Abstract
Background
This study evaluated the consequences in Europe of the COVID-19 outbreak on pathology laboratories orientated toward the diagnosis of thoracic diseases.
Materials and methods
A survey was sent to 71 pathology laboratories from 21 European countries. The questionnaire requested information concerning the organization of biosafety, the clinical and molecular pathology, the biobanking, the workload, the associated research into COVID-19, and the organization of education and training during the COVID-19 crisis, from 15 March to 31 May 2020, compared with the same period in 2019.
Results
Questionnaires were returned from 53/71 (75%) laboratories from 18 European countries. The biosafety procedures were heterogeneous. The workload in clinical and molecular pathology decreased dramatically by 31% (range, 3%-55%) and 26% (range, 7%-62%), respectively. According to the professional category, between 28% and 41% of the staff members were not present in the laboratories but did teleworking. A total of 70% of the laboratories developed virtual meetings for the training of residents and junior pathologists. During the period of study, none of the staff members with confirmed COVID-19 became infected as a result of handling samples.
Conclusions
The COVID-19 pandemic has had a strong impact on most of the European pathology laboratories included in this study. Urgent implementation of several changes to the organization of most of these laboratories, notably to better harmonize biosafety procedures, was noted at the onset of the pandemic and maintained in the event of a new wave of infection occurring in Europe.
Key words: COVID-19, pathology, biosafety, lung cancer, activity
Highlights
-
•
Biosafety measures used in the first wave of the COVID-19 crisis were heterogeneous in 53 European pathology laboratories.
-
•
A dramatic decrease of the workload in pathology laboratories was noted.
-
•
No case of healthcare workers contaminated with SARS-CoV-2 associated with samples handling was identified.
Introduction
After the initial emergence of the infection in China in early 2020, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus spread to Europe in February 2020, first to Italy and then rapidly in March-April 2020 to all European countries, notably to Spain, France, Belgium and the United Kingdom.1, 2, 3 None of the countries of the European Union were spared infection, and as of March/May 2020, Europe was declared as the epicenter of the world pandemic (https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/novel-coronavirus-2019-ncov). The contagiousness of SARS-CoV-2 and the uncertainty of human infection suddenly changed the functioning of hospitals and led to a new way of organizing the activity, as well as the setup of measures to protect the health of hospital workers.4, 5, 6, 7 In this context, the clinical and molecular pathology laboratories, notably those receiving specimens from the respiratory tract, were required to face up to the new constraints associated with the COVID-19 pandemic.8
This study aimed to evaluate in Europe the consequences of the COVID-19 pandemic on pathology laboratories that have a strong activity in thoracic diseases for the period between 15 March and 31 May 2020. Attention was given to (i) changes to organization and the establishment of protective measures, (ii) the clinical, molecular and biobanking activities, (iii) the setting up of research projects in the field of COVID-19 and the continuity of training programs for residents and pathologists, (iv) the planned projects for improvement of the management of these laboratories following the first wave of infection, and (v) the number of staff members who were potentially infected or developed COVID-19.
Material and methods
Study oversight
On behalf of the Pulmonary Pathology Working Group of the European Society of Pathology, a survey was sent to 71 pathologists, mainly experts in thoracic pathology, in several European countries (https://www.esp-pathology.org/working-groups/esp-working-groups/pulmonary-pathology.html). This survey contained questions focused globally on the consequences of the COVID-19 pandemic on the organization and the activities of the pathology laboratories between 15 March and 31 May 2020 (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2020.100024). The different parts of the survey included questions on: (i) the pre-existing or new setups for biosafety procedures, (ii) the clinical diagnostic activity in the area of thoracic cellular pathology, (iii) the molecular pathology applied to lung cancer samples, (iv) the collection of biospecimens for translational research purposes, (v) the development of research focused on COVID-19, (vi) the organization of the work time of staff members, (vii) the training and educational programs, (viii) the different measures considered following the first wave of the COVID-19 pandemic, and, (ix) information on the SARS-CoV-2-infected or suspected-infected staff members (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2020.100024). This study was registered with the ClinicalTrials.gov identifier, number NCT04476823. Moreover, the study complied with the law of January 1978 (78-17) relating to computing, data, and freedom known as ‘computing and freedoms’ and with rules 2016/679 of the European Parliament (of 25 May 2018) concerning the general data protection regulation (GDPR; MR004). The study was registered by the Nice University Hospital (R04-39) and filed on the Health Data Hub website (7 July 2020) (www.health-data-hub.fr/depot).
Statistical analysis
All the data were collected and entered into an Excel® file. Categorical data were determined as frequencies and percentages. Statistical comparisons were evaluated with the paired and unpaired chi-square or Fisher's exact tests when indicated. Quantitative data were determined as the mean, standard deviation, range, and quartile; the paired and unpaired Student's t or Wilcoxon tests were used to compare these data. All statistical analyses were two-sided and generated with R-3.5.1 software.
Results
A total of 53 out of 71 (75%) pathology laboratories from 42 cities of 18 European countries participated in the survey (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2020.100024). The number of participants per country varied from 1 to 19 (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2020.100024). The COVID-19 incidence during this period in the participating countries had no impact on the number of questionnaires returned, as we had as many surveys returned from countries with high COVID-19 incidence (mean = 3 returned questionnaires; e.g. Italy, Spain, France) as in countries with low COVID-19 incidence (mean = 3 returned questionnaires; e.g. Sweden, Germany, Finland). The main data are shown in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5.
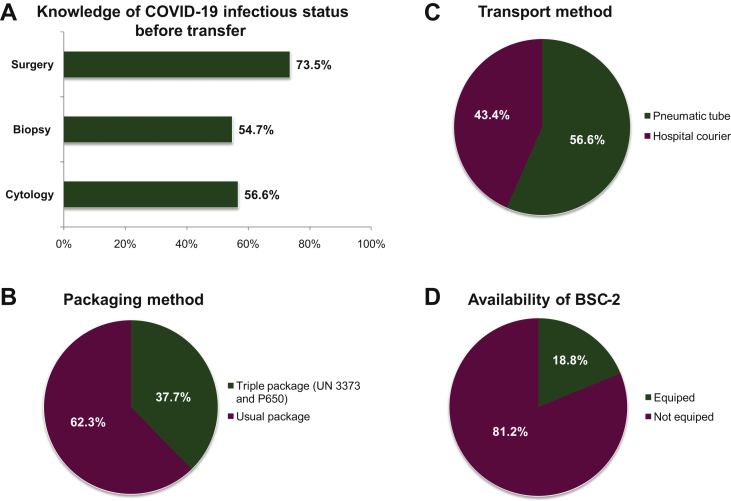
Figure 1.
Sample workflow from the clinical and surgical departments to the pathology department and procedures for handling biospecimens in the pathology laboratory.
(A) Knowledge of the COVID-19 infectious status before the transfer to the laboratory, according to the type of procedure. (B) Transport method. (C) Packaging method. (D) Class 2 biological safety cabinet (BSC-2) equipment in the room for gross macroscopy and frozen section.
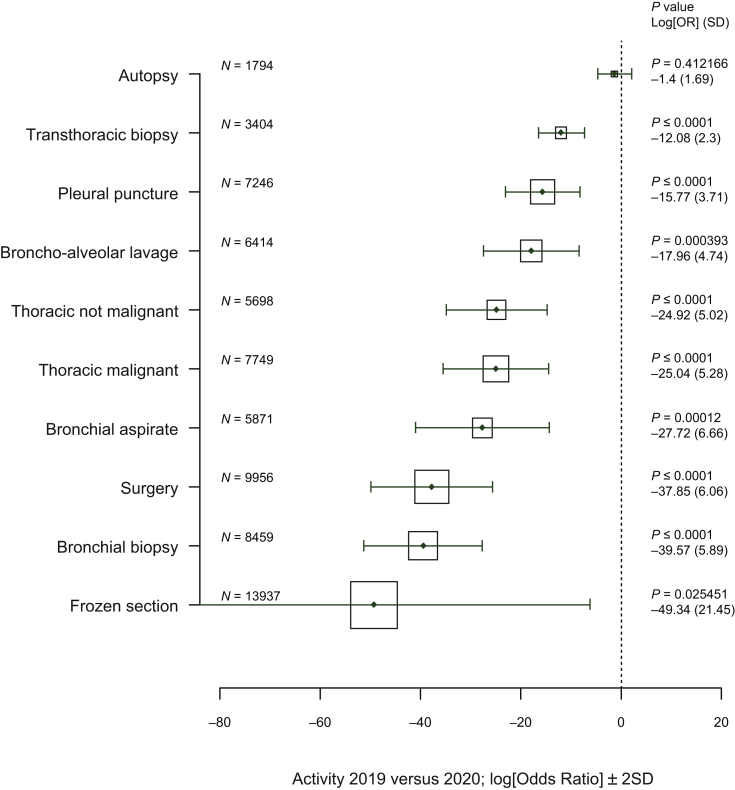
Figure 2.
Comparison of the clinical activity from 15 March to 31 May 2019 and from 15 March to 31 May 2020.
Vertical axis, type of analysis; horizontal axis, log[adjusted odds ratio] (0 = no effect, <0 = reduction in activity between 2019 and 2020); solid line, ±2 (standard deviation), the area of each square is proportional to the sample size of analysis 2019 and 2020; P value, P value for linear regression model adjusted to the center; log[OR], logarithm of the odds ratio (OR) for the linear regression model; SD, standard deviation of the log[OR].
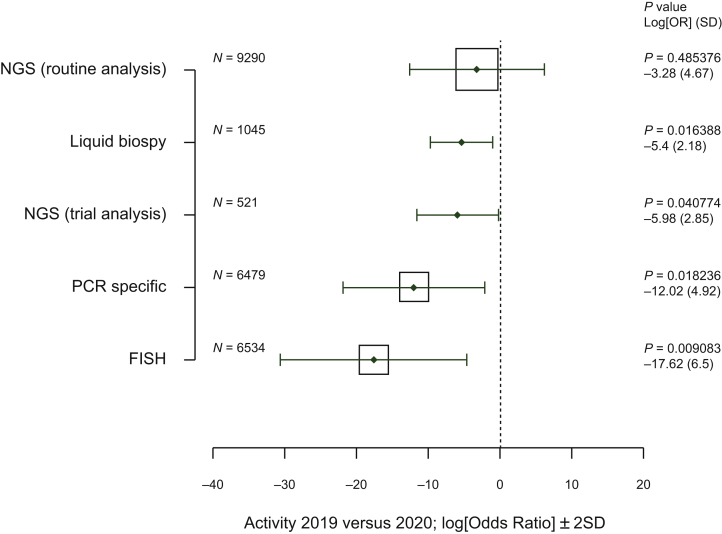
Figure 3.
Comparison of the molecular pathology activity from 15 March to 31 May 2019 and from 15 March to 31 May 2020.
Vertical axis, type of analysis; horizontal axis, log[adjusted odds ratio] (0 = no effect, <0 = reduction in activity between 2019 and 2020); solid line: ±2 (standard deviation), the area of each square is proportional to the sample size of analysis 2019 and 2020; P value, P value for linear regression model adjusted to the center; log[OR], logarithm of the odds ratio (OR) for the linear regression model; SD, standard deviation of the log[OR].
NGS, next-generation sequencing; PCR, polymerase chain reaction.
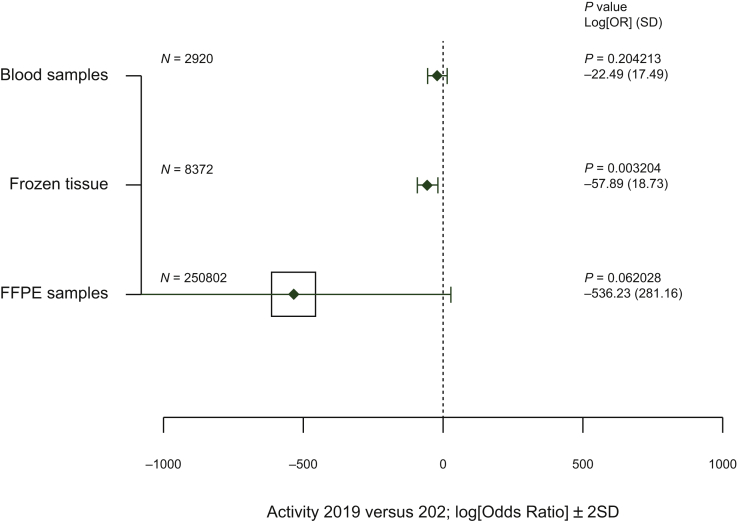
Figure 4.
Comparison of the biobanking activity between 15 March to 31 May 2019 and from 15 March to 31 May 2020.
Vertical axis. type of analysis; horizontal axis, log[adjusted odds ratio] (0 = no effect, <0 = reduction in activity between 2019 and 2020); solid line, ±2 (standard deviation), the area of each square is proportional to the sample size of analysis 2019 and 2020; P value, P value for linear regression model adjusted to the center; log[OR]: logarithm of the odds ratio (OR) for the linear regression model; SD, standard deviation of the log[OR].
FFPE, formalin-fixed paraffin-embedded.
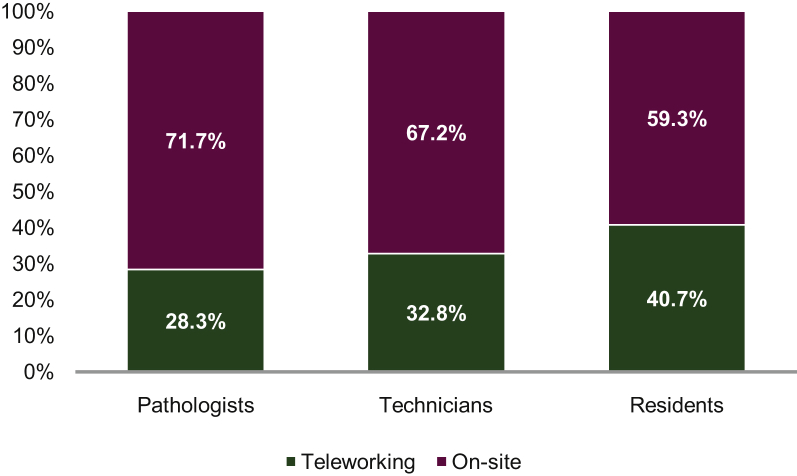
Figure 5.
Mean percentage of teleworking according to the occupational categories.
Pre-existing or new setups for biosafety procedures
Sample workflow from the clinical and surgical departments to the pathology department and procedures for handling biospecimens in the laboratory
The COVID-19 status of patients for whom cytological, biopsy, or surgical specimens were taken was known before the handling of the samples by the staff of the pathology laboratory in 30/53 (56.6%), 29/53 (54.7%), and 39/53 (73.5%) cases, respectively (Figure 1). Thirty out of 53 (56.6%) laboratories did not have pneumatic tubes and received samples by hospital couriers (Figure 1). Among the 23/53 (43.3%) remaining laboratories, 3/23 (13%) stopped using pneumatic tubes and received the samples by hospital courier only. Samples were sent to the laboratories in a triple package (as recommended by international guidelines according to UN 3373 and P650 norms) in 20/53 (37.7%) cases and in usual packages in 33/53 (62.3%) of cases (Figure 1). Ten out of 53 (18.8%) laboratories were equipped with a class 2 biosafety cabinet (BSC-2) in the room for gross macroscopy, which required handling of fresh tissue and frozen sections (Figure 1). Forty-two out of 53 (79.2%) laboratories had a BSC-2 for cytological samples, notably for handling and processing of fresh body fluids such as broncho-alveolar lavage (BAL) samples. The number of staff members allowed to work at the same time in the gross macroscopy room varied (from 2 to 16, median 2) or was not regulated by a consistent rule in 18/52 (34.6%) of cases.
Personal protective equipment
When handling tissue and cytological samples, 24/53 (45.3%) and 29/53 (54.7%) of the personnel (pathologists and technicians) wore a surgical or filtering face piece class 2 (FFP2)/N95 mask, respectively. Thirty-one out of 53 laboratories (58.5%) used one pair of gloves while 22 (41.5%) used two pairs. Twenty out of 52 laboratories (38.5%) wore surgical caps and 10/51 (19.6%) used surgical overshoes. The use of other personal protective equipment (PPE) varied according to the institution.
The clinical diagnostic activity of thoracic cellular pathology
The number of histological, cytological, and surgical samples decreased significantly for the considered period of analysis from 2019 to 2020 but was variable according to the type of biospecimen (Figure 2). The most significant decrease was recorded for the intraoperative frozen sections (Figure 2). The decrease in the number of surgical interventions could explain, at least partially, the decrease in the thoracic pathology activity, whereas the incidence of severe lung cancer cases dramatically increased for the same studied period (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2020.100024). Additionally, the mean number of autopsies slightly decreased from a mean of 27.47 (2019) to a mean of 25.29 (2020) (Figure 2). Moreover, only 19/53 (35.8%) institutions carried out autopsies on COVID-19-positive patients. Nine out of these 19 laboratories (47.3%) had a biosafety level 3 (BSL-3) autopsy room.
Molecular pathology for lung cancer patients
Some molecular pathology analyses were maintained, in particular for the next-generation sequencing (NGS) tests (Figure 3). However, a significant decrease from 2019 to 2020 was noted for the number of liquid biopsies (median decrease, 30.19%), the NGS analysis for clinical trials (median decrease, 17.54%), specific polymerase chain reaction (PCR) tests (median decrease, 14.06%), and the analyses using in situ fluorescent hybridization (median decrease, 10.84%) (Figure 3).
Collection of biospecimens for translational research purposes
The amount of biobanking of tumor tissues and liquid biopsies dropped from 2019 to 2020 (Figure 4). Only a few laboratories [3/53 (5.6%)] actively collected samples from COVID-19 patients (not shown). Twenty out of 53 (43.4%) pathology laboratories carried out or were associated with a research project into COVID-19. Overall, the activity of the 23 pathology laboratories led (to date) to 71 manuscripts accepted for publication (median 3, range 1-10) in the area of COVID-19.
Organization of the staff members' work time
The majority [47/53 (88.6%)] of pathology laboratories set up teleworking for at least one of the occupational categories, but mainly for the pathologists, the technicians, and the residents (Figure 5). The mean percentage of teleworking varied according to the occupational categories; notably, it was 28.3% (range, 0%-80%), 32.8% (range, 0%-80%), and 40.7% (range, 0%-100%), for the pathologists, the technicians, and the residents, respectively (Figure 5).
Training programs
Educational programs for residents and junior pathologists were maintained in the majority of cases [38/53 (71.7%)] using webinars (24.5%), digital pathology training and/or virtual courses (20.7%), and other activities (73.6%).
Different measures considered following the ‘first wave’ of the COVID-19 pandemic
The survey included a free text entry field to add information on different strategies to be considered after the first wave of the COVID-19 crisis. The questions focused notably on improvement of biosafety, development of training, and the intent to develop specific research into COVID-19 (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2020.100024). Globally, most of the laboratories considered improving their biosafety procedures [32/49 (65.3%)], and a few of them aimed to optimize the rooms for gross macroscopy, notably to set up a biosafety level 2 (BSL-2) environment and maintain the PPE. Some laboratories [28/49 (57.1%)] claimed that they would develop virtual training, but without giving any detail. Finally, a few laboratories carried out or participated in translational research projects on COVID-19.
Information into the SARS-CoV-2-infected or suspected-infected staff members
Of the personnel, 218/3368 (6.4%) and 136/3368 (4.9%) had fever or associated symptoms, respectively, which were suggestive of COVID-19. It is noteworthy that among these staff members, there was a higher number of technicians (110) than pathologists (65). A positive RT-PCR for SARS-CoV-2 was obtained in 64/777 (8.2%) of the tested personnel and 182/3368 (5.4%) staff members were in quarantine. Those with COVID-19 (64/64) were strongly suspected of having been infected outside of the workplace (infection by a family member known to be SARS-CoV-2-positive). No infection at the work site was identified, neither due to an inter-individual nor due to sample/aerosol contamination. Of the personnel, 547/3368 (16.2%) had a serological test for SARS-CoV-2 antibodies. A positive serological test was declared for only 36/547 (6.6%) of staff members.
Discussion
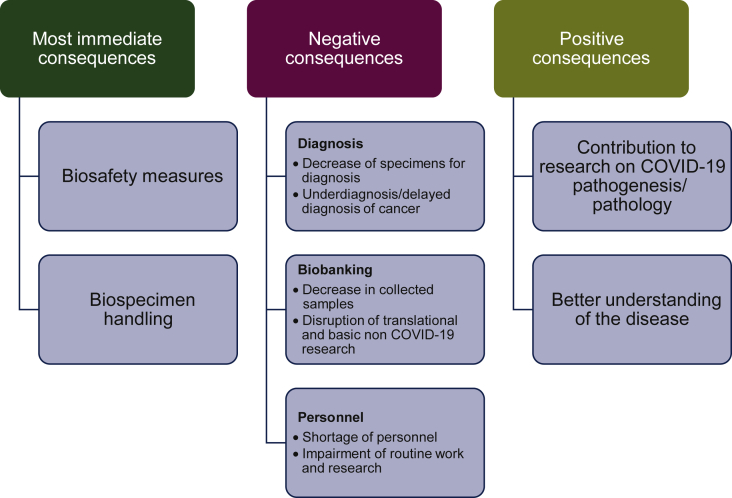
This study showed that the consequences of the COVID-19 pandemic in pathology laboratories in Europe were heterogeneous, notably for those in the field of thoracic pathology. Consequences concerned: (i) the biosafety measures and the introduction of changes to the organization of laboratory practices and (ii) the different activities including clinical and molecular pathology diagnoses as well as biobanking activities (Figure 6). This has been presented according to the different countries, cities, and institutions.
Figure 6.
The major consequences in the European thoracic pathology laboratories during the COVID-19 pandemic from 15 March to 31 May 2020.
The COVID-19 pandemic has a strong impact on clinical and molecular pathology in oncology (Figure 6).9, 10, 11 Delays due to COVID-19 had dramatic changes on the number of underdiagnosed cancer cases and additional cancer deaths, as recently reported by two modelling studies in England.12,13 This is highlighted in the present study by a substantial decrease in the activity in Italy, France, Spain, and the UK, and to a lesser degree in some of the other countries. Though the level of decline was not reported in this study, it could be explained, at least partially, by a decrease in the number of hospitalized patients with a thoracic pathology (other than a COVID-19 associated disease) during this period. For example, some patients with lung cancer seemed to have avoided hospitalization out of fear of being infected with SARS-CoV-2 or the care of these patients was delayed by the physicians.14,15 Moreover, some cancer patients were assumed not to be treated adequately due to extensive devotion of medical services to COVID-19 patients. In addition, in certain institutions, the staff members of pathology laboratories were reoriented to work in the emergency or other clinical departments to take part in the different activities against COVID-19 and to help take care of COVID-19-positive patients. A European decrease in the molecular pathology analyses for genomic alterations in lung cancer was noted during the studied period. This could be explained by the fact that molecular biology testing needed more staff members on-site and less social distancing between the personnel, which was mandatory in certain laboratories. Moreover, during the COVID-19 crisis, we assume that a minimum of molecular testing (such as EGFR mutations and ALK rearrangement) have been carried out as mandatory for the standard of care. In addition, a lower number of patients being included in clinical trials also led to the exclusive use of automatic targeted sequencing systems. Moreover, in the laboratories performing liquid biopsies, the number of respective molecular analyses decreased, including the number of targeted sequencing tests.
Sustaining clinical and molecular pathology during the COVID-19 pandemic, notably to perform the different diagnostic tests requested by the physicians for hospitalized lung cancer patients could be challenging in the pathology laboratories due to a possible reduced number of healthcare workers (HCWs).9,11,16,17 Thus, staff members may become infected with SARS-CoV-2 (notably after viral transmission among HCWs or from an external positive COVID-19 individual) and the staff members who came in contact with the infected HCW may be placed in quarantine. This could lead to reduced ability to perform critical steps of the molecular pathology analyses of cancer patients. In this context, to prevent the shutdown of clinical and molecular pathology, it is critical to adopt a strategy aimed at restricting the maximum number of personnel inside the laboratory, but also to keep a safe distance between operators. Hence technicians and medical staff should plan their daily routine based on the minimum number of individuals required for each room. Moreover, it is important to adopt as much as possible teleworking for all professional categories. Senior consultants, medical students, and residents should not be present in the laboratory during the COVID-19 crisis. The present study showed that, apart for some laboratories, most of them had a common strategy to reduce the number of personnel working at the same time in the same room, at least in the rooms dedicated to macroscopy and preparation of frozen sections with more than 80% of the laboratories having no more than two staff members working at the same time in these rooms. Moreover, teleworking was set up in most of the laboratories not only for the pathologists and the technicians but also for other professional categories.
We tried to evaluate the number of HCWs in a large number of European pathology laboratories who may have been SARS-CoV-2 positive or had some suggestive clinical symptom(s) of COVID-19 including fever. Thus, even if a small number of staff members became infected, as confirmed by a positive COVID-19 RT-PCR, more than 5% of the staff members showed a strong suspicion of COVID-19 as they had suggestive symptoms of this disease. Moreover, the impact of these different symptoms and results on the measures of quarantine was quite substantial since around 4%-6% of the staff members were required to stay at home due to a strong suspicion of COVID-19 or due to contact with another COVID-19-positive staff member. None of the cases of infection arose due to handling of SARS-CoV-2-positive samples and for a few HCWs, infection outside the work place was quite certainly due to infection via family members. One major limitation of this study is that the staff members were not regularly tested by RT-PCR for SARS-CoV-2 and thus infection of HCWs due to contact with asymptomatic subjects in the laboratory could not be ruled out.
It is noteworthy that despite the rapid introduction of international guidelines concerning the laboratory biosafety guidance relative to COVID-19 (https://www.who.int/docs/default-source/coronaviruse/laboratory-biosafety-novel-coronavirus-version-1-1.pdf?sfvrsn=912a9847_2; https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html; https://www.cap.org/advocacy/latest-news-and-practice-data/march-26-2020), the different protective measures for pathology laboratory HCWs were implemented in a variable fashion by the laboratories who participated in this study. One explanation for this could be a lack of understanding and some uncertainties of the contagiousness of the human samples handled in the pathology laboratory in the COVID-19 context.18, 19, 20, 21 The risk of infection of HCWs in the pathology laboratory is certainly much lower than in clinical departments (emergency and intensive care/reanimation units, pulmonology and other departments), and possibly in virology laboratories, although the actual risk remains to be determined.22,23 The main risk in a pathology laboratory probably comes from transmission among HCWs due to the non-compliance of social distancing in the laboratory space.24 At least in theory, the level of contagiousness of human samples is certainly variable depending on the nature and condition of the material (fresh, frozen or fixed samples), yet until now no case of SARS-CoV-2 infection has been reported due to sample handling in a pathology laboratory.25, 26, 27 However, specific measures need to be put in place to handle fresh thoracic tissue specimens, notably during procedures using frozen sections and non-fixed cytological samples (such as BAL, pleural effusions, bronchial aspirates, fine needle aspirate material) and processing and storage of frozen specimens. Thus, in the present study, it was noteworthy that most of the laboratories did not have a BSC-2 in the room for macroscopic analysis for handling of fresh tissue specimens and preparing frozen sections. Most of these pathology laboratories were only equipped with chemical hoods or with aspiration tables for gross macroscopy. Additionally, almost half of the HCWs working with fresh tissue specimens wore a surgical mask only and not an FFP2/NP95 protective system. The use of other PPE (goggles, face shields, surgical caps, etc.) varied from one laboratory to another. International guidelines have recommended abandoning the use of pneumatic tubes for tissue and cytological sample transportation, which has become mandatory for samples from COVID-19-positive patients (https://www.who.int/docs/default-source/coronaviruse/laboratory-biosafety-novel-coronavirus-version-1-1.pdf?sfvrsn=912a9847_2; https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html; https://www.cap.org/advocacy/latest-news-and-practice-data/march-26-2020). However, only 13% (3/23) of the pathology laboratories involved in this study stopped using pneumatic tubes for sample transportation. It was not clear if the remaining 20 laboratories knew the COVID-19 status of patients from whom samples were received through pneumatic tubes. Interestingly, the COVID-19 status of the patients' samples (fixed and/or fresh tissue and cytological samples) by the HCWs who handled samples was known in only around 50% of the laboratories. Thus, the biosafety measures and biospecimen handling need to be increased, adapted to the latest scientific data, and standardized and implemented (re-iterative cycle). These measures are required anyhow when handling fresh frozen tissue and unfixed liquid/cytological specimens in pathology laboratories to prevent infection by pathogens certainly more contagious for HCWs than SARS-CoV-2 (e.g. mycobacteria, human immunodeficiency virus, hepatitis B and C viruses, etc.). It is necessary to take into consideration that these procedures are associated with increased costs for the laboratories.
One of the consequences of the COVID-19 crisis was the absolute necessity to develop virtual educational programs, notably for residents and junior pathologists (digital pathology training, virtual courses, webinars, etc.).28, 29, 30 However, the results of the present survey showed that only around 25% of the laboratories set up these programs, highlighting the urgent need to become aware of the utility of these different tools. In addition, some information, in particular concerning the biosafety procedures to follow in a pathology laboratory should be established through virtual training, and this was the case in the present study for no more than 25% of the laboratories.
One of the more important negative consequences of the COVID-19 pandemic occurred in the biobanking field for translational research purposes, notably for the collection of samples from lung cancer patients (Figure 6).31,32 Biobanking in the field of thoracic pathology decreased substantially or was shut down in most of the pathology laboratories participating in this survey. Biobanking has been extremely important to provide material to solve the COVID-19 pathogenesis and a seminal contribution of pathology. Thus, pathologists should be strengthened to assure the continuity of the COVID-19 biobanking.33 Conversely, a number of the pathology laboratories included in the present work had the possibility to participate in and/or lead research projects into COVID-19.33, 34, 35, 36, 37 Thus, at the time of submission of this study, 71 manuscripts were published or submitted for publication in association with one or several of these pathology laboratories, which was a fruitful consequence occurring in only a short period of time.
Our study has a couple of limitations. Not all European countries were included. There is heterogeneity between the laboratories located in different countries, mostly with regard to the molecular pathology activity and the caseload (academic versus community hospitals). Because the survey herein was designed to address the measures taken from 15 March to 31 May 2020, the results of the current study might not apply to the later periods of the pandemic. Finally, it would be of interest to see if the different COVID-19 incidences according to the countries participating in this survey have a different impact on the pathology laboratories' activity.38 However, it was difficult to have regional incidence data inside each country (e.g. north versus south) given the scattered nature of the laboratories.
While Europe was the epicenter of the COVID-19 pandemic between February and May 2020, subsequently, the number of infected patients who were hospitalized decreased progressively. Thus, at the time of submission of this study, the aim was to sustain and/or to reinforce the different protective measures so far implemented, at least partially. Some of these procedures are costly, constrain the activity of HCWs, and modify the organization of the daily practice of the pathology laboratories. In this regard, the following questions may be asked: (i) is it possible to again use pneumatic tubes for sample transportation, (ii) can pathologists again use multi-head microscopes for collective observation of slides, (iii) should all the HCWs keep wearing masks when working in all the sectors of the laboratory, (iv) should teleworking become permanently established as a way of working, irrespective of the professional category, (v) is it now mandatory to use a BSC-2 for gross macroscopy of fresh tissue samples and frozen sections, in association with a chemical hood with a HEPA filter for handling formalin-fixed specimens, and (vi) is it always critical to know the COVID-19 status of patients before receiving tissue and cytological samples, notably fresh samples and before frozen section and/or biobanking procedures? The results of the present survey demonstrated that depending on the pathology laboratory participating in this study, the strategy to adopt still varied, despite the current international guidelines.39
Whatever the adopted strategy, the COVID-19 pandemic sparked the improvement of the biosafety procedures now in place in many European pathology laboratories participating in this study and to the re-evaluation of the organization of the work. It is noteworthy that although many pathology laboratories were not optimally equipped, the rate of infection among HCWs was low and COVID-19-positive staff members were infected outside of the workplace. This highlights that major prevention measures such as wearing surgical or FFP2/N95 masks and adopting a safe distance between operators were certainly effective in containing/reducing infection between staff members. We strongly believe that most of the different actions that have been set up must now be sustained and that the pathology laboratories need to rapidly adapt their working day to change the organization, notably in the event of futures COVID-19 waves.40
Acknowledgements
This study was commissioned by the Pulmonary Pathology Working Group of the European Society of Pathology. No financial funding was provided.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
Anonymized participant-level data are available for investigators.
Ethical approval
The study complied with the law of January 1978 (78-17) relating to computing, data, and freedom known as ‘computing and freedoms’ and with rules 2016/679 of the European Parliament (of 25 May 2018) concerning the general data protection regulation (GDPR; MR004). The study was registered by the Nice University Hospital (R04-39) and filed on the Health Data Hub website (7 July 2020) (www.health-data-hub.fr/depot).
Supplementary data
References
- 1.Contini C., Di Nuzzo M., Barp N. The novel zoonotic COVID-19 pandemic: an expected global health concern. J Infect Dev Ctries. 2020;14(3):254–264. doi: 10.3855/jidc.12671. [DOI] [PubMed] [Google Scholar]
- 2.Sironi M., Hasnain S.E., Rosenthal B. SARS-CoV-2 and COVID-19: a genetic, epidemiological, and evolutionary perspective. Infect Genet Evol. 2020;84:104384. doi: 10.1016/j.meegid.2020.104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudbjartsson D.F., Helgason A., Jonsson H. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382(24):2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black J.R.M., Bailey C., Przewrocka J., Dijkstra K.K., Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schunemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houlihan C.F., Vora N., Byrne T. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396(10246):e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluytmans-van den Bergh M.F.Q., Buiting A.G.M., Pas S.D. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3(5):e209673. doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosney J.R., Hofman P., Troncone G., Lopez-Rios F. Cellular pathology in the COVID-19 era: a European perspective on maintaining quality and safety. J Clin Pathol. 2021;74(1):64–66. doi: 10.1136/jclinpath-2020-206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malapelle U., Pisapia P., Iaccarino A. Predictive molecular pathology in the time of coronavirus disease (COVID-19) in Europe. J Clin Pathol. 2020 doi: 10.1136/jclinpath-2020-206957. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Rossi E.D., Pantanowitz L. International perspectives: impact of the COVID-19 pandemic on cytology. Cancer Cytopathol. 2020;128(5):307–308. doi: 10.1002/cncy.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troncone G., Hofman P. Pathologists and the coronavirus distraction effect. J Clin Pathol. 2020 doi: 10.1136/jclinpath-2020-206807. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Maringe C., Spicer J., Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sud A., Torr B., Jones M.E. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banna G., Curioni-Fontecedro A., Friedlaender A., Addeo A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5(2):e000785. doi: 10.1136/esmoopen-2020-000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo J., Rizvi H., Preeshagul I.R. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madrigal E. Going remote: maintaining normalcy in our pathology laboratories during the COVID-19 pandemic. Cancer Cytopathol. 2020;128(5):321–322. doi: 10.1002/cncy.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pambuccian S.E. The COVID-19 pandemic: implications for the cytology laboratory. J Am Soc Cytopathol. 2020;9(3):202–211. doi: 10.1016/j.jasc.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burki T.K. Testing for COVID-19. Lancet Respir Med. 2020;8(7):e63–e64. doi: 10.1016/S2213-2600(20)30247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y., Chen S., Yang Z. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201(11):1435–1438. doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lescure F.X., Bouadma L., Nguyen D. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng S., Shen C., Xia N., Song W., Fan M., Cowling B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8(5):434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese F., Pezzuto F., Fortarezza F. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477(3):359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso C., Calabrese F., Sbaraglia M. Feasibility of postmortem examination in the era of COVID-19 pandemic: the experience of a Northeast Italy University Hospital. Virchows Arch. 2020;477(3):341–347. doi: 10.1007/s00428-020-02861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cieri M., De Carlo C., Valeri M. How are we facing it? Dispatches from pathology residents in a COVID-19 Lombardy hospital. Front Public Health. 2020;8:259. doi: 10.3389/fpubh.2020.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna M.G., Reuter V.E., Ardon O. Validation of a digital pathology system including remote review during the COVID-19 pandemic. Mod Pathol. 2020;33:2115–2127. doi: 10.1038/s41379-020-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon R., Zhang M.L., VandenBussche C.J. Considerations for remote learning in pathology during COVID-19 social distancing. Cancer Cytopathol. 2020;128:642–647. doi: 10.1002/cncy.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofman P. Challenges and issues surrounding the use for translational research of human samples obtained during the COVID-19 pandemic from lung cancer patients. Transl Lung Cancer Res. 2020;9(4):1543–1553. doi: 10.21037/tlcr-20-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofman P., Puchois P., Brest P., Lahlou H., Simeon-Dubach D. Possible consequences of the COVID-19 pandemic on the use of biospecimens from cancer biobanks for research in academia and bioindustry. Nat Med. 2020;26(6):809–810. doi: 10.1038/s41591-020-0890-8. [DOI] [PubMed] [Google Scholar]
- 33.Tanga V., Leroy S., Fayada J. Establishment of a collection of blood derived products from COVID-19 patients for translational research. Experience of the LPCE Biobank (Nice, France) Biopreserv Biobank. 2020 doi: 10.1089/bio.2020.0055. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Huang S.F., Huang Y.C., Chang F.Y. Rapid establishment of a COVID-19 biobank in NHRI by National Biobank Consortium of Taiwan. Biomed J. 2020;43:314–317. doi: 10.1016/j.bj.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Washetine K., Heeke S., Bonnetaud C. Establishing a dedicated lung cancer biobank at the University Center Hospital of Nice (France). Why and how? Cancers (Basel) 2018;10(7):220. doi: 10.3390/cancers10070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wigginton N.S., Cunningham R.M., Katz R.H. Moving academic research forward during COVID-19. Science. 2020;368(6496):1190–1192. doi: 10.1126/science.abc5599. [DOI] [PubMed] [Google Scholar]
- 37.Stergachis A.B., Weiss S.T., Green R.C. Biobanks could identify medically actionable findings relevant for COVID-19 clinical care. Nat Med. 2020;26(7):991. doi: 10.1038/s41591-020-0953-x. [DOI] [PubMed] [Google Scholar]
- 38.Vigliar E., Cepurnaite R., Alcaraz-Mateos E. Global impact of the COVID-19 pandemic on cytopathology practice: results from an international survey of laboratories in 23 countries. Cancer Cytopathol. 2020;128(12):885–894. doi: 10.1002/cncy.22373. [DOI] [PubMed] [Google Scholar]
- 39.Hofman P., Lucas S., Jouvion G., Tauziede-Espariat A., Chretien F., Cathomas G. Pathology of infectious diseases: what does the future hold? Virchows Arch. 2017;470(5):483–492. doi: 10.1007/s00428-017-2082-6. [DOI] [PubMed] [Google Scholar]
- 40.Xu S., Li Y. Beware of the second wave of COVID-19. Lancet. 2020;395(10233):1321–1322. doi: 10.1016/S0140-6736(20)30845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.