Abstract
C-C chemokine receptor type 5 (CCR5) regulates the trafficking of various immune cells to sites of infection. In this study, we showed that expression of CCR5 and its ligands was rapidly increased in the kidney after systemic Candida albicans infection, and infected CCR5−/− mice exhibited increased mortality and morbidity, indicating that CCR5 contributes to an effective defense mechanism against systemic C. albicans infection. The susceptibility of CCR5−/− mice to C. albicans infection was due to impaired fungal clearance, which in turn resulted in exacerbated renal inflammation and damage. CCR5-mediated recruitment of NK cells to the kidney in response to C. albicans infection was necessary for the anti-microbial activity of neutrophils, the main fungicidal effector cells. Mechanistically, C. albicans induced expression of IL-23 by CD11c+ dendritic cells (DCs). IL-23 in turn augmented the fungicidal activity of neutrophils through GM-CSF production by NK cells. As GM-CSF potentiated production of IL-23 in response to C. albicans, a positive feedback loop formed between NK cells and DCs seemed to function as an amplification point for host defense. Taken together, our results suggest that CCR5-mediated recruitment of NK cells to the site of fungal infection is an important step that underlies innate resistance to systemic C. albicans infection.
Keywords: Candida albicans, CCR5, NK cells, IL-23, Dendritic cells, GM-CSF
INTRODUCTION
Candida albicans is the most prevalent human fungal pathogen and causes invasive candidiasis (1). This infectious disease presents a serious clinical challenge to immunocompromised patients and has a high mortality rate in these individuals, despite treatment with antifungal drugs (2,3). In particular, neutropenia is a major risk factor for invasive candidiasis (4,5). Systemic infections occur when colonizing or gut-residing C. albicans penetrates the mucocutaneous barriers. The kidney acts as the major site of fungal replication (6). Recognition of C. albicans by a variety of pattern recognition receptors initiates innate immunity (2,3). The C-type lectin receptor family is particularly important in anti-Candida defense (7,8). Signal transduction through these receptors orchestrates a complex series of molecular and cellular events that underlie innate resistance to C. albicans infection, including production of inflammatory mediators, inflammasome activation, and phagocytic and fungicidal activities (7,8). Failure of innate defense mechanisms frequently results in life-threatening Candida sepsis due to hyperactivation of the immune system (9,10).
Recent studies have clearly demonstrated that NK cells occupy a central position in an important defense pathway linked to the Candida-killing activity of neutrophils (11,12,13). During acute Candida infection, dendritic cells (DCs) and Ly6C+ monocytes produce IL-23 and IL-15, respectively, both of which stimulate NK cells to produce GM-CSF (12,13,14). Considering that NK cells are one of the cell types recruited most rapidly to sites of infection, it is surprising that Ly6C+ monocytes act upstream of NK cells. To understand the sequential events determining host defense, therefore, it is a prerequisite to define the regulatory immune networks that are created during C. albicans infection. One approach to this type of study is to dissect the chemotactic system that regulates recruitment of immune cells to the infection site. Although trafficking of Ly6C+ monocytes to the kidney is known to depend upon C-C chemokine receptor type 2 (CCR2) after systemic C. albicans infection (15), it has yet to be clarified how other types of immune cells are recruited to the infected kidney. We previously showed that injury of tubular epithelial cells (TECs) by ischemia and reperfusion induces recruitment of NK cells in a CCR5-dependent manner (16). In addition, host defense to some infections relies upon CCR5-mediated NK cell recruitment to infection sites (17). Based upon these observations, we hypothesized that ablation of CCR5 in NK cells would compromise innate resistance to systemic C. albicans infection and leads to death from fulminant candidiasis. Indeed, we showed that CCR5 was critical in NK cell recruitment and neutrophil activation during acute C. albicans infection. Unexpectedly, production of IL-23 was impaired in Candida-infected CCR5−/− mouse kidneys. GM-CSF secreted by NK cells was shown to promote IL-23 production, thereby forming a positive feedback loop for mutual regulation of these factors during acute C. albicans infection.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from Orient Bio-Charles River (Seoul, Korea). CCR5−/− mice with a C57BL/6 background were maintained in a specific pathogen-free facility and used between 7–8 wk of age. All experiments were conducted according to the regulations of the Animal Committee of the Ulsan University Hospital (UUH-0120-03)).
Fungal strains and growth conditions
C. albicans (ATCC26555) was grown in peptone dextrose extract at 30°C overnight, and aliquots were frozen at −80°C. To obtain hyphal forms, yeasts were suspended in Rosewell Park Memorial Institute (RPMI)-1640 medium (Welgene, Gyeongsan, Korea) at a final concentration of 5×106 cells/ml and were further cultured on a rotary shaker at 37oC for 120 min. To kill C. albicans hyphae, organisms were harvested by centrifugation, and pellets were washed twice in sterile PBS and resuspended at a density of 1×108 cells/ml before heat killing at 90°C for 30 min.
Experimental systemic candidiasis
C. albicans were inoculated intravenously into the lateral caudal tail veins with 3×105 colony forming units (CFUs) (lethal dose).
Counting CFUs
Mice were euthanized, and kidneys were removed aseptically to determine fungal burdens. Harvested kidneys were homogenized in 2 ml PBS, and serial dilutions of homogenates were plated on Sabouraud agar and incubated at 37°C for 24 h. Colonies were counted, and results were expressed as log10(CFUs/organ).
Phagocytosis assay
Bone marrow cells were collected from femurs and tibias, suspended in RPMI-1640 medium (Welgene) supplemented with 10% FBS, penicillin/streptomycin (100 U/ml), 2 mM L-glutamine (GIBCO, Grand Island, NY, USA), and 50 μM 2-mercaptoethanol. To purify neutrophils from bone marrow cells, anti-Ly6G magnetic activated cell sorting beads were used according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified neutrophils were resuspended in RPMI-1640 medium and adjusted to a concentration of 2×106 cells/ml. The cells were preincubated with GM-CSF (10 ng/ml) at 37°C for 4 h. After the addition of serum-opsonized FITC-labeled heat-killed (HK) C. albicans hyphae (multiplicity of infection [MOI]=10), the mixtures were incubated with slow rotation at 37°C for 30 min. Phagocytosis was stopped by the immediate transfer of cells onto ice, and the cells were washed thoroughly with cold FACS buffer. Extracellular fluorescence was quenched in a quenching solution containing 0.04% trypan blue and 1% formaldehyde, and cells containing fungi were analyzed using flow cytometry. Phagocytosis was expressed as the percentage of neutrophils phagocytosing FITC-labeled C. albicans.
Diff-Quit staining of neutrophils
Purified neutrophils were resuspended in PBS. Specimens were prepared using the Cytospin™ 4 Cytocentrifuge (Thermo Fisher Scientific, Waltham, MA, USA). The centrifuged specimens were then stained with a Diff-Quik™ staining set (Siemens Healthcare Diagnostics, Deerfield, IL, USA) and examined under microscope.
Histology
Kidneys were fixed in 10% (v/v) formalin, embedded in paraffin, sectioned (5 μm), stained with H&E or periodic acid-Schiff (PAS), and analyzed.
Depletion of neutrophils and NK cells
Neutrophil depletion was achieved by injecting 200 μg anti-Gr-1 (RB6-8C5) mAb intraperitoneally into mice 2 days before C. albicans infection. For NK cell depletion, anti-NK1.1 (PK136; eBioscience, San Diego, CA, USA) mAb was injected 1 days before and 2 days after infection.
Real-time RT-PCR
Total RNA was extracted from kidneys or cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Whole tissues were homogenized with a TissueLyzer tissue homogenizer (QUAGEN, Caldwell, NJ, USA), and cDNA was prepared with SuperScript reverse transcription (Invitrogen). Real-Time PCR was performed using SYBR Green PCR Master Mix (Qiagen, Hilden, Germany) on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The primers used in the experiments were as follows. Ccr5: 5′-AGATCTCTGCAGCTGCCCTCA-3′ (forward) and 5′-GGAGCACCTGCTGCTGGTGTAG-3′ (reverse); Il23a: 5′-CCAGCAGCTCTCTCG GAATC-3′ (forward) and 5′-TCATA GTCCCGCTGGTGC-3′ (reverse); Il12b: 5′-CCTGGTTTGCCATCGTTTTG-3′ (forward) and 5′-TCAGAGTCTCGCCTCCTTTGTG-3′ (reverse); Actb: 5′-CATTACTGCTCTGGCTCCTACC-3′ (forward) and 5′-GACTCATCGTACTCCTGCTTGC-3′ (reverse). All PCRs were performed in triplicate and normalized to internal control Actb mRNA. Relative expression was presented using the 2−△△CT method.
Measurement of cytokines, chemokines, and myeloperoxidase
Cytokines and chemokines present in total kidney homogenates and cell culture supernatants were measured using a Cytometric Bead Array kit (BD Biosciences, San Jose, CA, USA) or by ELISA (eBioscience), respectively, according to the manufacturers' protocols.
Preparation of kidney cells
Kidneys were perfused, minced, and placed in DMEM (Life Technologies, Carlsbad, CA, USA) containing 1 mg/ml collagenase IA and 100 ng/ml RNAse I (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30 min. Digested kidney tissues were passed through a 40-μm cell strainer (BD Falcon, San Diego, CA, USA), and the cell suspension obtained was centrifuged at 300 × g for 10 min. Cells were washed in PBS containing 2% BSA, suspended in 36% Percoll (Amersham Pharmacia Biotech, Little Chalfont, UK), and gently overlaid onto 72% Percoll. After centrifugation at 900 × g for 30 min at RT, cells were retrieved from the Percoll interface and washed twice in DMEM and once with staining buffer (PBS containing 2% BSA and 0.1% sodium azide).
Flow cytometry
The following FITC-, PE-, PE-Cy5, PerCP- or allophycocyanin-conjugated mAbs to mouse proteins were purchased from BD Biosciences or eBioscience and used for cell staining: CD45, CD11b, Ly6G, CD3, NK1.1, CD11c, CCR5, IL-23p19, GM-CSF, TCRβ chain, and Rat IgG2a κ chain. Prepared cells were blocked with 2.4G2 mAb in a staining buffer at 4°C for 20 min. Before washing twice with a staining buffer, cells were incubated with the relevant Ab at 4°C for 30 min. For intracellular cytokine staining, after staining of surface markers, cells were fixed and permeabilized using Cytofix/Cytoperm and Perm/wash buffer (BD Biosciences), followed by staining with mAb to mouse GM-CSF and IL-23p19 (eBioscience). Flow cytometric analysis was performed using a FACS Canto II (BD Biosciences) cytometer and data were analyzed using FACS Diva software (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR, USA).
Analysis of renal function
To determine kidney function, concentrations of creatinine and blood urea nitrogen (BUN) in sera were measured colorimetrically using the Quantichrom Urea Assay and the Quantichrom Creatine Assay kits (Bioassay Systems, Hayward, CA, USA).
Culture of bone marrow-derived DCs (BMDCs)
Bone marrow cells were collected from femurs and tibias, suspended in RPMI-1640 medium supplemented with 10% FBS, penicillin/streptomycin (100 U/ml), 2 mM L-glutamine (GIBCO), 50 μM 2-mercaptoethanol and Flt3L (150 ng/ml), were distributed into 6-well plates at a density of 1×106 cells/well for 7 days. At day 7, GM-CSF (10 ng/ml) was added, and cells were harvested at day 9. Cells was stimulated with GM-CSF (10 ng/ml) and HK Candida albicans hyphae (MOI=10).
Statistical analysis
All data were analyzed using GraphPad Prism Software version 4 (GraphPad Software, Inc., San Diego, CA, USA). Unpaired Student t-test or 1- or 2-way ANOVA with post hoc analysis were used to compare differences between the groups. The log-rank test and the Mann-Whitney U test were used to analyze survival curves and fungal counts, respectively. Error bars represent the SEM of the mean. A p-value below 0.05 was considered statistically significant.
RESULTS
Increased expression of CCR5 and its ligands in the kidney during C. albicans infection
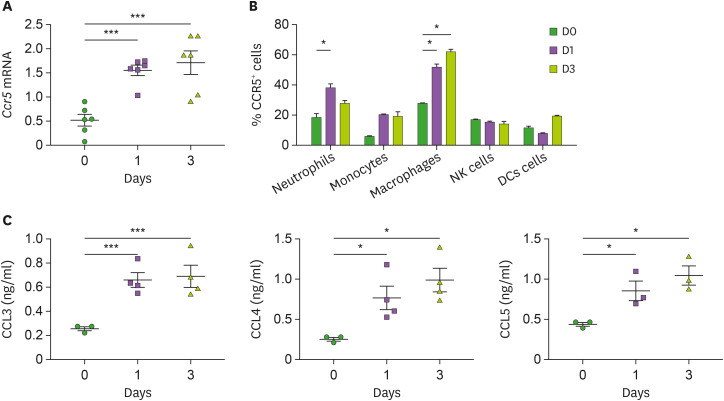
To investigate the role of CCR5 and its ligands in systemic C. albicans infection, we first investigated changes in their expression in the kidney with candidiasis. Expression of Ccr5 transcripts was significantly increased at days 1 and 3 after infection (Fig. 1A). FACS analysis showed that a higher percent of CCR5-expressing neutrophils was infiltrated into the kidney at day 1 after infection than before infection (Fig. 1B). The percentages of CCR5+ macrophages were significantly greater at either day 1 or day 3 after infection (Fig. 1B). In a similar context, production of CCR5 ligands, CCL3, CCL4, and CCL5, by infected kidneys was higher at days 1 and 3 after infection (Fig. 1C).
Figure 1. Upregulation of CCR5 and its ligands in the kidney during systemic candidiasis. C57BL/6 mice were intravenously injected with 3×105 CFUs of C. albicans and the kidneys were harvested at the indicated time points. (A) Levels of Ccr5 mRNAs in the kidneys were determined by RT-PCR (n=6 kidneys/group). (B) Percentages of CCR5-expressing kidney cells were determined using FACS at the indicated time points (n=3–5 kidneys/group). (C) Levels of CCR5 ligands (CCL3, CCL4, and CCL5) were measured from kidney lysates using ELISA (n=3–4 kidneys/group). Results are representative of 2-3 experiments and are presented as the mean±SEM. One-way ANOVA test was used.
*p<0.05; ***p<0.001.
Ccr5−/− mice are susceptible to systemic C. albicans infection
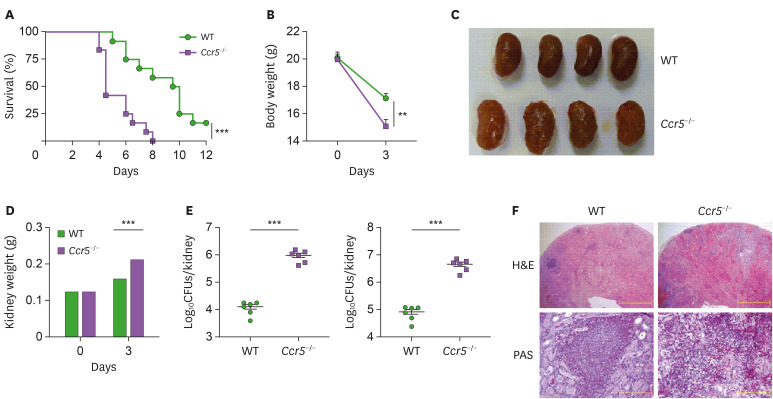
To define the role of CCR5 during systemic candidiasis, Ccr5−/− mice were challenged with a lethal dose of C. albicans and monitored for survival. Ccr5−/− mice succumbed to C. albicans infection more rapidly than wild-type (WT) mice (Fig. 2A). Ccr5−/− mice also experienced more severe body weight loss (Fig. 2B), and their kidneys appeared less pink, more swollen (Fig. 2C), and were significantly heavier than WT kidneys at day 3 post-infection (Fig. 2D). Gross observations also indicated that Ccr5−/− kidneys had many more distinguishable nodules than WT kidneys (Fig. 2C), indicating a more rapid proliferation of fungi and extensive abscess formation. Indeed, Ccr5−/− mice had a significantly increased fungal burden in the kidney at day 3 post-infection compared with WT mice (Fig. 2E). Histopathological analysis showed that Ccr5−/− kidneys had numerous multifocal areas of abscess formation as compared to WT kidneys (Fig. 2F). In particular, PAS staining revealed more prominent hyphae within abscesses of Ccr5−/− kidneys compared with those of WT mice (Fig. 2F).
Figure 2. CCR5−/− mice are susceptible to systemic candidiasis. WT and Ccr5−/− mice were intravenously injected with 3×105 CFUs of C. albicans. (A) Survival curves (n=12 mice/group). The log-rank test was used. (B) Changes in body weight (n=4 or 5 mice/group). Unpaired Student t-test was used. (C) Gross morphology of kidneys at day 3 post-infection. (D) Changes in kidney weight (n=8 or 10 kidneys/group). Two-way ANOVA test was used. (E) Fungal burdens at day 3 post-infection were presented per kidney or gram basis (n=6 kidneys/group). The Mann-Whitney U test was used. (F) Histopathologic findings: H&E staining (upper panels); PAS staining (lower panels). Results are representative of 2–4 experiments and are presented as the mean±SEM.
**p<0.01; ***p<0.001.
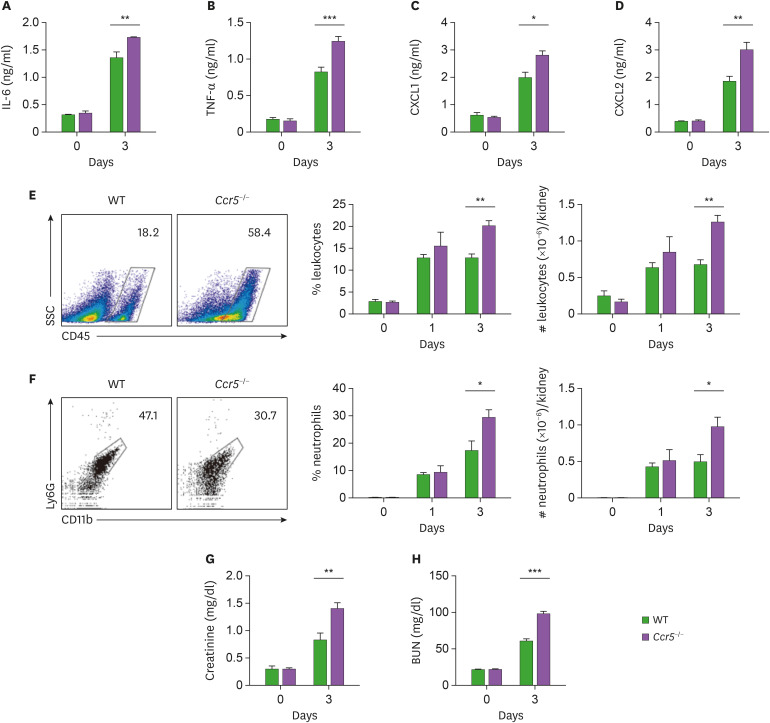
The data presented in Fig. 2 suggests that impaired suppression of fungal proliferation might result in uncontrolled inflammatory responses and subsequent tissue damage in Ccr5−/− mice. To test this hypothesis, we looked into the severity of renal inflammation. As expected, we detected higher levels of inflammatory cytokines and chemokines (IL-6, TNF-α, CXCL1, and CXCL2) in Ccr5−/− kidneys at day 3 post-infection than in those of WT mice (Fig. 3A-D). Consistent with these results, there was greater infiltration of CD45+ leukocytes, predominantly CD11b+Ly6G+ neutrophils, into infected Ccr5−/− kidneys (Fig. 3E and F). In addition, higher levels of serum creatinine and BUN indicated that CCR5−/− mice had more severe impairment of kidney function at day 3 post-infection (Fig. 3G and H). Taken together, our results suggest that uncontrolled fungal proliferation in Ccr5−/− mice might cause severe renal inflammation linked to fatal immunopathology.
Figure 3. Ccr5−/− mice have increased inflammatory responses to systemic C. albicans infection. WT and Ccr5−/− mice were intravenously injected with 3×105 CFUs of C. albicans. (A-D) Levels of IL-6 (A), TNF-α (B), CXCL1 (C), and CXCL2 (D) in kidney lysates at day 3 post-infection (n=4 or 6 kidneys/group). (E, F) Kidney cells were isolated, and stained with anti-CD45, anti-CD11b, and anti-Ly6G Abs. (E) Representative FACS plots for CD45+ leukocytes and their percentages and absolute numbers were presented (n=4 or 6 kidneys/group). (F) CD45+ leukocytes were gated and FACS plots for CD11b+ and Ly6G+ neutrophils and their percentages in leukocytes were presented (n=4 or 6 kidneys/group). (G, H) Serum creatinine and BUN levels (n=3 or 4 mice/group). Two-way ANOVA test was used. Results are representative of 2–4 experiments and are presented as the mean±SEM.
*p<0.05; **p<0.01; ***p<0.001.
Impaired recruitment of NK cells to the kidney is linked to the susceptibility of Ccr5−/− mice to C. albicans infection
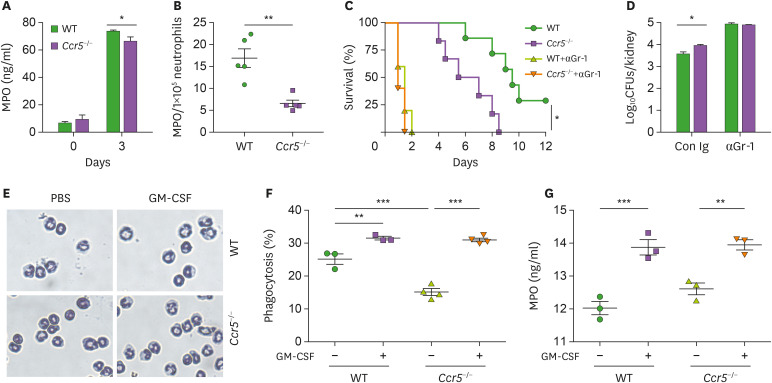
The increased presence of renal inflammation of Ccr5−/− mice after C. albicans infections seemed to suggest that although neutrophils had impaired function for fungal clearance, they induced severe immunopathology, presumably as a result of their increased presence in the kidney. Indeed, kidney neutrophils from Ccr5−/− mice had decreased levels of myeloperoxidase (MPO), a major constituent of azurophil granules necessary for generation of ROS, a key component of the neutrophils' killing arsenal (13,18) (Fig. 4A). Taking their numbers into account, the concentration of MPO per cell was approximately 2-fold lower compared to WT kidney neutrophils (Fig. 4B). Depletion of neutrophils using anti-Gr-1 Ab completely abrogated differences in survival and fungal clearance between Ccr5−/− and WT mice (Fig. 4C and D), confirming that neutrophils were important effector cells for fungal clearance. As anti-Gr-1 Ab depletes other types of Gr-1-expressing cells, however, we cannot exclude their contribution to fungal clearance. We next investigated the possibility that Ccr5−/− neutrophils have intrinsic functional defects which might cause the susceptible phenotype of Ccr5−/− mice to C. ablicans infection. Either WT or Ccr5−/− neutrophils isolated from the bone marrow had a similar premature neutrophil's nuclear morphology (Fig. 4E). Treatment with GM-CSF induced nuclear segmentation of either type of neutrophils to a similar extent (Fig. 4E). Consistent with this result, there was no difference in their phagocytic activity and MPO production after treatment with GM-CSF (Fig. 4F and G). However, it seems that Ccr5−/− bone marrow neutrophils have a lower phagocytic capacity in steady state (Fig. 4F).
Figure 4. Impaired recruitment of NK cells to the kidney results in characteristic phenotypic changes of Ccr5−/− mice after C. albicans infection. (A, B) Levels of MPO in kidney lysates obtained at day 3 post-infection. Data are presented as a concentration per kidney or normalized to 1×105 renal neutrophils (n=4 or 5 kidneys/group). (C, D) WT mice were administered anti-Gr-1 mAb (200 μg/mouse) or control IgG Ab 2 days before C. albicans infection. (C) Survival curves (n=5–7 mice/group). (D) Fungal CFUs at day 3 post-infection (n=4 or 5 mice/group). (E-G) Bone marrow neutrophils were isolated from WT and Ccr5−/− mice and were treated with GM-CSF for 4 h. (E) Diff-Quik staining. (F) Phaogcytosis assay. (G) Measurement of MPO (n=3 kidneys/group). Results are representative of 2–4 experiments and are presented as the mean±SEM.
*p<0.05; **p<0.01; ***p<0.001.
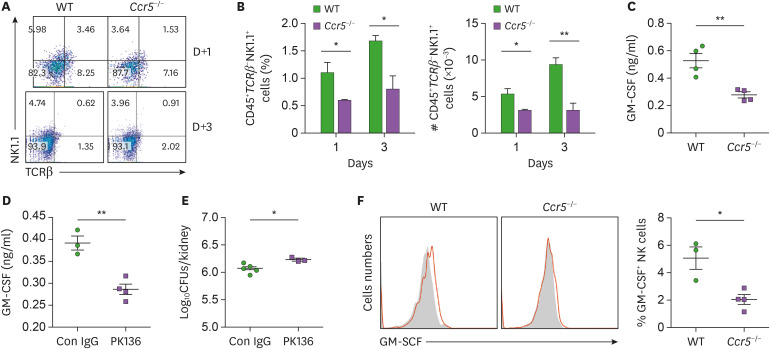
As NK cells have been shown to be indispensable for neutrophil activation (12,13), we hypothesized that CCR5 mediates recruitment of NK cells to the kidney during C. albicans infection. In accordance to our hypothesis, a significantly lower number of NK cells were present in Ccr5−/− kidneys at days 1 and 3 post-infection (Fig. 5A and B). Consistent with previous studies showing that GM-CSF is produced by NK cells for neutrophil activation (12,13), there were decreased levels of GM-CSF in CCR5−/− kidneys at day 3 post-infection (Fig. 5C). We further showed that depletion of NK cells in WT mice reduced levels of renal GM-CSF (Fig. 5D) and fungal clearance to some extent (Fig. 5E), confirming that NK cell numbers are associated with levels of GM-CSF and fungal clearance. Unexpectedly, individual kidney NK cells produced less GM-CSF in CCR5−/− mice (Fig. 5F), indicating that signaling upstream of NK cells may be defective in CCR5−/− mice during C. albicans infection.
Figure 5. Impaired recruitment of NK cells and their GM-CSF production in Ccr5−/− mice. (A, B) Renal cells were stained with anti-CD45, anti-TCRβ, and anti-NK1.1Abs. (A) CD45+ leukocytes were gated and analyzed for expression of NK1.1 and TCRβ. Representative FACS dot plots and percentages of CD45+TCRβ-NK1.1+ NK cells in leukocytes were presented. (B) Absolute numbers of CD45+TCRβ-NK1.1+ NK cells (n=4 or 6 kidneys/group). (C) Levels of GM-CSF in kidney lysates at day 3 post-infection (n=4 mice/group). (D, E) Mice were depleted of NK cells using anti-NK1.1 Ab (PK136). Kindeys were harvested at day 3 post-infection. (D) Levels of renal GM-CSF (n=3 or 4 kidneys/group). (E) Fungal CFUs (n=4 or 5 kidneys/group). (F) Intracellular staining was performed in kidney CD45+CD3-NK1.1+ NK cells at day 3 post-infection. Shown are FACS histograms and percentages of GM-CSF+ cells (n=3 or 4 mice/group). Results are representative of 2–4 experiments and are presented as the mean±SEM.
*p<0.05; **p<0.01.
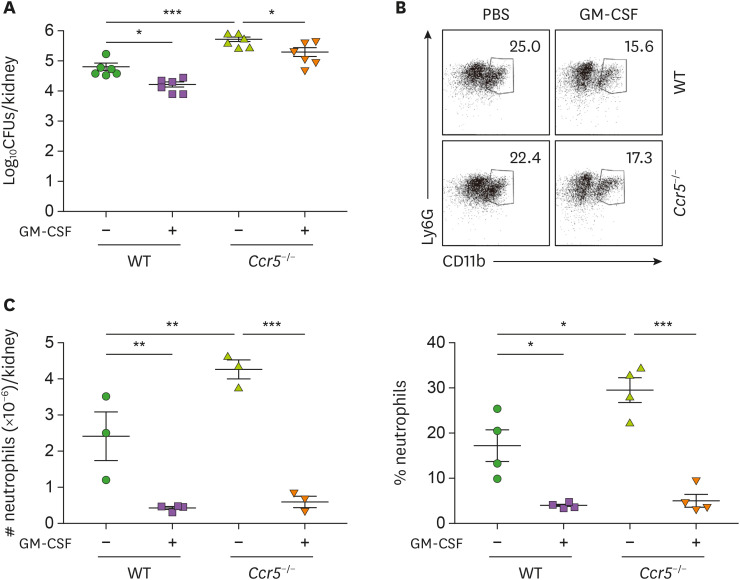
We next examined whether GM-CSF can displace NK cells in activating neutrophils and promoting their subsequent fungal clearance. Indeed, injection of GM-CSF significantly recovered the anti-fungal immunity of Ccr5−/− mice (Fig. 6A) but in our hands, exogenous GM-CSF didn't reduce fungal clearance to the extent observed in WT mice. Concomitantly, infiltrating neutrophil numbers were significantly reduced by injection of GM-CSF in Ccr5−/− mice (Fig. 6B and C). This result seems to support our previous observations that neutrophil activation is directly associated with effective fungal clearance and less severe renal inflammation. In sum, our data demonstrate that impaired recruitment of NK cells to the kidney in Ccr5−/− mice leads to defects in fungal clearance by neutrophils.
Figure 6. GM-CSF augments fungal clearance but reduces renal inflammation. Mice were intraperitoneally injected with GM-CSF (5 μg/mouse) 24 h and 36 h after infection. (A) Fungal CFUs were counted in kidneys at day 3 post-infection (n=6 kidneys/group). (B) Representative FACS dot plots for CD45+CD11b+Ly6G+ renal neutrophils and their percentages in leukocytes were presented (n=4 or 5). (C) Absolute numbers of renal neutrophils (n=3 or 4 kidneys/group). Results are representative of 3 experiments and are presented as the mean±SEM.
*p<0.05; **p<0.01; ***p<0.001.
DCs and NK cells reciprocally regulate production of IL-23 and GM-CSF during systemic C. albicans infection
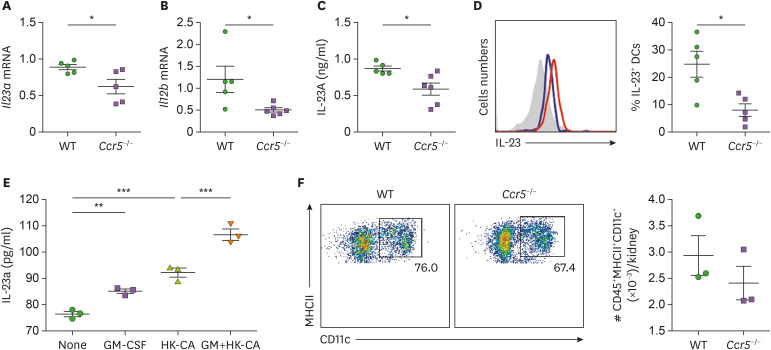
We next investigated why individual NK cells of Ccr5−/− mice have reduced levels of GM-CSF during systemic C. albicans infection. Ccr5−/− mice had decreased expression of IL-23 at the mRNA and protein levels in 3-day post-infection kidneys (Fig. 7A-C). We noticed that expression of Il12b was reduced in Ccr5−/− mouse kidneys to larger extent than that of Il23a (Fig. 7A and B), suggesting that this common subunit may be a better control target for inflammation regulation. Intracellular levels of IL-23 in CD11c+MHCII+ kidney DCs were significantly lower in Ccr5−/− mice at day 3 post-infection compared to WT mice (Fig. 7D). To verify that GM-CSF can affect C. albicans-induced production of IL-23 by DCs, we stimulated BMDCs with combination of GM-CSF and HK C. albicans hyphae. Combined treatment had a synergistic effect on IL-23 production compared to GM-CSF or HK C. albicans alone (Fig. 7E). There was no difference in renal DC numbers between naïve WT and Ccr5−/− mice (Fig. 7F). Thus, GM-CSF can increase its own production indirectly through DC stimulation of IL-23 production during systemic C. albicans infection.
Figure 7. GM-CSF enhances IL-23 production by DCs. (A-D) Kideys were harvested at day 3 post-infection and their expression of IL-23 was investigated. (A, B) Transcriptional levels of Il23a and Il12b (n=4 or 5 kidneys/group). (C) Concentrations of renal IL-23 protein (n=5 or 6 kidneys/group). (D) FACS hitograms for IL-23A intracellular staining in renal CD45+MHCII+CD11c+ DCs and percentages of IL-23+ cells. Red line: WT; blue line: CCR5−/− (n=5 kidneys/group). (E) BMDCs were cultured with combination of GM-CSF and hyphae forms of heat-kille (HK) C. albicans for 24 h. Concentrations of IL-23 in culture supernantants were determined using ELISA (n=3/group). (F) The percentage of CD45+MHCII+CD11c+ DCs in naïve mice (n=3 kidneys/group). Results are representative of 2–3 experiments and are presented as the mean±SEM.
*p<0.05; **p<0.01; ***p<0.001.
DISCUSSION
Innate immune cells play critical roles in host defense mechanisms against invasive candidiasis (18). Previous studies have reported chemotactic roles for CCR2 and CXCR2 in recruiting inflammatory monocytes and neutrophils, respectively, to infection sites during candidiasis (14,19), but little has been known regarding the chemokine/chemokine receptor systems involved in chemotaxis of other types of innate immune cells. In this study, we first showed that CCR5-mediated recruitment of NK cells to the kidney is important for innate resistance to systemic C. albicans infection. Although the percent of CCR5-expressing renal NK cells was lower compared to neutrophils and macrophages (Fig. 1B), CCR5 was highly specific for recruiting NK cells to the kidney (Fig. 5A and B). Secondly, our results support the previous findings that NK cells maintain a central position in an innate defense line established early after Candida infection (11,12,13,14). Finally, we demonstrated that there is a positive feedback loop between NK cells and DCs that amplifies fungal clearance by neutrophils. If this defense mechanism fails, uncontrolled fungal proliferation may result in uncontrolled renal inflammation and a life-threatening renal immunopathology (Fig. 7).
NK cells are a particularly important regulator for acute renal inflammation, regardless of its causes. During ischemia-reperfusion kidney injury, TECs that undergo hypoxic damage initiate acute inflammation by recruiting NK cells through secretion of CCR5 ligands (16). NK cells, in turn, stimulate TECs to produce CXCR2 ligands to recruit neutrophils (21). During systemic candidiasis, however, tissue-resident macrophages seem to rapidly respond to invading C. albicans and release CCR5 ligands to recruit NK cells (our unpublished data). Thus, NK cells contribute to acute renal inflammation by 2 mechanisms: recruitment of neutrophils (in ischemia-reperfusion kidney injury and candidiasis) and enhancement of their effector functions (in candidiasis). As effector cells, however, NK cells can kill damaged TECs during ischemia-reperfusion kidney injury (20,21). Interestingly, human NK cells recognize β-1,3-glucan, a component of the fungal cell wall, through the activating receptor NKp30 (22,23). This recognition triggers signaling that sequentially leads to degranulation of NK cells, delivers effector molecules, such as perforin, to the fungal wall, and finally kills C. albicans. This process requires phagocytosis of a pathogen (24). Therefore, defects in the fungicidal activity of NK cells in Ccr5−/− mice could contribute to their susceptibility to systemic C. albicans infection.
In the current study, our results suggest that the higher mortality rate of Ccr5−/− mice may be caused primarily by early neutrophil-mediated immunopathology, which occurs as a result of uncontrollable fungal proliferation. However, there is a second wave of neutrophil recruitment to the kidney in WT mice, whose peak time is around day 9 after infection (25). In this case, CCR1 but not CXCR2 is responsible for recruitment of neutrophils, whose main functions are to amplify late renal immunopathology without affecting fungal clearance (25) Thus, this phenotypic dichotomy of neutrophils, based on chemokine receptors, reflects their functional differences during invasive candidiasis.
Mutations in Cx3cr1 and Cxcr1 genes are associated with the susceptibility of humans to Candida infections (26,27,28). Loss of function mutations in these genes is related to neutrophil degranulation and survival of macrophages and monocytes, respectively. Considering that these defects commonly cause impaired fungal clearance, screening of human cohorts for Ccr5 gene mutations may reveal a relation between this gene and invasive candidiasis in humans.
GM-CSF is a pleiotropic cytokine produced by a variety of cell types, including lymphoid cells such as NK cells, Th17 cells, invariant NKT cells, type 2 innate lymphoid cells (ILC2s), and ILC3s (12,13,14,29,30,31,32,33). IL-1, IL-2, and IL-23 trigger GM-CSF production in Th17 cells, whereas IL-23 and IL-15 do so in NK cells. GM-CSF targets granulocytes, monocytes, macrophages, DCs, and glial cells, promoting tissue inflammation and host defense. During systemic C. albicans infection, GM-CSF secreted by NK cells is critical for fungal clearance for neutrophils (11,12,13) but additional targets are not known. Our observation showing that impairment of NK cell recruitment in CCR5−/− mice decreased production of IL-23 (Fig. 7) indicates that there is a mutual regulation between IL-23 and GM-CSF. Indeed, we showed that GM-CSF and C. albicans synergistically promote production of IL-23 by DCs (Fig. 7E). Although a mechanism underlying the crosstalk between PRRs and GM-CSF in DCs has yet to be explored, it is likely that GM-CSF enforces or co-stimulates PRR signaling. Thus, GM-CSF may be an important amplification point in the axis of resistance comprised of DCs, inflammatory monocytes, and neutrophils in the kidney and this resistance mechanism seems to override immunopathology incurred by GM-CSF-activated neutrophils, contributing to early survival. However, as mentioned previously, persistent fungal proliferation can induce later mortality in the survived host by recruitment of neutrophils that cause fatal renal immunopathology. Considering the diversity of GM-CSF source and its target, the immune network involving GM-CSF may be more complex than what has been known so far.
Overall, our results indicate that GM-CSF is a cytokine that plays a central role in activation of neutrophils to increase their phagocytic activity (Fig. 8). Nonetheless, injection of GM-CSF in Ccr5−/− mice did not induce fungal clearance as effectively as in WT mice (Fig. 6A). This observation suggests that there may be an impairment in GM-CSF target cells other than NK cells and neutrophils in Ccr5−/− mice. Another possibility is that CCR5 may be involved in chemotaxis of non-NK cells that contribute to fungal clearance. Further studies will be needed to clarify this aspect of CCR5 function during systemic C. albicans infection.
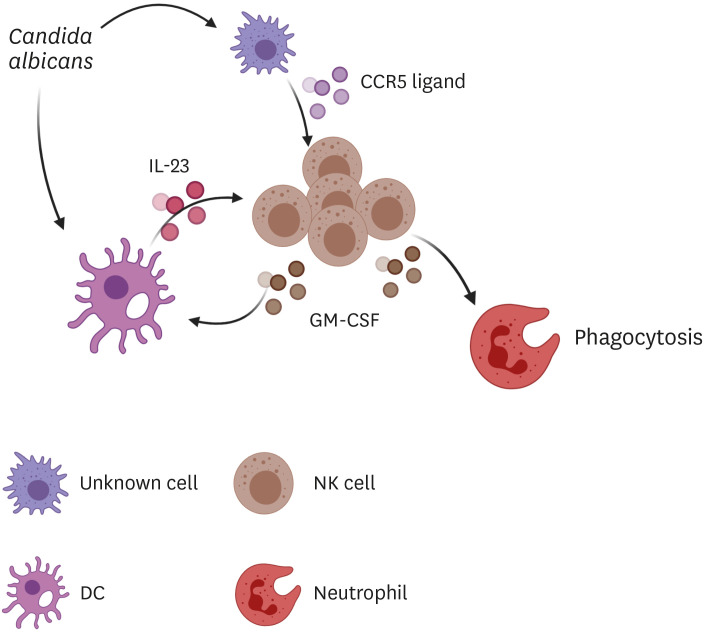
Figure 8. A schematic diagram showing CCR5 function in host defense against systemic C. albicans infection. CCR5 ligands are released by unidentified cells in response to C. albicans infection and induce recruitment of NK cells into the kidney. On the other hand, C. albicans triggers secretion of DC IL-23, which in turn stimulates NK cell to produce GM-CSF. Release of GM-CSF is amplified by a positive feedback regulation of IL-23 production by DCs. GM-CSF increases the phagocytic activity of neutrophils.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (NRF-2020M33A9D303789111 and NRF-2019R1A105584412).
Abbreviations
- BMDC
bone marrow-derived dendritic cell
- BUN
blood urea nitrogen
- CCR
C-C chemokine receptor
- CFU
colony-forming unit
- DC
dendritic cell
- HK
heat-killed
- ILC
innate lymphoid cell
- MOI
multiplicity of infection
- MPO
myeloperoxidase
- PAS
periodic acid-Schiff
- RPMI
Rosewell Park Memorial Institute
- TEC
tubular epithelial cell
- WT
wild-type
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Nguyen NZN, Tran VG, Kwon B.
- Data curation: Tran VG, Kang SW, Kim J, Kwon B.
- Formal analysis: Nguyen NZN, Tran VG, Kwon B.
- Funding acquisition: Cho HR, Kwon B.
- Investigation: Nguyen NZN, Tran VG, Lee S, Kim M, Kang SW, Kim J, Kim HJ.
- Project administration: Kwon B.
- Supervision: Cho HR, Kwon B.
- Validation: Lee JS, Kwon B.
- Writing - original draft: Nguyen NZN, Tran VG, Kwon B.
- Writing - review & editing: Nguyen NZN, Kwon B.
References
- 1.Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 2.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 3.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15:630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis MS. Genetic susceptibility to fungal infections in humans. Curr Fungal Infect Rep. 2012;6:11–22. doi: 10.1007/s12281-011-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006) Crit Care Med. 2009;37:1612–1618. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 6.Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun. 2011;3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geijtenbeek TB, Gringhuis SI. C-type lectin receptors in the control of T helper cell differentiation. Nat Rev Immunol. 2016;16:433–448. doi: 10.1038/nri.2016.55. [DOI] [PubMed] [Google Scholar]
- 8.Brown GD, Willment JA, Whitehead L. C-type lectins in immunity and homeostasis. Nat Rev Immunol. 2018;18:374–389. doi: 10.1038/s41577-018-0004-8. [DOI] [PubMed] [Google Scholar]
- 9.Legrand F, Lecuit M, Dupont B, Bellaton E, Huerre M, Rohrlich PS, Lortholary O. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis. 2008;46:696–702. doi: 10.1086/527390. [DOI] [PubMed] [Google Scholar]
- 10.Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Müller M, Kuchler K. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012;8:e1002811. doi: 10.1371/journal.ppat.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bär E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–127. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Whiteney PG, Bär E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog. 2014;10:e1004276. doi: 10.1371/journal.ppat.1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domínguez-Andrés J, Feo-Lucas L, Minguito de la Escalera M, González L, López-Bravo M, Ardavín C. Inflammatory Ly6Chigh monocytes protect against candidiasis through IL-15-driven NK cell/neutrophil activation. Immunity. 2017;46:1059–1072.e4. doi: 10.1016/j.immuni.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis. 2014;209:109–119. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Lee JS, Kim A, Koo S, Cha HJ, Han JA, Do Y, Kim KM, Kwon BS, Mittler RS, et al. TLR2 signaling in tubular epithelial cells regulates NK cell recruitment in kidney ischemia-reperfusion injury. J Immunol. 2013;191:2657–2664. doi: 10.4049/jimmunol.1300358. [DOI] [PubMed] [Google Scholar]
- 16.Khan IA, Thomas SY, Moretto MM, Lee FS, Islam SA, Combe C, Schwartzman JD, Luster AD. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2006;2:e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 18.Lionakis MS, Levitz SM. Host control of fungal infections: lessen from basic studies and human cohorts. Annu Rev Immunol. 2018;36:157–191. doi: 10.1146/annurev-immunol-042617-053318. [DOI] [PubMed] [Google Scholar]
- 19.Le HT, Tran VG, Kim W, Kim J, Cho HR, Kwon B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J Immunol. 2012;189:287–295. doi: 10.4049/jimmunol.1103564. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, Lee SC, Kwon BS, Mittler RS, Cho HR, et al. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proc Natl Acad Sci U S A. 2012;109:E13–E22. doi: 10.1073/pnas.1112256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang ZX, Shek K, Wang S, Huang X, Lau A, Yin Z, Sun H, Liu W, Garcia B, Rittling S, et al. Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury. J Immunol. 2010;185:967–973. doi: 10.4049/jimmunol.0903245. [DOI] [PubMed] [Google Scholar]
- 22.Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, Xiang RF, Oykhman P, Huston SM, Islam A, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe. 2013;14:387–397. doi: 10.1016/j.chom.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Li SS, Ogbomo H, Mansour MK, Xiang RF, Szabo L, Munro F, Mukherjee P, Mariuzza RA, Amrein M, Vyas JM, et al. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat Commun. 2018;9:751. doi: 10.1038/s41467-018-03014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voigt J, Hünniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, Löffler J, Kurzai O. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis. 2014;209:616–626. doi: 10.1093/infdis/jit574. [DOI] [PubMed] [Google Scholar]
- 25.Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog. 2012;8:e1002865. doi: 10.1371/journal.ppat.1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013;123:5035–5051. doi: 10.1172/JCI71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swamydas M, Gao JL, Break TJ, Johnson MD, Jaeger M, Rodriguez CA, Lim JK, Green NM, Collar AL, Fischer BG, et al. CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci Transl Med. 2016;8:322ra10. doi: 10.1126/scitranslmed.aac7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swamydas M, O'Hayre M, Sajib MS, Hoffman KW, Singh SP, Mourad A, Johnson MD, Ferre EM, Farber JM, Lim JK, et al. The homozygous CXCR1-M280 mutation impairs human monocyte survival. JCI Insight. 2018;3:e95417. doi: 10.1172/jci.insight.95417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 30.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dougan M, Dranoff G, Dougan SK. IL-2, and IL-5 family of cytokines: regulators of inflammation. Immunity. 2019;50:796–811. doi: 10.1016/j.immuni.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson C, Thornton EE, McKenzie B, Schaupp AL, Huskens N, Griseri T, West N, Tung S, Seddon BP, Uhlig HH, et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife. 2016;5:e10066. doi: 10.7554/eLife.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirota K, Hashimoto M, Ito Y, Matsuura M, Ito H, Tanaka M, Watanabe H, Kondoh G, Tanaka A, Yasuda K, et al. Autoimmune Th17 cells induced synovial stromal and innate lymphoid cell secretion of the cytokine GM-CSF to initiate and augment autoimmune arthritis. Immunity. 2018;48:1220–1232.e5. doi: 10.1016/j.immuni.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]