Highlights
-
•
This systematic review identified 8 randomized clinical trials investigating the effect of blood flow restriction (BFR) in patients with knee pain.
-
•
Resistance exercise with BFR did not show clinical benefits compared to resistance exercise.
-
•
Further high-quality studies are needed to clearly determine the clinical effectiveness of BFR in pain intensity and knee function in these patients.
Keywords: Anterior knee pain, Blood flow restriction, Knee, Osteoarthritis, Systematic review
Abstract
Background
Blood flow restriction (BFR) is an effective clinical intervention used to increase strength in healthy individuals. However, its effects on pain and function in individuals with knee pain are unknown.
Objective
To determine the effectiveness of adding BFR to resistance exercise for pain relief and improvement of function in patients with knee pain.
Methods
Systematic review with meta-analysis of randomized clinical trials. Medline, Central, Embase, PEDro, Lilacs, CINAHL, SPORTDiscus, and Web of Science databases were searched from inception to May 2019. Randomized clinical trials that compared resistance exercise with or without BFR to treat knee pain and function in individuals older than 18 years of age with knee pain were included.
Results
Eight randomized clinical trials met the eligibility criteria and for the quantitative synthesis, five studies were included. The pooled standardized mean difference (SMD) estimate showed that resistance exercises with BFR was not more effective than resistance exercises for reducing pain (SMD: −0.37 cm, 95% CI = −0.93, 0.19) and improving knee function (SMD = −0.23 points, 95% CI = −0.71, 0.26) in patients with knee pain.
Conclusion
In the short term, there is low quality of evidence that resistance exercise with BFR does not provide significant differences in pain relief and knee function compared to resistance exercises in patients with knee pain.
PROSPERO registration number: CRD42018102839.
Introduction
The prevalence of knee pain has increased exponentially in the last 20 years.1 It is estimated that approximately 50% of elderly people2, 3 and 19% to 31% of adolescents have knee pain.4, 5 The presentation of knee pain in 62% of cases has been reported to be in the tibiofemoral joint, while in 23% of cases the pain location is in the patellofemoral joint.6 This is consistent with most common diagnoses being knee osteoarthritis (OA) and patellofemoral pain (PFP).7
Knee pain is associated with high disability and loss of function as well as poor quality of life in the long term.4, 7, 8 These symptoms and activity limitations have been associated with unmodifiable risk factors, such as age and female sex, in addition to modifiable factors, such as obesity, low levels of physical activity, joint overloading, muscular imbalance in the hip joint, and reduced muscle mass and strength in the lower limbs.9, 10, 11 Weaknesses in the quadriceps, hip abductors, and hip external rotators have been found to be associated with knee pain.12, 13 Therefore, physical therapy programs focusing on hip and knee strengthening exercises with the use of orthosis or manual therapy, has proved to be effective in reducing symptoms in the short- and medium-term in individuals with knee OA and PFP.14, 15, 16, 17 Despite the favorable results, therapeutic exercise is limited by tolerance to load and variability in symptoms presented by patients with knee OA.18 Therefore, therapeutic exercise strategies must be adapted to each patient's clinical condition.15
Blood flow restriction (BFR) is a therapy which consists of applying continuous compression to the proximal portion of the limb using a pressure cuff with the aim to induce tissue hypoxia by reducing blood flow during exercise.19, 20 The external pressure applied to the proximal portion of the upper or lower limbs must be sufficiently low to maintain partial arterial flow in the muscle, but sufficiently high to occlude venous return from the muscle.21 BFR has proved to be a safe and effective training method for increasing muscle strength and mass.22, 23 The advantage of BFR is that loads close to 30% of maximum effort produce similar results to those achieved with loads equal to or greater than 80% of maximum effort.22, 24 The use of lower loads reduces joint loads and increases tolerance to exercise,19 and for this reason, BFR could be an alternative to high-load training in patients with musculoskeletal pain.19, 25 The use of BFR in patients with knee pain would appear to be a useful clinical approach, however it is not clear whether it produces clinical benefits for knee pain.16
A recent systematic review showed that the use of BFR with low load exercises produced a moderate effect in increasing muscle strength in people with musculoskeletal dysfunctions25; however, the effectiveness of adding BFR to resistance exercises, particularly in patients with knee pain, remains unclear. The aim of this systematic review with meta-analysis was to compare the effectiveness of resistance exercise alone versus resistance exercise with BFR for pain relief and functional improvement in patients with knee pain.
Methods
Protocol and registration
This systematic review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement and followed the recommendations of the Cochrane Collaboration Handbook.26, 27 The registration number in the International Prospective Register of Systematic Review (PROSPERO) is CRD42018102839.
Eligibility criteria
Studies were considered eligible based on the following inclusion criteria: (1) Population: participants were over 18 years of age and had knee pain (e.g. anterior knee pain, osteoarthritis, patellofemoral pain, post knee arthroscopy surgery); (2) Type of intervention: exercises incorporating restriction of blood flow or vascular occlusion in the lower limb; (3) Type of comparison: resistance exercise or other conventional exercise programs with short term intervention (i.e. treatment duration between two weeks to three months); (4) Type of outcome: pain intensity and knee function as primary or secondary outcomes; (5) Type of study: randomized clinical trial (RCT) or controlled clinical trial (CCT) published in English or Spanish. The exclusion criteria were: (1) studies including participants with experimental knee pain; (2) studies including participants with a history of acute trauma or previous fracture of the lower limb.
Electronic search
We systematically searched MEDLINE (via PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, the Physiotherapy Evidence Database (PEDro), the Latin American and the Caribbean Literature in Health Sciences (LILACS), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), SPORTDiscus, and Web of Science databases from inception until May 2019.
The search strategy used included a combination of the following Medical Subjects Headings (MeSH) terms: ‘Patellofemoral Pain Syndrome’; ‘Osteoarthritis knee’; with free-text terms: ‘Knee pain’; ‘Anterior knee pain’; ‘Blood flow restriction training’; ‘Kaatsu’; ‘Occlusion training’. To identify randomized trials in Medline, Central, and Embase databases, the Cochrane Highly Sensitive Search Strategies was used.27 We also manually searched the references of selected articles to identify additional potentially relevant studies. The literature search was independently conducted by three reviewers (ICV, FAQ, and HGE), we involved a fourth reviewer if a consensus could not be reached (ALS). The full search strategy for Medline is reported in the Supplemental Material.
Study selection
Three reviewers (ICV, FAQ, and HGE) independently screened the titles and abstracts retrieved from the searches. We obtained the full text of the articles that any of the 3 reviewers considered to be potentially relevant. We involved a fourth reviewer (ALS) if a consensus could not be reached.
Data collection process
The three same reviewers used a standardized form to independently extract the following data from each trial (i) author and year of publication; (ii) country; (iii) sample characteristics (sample size, age distribution, and sex); (iv) characteristics of BFR exercise; (v) characteristics of resistance exercise; (vi) length of follow-up and main outcomes; (vii) main results. To calculate the effect size from each study, we extracted means and standard deviation (i.e. final values) from each study. For three-arm trials, we chose as the control group the group that used the most similar exercise regimen to the BFR group.
Risk of bias of individual studies
The assessment of the risk of bias of individual studies was performed as recommended by the Cochrane Collaboration Handbook.27 This tool evaluates the risk of bias according to seven domains: generation of the random sequence; concealment of the randomization sequence; blinding of participants and treatments; blinding of the evaluation of the results; incomplete results data; selective reporting of results, and other biases. We consider as other bias: co-interventions or difference in the protocol intervention, contaminated participants in the studies (drugs), instruments that are not sensitive to measure the outcome measures. Each domain was considered as either low risk of bias, unclear risk of bias, or high risk of bias. Data extraction and quality assessment were independently performed by three reviewers (ICV, HGE, and ALS). We involved a fourth reviewer if a consensus could not be reached (FAQ). The assessment of risk of bias was performed by visual inspection in summary graph and the percentage calculated RevMan 5.3 program. The agreement rate between reviewers was calculated using kappa statistics.
Statistical methods
The random-effects models by DerSimonian and Laird were used.28 Pooled estimates of mean difference (MD) or standardized mean difference (SMD) were computed depending on the scales used in the included studies for the outcome measures. MD was used as a summary statistic in meta-analysis if outcome measurements in all studies had the same scale, and SMD was used as a summary statistic in meta-analysis if the included studies assessed the same outcome measured using different scales.27 We reported 95% confidence intervals (CI) for all pooled estimates. The heterogeneity of results across the studies was evaluated using the I2 statistic, interpreted as: might not be important (0%–40%), may represent moderate (30%–60%), may represent substantial (50%–90%), and considerable (75%–100%) heterogeneity.27 Meta-analyses were performed with the RevMan 5.3 program. Publication bias was planned to be evaluated through visual inspection of funnel plots, as well as using the method proposed by Sterne et al.29 The synthesis and quality of evidence for each outcome was performed with an adapted version of GRADE.30, 31 The GRADE system consists of five items: (1) study limitations (risk of bias); (2) inconsistency of results (heterogeneity); (3) indirectness of evidence; (4) imprecision of the effect estimates, and (5) reporting bias. The quality of the evidence was classified in four categories: high, moderate, low, and very low.32 This approach entails the downgrading of evidence from high to moderate to low and very low quality based on certain criteria. Downgrading the evidence one level: (1) for study limitation if the majority of studies (>50%) was rated as high risk of bias; (2) for inconsistency, if heterogeneity was greater than the accepted low level (I2 > 40%); (3) for indirectness, if the BFR session does not correspond to what is used in clinical practice; (4) for imprecision, if meta-analysis had small sample size (n < 300). The assessments were made by two reviewers (ICV and FAQ) with disagreements discussed as needed, if disagreement persisted, a third reviewer (HGE) arbitrated.
Results
Study selection
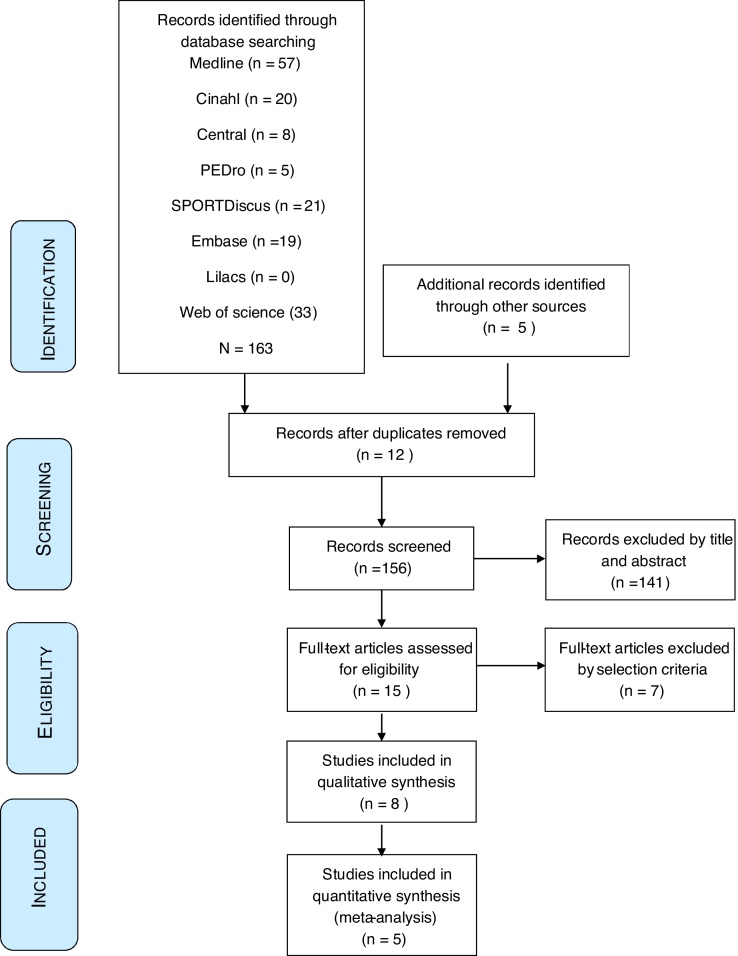
A total of 163 studies were found through electronic search and five through manual search. Eight studies met the eligibility criteria and were included in the systematic review.33, 34, 35, 36, 37, 38, 39, 40 The detailed steps of the article selection process for this systematic review are shown in the flow diagram in Fig. 1 and the excluded studies are shown in Supplemental Material. The kappa agreement rate between reviewers was 0.9.
Figure 1.
Flow chart diagram.
Study characteristics
Table 1 summarizes the characteristics of the included studies. The overall population was 340 patients (160 received an intervention with resistance exercise with BRF and 180 with resistance exercises without BRF), of which 140 were men and 200 women. The mean age was 49.5 years and only one study presented a 6-month follow-up.35 The studies comprised participants with the following diagnoses: five studies included patients with knee OA,33, 34, 37, 38, 39 one with PFP,35 one with anterior knee pain,40 and one included patients who had undergone knee arthroscopy.36
Table 1.
Characteristics of studies included in the systematic review and meta-analyses of the effects of adding blood flow restriction to resistance exercises in patients with knee pain.
| Author/Year | Patient characteristics | Intervention | Follow-up/results measurements | Results difference between groups |
|---|---|---|---|---|
| Ferraz et al. (2018)33 | Women diagnosed with knee OA, Randomized by a computer-generated code n: 48 patients Groups: 3 Age: 50–65 years CG: HI-RT n: 16 Age: 59.9 ± 4 CG: LI-RT n: 16 Age: 60.7 ± 4 EG: BFR n: 16 Age: 60.3 ± 4 |
All groups trained 2 days a week for 12 weeks. The first 4 weeks with 4 sets of 15 repetitions with 1 minute rest between sets; from the 5th week, the work was increased from 4 to 5 sets of 15 repetitions CG: “HI-RT” Work with high loads (80% of 1 – MR) CG: “LI–RT” Work with low load (30% of 1 – MR) EG: “LI-RT + BFR” Work with low load (30% of 1 – MR) plus partial restriction of blood flow. The pressure was personalized using 70% of total LOP, with a cuff 175 mm wide by 920 mm long (Pulse monitored by auscultation). |
No follow-up reported. Pain intensity: - WOMAC Function: - WOMAC - time up and go (TUG), - time stand test (TST). - SF-36 Muscle strength: - leg press - knee extension |
- WOMAC pain ↓ in LI-RT – 45% (p = 0.001) and BFR – 39% (p = 0.02) in comparison with HI - RT which ↓ 31% (p = 0.19) - WOMAC physical function ↑ in HI-RT – 42% (p = 0.002) and BFR – 49% (p = 0.019) in comparison with LI-RT which ↑ 42% (p = 0.09) - TST (p = 0.05). - TUG (p = 0.05). - SF-36 (p = 0.05). - Leg press HI-RT ↑ compared with LI-RT (p = 0.0001). BFR ↑ compared with LI-RT (p = 0.0004). - Knee extension HI-RT ↑ compared with LI-RT (p = 0.0004). BFR ↑ compared with LI-RT (p = 0.0005). HI-RT and BFR (p = 0.05). |
| Bryk (2016)34 | Women diagnosed with knee OA, grade 2 and 3 on the Kellgren and Lawrence scale. Randomization method not described. n: 34 Patients Groups: 2 Age: 53–69 CG: Conventional n: 17 Age: 60.4 ± 6.7 EG: BFR n: 17 Age: 62.3 ± 7 |
Both groups had 18 training sessions, spread over 6 weeks with 3 sessions per week. CG: “conventional” Stretching and strengthening of lower limb, with 70% of 1 MR quadriceps exercises (maximum extension from 90° flexion). EG: “BFR” Stretching and strengthening, but quadriceps strengthening exercise with knee extension was done with 30% of 1 MR combined with BFR (200 mmHg pressure), cuff characteristics not specified. |
No follow-up reported. Pain intensity: -NPRS Pain during exercise: - NPRS Function: - Lequesne functional scale - TUG Muscle strength: - Quadriceps (knee extension from 90° to 0°). |
- NPRS ↓ in EG 0.8 mm compared to CG (p = 0.001). - NPRS ↓ in EG 3.7 during exercise compared to CG (p = 0.01). - Lequesne ↓ in CG 1.0 compared to EG (p = 0.001) - TUG↓ in CG 0.4 s compared to EG (p = 0.006). - Quadriceps strength ↑ 7.4 kg in EG compared to CG (p = 0.001). |
| Giles et al. (2017)35 | Women and men, 18–40 years with patellofemoral pain Divided into 2 groups by simple randomization. n: 79 Patients Groups: 2 Age: 18–40 CG: standard n: 39 Age: 26.7 ± 5.5 EG: BFR n: 40 Age: 28.5 ± 5.2 |
The intervention in both groups lasted 8 weeks. They did 5 min “light” bicycling for a warm-up. Leg press from 0° to 60° flexion and knee extension from 90° to 45°. CG: “Standard” 7–10 repetitions with 70% of 1-MR and placebo BFR. EG: “BFR” Exercises at 30% of 1-MR and a set of 30 repetitions, followed by 3 sets of 15 repetitions and 30 s rest with the cuff in place. The pressure was personalized using 60% of the total LOP until loss of pulse (doppler ecography), cuff characteristics not specified. |
Follow-up for 6 months post study intervention Pain intensity: - VAS (0–100 mm) Function: - Kujala Patellofemoral Score (KPS) Muscle strength: - Torque in knee extension |
Pain intensity: - EG ↓ VAS “worse pain” compared to CG 6.0 mm (p = 0.237) - EG ↓ VAS by 12.1 mm in everyday activities compared to CG (p = 0.022) Function: - EG ↑ 2.4 points. Kujala patellofemoral score compared to CG (p = 0.308) Muscle strength: - EG ↑ torque of knee extension compared to CG 13 Nm (p = 0.073) |
| Tennent et al. (2017)36 | Post knee arthroscopy, aged between 18 and 65 years, authorized by their surgeon to perform physical activity. Randomization by computer. n: 17 patients Groups: 2 Age: 18–65 EG: BFR n: 10 Age: 37.0 ± 16.2 CG: standard n: 7 Age: 37.0 ± 15.0 |
Intervention commenced 2 weeks postsurgery, for 12 sessions over 6 weeks, supervised by a physiotherapist. EG: “BFR” 3 exercises performed with BFR with 30% of 1 MR and 30 s rest between sets with occlusion: leg press, leg extension, and reverse press. The occlusion pressure was personalized using 80% of the total LOP (doppler ecography), cuff characteristics not specified. CG: “standard” Exercises performed with 30% of 1 MR and 30 s rest between sets: leg press, leg extension, and reverse press. |
No follow-up reported. Pain intensity: - KOOS Function: - KOOS - walking velocity: SSWV - 4 square step test: FSST - sit to stand 5 times: STS5 - timed stair ascent: TSA Muscle strength: - knee extension and flexion with dynamometry |
Pain intensity: - KOOS pain scale (p = 0.1420) Function: - KOOS symptoms (p = 0.1711) - KOOS ADL (p = 0.2029) - KOOS QOL (p = 0.4612) - KOOS sports (p = 0.4054) - SSWV (p = 0.8073) - FSSt (p = 0.6256) - STS5 (p = 0.6256) Muscle strength: - stair climb ↓ EG 0.78 compared to CG (p = 0.0281). - strength in flexion and extension knee ↑ in EG compared to CG (p = 0.09). |
| Segal et al. (2015)37 | Women aged over 45 years with risk of symptomatic knee OA Randomization by computer n = 45 patients Groups = 2 EG: BFR n = 21 Age: 56.1 ± 5.9 CG: Control n = 24 Age: 54.6 ± 6.9 |
Both groups performed resistance training at 30% of 1-MR 3 times per week for 4 weeks EG: Using instrumented leg press with the BFR cuff in position. Occlusion pressure incremented by 140–200 mmHg week by week, with a cuff 65 mm wide by 650 mm long. CG: Using instrumented leg press without the BFR cuff in position. |
No follow-up reported. Pain intensity: - KOOS Function: - not evaluated Muscle strength: - power: leg press - stepped leg press - scaled isokinetic knee extensor torque - stair-climb power (W) |
Pain intensity: - KOOS pain scale (p = 0.9574) Muscle strength: - power on leg press (p = 0.6173) - strength in stepped leg press ↑ 0.2 kg/kg mass in EG compared to CG (p = 0.0385) - isokinetic strength of knee extensor ↑ 0.12 Nm/kg in EG compared to CG (p = 0.0048) - stair-climb power (p = 0.1520) |
| Segal et al. (2015)38 | Men with risk of symptomatic knee OA Randomized by computer, divided into 2 groups. n: 42 Patients Groups: 2 Age: 45–90 EG: BFR n: 20 Age: 58.4 ± 8.7 CG: Control group n: 22 Age: 56.1 ± 7.7 |
Both groups completed 4 sets of bilateral leg press at 30% of 1-MR 3 times per week for 4 weeks EG: “BFR” 3 exercises were performed under BFR with 30% of 1 MR. Occlusion pressure increased by 140–200 mmHg week by week, with a cuff 65 mm wide by 650 mm long. CG: “Control group” Exercises with 30% of 1 MR. |
No follow-up reported. Pain intensity: - KOOS Function: - not evaluated Muscle strength: - isometric leg press - knee extension on isokinetic machine |
Pain intensity: - knee pain KOOS (p = 0.254) Muscle strength: - isometric leg press (p = 0.322) - strength in knee extension ↑ 6.3 on isokinetic machine in CG (p = 0.066) |
| Korakakis et al. (2018)40 | Men diagnosed with anterior knee pain. Randomized by computer, Web Random page n: 40 Patients Groups: 2 EG: BFR n: 20 Age: 29 ± 6.6 CG: LLRT n: 20 Age: 29.7 ± 7.6 |
The two groups had different intervention times. The training protocol was knee extension with 90° flexion to total extension with maximum load 5 kg, personalized to the pain indicated on the NPRS 0–10. EG: training in 4 sets where the 1st set was the maximum number of repetitions followed by 3 sets of 15 repetitions, with 30 s rest between series. BFR 80% of LOP calculated with doppler, with a cuff 100 mm wide by 1160 mm long. Training lasted 3.2 ± 2.8 months. CG: training 4 sets where the first set was the maximum number of repetitions followed by 3 sets of 15 repetitions, with 30 s rest between sets. Training without BFR. Training lasted 4.3 ± 3.3 months. |
No follow-up reported. Pain intensity: - SLSs: pain on executing the movement measured by NPRS 0–10 - SLSd: pain on executing the movement measured by NPRS 0–10 - SDT: pain on executing the movement measured by NPRS 0–10 Function: - not evaluated Muscle strength: - not evaluated |
Pain intensity: - significant ↓ in pain in EG for SLSs immediately after training (p = <0.001). - significant ↓ in pain in EG for SLSd immediately after training (p = <0.001). - significant ↓ in pain in EG for SDT immediately after training (p = <0.001). - significant ↓ in pain in EG for SLS immediately after physical session (p = <0.001). - significant ↓ in pain in EG for SDT immediately after physical session (p = <0.001). - no significant differences were found between groups immediately after physical session in almost all the variables. - ↓ in pain in SLSd in EG compared to CG immediately after training (p = 0.052). - ↓ in pain in SLSs in EG compared to CG immediately after training (p = 0.374). - ↓ in pain in SDT for EG compared to CG immediately after training (p = 0.281). - ↓ in pain in SLSs for EG compared to CG after physical session (p = 0.428). - ↓ in pain in SLSd for EG compared to CG after physical session (p = 0.674). - ↓ in pain in SDT for EG compared to CG after physical session (p = 0.316). |
| Harper et al. (2019)39 | Elderly men and women with OA. RM: Randomization not specified. n: 35 Patients Groups: 2 EG: BFR n: 16 Age: 67.2 ± 5.2 CG: MIRT n: 19 Age: 69.1 ± 7.1 |
Both groups underwent the same training for 12 weeks with frequency 3 days per week. Both groups performed repetitions to failure. EG: Leg press, leg extension, leg curl, and calf flexion, all at 20% of 1-MR. BFR was personalized through an equation, LOP and cuff characteristics not specified. CG: Leg press, leg extension, leg curl, and calf flexion, all at 60% of 1-MR. No BFR |
No follow-up reported. Pain intensity: - WOMAC Function: - SPPB - Walking velocity (m/s) Function: LLFDI (0–100 pts). Muscle strength: - knee extension torque in Nm. |
Pain intensity: - ↓ 0.24 points on the WOMAC for CG compared to EG. Function: - ↓ in SPPB score of 0.66 for EG compared to CG. - ↑ in walking velocity of 0.01 m/s for CG compared to EG. - ↓ in LLFDI score of 0.79 for EG compared to CG. Muscle strength: - ↑ torque strength by 1.87 Nm for CG compared to EG. |
OA, osteoarthritis; EG, experimental group; CG, control group; SD, standard deviation; LOP, limb occlusion pressure; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; KOOS, Knee Injury and Osteoarthritis Outcome Score; MR, maximum repetition; TUG, time up and go; TST, time stand test; BFR, blood flow restriction; LI-RT, low-intensity resistance training; HI-RT, high-intensity resistance training; SF, Short Form Health Survey; NPRS, Numerical Pain Rating Scale; SSWV, self-selected walking velocity; FSST, 4 square step test; TSA, timed stair ascent; ADL, activities of daily living; QOL, quality of life; PCS, physical component score; MCS, mental component score; SLSs, shallow single leg squat; SLSd, deep single leg squat; SDT, 20 cm step down.
The duration of the interventions varied. There were two studies with protocols lasting four weeks37, 38 and two studies with protocols that lasted six weeks,34, 36 while the longer studies lasted eight35 and twelve weeks.33, 39, 40 For all 8 studies, BFR was applied at the proximal thigh region using a pneumatic cuff with a digital pressure manometer, while the patient performed isotonic exercises at 20%39 and 30% of 1 maximum repetition.33, 34, 35, 36, 37, 38 Only one study used a non-personalized load, which was determined arbitrarily.40 Four studies reported the width of the cuffs used: 17.5 cm,33 11.6 cm,40 and 6.5 cm37, 38; the other investigations did not describe this characteristic of the occlusion instrument.34, 35, 36, 39 Following the occlusion protocols used, four studies determined the total limb occlusion pressure (LOP) using an echo-doppler,33, 35, 36, 40 and then conducted training at 60%,35 70%,33 and 80%36, 40 of the total occlusion pressure. Only one study calculated the LOP indirectly with an equation.39 Three studies used a non-personalized LOP: two used a protocol with increments from 140 mmHg to 200 mmHg,37, 38 and only one study used a fixed pressure of 200 mmHg.34 All the interventions in the control groups were based on resistance exercises, which included protocols of moderate (60% of 1 maximum repetition)39 to high loads (70–80% of 1 maximum repetition),33, 34, 35 as well as low loads (30% of 1 maximum repetition),33, 36, 37, 38 except Korakakis et al.,40 which used an external weight of 5 kg.40 Only one study used stretching as a co-adjuvant modality, in both the control and experimental groups.34
Knee pain intensity was assessed using the numerical pain rating scale (NPRS)34 and visual analog scale (VAS)35, 39; three studies used the item related with the Knee Injury and Osteoarthritis Outcome Score (KOOS) pain variable,36, 37, 38 and two used the Western Ontario and McMaster Universities Arthritis Index (WOMAC) variable.33, 39 One study used NPRS to quantify the pain during three movements standing on one leg.40 The studies used five different questionnaires for self-reported assessment of knee function: WOMAC,33 Lequesne Index,34 Kujala Patellofemoral Score,35 Late Life Function and Disability Instrument (LLFDI),39 and KOOS.36, 37, 38 One study used the Short Physical Performance Battery (SPPB) to assess function of the lower limb by a third party.39
Risk of bias within studies
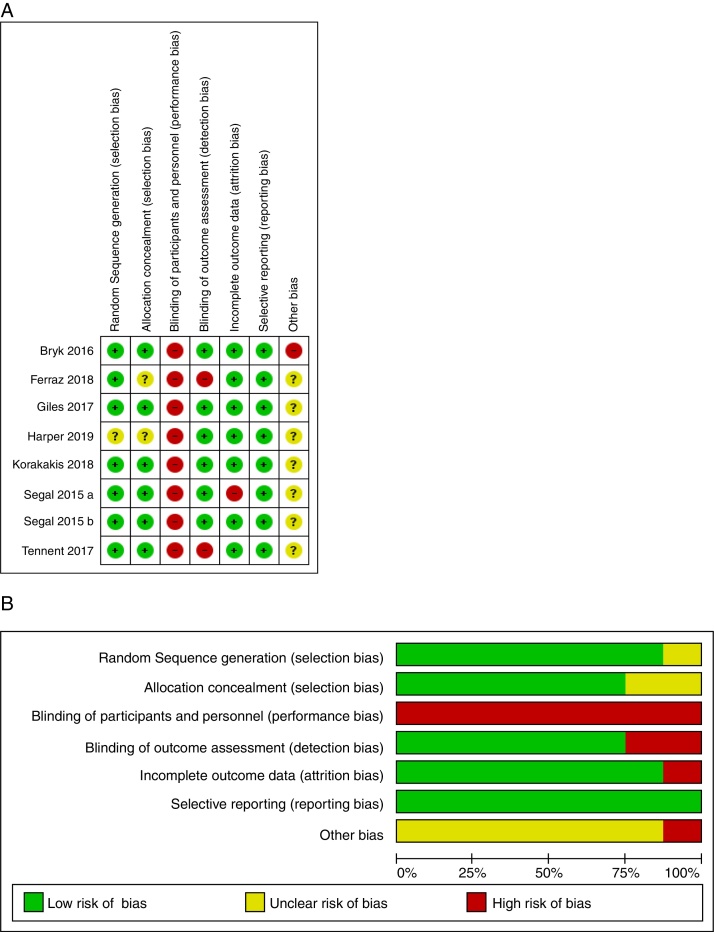
As evaluated by the Cochrane Collaborations tool for assessing the risk of bias for all clinical trials, 75.2% of the studies showed a low risk of bias,34, 35, 38, 40 and 24.8% showed a medium risk of bias.33, 36, 37, 39 When studies were analyzed by individual domains, the random sequence generation was suitable in 87.5% of the studies. Adequate allocation concealment was observed as low risk of bias in 75% of the studies and unclear in 25%. Outcome assessors were blinded in 75% of the studies, while selective reported data were observed in 100% of the studies as low risk of bias. Regarding other sources of bias, only one study34 (12.5%) was considered as “high risk of bias” because in the application of the exercise protocol a co-intervention, stretching exercises, was added to the intervention group. The risk of bias assessment is shown in Fig. 2.
Figure 2.
Summary of risk assessment of bias of included articles (A). Risk of bias graph presented as a percentage of all items included (B).
Synthesis of results
Primary outcome
Pain
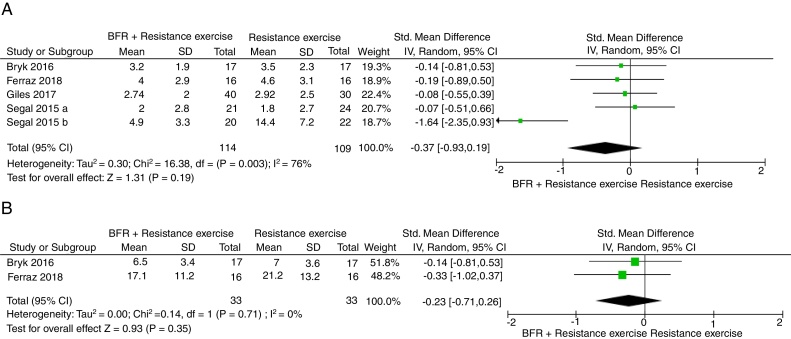
Five studies included data to conduct a meta-analysis for pain using the VAS, NPRS, and knee pain subscale from KOOS.33, 34, 35, 37, 38 The pooled SMD estimate showed that resistance exercise with BFR was not more effective than resistance exercise in reducing pain at the end of the intervention (SMD = −0.37, 95% CI = −0.93, 0.19, Fig. 3A), with results having considerable heterogeneity (I2 = 76%). The overall quality of evidence according to GRADE was rated as low (Table 2).
Figure 3.
Comparison of adding blood flow restriction to resistance exercises versus standard resistance exercises for pain intensity at six weeks (A). Comparison of adding blood flow restriction to resistance exercises versus standard resistance exercises for knee function at six weeks (B).
Table 2.
Summary of findings and quality of evidence (GRADE) for resistance exercise with or without blood flow restriction (BFR).
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Outcome | Number of patients BFR with resistance exercise | Number of patients with resistance exercise | Effect (absolute risk 95% CI) | Quality of evidence (GRADE) | Importance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | RCT | Not serious | Serious | Not serious | Serious | Pain intensity | 114 | 109 | SMD −0.37 (−0.93, 0.19) | ⊕⊕aLow | Critical |
| 2 | RCT | Serious | Not serious | Not serious | Serious | Knee function | 33 | 33 | SMD -0.23 (-0.71, 0.26) | ⊕⊕bLow | Important |
CI, confidence interval; RCT, randomized clinical trial; SMD, standard mean difference.
Quality of evidence:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
Reason for downgrade: I2 greater than 40% (serious inconsistency) and sample size lower than 300 (serious imprecision).
Reason for downgrade: majority of trials rated as high risk of bias (serious risk of bias) and sample size lower than 300 (serious imprecision).
Secondary outcome
Knee function
Two studies included data to conduct meta-analyses for knee function.33, 34 The pooled SMD estimate showed that resistance exercises with BFR was not more effective than resistance exercises in improving function at the end of treatment (SMD = −0.23, 95% CI = −0.71, 0.26, Fig. 3B), with results having no important heterogeneity (I2 = 0%). The overall quality of evidence according to GRADE was rated as low (Table 2).
Discussion
The aim of this systematic review with meta-analysis was to determine the effect of adding BFR to resistance exercise for pain relief and functional improvement in patients with knee pain. The results showed that, there is low quality of evidence that resistance exercise with BFR does not provide significant differences in pain relief and knee function compared to resistance exercises in patients with knee pain.
The lack of added effect of BFR in this systematic review could be explained by the heterogeneity in the occlusion protocols. The increase in protein synthesis and satellite cells following BFR training may depend on cuff width as well as the occlusion pressure.19, 41 Considerable heterogeneity was observed in the cuff width used as reported in four of the studies,33, 37, 38, 40 with the other four studies not describing the characteristics of the occlusion devices.34, 35, 36, 39 It has been shown that cuff width determines the need for higher or lower pressure to generate BFR42; thus, the use of standard, non-personalized pressures without calculating the LOP may favor the occurrence of secondary effects (especially pain, paresthesia, or swelling) or limit the clinical results by generating a sub-threshold therapeutic restriction (<40% LOP).41, 43 Some evidence suggests that the use of wide cuffs, using a lower pressure is needed to achieve the necessary occlusion, meaning less discomfort and greater safety.44, 45, 46 However, the width of the cuff does not seem to affect the degree of BFR when relative pressure is used.47
The LOP is calculated objectively using an echo-doppler; thus, clinical application is instrument-dependent.48 Only three of the included studies used an echo-doppler to calculate the LOP and determine the relative work pressure.33, 35, 36 It has now been shown that the LOP calculation is position-dependent, and that it should be calculated with the individual in the training position, since this affects the degree of BFR.49, 50 Bryk et al.34 did not describe the position adopted for the LOP evaluation, while Ferraz et al.33 described carrying out the evaluation in the supine position while the subsequent training was performed in a seated position; finally, Tennet et al.36 described performing the evaluation and the training in the sitting position. The LOP calculation studies used relative pressures ranging from 60% to 80%. It should be noted that the clinical results in hypertrophy and strength have been shown to be better with relative pressures close to 80%.51 It is possible that the studies which did not use relative pressures for BFR exercises may not have achieved the degree of occlusion necessary to produce any effect, especially the studies of Segal et al.37, 38 in which a relatively low pressure was applied for the width of the cuff used.
The studies included in this review present limitations in the phenotyping of the participants, preventing more detailed subgroup analysis as suggested in the recommendations of the IMMPACT group.52 Regarding phenotyping, it has been observed that certain groups of patients have a lower response to pain treatments, especially those with psychosocial problems, more complex symptoms, central sensitization, or dysfunction of the endogenous analgesic system.52, 53, 54, 55 From this perspective, it has been observed that exercise-induced hypoalgesia correlates strongly with normal conditioned pain modulation, thus, lowering the pain threshold under the pressure applied after a cold stimulus may be a predictor of a good response to treatment with physical exercise.56, 57 However, other studies have shown that healthy subjects with normal conditioned pain modulation present a reduction of hypoalgesia induced by exercise in the occluded limb58 and situations in which the same exercise causes pain.59 This is a practical limitation of BFR in the rehabilitation of pain, since the probabilities of feeling fatigue and pain are quite high.60, 61
Knee pain rehabilitation is multimodal,7, 15, 16 and strengthening of the hip musculature is an essential part of the exercise program14, 62, 63; however, none of the articles included in this review applied specific exercises for these muscle groups. It may be added that none of the control groups used a multimodal program for comparison as the evidence recommends.7, 15, 16 Three studies used comparisons with sub-threshold intensity (30% of 1 maximum repetition),36, 37, 38 which does not comply with the BFR comparison used in the literature41, 43, 46; the external validity of these studies is therefore limited. All clinical trials used an arbitrary, pre-established number of repetitions, or repetitions to failure, which may lead to increase pain due to overloading, or limitation of the results due to sub-threshold training. In terms of function, improvements were observed in muscular strength.25, 64 However it has been noted that in knee OA a gain of more than 30% in the strength of the knee extensors is necessary to result in changes in function and disability,65 which may explain our results.
The limitations of our study are as follows: (1) Although we searched in eight databases and included manual searches for references, we may have missed relevant articles; (2) There was a high degree of clinical and statistical heterogeneity between the studies included. Potential sources of heterogeneity include variations in the type and dose of the interventions used and in the form of the results measured; (3) Methodological limitations, such as an insufficient sample size, which may lead to the overestimation of the results of the interventions studied; (4) Due to the limited number of studies included, it was not possible to evaluate publication bias; (5) In the planning stages, we intended to conduct analyses of subgroups based on age, sex, and type of clinical condition; however, the results of the stratified analysis in the individual trials were not available.
Conclusion
This systematic review and meta-analysis demonstrates that in the short term, there is low quality of evidence that resistance exercise with BFR does not provide significant differences in pain relief and knee function compared to resistance exercises in patients with knee pain. Further research is needed to further clarify the clinical effectiveness of BFR exercise in these patients.
Funding
The authors did not receive any financial support for the research, authorship, and/or publication of this article.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Nguyen U.U.-S., Zhang Y.Y., Zhu Y. Increasing prevalence of knee pain and symptomatic knee osteoarthritis. Ann Intern Med. 2011;155(11):725–732. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jinks C., Jordan K., Ong B.N., Croft P. A brief screening tool for knee pain in primary care (KNEST). 2. Results from a survey in the general population aged 50 and over. Rheumatology. 2004;43(1):55–61. doi: 10.1093/rheumatology/keg438. [DOI] [PubMed] [Google Scholar]
- 3.Kim I.J., Kim H.A., Seo Y.-I. Prevalence of knee pain and its influence on quality of life and physical function in the Korean elderly population: a community based cross-sectional study. J Korean Med Sci. 2011;26(9):1140–1146. doi: 10.3346/jkms.2011.26.9.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masiero S., Carraro E., Sarto D., Bonaldo L., Ferraro C. Healthcare service use in adolescents with non-specific musculoskeletal pain. Acta Paediatr Int J Paediatr. 2010;99(8):1224–1228. doi: 10.1111/j.1651-2227.2010.01770.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith B.E., Selfe J., Thacker D. Incidence and prevalence of patellofemoral pain: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0190892. doi: 10.1371/journal.pone.0190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrokhi S., Chen Y.-F., Piva S.R., Fitzgerald G.K., Jeong J.-H., Kwoh C.K. The influence of knee pain location on symptoms, functional status, and knee-related quality of life in older adults with chronic knee pain: data from the osteoarthritis initiative. Clin J Pain. 2016;32(6):463–470. doi: 10.1097/AJP.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crossley K.M., Stefanik J.J., Selfe J. Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016;50(14):839–843. doi: 10.1136/bjsports-2016-096384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshpande B.R., Katz J.N., Solomon D.H. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 2016;68(12):1743–1750. doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheon Y.-H., Kim H.-O., Suh Y.S. Relationship between decreased lower extremity muscle mass and knee pain severity in both the general population and patients with knee osteoarthritis: findings from the KNHANES V 1–2. PLoS One. 2017;12(3):e0173036. doi: 10.1371/journal.pone.0173036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhdorfer A., Wirth W., Eckstein F. Association of knee pain with a reduction in thigh muscle strength – a cross-sectional analysis including 4553 osteoarthritis initiative participants. Osteoarthr Cartil. 2017;25(5):658–666. doi: 10.1016/j.joca.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass N.A., Torner J.C., Frey Law L.A. The relationship between quadriceps muscle weakness and worsening of knee pain in the MOST cohort: a 5-year longitudinal study. Osteoarthr Cartil. 2013;21(9):1154–1159. doi: 10.1016/j.joca.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnoff J.T., Hall M.M., Kyle K., Krause D.A., Lai J., Smith J. Hip strength and knee pain in high school runners: a prospective study. PM&R. 2011;3(9):792–801. doi: 10.1016/j.pmrj.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Long-Rossi F., Salsich G.B. Pain and hip lateral rotator muscle strength contribute to functional status in females with patellofemoral pain. Physiother Res Int. 2010;15(1):57–64. doi: 10.1002/pri.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lack S., Barton C., Sohan O., Crossley K., Morrissey D. Proximal muscle rehabilitation is effective for patellofemoral pain: a systematic review with meta-analysis. Br J Sports Med. 2015;49(21):1365–1376. doi: 10.1136/bjsports-2015-094723. [DOI] [PubMed] [Google Scholar]
- 15.Fransen M., McConnell S., Harmer A.R., Van der Esch M., Simic M., Bennell K.L. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–1557. doi: 10.1136/bjsports-2015-095424. [DOI] [PubMed] [Google Scholar]
- 16.Collins N.J., Barton C.J., van Middelkoop M. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br J Sports Med. 2018;52(18):1170–1178. doi: 10.1136/bjsports-2018-099397. [DOI] [PubMed] [Google Scholar]
- 17.Nascimento L.R., Teixeira-Salmela L.F., Souza R.B., Resende R.A. Hip and knee strengthening is more effective than knee strengthening alone for reducing pain and improving activity in individuals with patellofemoral pain: a systematic review with meta-analysis. J Orthop Sport Phys Ther. 2018;48(1):19–31. doi: 10.2519/jospt.2018.7365. [DOI] [PubMed] [Google Scholar]
- 18.Parry E., Ogollah R., Peat G. Significant pain variability in persons with, or at high risk of, knee osteoarthritis: preliminary investigation based on secondary analysis of cohort data. BMC Musculoskelet Disord. 2017;18(1):80. doi: 10.1186/s12891-017-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert B.S., Hedt C., Moreno M., Harris J.D., McCulloch P. Blood flow restriction therapy for stimulating skeletal muscle growth. Tech Orthop. 2018;33(2):89–97. [Google Scholar]
- 20.Loenneke J.P., Kim D., Fahs C.A. Effects of exercise with and without different degrees of BFR on torque and muscle activation. Muscle Nerve. 2015:1–32. doi: 10.1002/mus.24448. [DOI] [PubMed] [Google Scholar]
- 21.Loenneke J.P., Thiebaud R.S., Fahs C.A., Rossow L.M., Abe T., Bemben M.G. Effect of cuff type on arterial occlusion. Clin Physiol Funct Imag. 2013;33(4):325–327. doi: 10.1111/cpf.12035. [DOI] [PubMed] [Google Scholar]
- 22.Brandner C.R., May A.K., Clarkson M.J., Warmington S.A. Reported side-effects and safety considerations for the use of blood flow restriction during exercise in practice and research. Tech Orthop. 2018;33(2):114–121. [Google Scholar]
- 23.Slysz J., Stultz J., Burr J.F. The efficacy of blood flow restricted exercise: a systematic review & meta-analysis. J Sci Med Sport. 2016;19(8):669–675. doi: 10.1016/j.jsams.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Loenneke J.P., Wilson J.M., Marín P.J., Zourdos M.C., Bemben M.G. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849–1859. doi: 10.1007/s00421-011-2167-x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes L., Paton B., Rosenblatt B., Gissane C., Patterson S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51(13):1003–1011. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011; 2011. [Google Scholar]
- 28.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Sterne J.A., Egger M., Smith G.D. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umehara T., Tanaka R. Effective exercise intervention period for improving body function or activity in patients with knee osteoarthritis undergoing total knee arthroplasty: a systematic review and meta-analysis. Brazilian J Phys Ther. 2018;22(4):265–275. doi: 10.1016/j.bjpt.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martimbianco A.L.C., Torloni M.R.R., Andriolo B.N., Porfírio G.J.M., Riera R. Neuromuscular electrical stimulation (NMES) for patellofemoral pain syndrome. Cochrane Database Syst Rev. 2017;2017(12):CD011289. doi: 10.1002/14651858.CD011289.pub2. CD011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balshem H., Helfand M., Schünemann H.J. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Ferraz R.B., Gualano B., Rodrigues R. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc. 2018;50(5):897–905. doi: 10.1249/MSS.0000000000001530. [DOI] [PubMed] [Google Scholar]
- 34.Bryk F.F., dos Reis A.C., Fingerhut D. Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surg Sport Traumatol Arthrosc. 2016;24(5):1580–1586. doi: 10.1007/s00167-016-4064-7. [DOI] [PubMed] [Google Scholar]
- 35.Giles L., Webster K.E., Mcclelland J., Cook J.L. Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: a double-blind randomised trial. Br J Sports Med. 2017;51(23):1688–1694. doi: 10.1136/bjsports-2016-096329. [DOI] [PubMed] [Google Scholar]
- 36.Tennent D.J., Hylden C.M., Johnson A.E., Burns T.C., Wilken J.M., Owens J.G. Blood flow restriction training after knee arthroscopy: a randomized controlled pilot study. Clin J Sport Med. 2017;27(3):245–252. doi: 10.1097/JSM.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 37.Segal N.A., Williams G.N., Davis M.C., Wallace R.B., Mikesky A.E. Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. PMR. 2015;7(4):376–384. doi: 10.1016/j.pmrj.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segal N., Davis M.D., Mikesky A.E. Efficacy of blood flow-restricted low-load resistance training for quadriceps strengthening in men at risk of symptomatic knee osteoarthritis. Geriatr Orthop Surg Rehabil. 2015;6(3):160–167. doi: 10.1177/2151458515583088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harper S., Roberts L., Layne A. Blood-flow restriction resistance exercise for older adults with knee osteoarthritis: a pilot randomized clinical trial. J Clin Med. 2019;8(2):265. doi: 10.3390/jcm8020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korakakis V., Whiteley R., Giakas G. Low load resistance training with blood flow restriction decreases anterior knee pain more than resistance training alone. A pilot randomised controlled trial. Phys Ther Sport. 2018;34:121–128. doi: 10.1016/j.ptsp.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Mattocks K.T., Jessee M.B., Mouser J.G. The application of blood flow restriction. Curr Sports Med Rep. 2018;17(4):129–134. doi: 10.1249/JSR.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 42.Weatherholt A.M., Vanwye W.R., Lohmann J., Owens J.G. The effect of cuff width for determining limb occlusion pressure: a comparison of blood flow restriction devices. Int J Exerc Sci. 2019;12(3):136–143. [PMC free article] [PubMed] [Google Scholar]
- 43.Jessee M.B., Mattocks K.T., Buckner S.L. Mechanisms of blood flow restriction: the new testament. Tech Orthop. 2018;33(2):72–79. [Google Scholar]
- 44.Jessee M.B., Buckner S.L., Dankel S.J., Counts B.R., Abe T., Loenneke J.P. The influence of cuff width, sex, and race on arterial occlusion: implications for blood flow restriction research. Sport Med. 2016;46(6):913–921. doi: 10.1007/s40279-016-0473-5. [DOI] [PubMed] [Google Scholar]
- 45.Loenneke J.P., Fahs C.A., Rossow L.M. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol. 2012;112(8):2903–2912. doi: 10.1007/s00421-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanwye W.R., Weatherholt A.M., Mikesky A.E. Blood flow restriction training: implementation into clinical practice. Int J Exerc Sci. 2017;10(5):649–654. [PMC free article] [PubMed] [Google Scholar]
- 47.Mouser J.G., Dankel S.J., Mattocks K.T. Blood flow restriction and cuff width: effect on blood flow in the legs. Clin Physiol Funct Imaging. 2018;38(6):944–948. doi: 10.1111/cpf.12504. [DOI] [PubMed] [Google Scholar]
- 48.Laurentino G.C., Loenneke J.P., Mouser J.G. Validity of the handheld Doppler to determine lower-limb blood flow restriction pressure for exercise protocols. J Strength Cond Res. 2020;34(9):2693–2696. doi: 10.1519/JSC.0000000000002665. [DOI] [PubMed] [Google Scholar]
- 49.Hughes L., Jeffries O., Waldron M. Influence and reliability of lower-limb arterial occlusion pressure at different body positions. Peer J. 2018;6:e4697. doi: 10.7717/peerj.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sieljacks P., Knudsen L., Wernbom M., Vissing K. Body position influences arterial occlusion pressure: implications for the standardization of pressure during blood flow restricted exercise. Eur J Appl Physiol. 2018;118(2):303–312. doi: 10.1007/s00421-017-3770-2. [DOI] [PubMed] [Google Scholar]
- 51.Soligon S., Lixandrão M., Biazon T., Angleri V., Roschel H., Libardi C. Lower occlusion pressure during resistance exercise with blood-flow restriction promotes lower pain and perception of exercise compared to higher occlusion pressure when the total training volume is equalized. Physiol Int. 2018;105(3):276–284. doi: 10.1556/2060.105.2018.3.18. [DOI] [PubMed] [Google Scholar]
- 52.Edwards R.R., Dworkin R.H., Turk D.C. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157(9):1851–1871. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deveza L.A., Melo L., Yamato T.P., Mills K., Ravi V., Hunter D.J. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthr Cartil. 2017;25(12):1926–1941. doi: 10.1016/j.joca.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Fingleton C., Smart K., Moloney N., Fullen B.M., Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23(7):1043–1056. doi: 10.1016/j.joca.2015.02.163. [DOI] [PubMed] [Google Scholar]
- 55.Nijs J., Kosek E., Van Oosterwijck J., Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Phys. 2012;15(3 suppl):ES205–ES213. [PubMed] [Google Scholar]
- 56.Fingleton C., Smart K.M., Doody C.M. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain. 2017;33(5):395–404. doi: 10.1097/AJP.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy D.L., Kemp H.I., Ridout D., Yarnitsky D., Rice A.S.C. Reliability of conditioned pain modulation: a systematic review. Pain. 2016;157(11):2410–2419. doi: 10.1097/j.pain.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones M.D., Taylor J.L., Barry B.K. Occlusion of blood flow attenuates exercise-induced hypoalgesia in the occluded limb of healthy adults. J Appl Physiol. 2017;122(5):1284–1291. doi: 10.1152/japplphysiol.01004.2016. [DOI] [PubMed] [Google Scholar]
- 59.Gajsar H., Nahrwold K., Titze C., Hasenbring M.I., Vaegter H.B. Exercise does not produce hypoalgesia when performed immediately after a painful stimulus. Scand J Pain. 2018;18(2):311–320. doi: 10.1515/sjpain-2018-0024. [DOI] [PubMed] [Google Scholar]
- 60.Brandner C.R., May A.K., Clarkson M.J. Reported side-effects and safety considerations for the use of blood flow restriction during exercise in practice and research. Tech Orthop. 2018;33(2):114–121. [Google Scholar]
- 61.Husmann F., Mittlmeier T., Bruhn S., Zschorlich V., Behrens M. Impact of blood flow restriction exercise on muscle fatigue development and recovery. Med Sci Sports Exerc. 2018;50(3):436–446. doi: 10.1249/MSS.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 62.Hislop A.C., Collins N.J., Deasy M., Tucker K., Semciw A.I. Does adding hip exercises to quadriceps exercises result in superior outcomes in pain, function and quality of life for people with knee osteoarthritis?. A systematic review and meta-analysis. Br J Sports Med. 2020;54(5):263–271. doi: 10.1136/bjsports-2018-099683. [DOI] [PubMed] [Google Scholar]
- 63.Lun V., Marsh A., Bray R., Lindsay D., Wiley P. Efficacy of hip strengthening exercises compared with leg strengthening exercises on knee pain, function, and quality of life in patients with knee osteoarthritis. Clin J Sport Med. 2015;25(6):509–517. doi: 10.1097/JSM.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 64.Ladlow P., Coppack R.J., Dharm-Datta S. Low-load resistance training with blood flow restriction improves clinical outcomes in musculoskeletal rehabilitation: a single-blind randomized controlled trial. Front Physiol. 2018;9:1269. doi: 10.3389/fphys.2018.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartholdy C., Juhl C., Christensen R., Lund H., Zhang W., Henriksen M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: a systematic review and meta-regression analysis of randomized trials. Semin Arthritis Rheum. 2017;47(1):9–21. doi: 10.1016/j.semarthrit.2017.03.007. [DOI] [PubMed] [Google Scholar]