Abstract
Downregulation of human leukocyte antigen (HLA) class I has been postulated to be a mechanism of adaptive immune escape in various tumors, especially microsatellite instability–high (MSI‐H) colorectal cancer (CRC). In this study, we aimed to investigate HLA class I and β2‐microglobulin (β2M) expression in MSI‐H and microsatellite‐stable (MSS) CRCs and determine its prognostic impact. The representative areas from the tumor center (TC) and tumor periphery (TP) from 300 CRCs, including 161 MSI‐H and 139 MSS cases, were selected to construct a tissue microarray. Immunohistochemistry (IHC) for HLA A/B/C, β2M, CD3, and CD8 was performed. Reduced HLA A/B/C expression was detected in 113 (70.2%) MSI‐H and 54 (38.8%) MSS cases, while reduced β2M expression was observed in 69 (42.9%) MSI‐H and 17 (12.2%) MSS cases. Although reduced β2M expression was associated with higher pathological tumor (pT) stage in MSI‐H CRC with borderline significance, no association was found between HLA A/B/C and β2M expression and survival. Interestingly, reduced HLA A/B/C expression in MSS was associated with higher stage, and reduced HLA A/B/C and β2M expression was an independent prognostic factor in multivariate analysis. In conclusion, reduced HLA A/B/C and β2M expression was frequently observed in immunotherapy‐naive MSI‐H CRC, suggesting the possibility of primary resistance to immune checkpoint inhibitor. Interestingly, downregulation of HLA A/B/C and β2M was associated with poor prognosis in MSS cancers. Overall, IHC for HLA A/B/C and β2M might be a feasible predictive or prognostic tool in CRC.
Keywords: colorectal cancer, human leukocyte antigen class I, immunohistochemistry, microsatellite instability, β2‐microglobulin
Reduced HLA A/B/C and β2M expression is frequently observed in immunotherapy‐naive MSI‐H CRC, suggesting the possibility of primary resistance to immune checkpoint inhibitors. Interestingly, downregulation of HLA A/B/C and β2M was associated with poor survival in MSS cancers, but not in MSI‐H tumors, indicating that cell‐mediated antitumoral immune response may also play an important role in MSS CRC. Together, IHC for HLA A/B/C and β2M can be a predictive or prognostic tool in CRC.
![]()
1. INTRODUCTION
With the recent advent of immunotherapy, which attempts to block the immune evasion process of tumor cells, clinical outcome of various advanced cancers has improved substantially. 1 , 2 , 3 Although durable response is known to be a major advantage of immune checkpoint inhibitors, only a minority of patients benefit from the treatment. 4 , 5 , 6 To avoid unnecessary side effects and financial burden, selection of proper patient groups is required. Microsatellite instability (MSI), tumor mutation burden, and expression of PD‐L1 in tumor and tumor‐infiltrating immune cells have been suggested as predictive biomarkers. 7 Nevertheless, further validation and adjustment are needed for application. As the current immune checkpoint inhibitors mainly target the PD‐1/PD‐L1 axis, immune evasion through alteration of human leukocyte antigen (HLA) class I activity could be a possible explanation for the aforementioned limited response to treatment.
Human leukocyte antigen class I molecules form complexes with β2‐microglobulin (β2M) at the cell surface and present tumor‐derived neoantigens to cytotoxic T cells (CTLs), thereby contributing to a critical step in the cell‐mediated antitumor immune response. 8 Accordingly, downregulation of this HLA class I–associated antigen‐presenting process has been known as one of the important mechanisms via which tumor cells escape the immune response in various malignant tumors, including melanoma, 9 non–small cell lung cancer, 10 and breast cancer. 11 In colorectal cancer (CRC), tumors with MSI‐high (MSI‐H) phenotype are associated with increased mutational burden and significant tumor‐infiltrating immune cells, thereby eliciting prominent immune response. 12 Some researchers have suggested the downregulation of HLA class I as one of the immune evasion mechanism in MSI‐H CRC. 13 , 14 However, the extent of HLA class I downregulation in MSI‐H and microsatellite‐stable (MSS) CRC has not been investigated comprehensively.
A number of series have demonstrated that downregulation of HLA class I is associated with poor survival in diverse cancers, including pancreatic cancer, 15 non–small cell lung cancer, 16 esophageal cancer, 17 and gastric cancer. 18 However, in CRC, few studies have documented the prognostic impact of HLA class I, with contrasting results. 19 , 20 , 21 , 22 , 23 Generally, loss of HLA class I expression or mutation in β2M, which is one of the well‐known mechanisms leading to the loss of HLA class I, has been reported to be an indicator of good prognosis. 19 , 20 , 21 However, other studies have reported that downregulation of HLA class I was associated with poor outcome. 22 , 23 These previous studies were performed in mixed patient groups containing different proportion of MSI‐H and MSS CRC. Furthermore, investigation was usually performed for either HLA class I or β2M expression, and hence comprehensive analysis in both MSI‐H and MSS groups is required.
Therefore, in the present study, we aimed to investigate the expression of both HLA class I and β2M, and analyze their clinicopathological significance separately in MSI‐H and MSS CRC. We expect that our results will contribute to better understanding the potential role of HLA class I and β2M in CRC.
2. MATERIALS AND METHODS
2.1. Patients and specimens
Formalin‐fixed paraffin‐embedded (FFPE) surgically resected tissue specimens of CRC were obtained from 490 patients who were treated at Seoul National University Bundang Hospital from 2003 to 2012. The training set was composed of a total of 300 cases, including 161 MSI‐H tumors and 139 MSS tumors. An independent internal validation set was composed of 190 cases of MSS CRCs. Clinicopathological information, including sex, age, and stage was retrieved from the electronic medical records and pathology reports. Pathological staging was based on the eighth edition of the Cancer Staging Manual of the American Joint Committee on Cancer. The study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B‐1511/322‐306). The requirement for obtaining informed consent from patients was waived.
2.2. Construction of tissue microarray
Hematoxylin and eosin (H and E)‐stained slides were reviewed for confirming the original diagnosis and for selecting the most representative area. In the training set, the most representative tumor areas at the tumor center (TC) and tumor periphery (TP) were marked and a tissue microarray (TMA) was constructed as previously described using 2‐mm‐diameter cores derived from the FFPE tissue blocks. 24 In the validation set, the most representative tumor areas at TC were chosen to construct a TMA. 24
2.3. Microsatellite instability analysis
Microsatellite instability status was investigated by the fragmentation assay analysis, using a DNA autosequencer (ABI 3730 Genetic Analyzer, Applied Biosystems). Allele profiles of five markers (BAT‐26, BAT‐25, D5S346, D17S250, and D2S123) in tumor cells were compared with those of matched normal cells. The results were interpreted according to the Revised Bethesda Guidelines. 25
2.4. Immunohistochemistry ( IHC) analysis
In the training set, TMAs were sectioned at a thickness of 4 μm and stained using specific antibodies to HLA A/B/C (EMR8‐5, 1:200, Abcam), β2M (Β2M/961, 1:200, Abcam), CD3 (1:100; Dako), and CD8 (1:100; Dako). In the validation set, TMAs were stained using antibodies to HLA A/B/C (EMR8‐5, 1:200, Abcam) and β2M (Β2M/961, 1:200, Abcam) to confirm the prognostic significance of these proteins. Immunostaining was performed using the Ventana BenchMark XT staining system. For HLA A/B/C and β2M, both intensity and proportion (%) of tumor cell membrane were evaluated. The intensity of expression was classified into three categories: 0, negative; 1+, weakly positive; 2+, strongly positive. Weakly positive (1+) was defined as tumor cell membrane that stained weaker than lymphocytes or endothelial cells. Tumor cell membranes that showed staining equivalent or stronger than the internal control was considered as strongly positive (2+). For statistical analysis, retained expression was defined when 50% or more tumor cells showed strong positivity. Reduced expression was determined when <50% of the tumor cells were strongly positive, or ≥50% of the tumor cells were weakly positive. 22 , 26 The expression status was determined in TC and TP of each case. Finally, reduced expression in both TC and TP was defined as reduced expression. The open‐source software QuPath was utilized (https://qupath.github.io/) for the quantification of CD3‐positive and CD8‐positive lymphocytes. 27 The density of lymphocytes was calculated as the number of cells in a given area (mm2), and the mean density of TC and TP was used for statistical analysis.
2.5. Statistical analysis
The chi‐square test or Fisher exact test was used to assess the significance of the association of HLA A/B/C and β2M expression with clinicopathological parameters. Differences in the expression of HLA A/B/C and β2M and in TC and TP were analyzed using McNemar's test. Survival rates were calculated using the Kaplan‐Meier method, and statistical significance was assessed using the log‐rank test. The Cox proportional hazard regression model was used for multivariate analysis to determine the risk ratio and independent significance of individual factors for prognosis. All statistical tests were two‐sided, and P‐values < 0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences ver. 22 (IBM Corp.).
3. RESULTS
3.1. Clinicopathological characteristics
The clinicopathological features and results of IHC of the training set are summarized in Table 1. The median age of the patients was 64 years (range, 18‐90 years). The patients included 148 (49.7%) male and 151 (50.3%) female patients. In total, 168 (56%) and 132 (44%) patients were diagnosed as stage I and II, and stage III and IV, respectively. One (0.3%) patient received neoadjuvant and 192 (64.0%) patients received adjuvant chemotherapy, while four (1.3%) patients underwent both neoadjuvant and adjuvant chemotherapy. A total of 103 (34.3%) patients did not receive chemotherapy. The clinicopathological features of the validation set are summarized in Table S1.
Table 1.
Clinicopathologic characteristics according to microsatellite instability (MSI) status
| Characteristics | MSI‐H | MSS | P‐value | Total |
|---|---|---|---|---|
| Age (yr) | ||||
| Median (range) | 61 (18‐88) | 66 (30‐90) | .003* | 64 (18‐90) |
| Sex | ||||
| Male | 73 (45.3%) | 76 (54.7%) | .107 | 149 (49.7%) |
| Female | 88 (54.7%) | 63 (45.3%) | 151 (50.3%) | |
| Location | ||||
| Right | 135 (83.9%) | 49 (35.3%) | <.001* | 184 (61.3%) |
| Left | 26 (16.1%) | 90 (64.7%) | 116 (38.7%) | |
| Differentiation | ||||
| WD + MD | 122 (75.8%) | 134 (96.4%) | <.001* | 256 (85.3%) |
| PD | 39 (24.2%) | 5 (3.6%) | 44 (14.7%) | |
| Mucin | ||||
| Absent | 100 (62.1%) | 130 (93.5%) | <.001* | 230 (76.7%) |
| Present | 61 (37.9%) | 9 (6.5%) | 70 (23.3%) | |
| Lymphatic invasion | ||||
| Absent | 105 (65.2%) | 60 (43.2%) | <.001* | 165 (55.0%) |
| Present | 56 (34.8%) | 79 (56.8%) | 135 (45.0%) | |
| Perineural invasion | ||||
| Absent | 145 (90.1%) | 93 (66.9%) | <.001* | 238 (79.3%) |
| Present | 16 (9.9%) | 46 (33.1%) | 62 (20.7%) | |
| Venous invasion | ||||
| Absent | 153 (95%) | 116 (83.5%) | .001* | 269 (89.7%) |
| Present | 8 (5%) | 23 (16.5%) | 31 (10.3%) | |
| pT stage | ||||
| 1 + 2 | 27 (16.8%) | 20 (14.4%) | .571 | 47 (15.7%) |
| 3 + 4 | 134 (83.2%) | 119 (85.6%) | 253 (84.3%) | |
| pN stage | ||||
| 0 | 111 (68.9%) | 61 (43.9%) | <.001* | 172 (57.3%) |
| 1 + 2 | 50 (31.1%) | 78 (56.1%) | 128 (42.7%) | |
| pM stage | ||||
| 0 | 153 (95%) | 121 (87.1%) | .018* | 274 (91.3%) |
| 1 | 8 (5%) | 18 (12.9%) | 26 (8.7%) | |
| Stage | ||||
| I + II | 108 (67.1%) | 60 (43.2%) | <.001* | 168 (56%) |
| III + IV | 55 (34.2%) | 79 (56.8%) | 132 (44%) | |
| Chemotherapy | ||||
| None | 65 (40.4%) | 38 (27.3%) | .049* | 103 (34.3%) |
| Neoadjuvant | 1 (0.6%) | 0 (0.0%) | 1 (0.3%) | |
| Adjuvant | 92 (57.1%) | 100 (71.9%) | 192 (64.0%) | |
| Both | 3 (1.9%) | 1 (0.7%) | 4 (1.3%) | |
| HLA A/B/C | ||||
| Reduced | 113 (70.2%) | 54 (38.8%) | <.001* | 167 (55.7%) |
| Retained | 48 (29.8%) | 85 (61.2%) | 133 (44.3%) | |
| β2M | ||||
| Reduced | 69 (42.9%) | 17 (12.2%) | <.001* | 86 (28.7%) |
| Retained | 92 (57.1%) | 122 (87.8%) | 214 (71.3%) | |
| CD3+ lymphocytes (n/mm2) | ||||
| Median (range) | 582.3 (34.2‐2942.1) | 493.7 (26.5‐1633.4) | .001* | 512.9 (26.5‐2942.1) |
| CD8+ lymphocytes (n/mm2) | ||||
| Median (range) | 313.9 (21.0‐2495.5) | 213.8 (10.5‐769.5) | <.001* | 250.1 (10.5‐2495.5) |
| Total | 161 | 139 | 300 | |
Abbreviations: MD, moderately differentiated; MSI‐H, microsatellite instability–high; MSS, microsatellite stable; PD, poorly differentiated; pT, pathological tumor; pN, pathological node; pM, pathological metastasis; WD, well differentiated.
Statistically significant.
3.2. HLA A/B/C and β2M expression and clinicopathologic characteristics according to the MSI status
Compared with MSS CRCs, MSI‐H CRCs were associated with younger age (P = .003) and right‐sided disease (P < .001). Pathologically, poorly differentiated histology (P < .001) and mucin production (P < .001) were more common in MSI‐H tumors than in MSS tumors. Lymphatic (P < .001), perineural (P < .001) and venous invasion (P = .001), and higher stage (P < .001) were associated with the MSS phenotype (Table 1).
In MSI‐H CRC, reduced HLA A/B/C expression was observed in 113 (70.2%) cases, and reduced B2M expression was found in 69 (42.9%) cases, which was significantly more common than in MSS CRC (both P < .05) (Figure 1). MSI‐H CRCs showed significantly higher CD3‐positive and CD8‐positive lymphocyte density than MSS CRCs (both P < .05) (Table 1).
Figure 1.
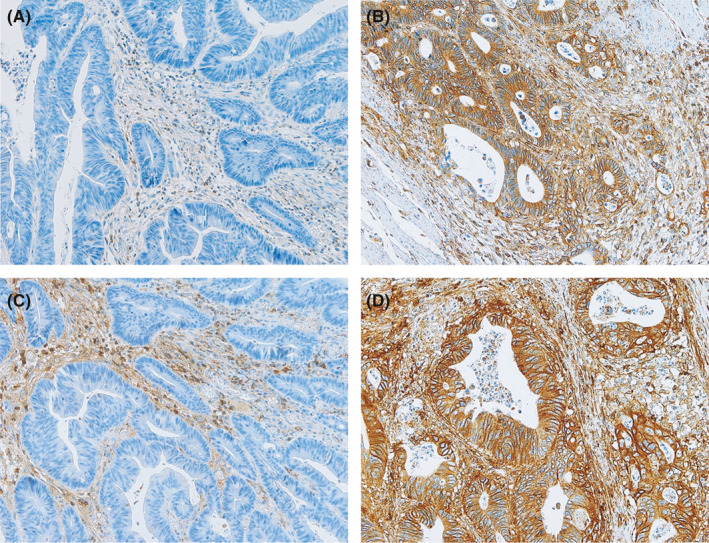
Representative examples of human leukocyte antigen (HLA) A/B/C and β2‐microglobulin (β2M) expression. A‐D, Tumor cells show reduced (A) and retained (B) HLA A/B/C expression, and reduced (C) and retained (D) β2M expression
3.3. Correlation of HLA A/B/C and β2M expression with clinicopathological features
In MSI‐H tumors, reduced HLA A/B/C was associated with the absence of mucin (P = .011). Reduced β2M expression was associated with higher pathological tumor (pT) stage with borderline significance (P = .051) (Table S2). In MSS tumors, reduced HLA A/B/C was associated with poorly differentiated histology (P = .008), higher pathological node (pN) stage (P = .019), and higher stage (P = .01). Reduced β2M was associated with left‐sided location (P = .03). (Table 2). In both MSI‐H and MSS CRCs, retained HLA A/B/C and β2M expression correlated with higher CD3‐positive and CD8‐positive lymphocyte density (all P < .05) (Figure 2). In the MSS validation set, both reduced HLA A/B/C and β2M expression were associated with higher pT and pM stage (all P < .05) (Table S1).
Table 2.
Humanleukocyteantigen(HLA) A/B/C and β2M expression and clinicopathologic characteristics in microsatellite‐stable (MSS) tumors
| Characteristics | HLA A/B/C | β2M | ||||
|---|---|---|---|---|---|---|
| Reduced | Retained | P‐value | Reduced | Retained | P‐value | |
| Sex | ||||||
| Male | 32 (23%) | 44 (31.7%) | 0.387 | 11 (7.9%) | 65 (46.8%) | .375 |
| Female | 22 (15.8%) | 41 (29.5%) | 6 (4.3%) | 57 (41%) | ||
| Location | ||||||
| Right | 21 (15.1%) | 28 (20.1%) | 0.474 | 10 (7.2%) | 39 (28.1%) | .030* |
| Left | 33 (23.7%) | 57 (41%) | 7 (5%) | 83 (59.7%) | ||
| Differentiation | ||||||
| WD + MD | 49 (35.3%) | 85 (61.2%) | 0.008* | 15 (10.8%) | 119 (85.6%) | .113 |
| PD | 5 (3.6%) | 0 (0%) | 2 (1.4%) | 3 (2.2%) | ||
| Mucin | ||||||
| Absent | 51 (36.7%) | 79 (56.8%) | >0.999 | 14 (10.1%) | 116 (83.5%) | .081 |
| Present | 3 (2.2%) | 6 (4.3%) | 3 (2.2%) | 6 (4.3%) | ||
| Lymphatic invasion | ||||||
| Absent | 19 (13.7%) | 41 (29.5%) | 0.13 | 6 (4.3%) | 54 (38.8%) | .484 |
| Present | 35 (25.2%) | 44 (31.7%) | 11 (7.9%) | 68 (48.9%) | ||
| Perineural invasion | ||||||
| Absent | 32 (23%) | 61 (43.9%) | 0.127 | 10 (7.2%) | 83 (59.7%) | .450 |
| Present | 22 (15.8%) | 24 (17.3%) | 7 (5%) | 39 (28.1%) | ||
| Venous invasion | ||||||
| Absent | 42 (30.2%) | 74 (53.2%) | 0.151 | 14 (10.1%) | 102 (73.4%) | >.999 |
| Present | 12 (8.6%) | 11 (7.9%) | 3 (2.2%) | 20 (14.4%) | ||
| pT stage | ||||||
| 1 + 2 | 7 (5%) | 13 (9.4%) | 0.703 | 3 (2.2%) | 17 (12.2%) | .713 |
| 3 + 4 | 47 (33.8%) | 72 (51.8%) | 14 (10.1%) | 105 (75.5%) | ||
| pN stage | ||||||
| 0 | 17 (12.2%) | 44 (31.7%) | 0.019* | 5 (3.6%) | 56 (40.3%) | .199 |
| 1 + 2 | 37 (26.6%) | 41 (29.5%) | 12 (8.6%) | 66 (47.5%) | ||
| pM stage | ||||||
| 0 | 44 (31.7%) | 77 (55.4%) | 0.119 | 15 (10.8%) | 106 (76.3%) | >.999 |
| 1 | 10 (7.2%) | 8 (5.8%) | 2 (1.4%) | 16 (11.5%) | ||
| Stage | ||||||
| 1 + 2 | 16 (11.5%) | 44 (31.7%) | 0.010* | 5 (3.6%) | 55 (39.6%) | .222 |
| 3 + 4 | 38 (27.3%) | 41 (29.5%) | 12 (8.6%) | 67 (48.2%) | ||
|
CD3+ lymphocytes (n/mm2) (median, range) |
357.1 (26.5‐1349.1) | 573.4 (136.3‐1633.4) | <0.001* | 180.1 (26.5‐900.3) | 512.9 (42.4‐1633.4) | .036* |
|
CD8+lymphocytes (n/mm2) (median, range) |
120.3 (10.6‐739.7) | 258.6 (58.6‐769.5) | 0.001* | 74.4 (10.5‐405.4) | 233.9 (19.1‐769.5) | .050* |
Abbreviations: MD, moderately differentiated; PD, poorly differentiated; pT, pathological tumor; pN, pathological node; pM, pathological metastasis.
Statistically significant.
Figure 2.
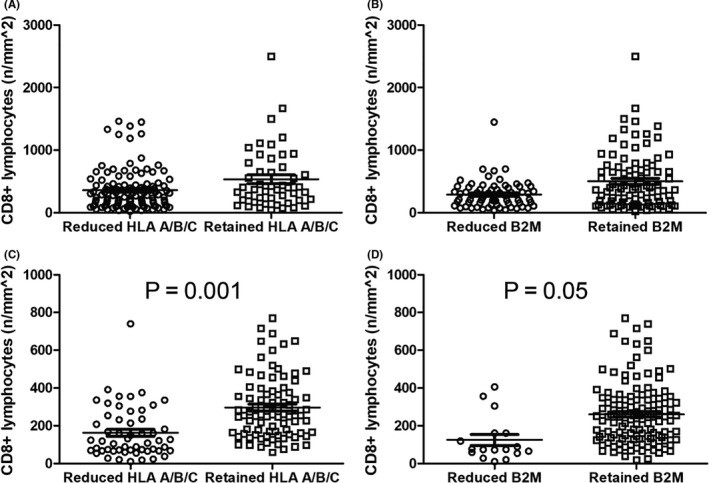
Differential CD8‐positive lymphocyte density according to human leukocyte antigen (HLA) A/B/C and β2‐microglobulin (β2M) expression. A and B, MSI‐high (MSI‐H) colorectal cancer (CRC). Retained HLA A/B/C (A) and retained β2M (B) correlated with higher CD8‐positive lymphocyte density (all P < .05). C and D, microsatellite‐stable (MSS) CRC. Retained HLA A/B/C (C) and retained β2M (D) also correlated with higher CD8‐positive lymphocyte density in MSS tumors (all P < .05)
3.4. Survival analysis
Kaplan‐Meier analysis revealed that reduced HLA A/B/C and β2M was associated with significantly inferior overall survival (OS) (P = .005 and P = .015) and disease‐free survival (DFS) (P = .028 and P = .005) (Figure 3) in MSS tumors. The expression of HLA A/B/C and β2M did not show statistically significant association with OS or DFS in MSI‐H cancers (Table S3). In multivariate analysis, reduced HLA A/B/C and β2M expression remained independent prognostic factors for shorter OS and DFS in MSS tumors (all P < .05) (Table 3). In contrast, lymphatic invasion, perineural invasion, and pathological metastasis (pM) stage, but not HLA A/B/C and β2M expression, were independent prognostic factors in MSI‐H CRC (Table S3). These results were confirmed by the MSS validation set, which revealed that reduced HLA A/B/C and β2M expression was associated with inferior OS in Kaplan‐Meier survival analysis (P = .04 and P = .018) (Figure 4). However, poorly differentiated histology, venous invasion, and pM stage, but not reduced HLA A/B/C and β2M expression, were independent prognostic factors in multivariate analysis (all P < .05) (Table S4).
Figure 3.
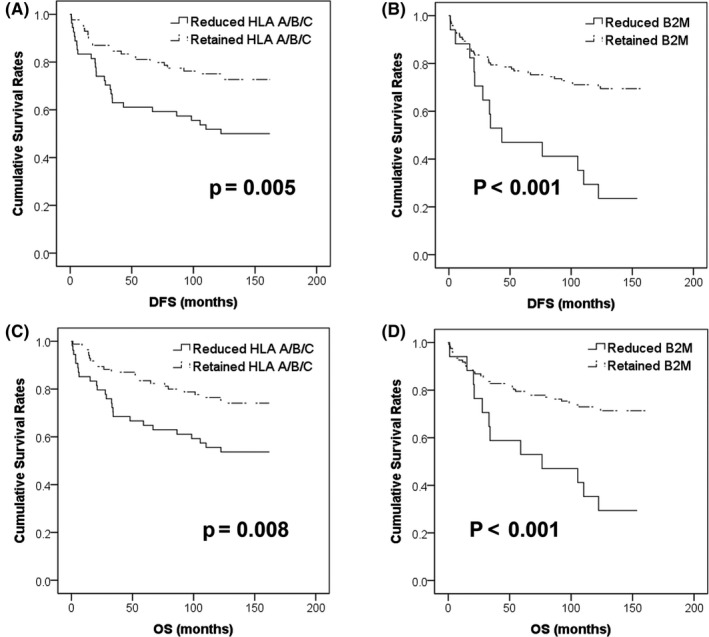
Kaplan‐Meier survival analyses in microsatellite‐stable (MSS) colorectal cancer (CRC). Reduced human leukocyte antigen (HLA) A/B/C (A) and β2‐microglobulin (β2M) (B) was significantly associated with poor disease‐free survival (DFS) (all P < .05). Similarly, reduced HLA A/B/C (C) and β2M (D) was also associated with shorter overall survival (OS) (all P < .05)
Table 3.
Univariate and multivariate Cox regression analysis in microsatellite‐stable (MSS) colorectal cancer patients
| Clinicopathologic variables | DFS | OS | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| P‐value | P‐value | HR (95% CI) | P‐value | P‐value | HR (95% CI) | |
| HLA A/B/C | .028* | .007* | 0.464 (0.265‐0.813) | .032* | 0.518 (0.283‐0.946) | |
| Differentiation | <.001* | NS | — | <.001* | NS | — |
| Lymphatic invasion | .01* | NS | — | .005* | NS | — |
| Perineural invasion | .001* | .007* | 2.169 (1.233‐3.816) | .004* | .019* | 2.020 (1.124‐3.631) |
| Venous invasion | .001* | NS | — | <.001* | NS | — |
| pT stage | .046* | NS | — | .068 | — | — |
| pN stage | .001* | .002* | 2.606 (1.409‐4.821) | .001* | .027* | 2.110 (1.089‐4.088) |
| pM stage | <.001* | <.001* | 4.011 (2.042‐7.878) | <.001* | <.001* | 5.443 (2.754‐10.759) |
| B2M | .001* | <.001* | 0.252 (0.130‐0.491) | .001* | <.001* | 0.285 (0.143‐0.568) |
| Differentiation | <.001* | .033* | 3.510 (1.105‐11.148) | <.001* | .030* | 3.607 (1.135‐11.460) |
| Lymphatic invasion | .01* | NS | — | .005* | NS | — |
| Perineural invasion | .001* | .004* | 2.323 (1.310‐4.117) | .004* | .025* | 1.958 (1.088‐3.521) |
| Venous invasion | .001* | NS | — | <.001* | NS | — |
| pT stage | .046* | NS | — | .068 | — | — |
| pN stage | .001* | .006* | 2.429 (1.294‐4.560) | .001* | .024* | 2.109 (1.102‐4.035) |
| pM stage | <.001* | <.001* | 4.607 (2.358‐9.003) | <.001* | <.001* | 5.080 (2.573‐10.031) |
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HLA, human leukocyte antigen; HR, hazard ratio; NS, not significant; OS, overall survival; pT, pathological tumor; pN, pathological node; pM, pathological metastasis.
Statistically significant.
Figure 4.
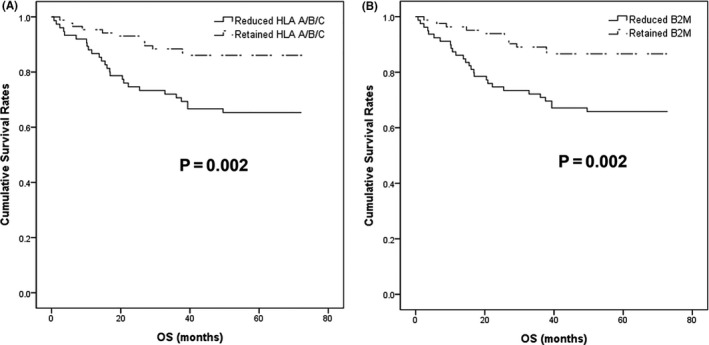
Kaplan‐Meier survival analyses in the microsatellite‐stable (MSS) validation set. Reduced human leukocyte antigen (HLA) A/B/C (A) and β2‐microglobulin (β2M) (B) was associated with poor overall survival (OS) (all P < .05)
4. DISCUSSION
In this study, we thoroughly evaluated the expression of HLA A/B/C and β2M in immunotherapy‐naive CRCs. Reduced HLA A/B/C and β2M expression was more often observed in MSI‐H CRCs than in MSS CRCs. Of note, reduced HLA A/B/C and β2M was associated with inferior survival in the MSS group (all P < .05), but not in the MSI‐H group, which was also confirmed in the validation set. Multivariate analysis in MSS CRC revealed reduced HLA A/B/C and β2M expression as independent prognostic factors.
Reduced HLA class I expression in MSI‐H CRCs was previously reported in 23%‐80%, whereas it accounted for 23%‐25% of MSS tumors. 21 , 28 , 29 Published data regarding β2M expression are limited, and the frequency of β2M downregulation in MSI‐H CRC was reported as 80% in one study. 28 The prevalence of β2M mutations in MSI‐H CRC was reported to be 7.5% to 33.9%, while that of MSS tumors was considerably low (<1%). 14 , 20 , 30 , 31 , 32 , 33 , 34 , 35 , 36 Considering that approximately 70% of β2M‐mutated MSI‐H CRC showed complete loss of expression in IHC according to the study done by Middha et al, 30 the frequency of HLA A/B/C and β2M downregulation detected in the current study is presumed to be higher in both MSI‐H (70.2% and 42.9%) and MSS (38.8% and 12.2%) cases than in previous studies. There are several possible explanations for this discrepancy. First, different types of antibodies with different cut‐off values were applied. Second, MSI status was variably determined by either IHC or fragment analysis among studies. Finally, post‐translational or epigenetic alteration can play a role in the downregulation.
The present study revealed that MSI‐H CRCs showed higher CD3‐positive and CD8‐positive lymphocyte density and more frequent reduced HLA A/B/C and β2M expression compared with MSS tumors. MSI which is caused by epigenetic silencing or mutation of DNA mismatch repair genes leads to accumulation of mutations in genes enriched with microsatellite regions. 37 These mutations are known to create tumor neoantigens, which can be presented to CTLs by MHC class I molecules and elicit brisk antitumor immune response. 38 Recently, downregulation of HLA class I molecules, including β2M, has been suggested as one of possible mechanisms of immune evasion in these immunogenic tumors. 4 , 13 , 14 The presence of β2M mutation has been reported in both immunotherapy‐naïve 32 , 33 , 34 , 35 , 36 , 39 and immunotherapy‐resistant MSI‐H CRCs, 4 suggesting that it can cause both intrinsic and acquired resistance. Our results are in line with these previous studies, suggesting immune evasion through altered HLA class I molecules might take place in a considerable proportion of MSI‐H CRCs even before immunotherapy. To date, it is unclear whether downregulated HLA class I in preexisting subclone leads to treatment failure or new clones appear secondary to immunotherapy exposure. 40 There are limited data regarding immunotherapy response in β2M mutated immunotherapy‐naïve MSI‐H CRCs; however, loss of β2M was more common in immunotherapy nonresponder melanoma patients. 34 Although further investigation including treatment response is required, β2M IHC could be a feasible tool for selecting the correct patient subgroup in MSI‐H CRC.
Although there was no association between HLA A/B/C and β2M expression and survival in MSI‐H CRCs, reduced β2M was associated with higher pT stage with borderline significance (Table S2). Similarly, Viktor et al 41 have demonstrated that HLA class I loss was frequently associated with higher tumor budding at the invasive front, suggesting that the tumor cells might acquire invasive ability via immune evasion. Considering that high mutation burden of MSI‐H CRC triggers massive immune cell reaction, complex immune context, including NK cells and possibly the PD‐1/PD‐L1 axis, might be the reason why we did not observe any prognostic significance in this group.
The prognostic impact of HLA class I downregulation differs highly among different tumors. Intact HLA class I expression has been reported to be associated with better survival in melanoma, non–small cell lung cancer, pancreatic cancer, and esophageal squamous cell carcinoma. 15 , 16 , 17 Conversely, retained HLA class I was described as an independent prognostic factor for poor outcome in gastric cancer. 18 In CRC, retained HLA class I expression or β2M mutation has generally been reported to be associated with poor prognosis. 20 , 21 , 23 However, Speetjens et al 22 have reported that loss of HLA class I was associated with inferior survival in rectal cancer. These contradicting results might be partially due to the inhibitory role of HLA‐B/C on natural killer (NK) cells, which, when downregulated, renders tumor cells more vulnerable to the cytotoxicity of NK cells. 42 , 43 However, more importantly, there appears to be a selection bias. As reduced HLA class I is more frequently observed in MSI‐H CRCs than in MSS CRCs, and MSI‐H cases are associated with better clinical outcome, different proportions of MSI‐H CRC among the studies might have resulted in these contrasting observations. In rectal cancer cases mostly composed of the MSS type, loss of HLA class I was associated with poor survival, 22 while contrasting results were reported in other studies that included higher proportion of MSI‐H CRCs. 21 Similar to the results from the rectal cancer cohort, 22 we demonstrated that reduced HLA A/B/C and β2M was associated with inferior survival in MSS CRCs. Of note, reduced β2M was an independent prognostic factor for DFS and OS in the MSS group, suggesting the clinical importance of β2M in this group as well as in MSI‐H CRCs. Furthermore, reduced HLA A/B/C and β2M correlated with lower CD8‐positive lymphocyte density, suggesting the potential role of T‐cell–mediated antitumor immunity in MSS tumors as well as MSI‐H counterpart.
In this study, β2M expression tended to be more preserved than that of HLA A/B/C in both MSI‐H and MSS cases. These are in agreement with the fact that complex mechanisms other than β2M impairment are involved in HLA class I downregulation. 44 , 45 , 46 Indeed, several phenotypes of HLA class I alteration are known, including total loss, haplotype loss, locus loss, and allelic loss, which result from changes at various steps of HLA class I expression such as protein synthesis, assembly, transport, or cell surface expression. 44 , 46 Moreover, these changes can occur at genetic, epigenetic, transcriptional, and/or posttranscriptional levels. 45 Among various alterations, β2M mutation has been known to be one of the most common mechanisms of HLA class I downregulation in MSI‐H CRC, while its frequency is considerably lower in MSS tumors. 14
Our study has certain limitations. This was a retrospective study, and IHC was conducted on the TMA slides, raising the possibility of sampling bias. In addition, clinical information regarding immune checkpoint blockade was not available. Nonetheless, compared with previous studies, the patient cohort in this study is large and homogenous, with few confounding factors, rendering the results reliable.
In conclusion, reduced HLA A/B/C and β2M expression is frequently observed in immunotherapy‐naive MSI‐H CRC, suggesting the possibility of primary resistance to immune checkpoint inhibitors. Interestingly, downregulation of HLA A/B/C and β2M was associated with poor survival in MSS cancers, but not in MSI‐H tumors. These results indicated that cell‐mediated antitumoral immune response may also play an important role in MSS CRC. Together, IHC for HLA A/B/C and β2M can be a predictive or prognostic tool in CRC. Further investigations regarding the detailed molecular mechanisms and correlation with clinical response to immunotherapy are required.
DISCLOSURE
The authors have no conflicts of interest to disclose.
Supporting information
Table S1–S4
ACKNOWLEDGMENT
This work was supported by grant no 14‐2017‐002 from the SNUBH Research Fund. Our study was presented as “oral presentation” at the 2020 annual meeting of the United States & Canadian Academy of Pathology (USCAP), but the whole paper was not published in any other journal.
Na HY, Park Y, Nam SK, et al. Expression of human leukocyte antigen class I and β2‐microglobulin in colorectal cancer and its prognostic impact. Cancer Sci. 2021;112:91–100. 10.1111/cas.14723
DATA AVAILABILITY STATEMENT
All data are available upon request.
REFERENCES
- 1. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 2. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non‐small‐cell lung cancer: two‐year outcomes from two randomized, open‐label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924‐3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521‐2532. [DOI] [PubMed] [Google Scholar]
- 4. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science. 2017;357:409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair‐deficient/microsatellite instability‐high metastatic colorectal cancer. J Clin Oncol. 2018;36:773‐779. [DOI] [PubMed] [Google Scholar]
- 6. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor‐based immunotherapy. Lancet Oncol. 2016;17:e542‐e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Townsend A, Bodmer H. Antigen recognition by class I‐restricted T lymphocytes. Annu Rev Immunol. 1989;7:601‐624. [DOI] [PubMed] [Google Scholar]
- 9. Chang CC, Pirozzi G, Wen SH, et al. Multiple structural and epigenetic defects in the human leukocyte antigen class I antigen presentation pathway in a recurrent metastatic melanoma following immunotherapy. J Biol Chem. 2015;290:26562‐26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGranahan N, Rosenthal R, Hiley CT, et al. Allele‐specific HLA loss and immune escape in lung cancer evolution. Cell. 2017;171:1259‐1271 e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M, Zhang C, Song Y, et al. Mechanism of immune evasion in breast cancer. Onco Targets Ther. 2017;10:1561‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kloor M, von Knebel DM. The immune biology of microsatellite‐unstable cancer. Trends Cancer. 2016;2:121‐133. [DOI] [PubMed] [Google Scholar]
- 13. Grasso CS, Giannakis M, Wells DK, et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018;8:730‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozcan M, Janikovits J, von Knebel DM, Kloor M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology. 2018;7:e1445453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imai D, Yoshizumi T, Okano S, et al. The prognostic impact of programmed cell death ligand 1 and human leukocyte antigen class I in pancreatic cancer. Cancer Med. 2017;6:1614‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kikuchi E, Yamazaki K, Torigoe T, et al. HLA class I antigen expression is associated with a favorable prognosis in early stage non‐small cell lung cancer. Cancer Sci. 2007;98:1424‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizukami Y, Kono K, Maruyama T, et al. Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2008;99:1462‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ueda Y, Ishikawa K, Shiraishi N, Yokoyama S, Kitano S. Clinical significance of HLA class I heavy chain expression in patients with gastric cancer. J Surg Oncol. 2008;97:451‐455. [DOI] [PubMed] [Google Scholar]
- 19. Menon AG, Morreau H, Tollenaar RA, et al. Down‐regulation of HLA‐A expression correlates with a better prognosis in colorectal cancer patients. Lab Invest. 2002;82:1725‐1733. [DOI] [PubMed] [Google Scholar]
- 20. Tikidzhieva A, Benner A, Michel S, et al. Microsatellite instability and Beta2‐Microglobulin mutations as prognostic markers in colon cancer: results of the FOGT‐4 trial. Br J Cancer. 2012;106:1239‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeestraten EC, Reimers MS, Saadatmand S, et al. Combined analysis of HLA class I, HLA‐E and HLA‐G predicts prognosis in colon cancer patients. Br J Cancer. 2014;110:459‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Speetjens FM, de Bruin EC, Morreau H, et al. Clinical impact of HLA class I expression in rectal cancer. Cancer Immunol Immunother. 2008;57:601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watson NF, Ramage JM, Madjd Z, et al. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118:6‐10. [DOI] [PubMed] [Google Scholar]
- 24. Lee KS, Kwak Y, Ahn S, et al. Prognostic implication of CD274 (PD‐L1) protein expression in tumor‐infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol Immunother. 2017;66:927‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chew SF, Kanaan C, Tait BD. HLA expression and cancer–14th IHIWS immunohistochemistry quality control exercise exchange results. Tissue Antigens. 2007;69(Suppl 1):248‐251. [DOI] [PubMed] [Google Scholar]
- 27. Bankhead P, Loughrey MB, Fernandez JA, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dierssen JW, de Miranda NF, Mulder A, et al. High‐resolution analysis of HLA class I alterations in colorectal cancer. BMC Cancer. 2006;6:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kubo T, Hirohashi Y, Matsuo K, et al. Mismatch repair protein deficiency is a risk factor for aberrant expression of HLA Class I molecules: a putative, "adaptive immune escape" phenomenon. Anticancer Res. 2017;37:1289‐1295. [DOI] [PubMed] [Google Scholar]
- 30. Middha S, Yaeger R, Shia J, et al. Majority of B2M‐mutant and ‐deficient colorectal carcinomas achieve clinical benefit from immune checkpoint inhibitor therapy and are microsatellite instability‐high. JCO Precis Oncol. 2019;3:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kašubová I, Sňahničanová Z, Kalman M, Plank L, Lasabová Z. 646PMutation analysis of B2M gene in colorectal cancer patients with microsatellite instability. Ann Oncol. 2019;30:v243. [Google Scholar]
- 32. Clendenning M, Huang A, Jayasekara H, et al. Somatic mutations of the coding microsatellites within the beta‐2‐microglobulin gene in mismatch repair‐deficient colorectal cancers and adenomas. Fam Cancer. 2018;17:91‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janikovits J, Muller M, Krzykalla J, et al. High numbers of PDCD1 (PD‐1)‐positive T cells and B2M mutations in microsatellite‐unstable colorectal cancer. Oncoimmunology. 2018;7:e1390640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sade‐Feldman M, Jiao YJ, Chen JH, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snahnicanova Z, Kasubova I, Kalman M, et al. Genetic and epigenetic analysis of the beta‐2‐microglobulin gene in microsatellite instable colorectal cancer. Clin Exp Med. 2020;20:87‐95. [DOI] [PubMed] [Google Scholar]
- 36. Yeon Yeon S, Jung SH, Jo YS, et al. Immune checkpoint blockade resistance‐related B2M hotspot mutations in microsatellite‐unstable colorectal carcinoma. Pathol Res Pract. 2019;215:209‐214. [DOI] [PubMed] [Google Scholar]
- 37. Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor‐infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417‐2422. [PubMed] [Google Scholar]
- 38. Tougeron D, Fauquembergue E, Rouquette A, et al. Tumor‐infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009;22:1186‐1195. [DOI] [PubMed] [Google Scholar]
- 39. Kloor M, Becker C, Benner A, et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418‐6424. [DOI] [PubMed] [Google Scholar]
- 40. Sahin IH, Akce M, Alese O, et al. Immune checkpoint inhibitors for the treatment of MSI‐H/MMR‐D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. 2019;121:809‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koelzer VH, Dawson H, Andersson E, et al. Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Transl Res. 2015;166:207‐217. [DOI] [PubMed] [Google Scholar]
- 42. Ljunggren HG, Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990;11:237‐244. [DOI] [PubMed] [Google Scholar]
- 43. Long EO. Tumor cell recognition by natural killer cells. Semin Cancer Biol. 2002;12:57‐61. [DOI] [PubMed] [Google Scholar]
- 44. Garcia‐Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346‐355. [DOI] [PubMed] [Google Scholar]
- 45. Garrido F, Cabrera T, Aptsiauri N. "Hard" and "soft" lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249‐256. [DOI] [PubMed] [Google Scholar]
- 46. Maleno I, Cabrera CM, Cabrera T, et al. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics. 2004;56:244‐253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Data Availability Statement
All data are available upon request.


