and the timing of our assessment might all have contributed in evoking higher decisional conflict [31]. Highest baseline (before decision making) decisional conflict has been found among individuals who were ill and were making decisions for themselves [31], which is the case for our population. Furthermore, as the majority of participants in our study had only recently been diagnosed with BC and introduced to the possibility of BR, and all were waiting to be informed by a plastic surgeon, this will likely have contributed to the high levels in our sample.
Although we found a significant positive association between anxiety and CSDC, the association was weak. This is in line with the study of Manne et al. (2016) [38], in which they did not find any association between anxiety and decisional conflict regarding immediate BR. However, we do think it is a factor worth considering in future research, as we did find anxiety levels to differ between BR preference groups. Thus, the weak association could be attributed to effect modification in which the degree of association between anxiety and CSDC differs among different BR preference groups. Additionally, the association between anxiety and decisional conflict has been reported in other populations [[45], [46], [47]], and is in line with the conceptual framework of decisional conflict [23].
Intuitively, one would expect that patients with a strong preference for a certain option would experience less decisional conflict than patients with either a slight preference for - or with no preference for a certain option. However, this was only partially true in our results. Surprisingly, patients with a strong preference for no BR more frequently experienced CSDC than patients with a strong preference for BR. Consistent with our finding, Manne et al. (2016) found that BC patients who reported a greater number of reasons not to choose BR had higher decisional conflict [38], suggesting that patients who may tend to decline BR feel more conflicted about their decision. Although speculative, based on clinical experience and interviews with patients about their experiences with decision-making about BR (manuscript submitted), the possibility of BR is often communicated as something positive. Thus, patients who prefer not to have BR might perceive information provision as ‘favoring BR over no BR’, which may have contributed to increased decisional conflict.
Besides anxiety and BR preference, no factors were associated with CSDC. It is difficult to compare our results with prior findings, as literature on predictors of decisional conflict is scarce and heterogeneous in studied predictors and populations (e.g. individuals with diabetes [40], prostate cancer [41,46], or tested for hereditary cancer [47]). However, the absence of associations with sociodemographic and clinical characteristics is largely consistent with a prior study in a comparable population [39], with the exception that we did not find an association with time since diagnosis. Furthermore, the absence of any association of decisional conflict with knowledge is in contrast to the conceptual model of decisional conflict [22]. Possibly, decisional conflict might be more strongly related to a patients’ perceived knowledge than their actual knowledge [24].
This study has several limitations. Our sample consisted of highly educated patients limiting the generalizability of our findings. Furthermore, since data were collected as part of a trial evaluating the impact of a patient decision aid, we might have included patients with relatively higher decision support needs, potentially leading to an overestimation of the levels of decisional conflict. Another limitation is that data on the psychometric properties of the total score of the Decisional Conflict Scale without the assessment of the effective decision-making subscale is lacking. Although the developers of the instrument indicated that this subscale should only be assessed in circumstances where a decision has already been made [24], and other studies also omitted it [48,49], the reliability and validity of the instrument without the effective decision-making subscale needs to be confirmed. Finally, although the cutoff (>37.5) for CSDC has been used in prior research [24,31,50,51], and a review that examined decisional conflict over time including 253 studies found support for it [31], more evidence on its validity in the context of BR decision-making seems warranted.
The large sample size is considered a major strength of this study. Additionally, the timing of our assessment, namely during the short period between diagnosis and mastectomy, is highly relevant and rarely studied. While immediate BR after mastectomy is increasingly performed [[1], [2], [3]], previous studies have mainly focused on decisional conflict in patients who had already undergone mastectomy and were considering BR after completion of their oncological treatment [44,45,52].
Our results emphasize the need for support for BC patients in making this complex decision, especially for those patients without a strong preference for BR. Decisional conflict may be reduced by addressing contributors to uncertainty, such as providing information about benefits and risks for each option and helping patients understand their own values [32]. The use of decision aids as an addition to standard clinical counseling has been found to reduce decisional conflict, also in patients deciding about BR [[52], [53], [54], [55]].
We conclude that the majority of BC patients who consider immediate BR after mastectomy experienced CSDC. Our results emphasize the need for support for BC patients in making this complex decision, especially in patients without a strong preference for BR.
Funding source
This trial is funded by the Dutch Cancer Society (Delflandlaan 17, 1062 EA, Amsterdam, The Netherlands) (grant number A6C/NKI 2014–7031). The funding body had no role in the design of the study, the collection, analysis and interpretation of the data, in writing the manuscript nor in the decision to submit the manuscript for publication.
Ethical approval
The research protocol of the RCT was examined by the accredited Medical Research Ethics Committee of the Dutch Cancer Institute. They concluded that, considering the length and nature of the questionnaires, the obligation to fulfil the specific requirements of the Dutch law for Medical Research Involving Human Subjects was waived (reference: METC17.0652). The review boards of all participating hospitals approved the study protocol. All participating patients signed an online IC form.
Availability of data and materials
The dataset will be available from the corresponding author (stored in a data repository at the Netherlands Cancer Institute) on reasonable request.
Declaration of competing interest
None.
Acknowledgements
We would like to thank all participating patients and all involved healthcare professionals in the participating centers. Also, we would like to thank the Dutch Cancer Society for funding this study (grant number A6C/NKI 2014–7031).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.12.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Panchal H., Matros E. Current trends in postmastectomy breast reconstruction. Plast Reconstr Surg. 2017;140:7s–13s. doi: 10.1097/PRS.0000000000003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mennie J.C., Mohanna P.N., O’Donoghue J.M., Rainsbury R., Cromwell D.A. National trends in immediate and delayed post-mastectomy reconstruction procedures in England: a seven-year population-based cohort study. Eur J Surg Oncol. 2017;43:52–61. doi: 10.1016/j.ejso.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 3.van Bommel A., Spronk P., Mureau M., Siesling S., Smorenburg C., Tollenaar R. Breast-contour-preserving procedure as a multidisciplinary parameter of esthetic outcome in breast cancer treatment in The Netherlands. Ann Surg Oncol. 2019;26:1704–1711. doi: 10.1245/s10434-019-07265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutch Society for Plastic Surgery . 2015. Guideline breast reconstruction.www.nvpc.nl [Google Scholar]
- 5.Cordeiro P.G. Breast reconstruction after surgery for breast cancer. N Engl J Med. 2008;359:1590–1601. doi: 10.1056/NEJMct0802899. [DOI] [PubMed] [Google Scholar]
- 6.Rowland J.H., Holland J.C., Chaglassian T., Kinne D. Psychological response to breast reconstruction. Expectations for and impact on postmastectomy functioning. Psychosomatics. 1993;34:241–250. doi: 10.1016/S0033-3182(93)71886-1. [DOI] [PubMed] [Google Scholar]
- 7.Al-Ghazal S.K., Fallowfield L., Blamey R.W. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Canc. 2000;36:1938–1943. doi: 10.1016/s0959-8049(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 8.Al-Ghazal S.K., Sully L., Fallowfield L., Blamey R.W. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol. 2000;26:17–19. doi: 10.1053/ejso.1999.0733. [DOI] [PubMed] [Google Scholar]
- 9.Sherman K.A., Woon S., French J., Elder E. Body image and psychological distress in nipple-sparing mastectomy: the roles of self-compassion and appearance investment. Psycho Oncol. 2017;26:337–345. doi: 10.1002/pon.4138. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins E.G., Cederna P.S., Lowery J.C., Davis J.A., Kim H.M., Roth R.S. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106:1014–1025. doi: 10.1097/00006534-200010000-00010. discussion 26-7. [DOI] [PubMed] [Google Scholar]
- 11.Dauplat J., Kwiatkowski F., Rouanet P., Delay E., Clough K., Verhaeghe J.L. Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg. 2017;104:1197–1206. doi: 10.1002/bjs.10537. [DOI] [PubMed] [Google Scholar]
- 12.Kouwenberg C.A.E., de Ligt K.M., Kranenburg L.W., Rakhorst H., de Leeuw D., Siesling S. Long-term health-related quality of life after four common surgical treatment options for breast cancer and the effect of complications: a retrospective patient-reported survey among 1871 patients. Plast Reconstr Surg. 2020;146:1–13. doi: 10.1097/PRS.0000000000006887. [DOI] [PubMed] [Google Scholar]
- 13.Jeevan R., Cromwell D.A., Browne J.P., Caddy C.M., Pereira J., Sheppard C. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. J Plast Reconstr Aesthetic Surg. 2014;67:1333–1344. doi: 10.1016/j.bjps.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Pinsolle V., Grinfeder C., Mathoulin-Pelissier S., Faucher A. Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthetic Surg. 2006;59:1017–1024. doi: 10.1016/j.bjps.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Zhong T., Hofer S.O., McCready D.R., Jacks L.M., Cook F.E., Baxter N. A comparison of surgical complications between immediate breast reconstruction and mastectomy: the impact on delivery of chemotherapy--an analysis of 391 procedures. Ann Surg Oncol. 2012;19:560–566. doi: 10.1245/s10434-011-1950-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee G.K., Sheckter C.C. Breast reconstruction following breast cancer treatment-2018. J Am Med Assoc. 2018;320:1277–1278. doi: 10.1001/jama.2018.12190. [DOI] [PubMed] [Google Scholar]
- 17.Burgess C., Cornelius V., Love S., Graham J., Richards M., Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegel M.T., Moore C.P., Collins E.D., Kearing S., Gillock K.L., Riggs R.L. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006;107:2924–2931. doi: 10.1002/cncr.22335. [DOI] [PubMed] [Google Scholar]
- 19.Mertz B.G., Bistrup P.E., Johansen C., Dalton S.O., Deltour I., Kehlet H. Psychological distress among women with newly diagnosed breast cancer. Eur J Oncol Nurs. 2012;16:439–443. doi: 10.1016/j.ejon.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs. 1999;22:185–194. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Scott D.W. Anxiety, critical thinking and information processing during and after breast biopsy. Nurs Res. 1983;32:24–28. [PubMed] [Google Scholar]
- 22.O’Connor A.M. Decisional conflict. In: McFarland G.K., McFarlane E.A., editors. Nursing: diagnosis and intervention. second ed. C.V. Mosby; St. Lois, MO: 1993. p. 468Y77. [Google Scholar]
- 23.Hoefel L., O’Connor A.M., Lewis K.B., Boland L., Sikora L., Hu J. 20th anniversary update of the ottawa decision support framework Part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555–581. doi: 10.1177/0272989X20936209. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor A.M. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor A.M. Ottawa Hospital Research Institute; Ottawa: 1993. User manual - decisional conflict scale.https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf Available from: [Google Scholar]
- 26.Becerra-Perez M.-M., Menear M., Turcotte S., Labrecque M., Légaré F. More primary care patients regret health decisions if they experienced decisional conflict in the consultation: a secondary analysis of a multicenter descriptive study. BMC Fam Pract. 2016;17:156. doi: 10.1186/s12875-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ter Stege J.A., Woerdeman L.A.E., Hahn D.E.E., van Huizum M.A., van Duijnhoven F.H., Kieffer J.M. The impact of an online patient decision aid for women with breast cancer considering immediate breast reconstruction: study protocol of a multicenter randomized controlled trial. BMC Med Inf Decis Making. 2019;19:165. doi: 10.1186/s12911-019-0873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (Us) 2000 Feb 29. Identifier: NCT03791138, the impact of a web-based patient decision aid for women considering breast reconstruction (TANGO);2019 jan 2.https://clinicaltrials.gov/ct2/show/NCT03791138?term=TANGO&cond=Breast+Cancer&rank=2 Available from: [Google Scholar]
- 29.Exploratio https://explora-zorg.nl/
- 30.Koedoot N., Molenaar S., Oosterveld P., Bakker P., de Graeff A., Nooy M. The decisional conflict scale: further validation in two samples of Dutch oncology patients. Patient Educ Counsel. 2001;45:187–193. doi: 10.1016/s0738-3991(01)00120-3. [DOI] [PubMed] [Google Scholar]
- 31.Garvelink M.M., Boland L., Klein K., Nguyen D.V., Menear M., Bekker H.L. Decisional conflict scale findings among patients and surrogates making health decisions: part II of an anniversary review. Med Decis Making. 2019;39:315–326. doi: 10.1177/0272989X19851346. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor A.M., Tugwell P., Wells G.A., Elmslie T., Jolly E., Hollingworth G. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Counsel. 1998;33:267–279. doi: 10.1016/s0738-3991(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 33.Ferron Parayre A., Labrecque M., Rousseau M., Turcotte S., Légaré F. Validation of SURE, a four-item clinical checklist for detecting decisional conflict in patients. Med Decis Making. 2014;34:54–62. doi: 10.1177/0272989X13491463. [DOI] [PubMed] [Google Scholar]
- 34.Sherman K.A., Kilby C.J., Shaw L.K., Winch C., Kirk J., Tucker K. Facilitating decision-making in women undergoing genetic testing for hereditary breast cancer: BRECONDA randomized controlled trial results. Breast. 2017;36:79–85. doi: 10.1016/j.breast.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Degner L.F., Sloan J.A., Venkatesh P. The Control preferences scale. Can J Nurs Res. 1997;29:21–43. [PubMed] [Google Scholar]
- 36.van Zuuren F.J., de Groot K.I., Mulder N.L., Muris P. Coping with medical threat: an evaluation of the threatening medical situations inventory (TMSI) Pers Indiv Differ. 1996;21:21–31. [Google Scholar]
- 37.Marteau T.M., Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31(Pt 3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 38.van der Bij A.K., de Weerd S., Cikot R.J., Steegers E.A., Braspenning J.C. Validation of the Dutch short form of the state scale of the Spielberger State-Trait Anxiety Inventory: considerations for usage in screening outcomes. Community Genet. 2003;6:84–87. doi: 10.1159/000073003. [DOI] [PubMed] [Google Scholar]
- 39.Manne S.L., Topham N., Kirstein L., Virtue S.M., Brill K., Devine K.A. Attitudes and decisional conflict regarding breast reconstruction among breast cancer patients. Cancer Nurs. 2016;39:427–436. doi: 10.1097/NCC.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bravo P., Dois A., Hernández M.J., Villarroel L. Decisional conflict among people with diabetes mellitus or hypertension attending primary care. Rev Med Chile. 2018;146:1286–1293. doi: 10.4067/S0034-98872018001101286. [DOI] [PubMed] [Google Scholar]
- 41.Chien C.H., Chuang C.K., Liu K.L., Li C.L., Liu H.E. Changes in decisional conflict and decisional regret in patients with localised prostate cancer. J Clin Nurs. 2014;23:1959–1969. doi: 10.1111/jocn.12470. [DOI] [PubMed] [Google Scholar]
- 42.Ottawa Decision Support Tutorial (Odst): Improving Practitioners’ Decision Support Skills. https://decisionaid.ohri.ca/ODST/.
- 43.Cohen J. second ed. Lawrence Erlbaum Associates; Hillsdale, New York: 1998. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 44.Causarano N., Platt J., Baxter N.N., Bagher S., Jones J.M., Metcalfe K.A. Pre-consultation educational group intervention to improve shared decision-making for postmastectomy breast reconstruction: a pilot randomized controlled trial. Support Care Canc. 2015;23:1365–1375. doi: 10.1007/s00520-014-2479-6. [DOI] [PubMed] [Google Scholar]
- 45.Metcalfe K., Zhong T., O’Neill A.C., McCready D., Chan L., Butler K. Development and testing of a decision aid for women considering delayed breast reconstruction. J Plast Reconstr Aesthetic Surg. 2018;71:318–326. doi: 10.1016/j.bjps.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Underhill M.L., Hong F., Berry D.L. When study site contributes to outcomes in a multi-center randomized trial: a secondary analysis of decisional conflict in men with localized prostate cancer. Health Qual Life Outcome. 2014;12:159. doi: 10.1186/s12955-014-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sie A.S., Prins J.B., Spruijt L., Kets C.M., Hoogerbrugge N. Can we test for hereditary cancer at 18 years when we start surveillance at 25? Patient reported outcomes. Fam Cancer. 2013;12:675–682. doi: 10.1007/s10689-013-9644-9. [DOI] [PubMed] [Google Scholar]
- 48.Garvelink M.M., Boland L., Klein K., Nguyen D.V., Menear M., Bekker H.L. Decisional conflict scale use over 20 years: the anniversary review. Med Decis Making. 2019;39:301–314. doi: 10.1177/0272989X19851345. [DOI] [PubMed] [Google Scholar]
- 49.Reumkens K., Tummers M.H.E., Gietel-Habets J.J.G., van Kuijk S.M.J., Aalfs C.M., van Asperen C.J. Online decision support for persons having a genetic predisposition to cancer and their partners during reproductive decision-making. J Genet Counsel. 2019;28:533–542. doi: 10.1002/jgc4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldé A., Légaré F., Labrecque M. Assessment of needs of men for decision support on male sterilization. Patient Educ Counsel. 2006;63:301–307. doi: 10.1016/j.pec.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Labrecque M., Paunescu C., Plesu I., Stacey D., Légaré F. Evaluation of the effect of a patient decision aid about vasectomy on the decision-making process: a randomized trial. Contraception. 2010;82:556–562. doi: 10.1016/j.contraception.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Sherman K.A., Shaw L.K., Winch C.J., Harcourt D., Boyages J., Cameron L.D. Reducing decisional conflict and enhancing satisfaction with information among women considering breast reconstruction following mastectomy: results from the BRECONDA randomized controlled trial. Plast Reconstr Surg. 2016;138:592e–602e. doi: 10.1097/PRS.0000000000002538. [DOI] [PubMed] [Google Scholar]
- 53.Manne S.L., Topham N., D’Agostino T.A., Myers Virtue S., Kirstein L., Brill K. Acceptability and pilot efficacy trial of a web-based breast reconstruction decision support aid for women considering mastectomy. Psycho Oncol. 2016;25:1424–1433. doi: 10.1002/pon.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam W.W., Chan M., Or A., Kwong A., Suen D., Fielding R. Reducing treatment decision conflict difficulties in breast cancer surgery: a randomized controlled trial. J Clin Oncol. 2013;31:2879–2885. doi: 10.1200/JCO.2012.45.1856. [DOI] [PubMed] [Google Scholar]
- 55.Stacey D., Légaré F., Lewis K., Barry M.J., Bennett C.L., Eden K.B. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:Cd001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset will be available from the corresponding author (stored in a data repository at the Netherlands Cancer Institute) on reasonable request.