Abstract
Metastasis is a primary reason related to the mortality of oral squamous cell carcinoma (OSCC) patients. A program called epithelial‐mesenchymal transition (EMT) has been shown to play a critical role in promoting metastasis in epithelium‐derived carcinoma. During EMT, epithelial cancer cells acquire motile mesenchymal phenotypes and detach from primary tumors. Recent lines of evidence have suggested that EMT confers cancer cells with tumor‐initiating ability. Therefore, selective targeting of EMT would lead to the development of effective therapeutic agents. In this study, using a chemical biology approach, we identified isoxsuprine, a β2‐adrenergic receptor (β2‐AR) agonist as a low‐molecular‐weight compound that interferes with the acquisition of mesenchymal phenotypes of oral cancer cells. Treatment of multiple types of oral cancer cells with isoxsuprine led to the downregulation of mesenchymal cell markers that was accompanied by reduced cell motility. Similar inhibitory effects were also observed for isoprenaline, a non‐selective β‐adrenergic receptor (β‐AR) agonist. In addition, inhibition of cell migration upon treatment with isoxsuprine was reverted by a non‐selective β‐AR antagonist, propranolol, and the CRISPR/Cas9 system‐mediated deletion of the β2‐AR gene, suggesting that the effects exerted by isoxsuprine involved signals mediated by β2‐AR. In addition, in a subcutaneous xenograft model of oral cancer cells, the administration of isoxsuprine effectively suppressed primary tumor growth, suggesting β2‐AR signals to be a promising cancer therapeutic target for treatment of OSCC.
Keywords: β2‐adrenergic receptor, isoxsuprine, MET (mesenchymal‐epithelial transition), oral squamous cell carcinoma, tumor growth
In this study we identified isoxsuprine, a β2‐adrenergic receptor agonist as an effective inhibitor of mesenchymal phenotypes and migration of oral squamous cell carcinoma cells suggesting that β2‐adrenergic receptor signal is a new promising therapeutic target for treatment of oral cancer.
Abbreviations
- ADRB2
β2‐adrenergic receptor gene
- β‐AR
β‐adrenergic receptor
- EMT
epithelial‐mesenchymal transition
- HNSCC
head and neck squamous cell carcinoma
- IL‐6
interleukin‐6
- MET
mesenchymal‐epithelial transition
- OSCC
oral squamous cell carcinoma
- PKA
protein kinase A
- SM22α
smooth muscle protein 22α
- TGF‐β
transforming growth factor‐β
1. INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is a common type of cancer affecting the oral cavity and the pharynx. Worldwide, HNSCC accounts for more than 650 000 cases with and overall survival rate of 50%. 1 More than 90% of oral and oropharyngeal cancers are represented by OSCC. The poor prognosis of OSCC patients is associated with a lack of therapeutic methods that could effectively prevent metastasis to the sentinel lymph nodes and colonization of distant organs. The molecular mechanisms underlying OSCC progression remain largely uncharacterized, urging the identification of new potential therapeutic targets and the development of new therapeutic methods.
Metastasis involves many steps that can be targeted, but most approaches would rely on both the prevention of cancer cell detachment from primary tumors and suppression of colonization. 2 EMT is believed to play a major role in dissemination. During EMT, epithelial cancer cells reduce their cell‐cell contacts and acquire mesenchymal characteristics that result in motile and invasive phenotypes. 3 However, particular cancer cells still would retain some epithelial features, suggesting that they could undergo partial EMT. Recent studies have shown that partial EMT not only enhances cancer cell invasiveness, but also confers cancer cells with high tumor‐initiating potential by inducing the phenotypes of cancer stem cells. 4 , 5 , 6
Various types of signals have been implicated in the activation of metastatic pathways, endowing cancer cells with mesenchymal traits. Transforming growth factor‐β (TGF‐β), in particular, as an EMT driver has been widely studied. TGF‐β creates a favorable environment for facilitating cancer cell metastasis that confers epithelial cells with increased cell motility and invasive properties. 7 , 8 We have previously reported that TGF‐β signals enhance oral cancer cell migration, 9 suggesting the involvement of TGF‐β in OSCC progression. MET is a process reverse to EMT, during which cells with mesenchymal phenotype regain their epithelial identity. MET is believed to participate in the formation and stabilization of distant metastases. 10 However, recent reports have shown that the induction of MET in squamous cell carcinoma reduced its invasiveness, suggesting that MET‐inducing compounds could become potential therapeutic agents for prevention of metastases. 11
The signals from α‐ and β‐adrenergic receptors have been reported to control various cellular processes involved in tumorigenesis, such as cell motility, 12 cell growth, 13 inflammation, 14 and angiogenesis. 15 In particular, signals from β‐adrenergic receptors (β‐ARs) have been suggested to play a major role in tumor progression. The β‐ARs belong to the family of G‐protein‐coupled receptors. There are three subtypes of β‐ARs, β1‐, β2‐ and β3‐ARs, but mostly the signals from β2‐AR have been implicated in the progression of multiple types of cancer, such as pancreatic, 16 gastric, 17 or ovarian cancers. 18 The β2‐AR signals have been reported to regulate tumor immunosuppressive activities 19 , 20 and stimulate tumor angiogenesis. 18 Although it has been considered that activation of β2‐AR signals stimulates tumor progression, previous reports have indicated that the activation of β2‐AR may suppress tumor growth. 21 , 22
In addition, β2‐AR‐dependent signals have been implicated in the progression of OSCC, but the exact roles of β2‐AR remain controversial. While some studies have suggested the activation of β2‐AR signaling to promote oral cancer growth 23 or to induce EMT in OSCC, 24 other studies have demonstrated that OSCC patients who expressed high levels of β2‐AR expression had a higher overall survival rate compared with the patients with weak or negative β2‐AR expression levels. 25 Thus, the exact roles of β2‐AR signals in progression of OSCC remain to be elucidated.
In the present study, we demonstrated that the β2‐AR agonist isoxsuprine downregulated the expression of mesenchymal cell markers of multiple types of oral cancer cells and suppressed their motility. In addition, pharmacological and genetic approaches have shown that these inhibitory effects of isoxsuprine involved the signals mediated by β2‐AR. Furthermore, we also found that administration of isoxsuprine resulted in the inhibition of tumor growth in a mouse subcutaneous xenograft model of OSCC, suggesting a novel application for isoxsuprine as a drug for treatment of OSCC progression.
2. MATERIALS AND METHODS
2.1. Reagents
TGF‐β1 was purchased from Peprotech (Rocky Hill, NJ, USA) and used at a concentration of 1 ng/mL. SB431542 was obtained from FUJIFILM Wako Pure Chemical (Osaka, Japan) and used at a concentration of 10 μmol/L. Isoxsuprine hydrochloride (isoxsuprine), isoprenaline hydrochloride (isoprenaline), and propranolol hydrochloride (propranolol) were purchased from Sigma‐Aldrich (St. Louis, MO, USA) and used at concentrations of 0.01, 0.1, 1, or 10 µmol/L depending on the experiment.
2.2. RNA isolation and quantitative RT‐PCR
Total RNA was isolated using Sepasol RNA I Super G Reagent (Nacalai Tesque, Kyoto, Japan) and reverse‐transcribed using the ReverTraAce qPCR RT Master Mix (TOYOBO, Osaka, Japan). Quantitative RT‐PCR (qRT‐PCR) analysis was performed using SYBR Green (Roche, Basel, Switzerland) on a Step One Plus Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). All expression data were normalized to the expression of β‐actin. The genes and corresponding primer sequences are listed in Table S1.
2.3. Chamber migration assay
Cells were cultured in the absence or presence of TGF‐β1, SB431542, isoxsuprine, isoprenaline, and/or propranolol for 72 h. After the indicated period, cells were collected, seeded into the upper chamber of a 24‐well transwell with 8‐μm pore filter (BD Bioscience, New York, NY, USA) and allowed to migrate for 48 h. The filters were then stained with DiffQuik (Sysmex, Kobe, Japan), followed by removal of non‐migrated cells with a cotton swab. Migrated cells that adhered to the bottom side of the chamber were then photographed and counted using a light microscope at ×10 magnification. The experiments were performed in duplicate.
2.4. Cell proliferation assay
Cells were seeded into 6‐well culture plates and grown in a presence of TGF‐β1, SB431542, isoxsuprine, isoprenaline, and/or propranolol for 72 h, followed by direct counting of trypsinized cells using an hemocytometer. The experiments were performed in triplicate.
2.5. Generation of β2‐adrenergic receptor gene (ADRB2) knockout SAS oral cancer cells
The experimental procedures were approved by the Genetically Modified Organisms Safety Comittee of Tokyo Medical and Dental University (registration number C2019‐026C). ADRB2 knockout (ADRB2 KO) SAS cells were established using the clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9 (CRISPR/CAS9)‐mediated genome editing system. 26 Short guide RNA (sgRNA) target sequences for ADRB2: 5′‐CACAAAGCCCTCAAGACGTTAGG‐3′ (sgRNA #1) and 5′‐CCCTGTGCGTGATCGCAGTGGAT‐3′ (sgRNA #2) were designed with CRISPRdirect (https://crispr.dbcls.jp). The gRNAs were inserted into the pX459 plasmid (a gift from Feng Zhang; Addgene plasmid #62988; http://n2t.net/addgene:62988; RRID: Addgene_62988), carrying the human Cas9 expression cassette. SAS cells were transfected with the pX459‐sgADBR2 #1 or pX459‐sgADBR2 #2 plasmids and knockout clones were isolated by limiting dilution. The effective knockout of ADRB2 was confirmed by genome sequencing. SAS cells transfected with empty pX459 were used as the control (wild type: WT) cells.
2.6. Subcutaneous xenograft model and isoxsuprine administration
Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University (registration number: A2019‐251C2) and performed in accordance with the guidelines of the Animal Care Standards of Tokyo Medical and Dental University. SAS cells (1 × 103 cells in 100 μL of Matrigel; BD Bioscience) were inoculated subcutaneously into the left abdominal wall of BALB/c 6‐wk‐old male immunodeficient nude mice (Sankyo Labo Service, Tokyo, Japan). Once the tumor volume reached 50 mm3, the mice were randomly divided into 2 groups, a control group (n = 8) and an experimental group (n = 8). Mice were then administered daily by intraperitoneal injection with either vehicle (DMSO in PBS) or isoxsuprine (isoxsuprine, DMSO in PBS; dose: 6 mg/kg per mouse). Tumor growth was assessed at the indicated times with calipers and calculated from the minor axis and major radius using the following formula: V (mm3) = L × W2 × 0.5, where V corresponds to tumor volume (mm3), L to the length of the tumor (mm), and W to the tumor width (mm).
2.7. Statistical analyses
Significant differences between means were determined using one‐tailed unpaired Student t test or one‐way ANOVA with post hoc Tukey test using EZR software. 27 Values are presented as the mean ± standard deviation (SD) or standard error (SE). Differences between means were considered statistically significant at P values < 0.05.
Additional materials and methods are described in Supplementary information Documents S1 and S2.
3. RESULTS
3.1. Establishment of an oral cancer cell‐based model to screen for low‐molecular‐weight compounds stimulating mesenchymal‐epithelial transition
In the early steps of metastasis, epithelial cells are endowed with mesenchymal traits. Saito and colleagues reported that SAS oral cancer cells acquired mesenchymal features in response to TGF‐β, while their characteristics were shifted toward a more epithelial phenotype upon a treatment with SB431542, an inhibitor of TGF‐β receptor type I kinase. 28 As shown in Figure S1A, SAS cells incubated with TGF‐β underwent EMT, characterized by decreased staining for epithelial cell marker, E‐cadherin that was accompanied by increased expression of a mesenchymal cell marker, SM22α compared with untreated control cells. Conversely, in the presence of SB431542, SAS cells showed a more epithelial phenotype, as revealed by increased staining for E‐cadherin. These results suggested that SAS cells exhibited hybrid a epithelial‐mesenchymal phenotype, a so‐called partial EMT, and were able to undergo MET. Therefore, we utilized this SAS cell characteristics and established SAS cells that expressed green fluorescent protein (ZsGreen1) under the control of the CDH1/E‐cadherin promoter (SAS‐A3 clone) by introducing the PEcadZsG reporter construct 29 to screen for MET‐inducing agents. Treatment of the SAS‐A3 clone with TGF‐β led to the almost complete loss of fluorescence (Figure S1B) that resulted from the downregulation of the E‐cadherin promoter, as observed in cells undergoing the EMT program. Conversely, a significant increase in fluorescence intensity was seen in the presence of SB431542, indicating that SAS‐A3 cells can be used to visualize MET‐like changes (Figure S1B).
3.2. Isoxsuprine suppresses the mesenchymal features of multiple types of oral cancer cells
We utilized SAS‐A3 cells to screen a chemical library comprising 1600 low molecular weight well‐characterized compounds to search for compounds with MET‐inducing activity. As shown in Figure S2A, isoxsuprine, a vasodilating agent induced the expression of green fluorescent protein and increased cell‐cell contact in SAS‐A3 cells (Figure S2B), suggesting that it exhibited putative MET‐inducing activity. To confirm this finding, we examined the expression of various mesenchymal markers in SAS cells using qRT‐PCR analysis. As expected, the expression levels of vimentin (Figure 1A), N‐cadherin (Figure 1B), fibronectin (Figure 1C), and SM22α (Figure 1D) were upregulated by treatment with TGF‐β for 72 h (Figure 1A‐D). Concomitantly, SB431542 significantly decreased expression levels of vimentin (Figure 1A), fibronectin (Figure 1C), and SM22α (Figure 1D). Consistent with the screening results, the decreased expression of all mesenchymal markers was observed in SAS cells cultured in the presence of isoxsuprine (Figure 1A‐D), with the significant downregulation of vimentin (Figure 1A), N‐cadherin (Figure 1B) and fibronectin (Figure 1C). Isoxsuprine exhibited a stronger suppressive effect toward expression of vimentin (Figure 1A) and N‐cadherin (Figure 1B) compared with SB431542, suggesting that isoxsuprine suppressed the mesenchymal features of SAS cells. We also examined the effects of isoxsuprine at the protein level using immunocytochemical analysis. In agreement with the qRT‐PCR results, SAS cells cultured in the presence of TGF‐β exhibited a higher level of vimentin protein compared with the untreated control (Figure 1E,F). Treatment with isoxsuprine or SB431542 decreased vimentin protein expression (Figure 1E,F), suggesting that isoxsuprine inhibited the mesenchymal features of SAS cells.
FIGURE 1.
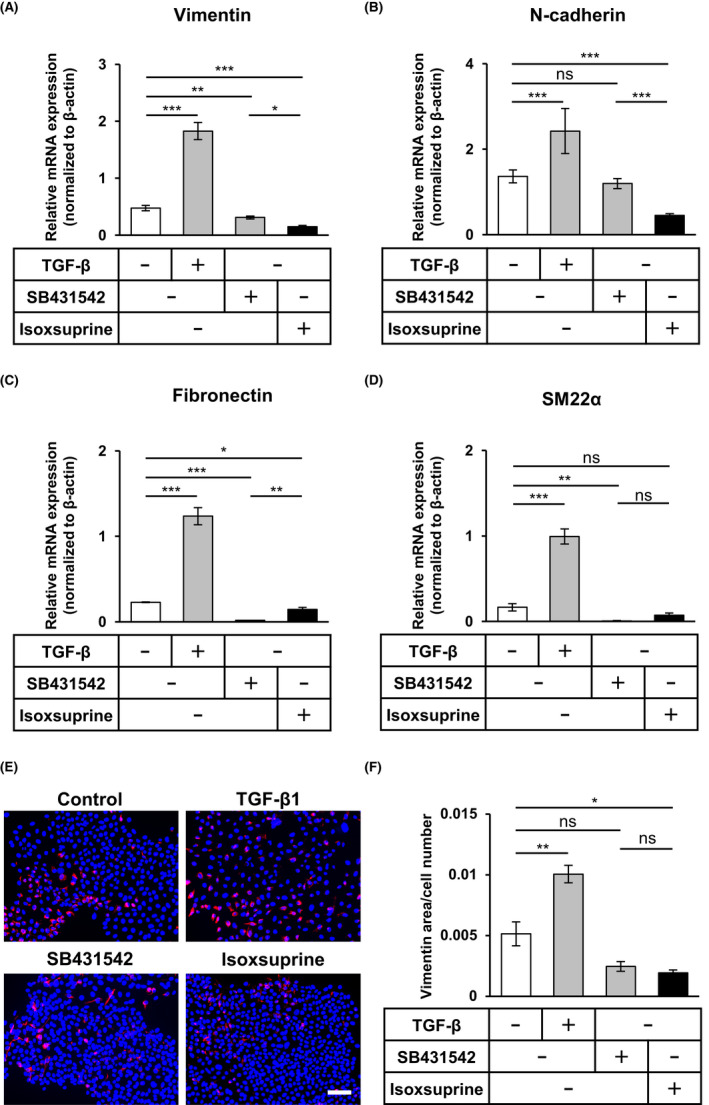
Effect of SB431542 and isoxsuprine, a β2‐adrenergic receptor agonist, on mesenchymal characteristics of SAS oral cancer cells. SAS cells were cultured in the absence (−/control) or presence of 1 ng/mL TGF‐β1, 10 μmol/L SB431542, or 10 μmol/L isoxsuprine for 72 h, followed by qRT‐PCR analyses for the expression of vimentin (A), N‐cadherin (B), fibronectin (C) SM22α (D) and fluorescence immunostaining (E) for vimentin (red) and nuclei (blue) and quantification of vimentin‐positive area (F). Scale bar: 100 μm. Data are represented as mean ± SD *P < 0.05 **P < 0.01 ***P < 0.001; ns, not significant
To generalize our findings, we utilized another human oral cancer cell line, HSC‐4. As already observed in SAS cells, SB431542 significantly decreased the expression of vimentin (Figure S3A), N‐cadherin (Figure S3B), fibronectin (Figure S3C) and SM22α (Figure S3D) in HSC‐4 cells. Isoxsuprine also significantly decreased the expression of all mesenchymal markers in HSC‐4 cells (Figure S3A‐D). These results suggested that isoxsuprine suppresses mesenchymal phenotypes in multiple types of oral cancer cell lines.
3.3. Isoxsuprine inhibits the migration of multiple types of oral cancer cells
Although the motility of cancer cells that exhibit an epithelial phenotype is low, cells undergoing EMT in response to TGF‐β display increased cell motility. 7 , 8 As shown in Figures S1C,D, TGF‐β significantly enhanced SAS cell migration, whereas treatment with SB431542 significantly reduced it; this was in agreement with a previous report. 28 As our results suggested that isoxsuprine induced a MET‐like program, we examined the effect of isoxsuprine on the migration of SAS cells. SAS cells were exposed to SB431542 or isoxsuprine for 72 h and subjected to a chamber migration assay. Isoxsuprine decreased the migration of SAS cells in a dose‐dependent fashion, and the effect of 10 μmol/L of isoxsuprine was comparable with 10 μmol/L of SB431542 (Figure 2A,B). Suppressed migration did not result from lower cell viability, as none of isoxsuprine concentrations significantly affected SAS cell proliferation (Figure 2C).
FIGURE 2.
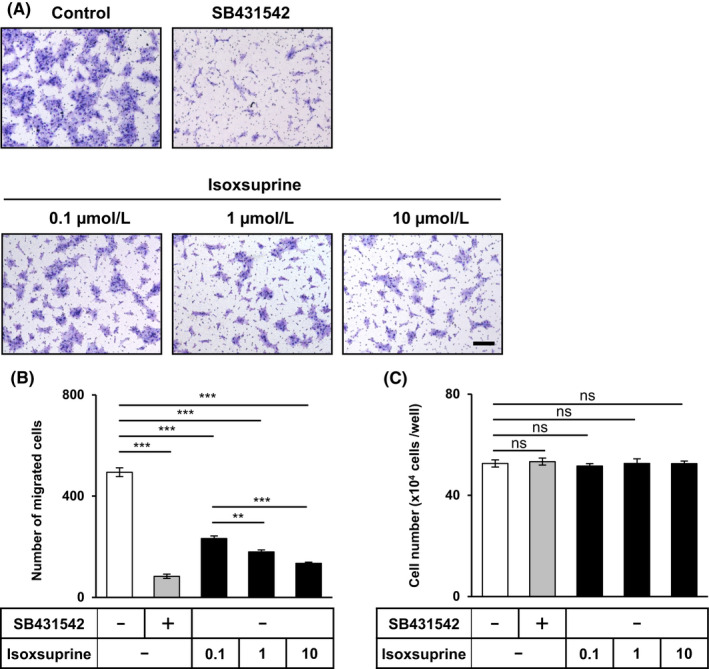
Effect of SB431542 and isoxsuprine on the migration and proliferation of SAS cells. A, B, SAS cells were cultured in the absence (−/control) or presence of 10 μmol/L SB431542 or various concentrations of isoxsuprine (0.1, 1, and 10 μmol/L) for 72 h. SAS cells migrated for 48 h were stained (A) and counted (B). Scale bar: 200 µm. (C) SAS cells were grown for 72 h in the absence (−) or presence of 10 μmol/L SB431542 or 10 μmol/L isoxsuprine and counted. Data are represented as mean ± SD **P < 0.01, ***P < 0.001; ns, not significant
The inhibitory effect of isoxsuprine on cell migration was also examined in HSC‐4 cells. As expected, isoxsuprine‐dependent inhibition of cell motility was also observed in HSC‐4 cells, however its effect was weaker than that induced by SB431542 (Figure S3E,F). As already observed in SAS cells, isoxsuprine did not show any inhibitory effect on proliferation of HSC‐4 cells (Figure S3G).
3.4. Isoxsuprine suppresses the mesenchymal phenotypes of SAS cells without affecting TGF‐β signaling
As both SB431542 and isoxsuprine suppressed mesenchymal phenotypes in a similar fashion, there was a possibility that isoxsuprine may interfere with TGF‐β signaling and in this way exhibit suppressive activities. To test this possibility, we used a TGF‐β signal‐responsive HEK‐Blue reporter cell system. This system allows direct monitoring of TGF‐β signaling by quantification of secreted alkaline phosphatase (SEAP) expressed under the control of Smad2/3/4‐inducible elements. Although HEK‐Blue cells treated with TGF‐β showed the activation of the Smad signal transduction pathway, as revealed by increased SEAP activity, this TGF‐β‐dependent activation of Smad signals was not affected by isoxsuprine (Figure S4A). These results were confirmed by the finding that TGF‐β‐induced expression of transmembrane prostate androgen‐induced protein (TMEPAI), a direct downstream target of TGF‐β was not affected (Figure S4B) by isoxsuprine. These results indicated that isoxsuprine‐mediated suppression of mesenchymal phenotypes did not involve inhibition of TGF‐β signals.
3.5. Activation of β2‐adrenergic receptor signals suppresses mesenchymal phenotypes of SAS cells
Isoxsuprine was originally developed as a β2‐AR agonist. 30 Therefore, we examined whether the isoxsuprine‐induced suppression of mesenchymal phenotypes of SAS cells involved β2‐AR signals. As it had been reported that activation of β2‐AR signaling induced the transcriptional response of interleukin‐6 (IL‐6), 31 we examined the effect of isoxsuprine on IL‐6 expression in SAS cells. As shown in Figure 3A, treatment of SAS cells with isoxsuprine significantly upregulated IL‐6 expression, suggesting that isoxsuprine activated β2‐AR signals. To examine whether the inhibitory effect was isoxsuprine‐specific or could be attributed to other β‐AR agonists, we performed the same set of experiments with isoprenaline (Figure S2C), the most commonly used β‐AR agonist that activates all β‐ARs. 32 Treatment with isoprenaline also upregulated IL‐6 expression (Figure 3B). Isoprenaline also inhibited the migration of SAS cells in a dose‐dependent manner (Figure 3C,D). Concomitantly, as for isoxsuprine, isoprenaline did not affect cell proliferation at any concentration tested (Figure 3E). Taken together, these results suggested that suppression of mesenchymal phenotypes of oral cancer cells by isoxsuprine relied on the adrenergic signals.
FIGURE 3.
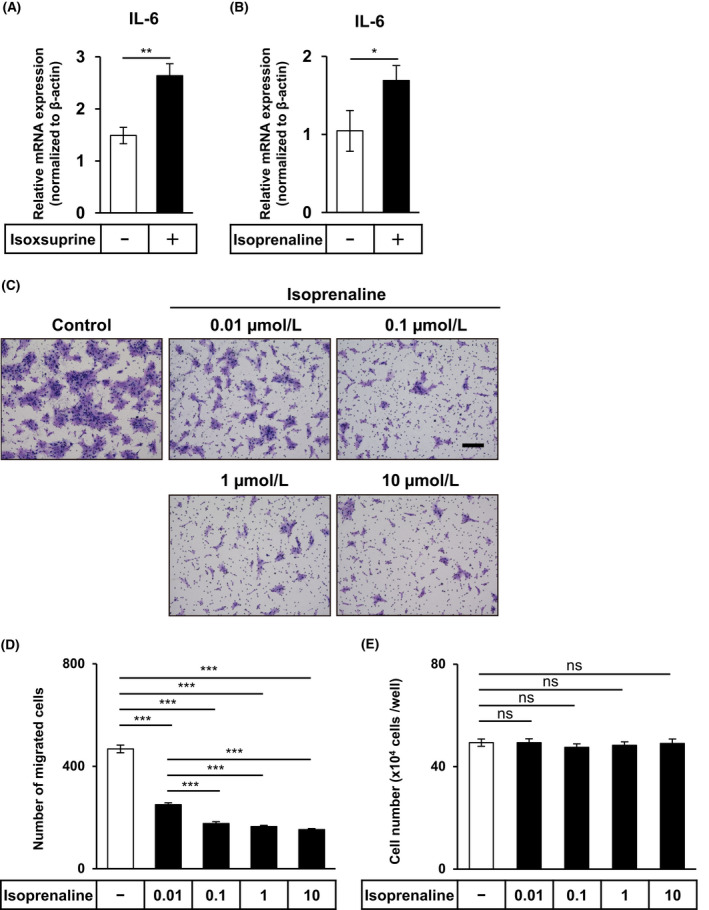
Effect of isoxsuprine and isoprenaline, a β‐adrenergic receptor agonist, on the activation of β‐adrenergic signals, migration, and proliferation of SAS cells. A, B, SAS cells were cultured in the absence (−) or presence of 10 μmol/L isoxsuprine (A) or 10 μmol/L isoprenaline (B) followed by qRT‐PCR analysis for the expression of IL‐6. C, D, SAS cells were cultured in the absence (−/control) or presence of various concentrations of isoprenaline (0.01, 0.1, 1, and 10 μmol/L). The cells migrated for 48 h were stained (C) and counted (D). Scale bar: 200 µm. E, SAS cells were grown for 72 h in the absence (−) or presence of various concentrations of isoprenaline and counted. Data are represented as mean ± SD *P < 0.05 **P < 0.01 ***P < 0.001; ns, not significant
3.6. Isoxsuprine‐induced suppression of mesenchymal phenotypes of SAS cells is inhibited by a β‐adrenergic receptor antagonist
We next examined whether the effects of isoxsuprine on SAS cells were altered by pharmacological inhibition of β‐AR signals by using propranolol, a non‐selective β‐AR antagonist. 33 To study whether propranolol could inhibit the anti‐migratory effects of isoxsuprine and isoprenaline, SAS cells were pretreated with propranolol and incubated for 72 h in the absence or presence of either isoxsuprine or isoprenaline, followed by chamber migration assay. Addition of propranolol almost completely abrogated the inhibitory effect of isoxsuprine on SAS cell migration (Figure 4A,B). Pretreatment with propranolol also exerted similar effects on isoprenaline‐induced inhibition of cell motility (Figure 4A,B), suggesting that isoxsuprine‐induced and isoprenaline‐induced inhibition of SAS cell migration was regulated by β‐adrenergic signals. Concomitantly, the proliferation of SAS cells cultured in the presence of propranolol alone or in combination with isoxsuprine or isoprenaline did not differ from proliferation of untreated cells (Figure 4C).
FIGURE 4.
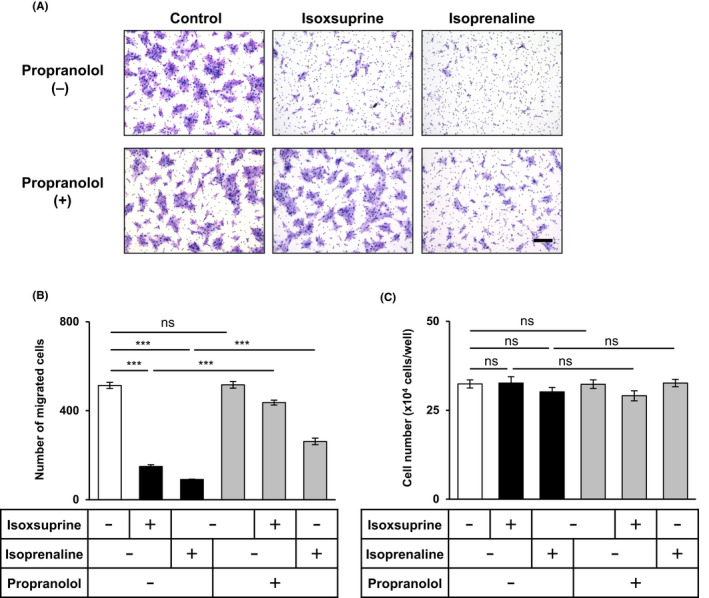
Effect of propranolol, a β‐adrenergic receptor antagonist, on the suppression of mesenchymal phenotypes of SAS cells by isoxsuprine and isoprenaline. The SAS cells were cultured in the absence (−/control) or presence of 10 μmol/L isoxsuprine or 10 μmol/L isoprenaline in combination with 10 μmol/L propranolol for 72 h followed by chamber migration (A, B) and cell proliferation assays (C). A, B, SAS cells migrated for 48 h were stained (A) and counted (B). Scale bar: 200 µm. (C) SAS cells cultured for 72 h in the absence (−) or presence of 10 μmol/L isoxsuprine or 10 μmol/L isoprenaline in combination with 10 μmol/L propranolol were counted. Data are represented as mean ± SD ***P < 0.001; ns, not significant
3.7. β2‐adrenergic receptor is indispensable for isoxsuprine‐induced suppression of mesenchymal phenotypes of SAS cells
Our finding that propranolol inhibited isoxsuprine‐induced suppression of mesenchymal phenotypes of SAS cells suggested that the mechanisms of action of isoxsuprine relied on the activation of β‐adrenergic signals that are mediated by β‐ARs (β1‐AR, β2‐AR and β3‐AR). As isoxsuprine was developed as a β2‐AR‐specific agonist, 30 we next examined whether β2‐AR played an essential role in isoxsuprine‐induced effects. We performed CRISPR/CAS9‐mediated genome editing 26 to establish SAS cells in which the β2‐AR gene (ADRB2) was deleted (Figure S5). As revealed by qRT‐PCR analysis, knocking out ADRB2 affected signals that induced the transcriptional response of IL‐6. Treatment with isoxsuprine did not upregulate IL‐6 expression (Figure 5A), suggesting effective genome editing that led to the inhibition of β2‐AR signals. In addition, knocking out of ADRB2 significantly upregulated the expression of mesenchymal markers, vimentin (Figure 5B) and N‐cadherin (Figure 5C) in comparison with the control (wild type: WT) cells that were established from parental SAS cells by transfection with an empty vector. Although incubation of WT cells with isoxsuprine downregulated the expression of both vimentin and N‐cadherin (Figure 5B,C), the expression levels of both markers in the ADRB2 knockout (ADRB2 KO #1) cells were not significantly decreased by isoxsuprine (Figure 5B,C) suggesting that β2‐AR was involved in the exerting inhibitory effects of isoxsuprine. We also performed immunocytochemical analysis to confirm our findings at the protein level. Consistent with qRT‐PCR data, in the ADRB2 KO #1 cells, increased expression of vimentin protein was observed compared with WT cells (Figure 5D,E). In addition, ADRB2 KO #1 cells did not respond to isoxsuprine treatment, as no changes in vimentin expression could be seen (Figure 5D,E). This upregulated expression of vimentin in ADRB2 KO #1 cells was also accompanied by increased cell motility. We observed a 2‐fold increase in the motility of ADRB2 KO #1 cells compared with WT cells (Figure 5F,G). Of note, incubation of ADRB2 KO #1 cells with isoxsuprine did not suppress cell motility (Figure 5F,G). To exclude the possibility of an off‐target effect, we established another SAS ADRB2 knockout (ADRB2 KO #2) clone using different guide RNA (Figure S5). In general, similar phenotypes, such as upregulated expression of mesenchymal markers and unresponsiveness to isoxsuprine were observed (Figure S6). These results indicated that β2‐AR is indispensable for isoxsuprine‐induced MET‐like changes in SAS cells.
FIGURE 5.
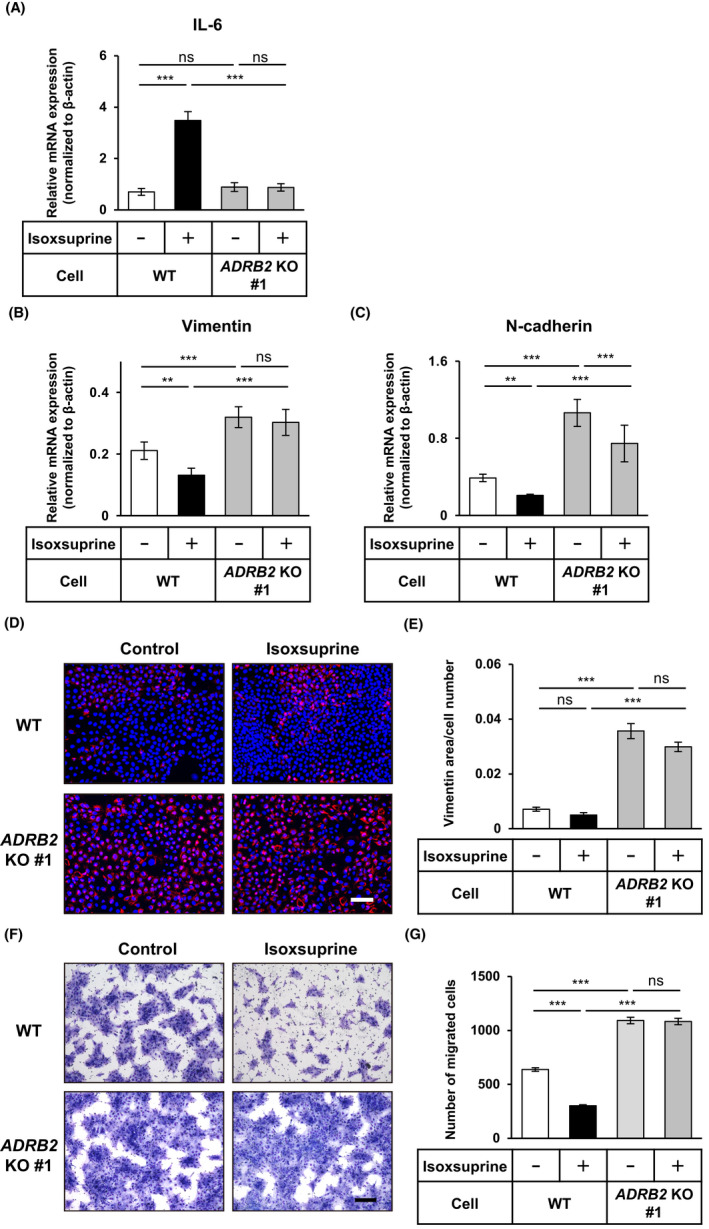
Roles of β2‐adrenergic receptor in isoxsuprine‐induced inhibition of mesenchymal traits. The control (wild type: WT) and β2‐AR gene (ADRB2) knockout (ADRB2 KO #1) SAS cells were cultured in the absence (−/control) or presence of 10 μmol/L isoxsuprine for 72 h, followed by the qRT‐PCR analyses for the expression of IL‐6 (A), vimentin (B), N‐cadherin (C), fluorescence immunostaining (D) for vimentin (red) and nuclei (blue) and quantification of vimentin‐positive area (E). Scale bar: 100 μm. F, G, WT and ADRB2 KO #1 cells were cultured in the absence (−/control) or presence of 10 μmol/L isoxsuprine for 72 h. The cells migrated for 48 h were stained (F) and counted (G). Scale bar: 200 μm. Data are represented as mean ± SD **P < 0.01 ***P < 0.001; ns, not significant
3.8. Isoxsuprine inhibits OSCC tumor growth in vivo
Recent lines of evidence have indicated that EMT programs confer cancer cells with high tumor‐initiating potential. 4 Therefore, the putative MET‐inducing properties of isoxsuprine in vitro prompted us to examine its effect on tumor growth in vivo. SAS cells were injected subcutaneously into the abdominal wall of immunodeficient mice. Once the tumor masses were palpable, the mice were grouped into 2 groups of 8 animals each, and were administered daily with isoxsuprine or vehicle. Administration with isoxsuprine resulted in significantly smaller primary tumors compared with the tumors developed by mice administered with vehicle (Figure 6A), suggesting a tumor‐suppressive effect of isoxsuprine. No significant changes in body weight of the group administrated with isoxsuprine was observed compared with the control group (Figure 6B).
FIGURE 6.
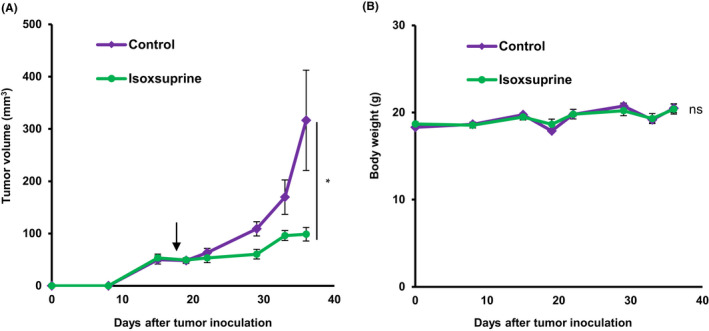
Effect of isoxsuprine on OSCC tumor growth in vivo. SAS cells were injected into the left abdominal wall of BALB/c nude mice. The mice were administered daily with the intraperitoneal injection of vehicle alone (control) or isoxsuprine (6 mg/kg per mouse) starting from the day 19 (arrow). Tumor growth and body weight were monitored for 5 wk. A, Tumor growth curves. B, Changes in body weight of mice with SAS cell xenotransplants. Data are represented as mean ± SE. *P < .05; ns, not significant
4. DISCUSSION
The poor prognosis of OSCC patients has not improved over the last decade and the lack of effective treatment rises the demand for development of new therapeutics. In this study, we revealed that a β2‐AR agonist, isoxsuprine, inhibited cell motility and induced MET‐like changes in multiple types of oral cancer cell lines through the activation of β2‐ARs. Administration of isoxsuprine in vivo effectively suppressed primary tumor growth, suggesting that isoxsuprine is a promising OSCC therapeutic agent.
In the present study, we used SAS oral cancer cells that exhibited both epithelial and mesenchymal phenotypes, representing a partial EMT state. The activation of the partial EMT program enables cancer cells to acquire stem‐like properties 34 that are often associated with increased invasiveness and drug resistance. 5 , 6 We found that isoxsuprine suppressed the mesenchymal features of SAS cells in vitro (Figures 1 and 2), suggesting that isoxsuprine shifted the cell phenotype toward a more epithelial phenotype and possibly induced a MET‐like state. The MET‐inducing activity of isoxsuprine seemed to differ from the one exerted by SB431542 and did not involve the direct inhibition of TGF‐β signals. Considering the dual roles of TGF‐β in the process of tumorigenesis, 4 isoxsuprine may represent a promising compound that inhibits only the mesenchymal properties of OSCC, without affecting tumor‐suppressive TGF‐β signals.
Multiple studies have shown that the activation of β2‐AR signals was associated with the progression of several types of cancers. 16 , 17 , 18 Zhang and colleagues claimed that treatment of oral cancer cells with a β2‐AR agonist, norepinephrine, resulted in the activation of β2‐AR signals, and stimulated oral cancer cell proliferation and invasion in vitro. 23 In contrast, we observed that two β2‐AR agonists, isoxsuprine and isoprenaline, inhibited the migration of SAS cells (Figures 2 and 3). This discrepancy may result from the cell model used in the previous study. Cal27 cells used by Zhang and colleagues in their study represents an adenosquamous carcinoma. Adenosquamous carcinoma shows a different growth pattern in vivo compared with other typical OSCCs 35 and therefore its response to both catecholamines may differ from the one exhibited by SAS and HSC‐4 cells.
Our results suggested that activation of β2‐AR signals have a beneficial effect on OSCC therapies. In support to our findings, Bravo‐Calderón and colleagues reported that higher levels of β2‐AR expression were positively correlated with a higher rate of overall survival in OSCC patients. 25 Moreover, the anti‐tumor effect of catecholamine‐derivative had been also found in other types of cancers, for example in breast and colon cancer models, in which administration of β2‐AR agonist, ARA‐211 (pirbuterol), resulted in tumor regression in mouse xenograft models. 22
The regulatory roles of β2‐AR in EMT progression under both physiological and pathological conditions have been reported. While some reports showed that EMT was induced by activation of β‐adrenergic signals, 36 others pointed out that blocking of β2‐AR would in contrast enhance EMT 37 and cancer progression. 38 Such differential effects of β2‐AR signals seem to be dependent on cellular contexts and the levels of β2‐AR expression, because decreased expression of β2‐AR has been reported to promote aggressiveness of prostate cancer. 39 Yu and colleagues observed that silencing of β2‐AR in transformed prostatic epithelial cells resulted in an increased expression of vimentin and N‐cadherin. These changes were accompanied by decreased expression of integrin‐β4, suggesting that cells had acquired a mesenchymal phenotype. In addition, silencing of β2‐AR was shown to be associated with increased motility and cancer invasion. 39 The EMT‐like changes upon suppression of β2‐AR signals appeared to be associated with altered activity of Rap1, a small GTPase that promotes cell‐matrix and cell‐cell adhesion and maintains the epithelial phenotype of pancreatic cells. 40 Therefore the suppression of β2‐AR signals resulted in decreased levels of cyclic adenosine monophosphate (cAMP) and subsequent inactivation of Rap1, leading to the loss of cell‐cell adhesion and EMT. Our present data also suggested crosstalk between β2‐AR and cellular programs that regulated mesenchymal traits of oral cancer cells. Knocking out ADRB2 in SAS cells upregulated the expression of mesenchymal markers and enhanced cell migration (Figure 5 and S6), suggesting that activation of β2‐AR suppressed OSCC progression. Whether the phenotype observed in SAS cells is controlled by similar mechanisms as the one reported by Yu and colleagues requires further study.
The present study revealed that the inhibitory effect of isoxsuprine on mesenchymal phenotype involved β‐AR signals. Canonical β‐ARs signaling involves binding of ligand to β‐ARs that triggers the synthesis of cAMP, resulting in the activation of protein kinase A (PKA). 41 A previous report by Pattabiraman and colleagues suggested that activation of PKA led to MET and loss of the tumor‐initiating ability in mesenchymal human mammary epithelial cells. 42 In addition, another report showed that treatment of melanoma cells with (R,R′)‐4′‐methoxy‐1‐naphthylfenoterol, a β2‐AR agonist, inhibited melanoma cell migration via activation of cAMP/PKA signals. 43 Whether the isoxsuprine‐induced suppression of mesenchymal phenotype observed in oral cancer cells also involved the activation of PKA requires additional study.
Previous studies have suggested that activation of β2‐AR signals promoted tumor growth by suppressing anti‐tumor immunity. 44 , 45 In contrast, our data revealed that a daily intraperitoneal administration of isoxsuprine retarded tumor growth in vivo (Figure 6), suggesting that β2‐AR signals play protective roles in oral cancer. In our study, we performed experiments using an immunodeficient mouse model, whereas other groups used immunocompetent mouse models, suggesting that the outcomes of targeting the β2‐AR signals may be influenced by the immunocompetency of the host. Considering this fact, the effect of isoxsuprine on systemic anti‐tumor immunity needs further evaluation.
Partial EMT is believed to promote collective cell migration. 6 In HNSCC patients, cancer cells that exhibited partial EMT were found at the leading edge of primary tumors, suggesting that partial EMT plays a role in local invasion and lymph node metastasis. 46 Isoxsuprine efficiently inhibited the motility of oral cancer cells in vitro, probably by shifting their phenotype toward more a epithelial phenotype. Such effect would be beneficial, as Takaishi and colleagues demonstrated that induction of MET in oral cancer cells by overexpressing reprogramming factors, Oct3/4, Sox2, Klf4, and Myc reduced the malignant potential of oral cancer cells in vivo. 11 Although the detailed mechanism by which isoxsuprine induces MET‐like changes remains to be identified, considering the fact that isoxsuprine represents an already existing approved drug, it might be considered as a promising compound for good therapeutic intervention in OSCC.
DISCLOSURE
The authors declare no competing interests.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr. Johji Inazawa (TMDU) for the kind gift of the PEcadZsG reporter plasmid; Megumi Naito, Hitomi Takahashi, and Ikumi Wakabayashi (TMDU) for technical assistance; and Dr. Yasuhiro Yoshimatsu (Niigata University) and members of the Department of Biochemistry, TMDU for critical discussion. This study was conducted through a research program of the Project for Cancer Research and Therapeutic Evolution (P‐CREATE), the Japan Agency for Medical Research and Development (AMED) (20cm0106253h0002 to TW). This work was also supported in part by a grant for the Grant‐in‐Aid for Scientific Research (C) (17K11828 to KAPI) from the Japan Society for the Promotion of Science (JSPS). Part of this research is based on the Project for Promoting Leading‐edge Research in Oral Science (to KAPI and TW) and Cooperative Research Project of Research Center for Biomedical Engineering (to MIY and HK) at Tokyo Medical and Dental University (TMDU).
Sakakitani S, Podyma‐Inoue KA, Takayama R, et al. Activation of β2‐adrenergic receptor signals suppresses mesenchymal phenotypes of oral squamous cell carcinoma cells. Cancer Sci. 2021;112:155–167. 10.1111/cas.14670
Funding informationJapan Agency for Medical Research and Development (Grant/ Award Number: 'P‐CREATE/20cm0106253h0002'); Japan Society for the Promotion of Science (Grant/ Award Number: 'Grant‐in‐Aid for Scientific Research (C)/17K1182'); and Tokyo Medical and Dental University (Grant/Award Number: 'Cooperative Research Project of Research Center for Promoting Leading‐edge Research in Oral Science').
Contributor Information
Katarzyna A. Podyma‐Inoue, Email: kapobch@tmd.ac.jp.
Tetsuro Watabe, Email: t-watabe@umin.ac.jp.
REFERENCES
- 1. Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 Cancer Groups, 1990 to 2017: a systemic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21‐45. [DOI] [PubMed] [Google Scholar]
- 4. Miyazono K, Katsuno Y, Koinuma D, Ehata S, Morikawa M. Intracellular and extracellular TGF‐β signaling in cancer: some recent topics. Front Med. 2018;12:387‐411. [DOI] [PubMed] [Google Scholar]
- 5. Saitoh M. Involvement of partial EMT in cancer progression. J Biochem. 2018;164:257‐264. [DOI] [PubMed] [Google Scholar]
- 6. Jolly MK, Somarelli JA, Sheth M, et al. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol Ther. 2019;194:161‐184. [DOI] [PubMed] [Google Scholar]
- 7. Katsuno Y, Lamouille S, Derynck R. TGF‐β signaling and epithelial‐mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76‐84. [DOI] [PubMed] [Google Scholar]
- 8. Moustakas A, Heldin CH. Mechanisms of TGFβ‐induced epithelial‐mesenchymal transition. J Clin Med. 2016;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi K, Podyma‐Inoue KA, Takao C, et al. Regulatory role of transforming growth factor‐β signals in the migration and tumor formation of HOC313‐LM cells, an oral squamous cell carcinoma. J Stomatolog Soc Jpn. 2018;85:52‐61. [Google Scholar]
- 10. Yao D, Dai C, Peng S. Mechanism of the mesenchymal‐epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608‐1620. [DOI] [PubMed] [Google Scholar]
- 11. Takaishi M, Tarutani M, Takeda J, Sano S. Mesenchymal to epithelial transition induced by reprogramming factors attenuates the malignancy of cancer cells. PLoS One. 2016;11:e0156904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim TH, Gill NK, Nyberg KD, et al. Cancer cells become less deformable and more invasive with activation of β‐adrenergic signaling. J Cell Sci. 2016;129:4563‐4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coelho M, Soares‐Silva C, Brandão D, Marino F, Cosentino M, Ribeiro L. β‐Adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol. 2017;143:275‐291. [DOI] [PubMed] [Google Scholar]
- 14. Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63:1115‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zahalka AH, Arnal‐Estapé A, Maryanovich M, et al. Adrenergic nerves activate an angio‐metabolic switch in prostate cancer. Science. 2017;358:321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Renz BW, Takahashi R, Tanaka T, et al. β2 Adrenergic‐neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33: 75‐90.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Zhang Y, He Z, et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 2019;10:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939‐944. [DOI] [PubMed] [Google Scholar]
- 19. Qin JF, Jin FJ, Li N, et al. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015;48:295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohammadpour H, MacDonald CR, Qiao G, et al. β2 adrenergic receptor‐mediated signaling regulates the immunosuppressive potential of myeloid‐derived suppressor cells. J Clin Invest. 2019;129:5537‐5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pérez Piñero C, Bruzzone A, Sarappa MG, Castillo LF, Lüthy IA. Involvement of α2‐ and β2‐adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br J Pharmacol. 2012;166:721‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carie AE, Sebti SM. A chemical biology approach identifies a beta‐2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf‐1/Mek‐1/Erk1/2 pathway. Oncogene. 2007;26:3777‐3788. [DOI] [PubMed] [Google Scholar]
- 23. Zhang B, Wu C, Chen W, et al. The stress hormone norepinephrine promotes tumor progression through β2‐adrenoreceptors in oral cancer. Arch Oral Biol. 2020;113:104712. [DOI] [PubMed] [Google Scholar]
- 24. Liu H, Wang C, Xie N, et al. Activation of adrenergic receptor β2 promotes tumor progression and epithelial mesenchymal transition in tongue squamous cell carcinoma. Int J Mol Med. 2018;41:147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bravo‐Calderón DM, Oliveira DT, Marana AN, Nonogaki S, Carvalho AL, Kowalski LP. Prognostic significance of beta‐2 adrenergic receptor in oral squamous cell carcinoma. Cancer Biomark. 2011;10:51‐59. [DOI] [PubMed] [Google Scholar]
- 26. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR‐Cas9 system. Nat Protoc. 2013;8:2281‐2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito D, Kyakumoto S, Chosa N, et al. Transforming growth factor‐β1 induces epithelial‐mesenchymal transition and integrin α3β1‐mediated cell migration of HSC‐4 human squamous cell carcinoma cells through Slug. J Biochem. 2013;153:303‐315. [DOI] [PubMed] [Google Scholar]
- 29. Harazono Y, Muramatsu T, Endo H, et al. miR‐655 is an EMT‐suppressive microRNA targeting ZEB1 and TGFBR2. PLoS One. 2013;8:e62757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ariens EJ, Waelen MJ, Sonneville PF, Simonis AM. The pharmacology of catecholamines and their derivatives. I. Relationship between structure and activity especially as far as the vascular system is concerned. Arzneimittelforschung. 1963;13:541‐546. [PubMed] [Google Scholar]
- 31. Madden KS, Szpunar MJ, Brown EB. β‐Adrenergic receptors (β‐AR) regulate VEGF and IL‐6 production by divergent pathways in high β‐AR‐expressing breast cancer cell lines. Breast Cancer Res Treat. 2011;130:747‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Billington CK, Penn RB, Hall IP. β2 Agonists. Handb Exp Pharmacol. 2017;237:23‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peixoto R, Pereira ML, Oliveira M. Beta‐blockers and cancer: where are we? Pharmaceuticals (Basel). 2020;13:E105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mani SA, Guo W, Liao MJ, et al. The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang L, Ji N, Zhou Y, et al. CAL 27 is an oral adenosquamous carcinoma cell line. Oral Oncol. 2009;45:e204‐e207. [DOI] [PubMed] [Google Scholar]
- 36. Shan T, Cui X, Li W, et al. Novel regulatory program for norepinephrine‐induced epithelial‐mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 2014;105:847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pagano F, Angelini F, Siciliano C, et al. Beta2‐adrenergic signaling affects the phenotype of human cardiac progenitor cells through EMT modulation. Pharmacol Res. 2018;127:41‐48. [DOI] [PubMed] [Google Scholar]
- 38. Gargiulo L, Copsel S, Rivero EM, et al. Differential β₂‐adrenergic receptor expression defines the phenotype of non‐tumorigenic and malignant human breast cell lines. Oncotarget. 2014;5:10058‐10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu J, Cao Q, Mehra R, et al. Integrative genomics analysis reveals silencing of beta‐adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419‐431. [DOI] [PubMed] [Google Scholar]
- 40. Jaśkiewicz A, Pająk B, Orzechowski A. The many faces of Rap1 GTPase. Int J Mol Sci. 2018;19:2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson GP. Current issues with β2‐adrenoceptor agonists: pharmacology and molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2006;31:119‐130. [DOI] [PubMed] [Google Scholar]
- 42. Pattabiraman DR, Bierie B, Kober KI, et al. Activation of PKA leads to mesenchymal‐to‐epithelial transition and loss of tumor‐initiating ability. Science. 2016;351:aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wnorowski A, Sadowska M, Paul RK, et al. Activation of β2‐adrenergic receptor by (R, R')‐4'‐methoxy‐1‐naphthylfenoterol inhibits proliferation and motility of melanoma cells. Cell Signal. 2015;27:997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bucsek MJ, Qiao G, MacDonald CR, et al. β‐adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T Cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77:5639‐5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen M, Qiao G, Hylander BL, et al. Adrenergic stress constrains the development of anti‐tumor immunity and abscopal responses following local radiation. Nat Commun. 2020;11:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puram SV, Tirosh I, Parikh AS, et al. Single‐cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171: 1611‐1624.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


