Abstract
Reliable biomarkers for upper‐tract urothelial carcinoma (UTUC) have yet to be found. Plasma cell‐free DNA (cfDNA) has been clinically applied as a minimally invasive blood biomarker for various types of cancer. We investigated the utility of plasma cfDNA as a blood biomarker in UTUC patients. The fragment size of plasma cfDNA was shorter and the concentration of plasma cfDNA was higher in UTUC patients than in healthy controls. The fragment size of plasma cfDNA had a moderate accuracy of diagnosing UTUC (area under the curve [AUC] = 0.72), and multivariate analysis indicated that the fragment size of plasma cfDNA was significantly associated with the presence of UTUC (odds ratio = 0.807, 95% confidence interval [CI] 0.653‐0.955, P = .024). Furthermore, we found that the size of plasma cfDNA shortens alongside disease progression (P < .001). The fragment size of plasma cfDNA in UTUC patients may be an auxiliary tool for the diagnosis of UTUC patients. We also found a high correlation between the fragmentation of plasma cfDNA and serum levels of three inflammatory cytokines (TNFα [r = −.837], interleukin‐6 [IL‐6] [r = −.964], interleukin‐1 receptor antagonist [IL‐1ra] [r = −.911]), which were reported to associate with poor prognosis. Also, we found that the proportion of short fragments of cfDNA was significantly increased in the supernatant of peripheral blood mononuclear cells (PBMCs) from healthy controls cultured in media containing TNFα. These results supposed that cancer‐associated systemic inflammation, especially tumor necrosis factor‐α (TNFα), may contribute to the fragmentation of plasma cfDNA in UTUC patients.
Keywords: cell‐free DNA, fragment size, systemic inflammation, TNFα, upper‐tract urothelial carcinoma
We found that the fragment size of plasma cell‐free DNA (cfDNA) had moderate accuracy for the diagnosis of upper‐tract urothelial carcinoma (UTUC). We also found that the size of plasma cfDNA shortens alongside disease progression and clarified that the fragmentation of cfDNA was caused by cancer‐related systemic inflammation, especially tumor necrosis factor‐α (TNFα).

Abbreviations
- Alb
albumin
- cfDNA
cell‐free DNA
- CI
confidence interval
- CRP
C‐reactive protein
- EMEM
Eagle's minimal essential medium
- Hb
hemoglobin
- IL‐6
interleukin‐6
- IL‐1ra
interleukin‐1 receptor antagonist
- NLR
neutrophil to lymphocyte ratio
- OS
overall survival
- PBMCs
peripheral blood mononuclear cells
- Plt
platelet
- OR
odds ratio
- ROC
receiver operating characteristic
- RPMI
Roswel Park Memorial Institute
- TNFα
tumor necrosis factor‐α
- UBC
urothelial bladder cancer
- UTUC
upper‐tract urothelial carcinoma
- WBC
white blood cell
1. INTRODUCTION
Urothelial carcinoma (UC) is the fourth most common prevalent cancer globally. 1 These tumors may be located in the lower (bladder and urethra) or the upper (pyelocaliceal cavities and ureter) urinary tract. In the diagnosis of UC, urinary cytology is routinely used in combination with endoscopy, ultrasound, computed tomography (CT), and so on, but the accuracy of urine cytology is not so high. Lower‐tract urothelial carcinoma (LTUC), mainly urothelial bladder cancer (UBC), is relatively easily diagnosed using cystoscopy or abdominal ultrasound. On the other hand, it is difficult to diagnose upper‐tract urothelial carcinoma (UTUC) because of the lack of sufficient imaging methods, and because urinary cytology is the only reliable method to diagnose UTUC. UTUC is uncommon and accounts for only 5%‐10% of UC. 1 , 2 Overall, more than half of UTUCs are invasive at the time of diagnosis compared with 15‐25% in UBC, 2 , 3 and its prognosis is extremely poor. 4 So, early diagnosis of UTUC is an important issue. CT urography has the highest diagnostic accuracy of the available imaging techniques, but it is limited to patients with normal renal function and without contrast media allergy. Other tests, such as retrograde pyelography or ureteroscopy, are more invasive. 1 To improve the prognosis of UTUC, minimally invasive diagnostic procedures other than urinary cytology are urgently needed.
Some kinds of human cells release DNA into the blood circulation, which is referred to as cell‐free DNA (cfDNA), including circulating tumor DNA (ctDNA) derived from tumor cells. In healthy individuals, plasma cfDNA is mainly derived from hematopoietic cells such as lymphocytes. 5 , 6 Plasma cfDNA is released into the circulation by cell death, necrosis, and secretion, 7 and it provides a minimally invasive diagnostic and monitoring avenue for cancer patients. 7
It has been reported that some cancer types have a shorter fragment size of plasma cfDNA compared with healthy controls. 8 , 9 Recent studies have also attempted to identify cancer types from the fragmentation profiles of plasma cfDNA. 10 The fragmentation and increased concentration of plasma cfDNA are thought to be due to an increase in ctDNA. 9 , 11 However, as the content of ctDNA in plasma cfDNA is considered to be very low, 12 , 13 we hypothesized that hematopoietic cell–derived cfDNA, which is thought to account for the majority of plasma cfDNA, is also somehow affected in the cancer environment.
It is well known that several inflammatory cytokines such as tumor necrosis factor‐α (TNFα) and interleukin‐6 (IL‐6) play important roles in both cancer initiation and progression. 14 , 15 However, the relationship between the dynamics of cfDNA and systemic inflammation in cancer patients remains unclear. So here, we investigated the usefulness of plasma cfDNA as a blood biomarker in UTUC patients and also examined the association between plasma cfDNA fragmentation and systemic inflammation.
2. MATERIALS AND METHODS
2.1. Study design
Between January 2016 and December 2019, a total of 50 patients with UTUC and 19 patients with UBC were enrolled in this study. None of the patients had received systemic chemotherapy. This study was approved by the Institutional Review Board of Osaka University Hospital (# 3397‐12). All patients had provided written informed consent for the collection and analysis of blood samples. All patients were pathologically diagnosed using surgical resection samples or ureteroscopic biopsy. Histological diagnosis was determined based on standard hematoxylin‐ and eosin‐stained sections. Two or more experienced senior pathologists provided pathological diagnoses according to the 8th American Joint Committee on Cancer TNM staging system. 16 Urine cytology was also evaluated by specialists according to our institutional criteria, in which negative urine cytology is defined to be no more than class III and positive urine cytology to be class IV and class V. We used the highest urine cytology class for data analysis if patients received several cytology tests before treatment.
Clinically, overall survival (OS) was evaluated from the first blood sampling day to the last follow‐up point. We retrospectively assessed the blood levels of white blood cells (WBC), hemoglobin (Hb), platelet (Plt), albumin (Alb), C‐reactive protein (CRP), and neutrophil to lymphocyte ratio (NLR).
2.2. Preparation of blood samples and cfDNA extraction from plasma
Whole blood (2.0‐7.0 mL) was collected directly into EDTA tubes. Within 3 hours after collection, all blood samples were centrifuged sequentially at 900 and 20 000 gravity for 10 minutes each, and supernatants were stored at −80°C as plasma. cfDNA was isolated from 0.6‐2.8 mL plasma samples using the QIAamp® Circulating Nucleic Acid Kit (QIAGEN) according to the manufacturer's protocol.
2.3. Quantification of cfDNA concentration
We quantified cfDNA concentration as previously reported. 17 Quantitative real‐time PCR analysis was performed using a CFX Connect™ real‐time system (Bio‐Rad Laboratories) to detect the concentration of plasma cfDNA. A 106‐bp amplicon of ACTB was used as the target for the quantification of cfDNA fragments as previously reported. 17 , 18 ACTB is a housekeeping gene and is expressed in both cancer cells and normal cells such as blood cells. We have measured the concentration of total cfDNA by measuring the copy number of ACTB in plasma cfDNA.
2.4. Measurement of the fragment size of plasma cfDNA
We measured the fragment size of plasma cfDNA as previously reported. 17 The cfDNA fragment size was measured using a microfluidics‐based platform, the Agilent 2100 Bioanalyzer with the High Sensitivity DNA Kit (Agilent Technologies). The Agilent 2100 Expert software (version B.02.08) automatically determines the mean size for each defined smear region of plasma cfDNA. The results of the Agilent 2100 Bioanalyzer were shown with a range of around 100 bp. We defined the value at the top of that range, which represents the average of all fragment sizes, as the fragment size of each individual.
2.5. PBMC isolation
Peripheral blood mononuclear cells (PBMCs) from individuals were isolated from whole‐blood samples (21 mL) and collected into BD Vacutainer CPT tubes with sodium citrate (Becton Dickinson) according to the manufacturing procedure.
2.6. Cell culture and cfDNA extraction from the culture supernatant
We cultured PBMCs (1.0 × 106) derived from healthy controls for 48 hours in Biotarget medium supplemented with 10% FBS and 4 mmol/L L‐glutamine (Biological Industries) in a humidified incubator set to 37°C and 5% CO2. Human bladder cancer cell lines T24, J82, and 5637 were purchased from ATCC and maintained in basal media (Eagle's minimal essential medium [EMEM] for T24 and J82, Roswel Park Memorial Institute [RPMI] for 5637) supplemented with 10% FBS and 1% gentamicin‐tyrosine solution (Invitrogen) in a humidified incubator set to 37°C and 5% CO2. PBMCs cultures were incubated with the three cytokines as follows: TNFα (1‐100 ng/mL), IL‐6 (10‐100 ng/mL), or IL‐1ra (10‐100 ng/mL) (FUJIFILM). We used R‐7050 (10 μmol/L) (Selleck Chemicals LLC), a TNFα receptor antagonist for the inhibition assay of TNFα. All mediums were centrifuged sequentially at 400 gravity for 5 minutes and 20 000 gravity for 10 minutes, and supernatants were stored at −80°C. cfDNA was isolated from 1.0 mL culture supernatants using the QIAamp® Circulating Nucleic Acid Kit (QIAGEN) according to the manufacturer's protocol.
2.7. Quantification of serum cytokines
To measure the cytokine levels in the serum, we used a Human TNFα Quantikine ELISA kit, a Human IL‐6 Quantikine ELISA kit, and a Human IL‐1ra Quantikine ELISA kit (R&D Systems) and read the absorbance in a microplate reader at 450/570 nm. Cytokine concentrations were calculated by spiking recombinant protein into 0.1 mL aliquots of serum.
2.8. Statistical analysis
All statistical analyses were performed using JMP® Pro 13.2.0 (SAS Institute Inc), GraphPad Prism 5 (GraphPad Software), and R version 2.13.0 with the RcmdrPlugin, EZR package (Saitama Medical Center, Jichi Medical University). Results on patient and cfDNA characteristics are presented as median, and data were compared using the Wilcoxon test or Dunn's multiple comparison test. Receiver operating characteristic (ROC) curve analysis was used to generate AUC values to evaluate the diagnostic capability of cfDNA for UTUC. Stepwise associations between the features of plasma cfDNA and pathological findings (pTa/1, pT2, pT3, pT4/M+) were compared using the Jonckheere‐Terpstra test. The OS rate was calculated using the Kaplan‐Meier method. Differences between the two groups were assessed by the log‐rank test. Pearson's correlation coefficient was used to analyze associations between the fragment size of plasma cfDNA and peripheral blood test items (CRP, NLR, Alb) and serum cytokines (TNFα, IL‐6, IL‐1ra). All p values were two‐sided, with statistical significance being accepted at P < .05. Univariate and multivariate logistic regression analysis was performed to assess the relative contributions of various factors (age, gender, WBC, Hb, Plt, NLR, Alb, and CRP) and plasma cfDNA characteristics such as fragment size and concentration for the diagnosis of UTUC.
3. RESULTS
3.1. Patient characteristics
The clinical and pathological characteristics are summarized in Table 1. In total, 50 patients were histologically diagnosed with UC. The UTUC cohort consisted of 42 males and 8 females, and the median age was 73 years (range 51‐88 years). In UTUC patients, the median serum levels of Alb/CRP/NLR were 3.75/0.14/2.56 (range 1.90‐4.60, 0.04‐22.6, 1.28‐14.05), respectively. The clinical T stage of 0/a/1 and 2, 3/4 was 16/21/13 patients, respectively, and 9 patients had metastases at the time of diagnosis. Forty patients underwent nephroureterectomy, and the pathological T stage of a and 1/2/3 and 4 was 18/7/15 patients, respectively.
Table 1.
Characteristics of upper‐tract urothelial carcinoma (UTUC) patients and healthy controls (n = 76)
| Total (n = 76) | UTUC patients (n = 50) | Healthy controls (n = 26) | P‐value | ||||
|---|---|---|---|---|---|---|---|
| Age (y) (median [range]) | 71 | (50‐88) | 73 | (51‐88) | 68 | (50‐77) | <.001 |
| Gender, n (%) | |||||||
| Male/female | 52/24 | (68/32) | 42/8 | (84/16) | 10/16 | (38/62) | <.001 |
| WBC: median (range) (cells/mm3) | 5470 | (2810‐54 900) | 6180 | (3770‐21 990) | 4960 | (2810‐9820) | .012 |
| Hb: median (range) (g/dL) | 13 | (6.4‐16.8) | 12.6 | (6.4‐16.8) | 13.4 | (11.6‐16.8) | .037 |
| Plt: median (range) (* 10e3 cells/mm3) | 227 | (95‐524) | 228 | (95‐524) | 226 | (154‐366) | .519 |
| Alb: median (range) (g/dL) | 3.9 | (1.9‐4.8) | 3.75 | (1.90‐4.60) | 4.10 | (3.30‐4.80) | .003 |
| CRP: median (range) (mg/dL) | 0.08 | (0.04‐22.6) | 0.14 | (0.04‐22.6) | 0.04 | (0.04‐5.46) | <.001 |
| NLR: median (range) | 2.41 | (0.79‐14.05) | 2.56 | (1.28‐14.05) | 1.90 | (0.79‐13.4) | .001 |
| Tumor location, n (%) | |||||||
| Renal pelvis | 23 | (46) | |||||
| Ureter | 24 | (48) | |||||
| Multiple | 3 | (6) | |||||
| Previous bladder cancer, n (%) | |||||||
| No | 16 | (32) | |||||
| Yes | 34 | (68) | |||||
| Urinary cytology, n (%) | |||||||
| Negative | 22 | (44) | |||||
| Positive | 24 | (48) | |||||
| Unknown | 4 | (8) | |||||
| Clinical T, n (%) | |||||||
| 0, a, 1 | 16 | (32) | |||||
| 2 | 21 | (42) | |||||
| 3, 4 | 13 | (26) | |||||
| Clinical N, n (%) | |||||||
| 0 | 43 | (86) | |||||
| 1 | 2 | (4) | |||||
| 2 | 5 | (10) | |||||
| 3 | 0 | (0) | |||||
| Clinical M, n (%) | |||||||
| 0 | 44 | (88) | |||||
| 1 | 6 | (12) | |||||
| Clinical stage, n (%) | |||||||
| I | 16 | (32) | |||||
| II | 20 | (40) | |||||
| III | 5 | (10) | |||||
| IV | 9 | (18) | |||||
| Treatment, n (%) | |||||||
| Nephroureterectomy | 40 | (80) | |||||
| Others | 10 | (20) | |||||
| Pathological T, n (%) | |||||||
| a, 1 | 18 | (45) | |||||
| 2 | 7 | (18) | |||||
| 3, 4 | 15 | (37) | |||||
Abbreviations: Alb, albumin; CRP, C‐reactive protein; Hb, hemoglobin; NLR, neutrophil to lymphocyte ratio; Plt, platelet; WBC, white blood cell.
3.2. Confirmation of plasma cfDNA
To confirm that cfDNA was extracted, we used a microfluidics‐based platform. In all cases, we confirmed the presence of plasma cfDNA. The fragment size of plasma cfDNA was found to be approximately 167 bp (Figure 1A), which is reported to correspond to the size of the DNA wrapped around the nucleosome (approximately 147 bp) and the linker DNA associated with histone H1. 7 Within the 35‐10 380 bp range, the median proportion of 100‐200 bp fractions was 65% (40‐98%). From these results, the fragment size of the majority of plasma cfDNA was found to be 100‐200 bp, as previously reported (Figure 1B).
FIGURE 1.
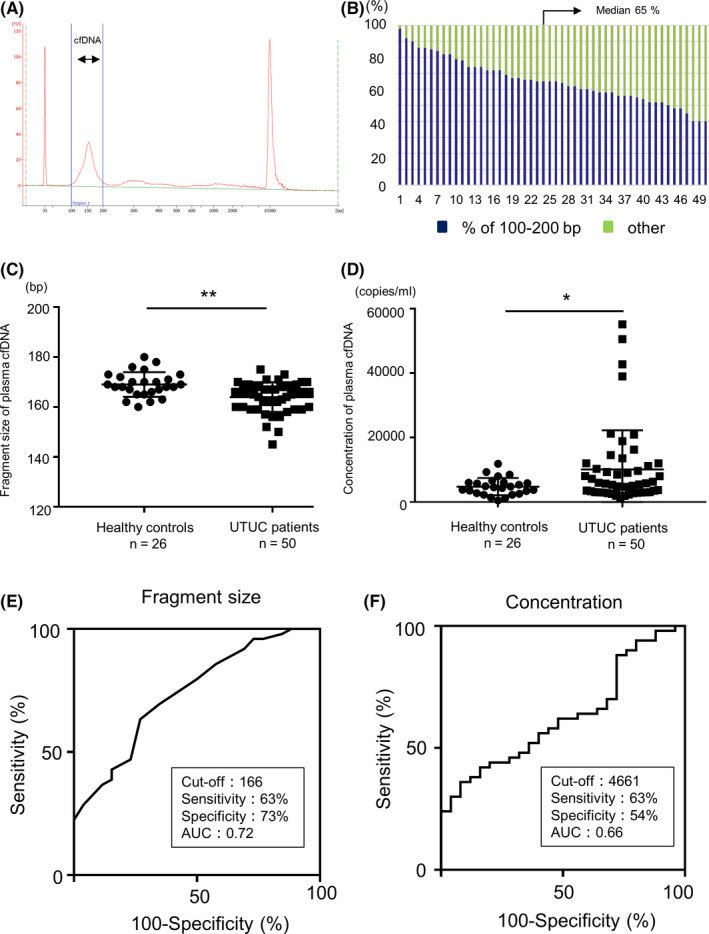
Characterization of plasma cell‐free DNA (cfDNA) fragment size using a microfluidics device and concentration using qPCR between upper‐tract urothelial carcinoma (UTUC) patients and healthy controls. A, Capillary electropherograms showing the presence of plasma cfDNA in all cases. The lower marker indicates 35 bp, and the upper marker indicates 10 380 bp. B, Bar chart showing the proportion of size fraction of plasma cfDNA. Blue represents the fraction of 100‐200 bp and green represents the fraction of other sizes (35‐100 bp, 200‐10 380 bp). C, The comparison of the fragment size of plasma cfDNA in UTUC patients (n = 50) and healthy controls (n = 26). D, The comparison of the concentration of plasma cfDNA in UTUC patients (n = 50) and healthy controls (n = 26). A comparison between the two groups was performed by Wilcoxon test, *P < .05, **P < .01. E, ROC curve analysis for the diagnosis of UTUC using the fragment size of plasma cfDNA. The unit for the cutoff value is bp. F, ROC curve analysis for the diagnosis of UTUC using the concentration of plasma cfDNA. The unit for the cutoff value is copies/mL
3.3. Clinical utility of cfDNA in UTUC patients
Furthermore, to evaluate the diagnostic potential of plasma cfDNA for UTUC, we compared the fragment size and concentration of plasma cfDNA in UTUC patients and healthy controls. The fragment size of plasma cfDNA from UTUC patients (median 166, range 145‐175) was significantly shorter than that from healthy controls (median 168.5, range 160‐180 bp) (P = .007) (Figure 1C). Overall, the concentration of plasma cfDNA from UTUC patients (median 5597, range 1062‐55 113 copies/mL) was significantly higher than that from healthy controls (median 4586, range 522‐11 876 copies/mL) (P = .042) (Figure 1D). However, neither the fragment size nor the concentration of plasma cfDNA was significantly different in patients with UBC, a lower‐tract UC, compared with healthy controls (Figure S1).
ROC curve analysis revealed that the fragment size of plasma cfDNA showed a sensitivity of 63% and a specificity of 73% to diagnose UTUC (area under the curve [AUC] = 0.72, cutoff value 166 bp, Figure 1E), and the concentration of plasma cfDNA demonstrated a sensitivity of 63% and a specificity of 54% to diagnose UTUC (AUC = 0.66, cutoff value 4661 copies/ml, Figure 1F), respectively. UTUC diagnostic sensitivity was higher for plasma cfDNA fragment size than for urine cytology (Figure S2). Multivariate logistic regression analysis revealed that shorter fragment size of plasma cfDNA was significantly associated with the diagnosis of UTUC (odds ratios 0.81, 95% confidence interval [CI] 0.65‐0.96, P = .024, Table 2). The above results show that the fragment size of plasma cfDNA could be an auxiliary tool for diagnosing UTUC.
Table 2.
Univariate and multivariate logistic regression analysis for the diagnosis of upper‐tract urothelial carcinoma (UTUC) (n = 76)
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Age (y) | 1.115 | 1.046‐1.204 | .002 | 1.156 | 1.034‐1.344 | .026 |
| Gender | 8.200 | 2.744‐24.501 | .000 | 11.863 | 2.224‐89.297 | .007 |
| Fragment size of plasma cfDNA | 0.829 | 0.728‐0.919 | .001 | 0.807 | 0.653‐0.955 | .024 |
| Concentration of plasma cfDNA (* 10e3) | 1.163 | 1.034‐1.380 | .045 | 1.058 | 0.903‐1.359 | .610 |
| WBC (* 10e3) | 0.997 | 0.926‐1.073 | .928 | |||
| Hb | 0.736 | 0.553‐0.947 | .024 | 0.734 | 0.359‐1.374 | .367 |
| Plt (* 10e4) | 1.043 | 0.980‐1.124 | .214 | |||
| NLR | 1.214 | 0.917‐1.607 | .175 | |||
| Alb | 0.130 | 0.032‐0.529 | .004 | 0.663 | 0.049‐8.293 | .749 |
| CRP (* 0.1) | 1.044 | 0.984‐1.109 | .156 | |||
Abbreviations: Alb, albumin; cfDNA, cell‐free DNA; CI, confidence interval; CRP, C‐reactive protein; Hb, hemoglobin; NLR, neutrophil to lymphocyte ratio; OR, odds ratio; Plt, platelet; WBC, white blood cell.
3.4. The fragmentation of plasma cfDNA was more pronounced in advanced cancer
Subsequently, we examined the association between clinicopathological characteristics and plasma cfDNA in UTUC patients. Interestingly, the fragment size of plasma cfDNA tended to shorten as the pathological stage progressed (P‐value for trend < .001, Figure 2A). On the other hand, the concentration of plasma cfDNA did not show a stepwise change with the progression of the pathological stage (P‐value for trend = .193, Figure 2B). Also, we found significant relationships between the shorter fragment sizes of plasma cfDNA in patients and reduced Alb (r = .618, P < .001) and elevated CRP (r = −0.612, P < .001) and NLR (r = −0.530, P < .001), which were previously reported as prognostic factors in UTUC patients 19 , 20 (Figure 2C). Regarding a prognostic value of the fragment size of plasma cfDNA, patients with shorter plasma cfDNA fragment size (≤166 bp) tended to have worse OS compared with those with a longer fragment (>166 bp) in our cohort (P = .083, Figure 2D).
FIGURE 2.
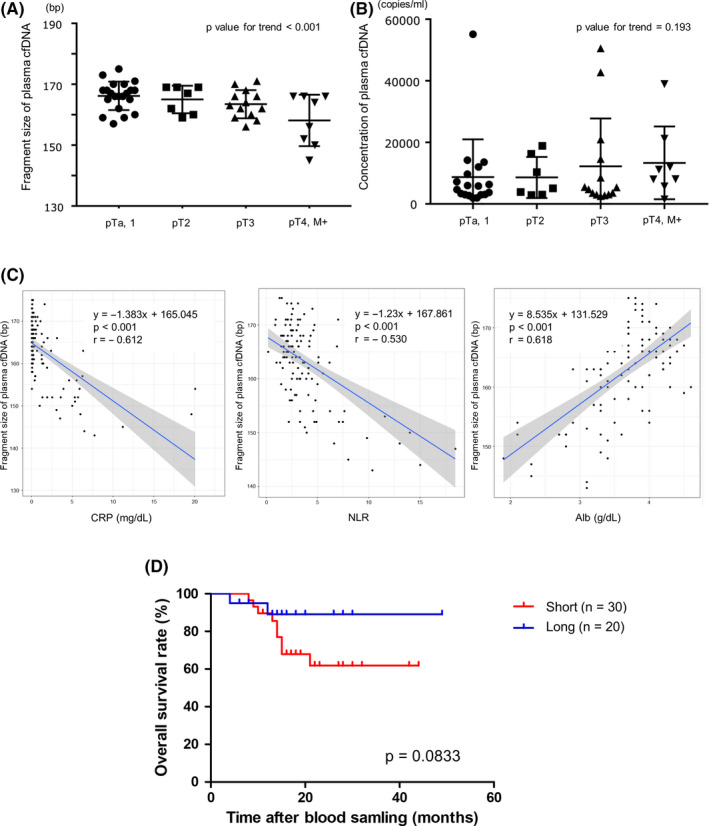
A, The fragment size of plasma cell‐free DNA (cfDNA) at each pathological stage. Stepwise associations between the fragment of plasma cfDNA and pathological stage were analyzed using the Jonckheere‐Terpstra test. B, The concentration of plasma cfDNA at each pathological stage. Stepwise associations between the concentration of plasma cfDNA and pathological stage were analyzed using the Jonckheere‐Terpstra test. C, The scatterplot of the fragment size of plasma cfDNA and the inflammatory markers (C‐reactive protein [CRP], neutrophil to lymphocyte ratio [NLR], albumin [Alb]). The blue line shows the correlation line and the grey area represents a 95% confidence interval (CI). D, The association of overall survival (OS) and cfDNA fragment size, with ≤166 bp being “short” and >166 bp being “long,” as assessed by the Kaplan‐Meier and the log‐rank test
3.5. The fragmentation of plasma cfDNA is associated with inflammatory cytokines
Based on the above results, we hypothesized that systemic inflammation induced fragmentation of plasma cfDNA. We focused on three inflammatory cytokines (TNFα, IL‐6, IL‐1ra) as representatives of systemic inflammation. These cytokines have already been reported to be associated with prognosis in UBC. 21 When three inflammatory cytokines were measured in the serum of UTUC patients and compared with the fragment size of plasma cfDNA, we found significantly higher concentrations of each cytokine in the serum in patients with shorter plasma cfDNA fragment size, as specified below. The patients with shorter plasma cfDNA fragment size (<166 bp) had significantly higher concentrations of serum TNFα, IL‐6, and IL‐1ra (median 23.7, 11.8, 510.7 pg/mL, range 3.4‐116.4, 0.0‐79.3, 155.9‐2000.0, respectively) than the patients with longer plasma cfDNA fragment size (166 bp≤) (median 7.5, 3.5, 281.9 pg/mL, range 1.0‐25.9, 0.0‐16.7, 44.2‐577.3, respectively) (P = .007, .002, .013, respectively, Figure 3A). Additionally, the fragment size of plasma cfDNA is highly correlated with serum TNFα, IL‐6, and IL‐1ra concentration (r = −.837, −.964, −.911, respectively, Figure 3B). These results indicate that the fragmentation of cfDNA is significantly associated with the serum levels of inflammatory cytokines.
FIGURE 3.
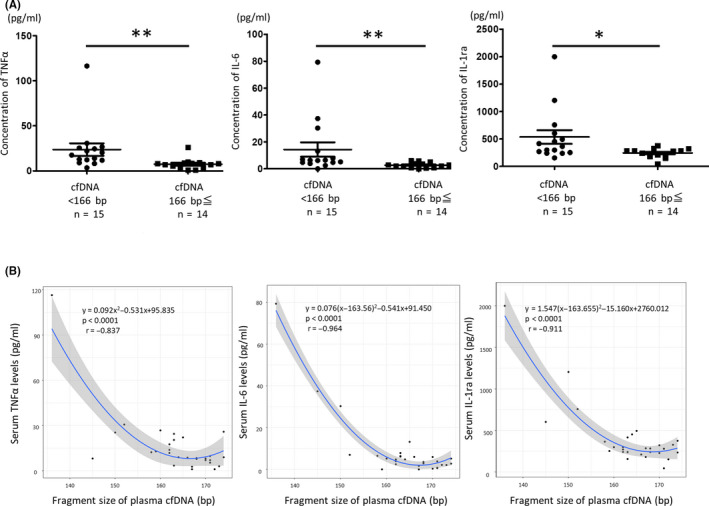
A, The comparison of the concentration of each cytokine between the group with shorter plasma cell‐free DNA (cfDNA) fragment size (<166 bp) and the group with longer plasma cfDNA fragment size (166 bp≤). A comparison between the two groups was performed by Wilcoxon test, *P < .05, **P < .01. B, The scatterplot of the fragment size of plasma cfDNA and the serum level of each cytokine (tumor necrosis factor‐α [TNFα], interleukin‐6 [IL‐6], interleukin‐1 receptor antagonist [IL‐1ra]) in upper‐tract urothelial carcinoma (UTUC) patients. The blue line shows the correlation curve, and the grey area represents a 95% confidence interval [CI]
3.6. In vitro study of cfDNA size in cancer cell line and cytokine‐treated PBMCs
To further investigate which cytokine plays an important role in the fragmentation of cfDNA, we performed a verification in the following experimental system. First, PBMCs from healthy controls were cultured for 48 hours in media containing one of the three inflammatory cytokines (TNFα, IL‐6, IL‐1ra). Subsequently, we extracted the cfDNA from the culture supernatant and assessed the proportion of short (50‐166 bp) cfDNA fractions by a microfluidics‐based platform. The proportions of the short fractions of cfDNA extracted from the PBMCs were 24% (range 11‐49%) in culture supernatant untreated with cytokines, 31% (range 19‐56%) in the culture supernatant with 10 ng/mL TNFα, and 39% (range 12‐49%) in the culture supernatant with 100 ng/mL TNFα, respectively. In the cfDNA extracted from the culture supernatant with added TNFα, the short cfDNA was significantly increased (P = .045 [10 ng/mL], 0.030 [100 ng/mL]). Although there was no significant difference between the cfDNA extracted from the culture supernatant with the addition of 10 ng/mL TNFα and 100 ng/mL TNFα (Figure 4A, Figure S3), TNFα less than 10 ng/mL promoted the fragmentation of cfDNA in a dose‐dependent manner (Figure 5A).No change in fragmentation was observed in cfDNA in the culture supernatant with the other cytokines (IL‐6, IL‐1ra) (Figure 4B,C and Figure S3). We also confirmed that the effect of TNFα on the increase of short fragment size of cfDNA was canceled by the TNFα inhibitor (R‐7050) (Figure 5B). These results suggest that the increase of short fragment size of cfDNA is a direct effect of TNFα.
FIGURE 4.
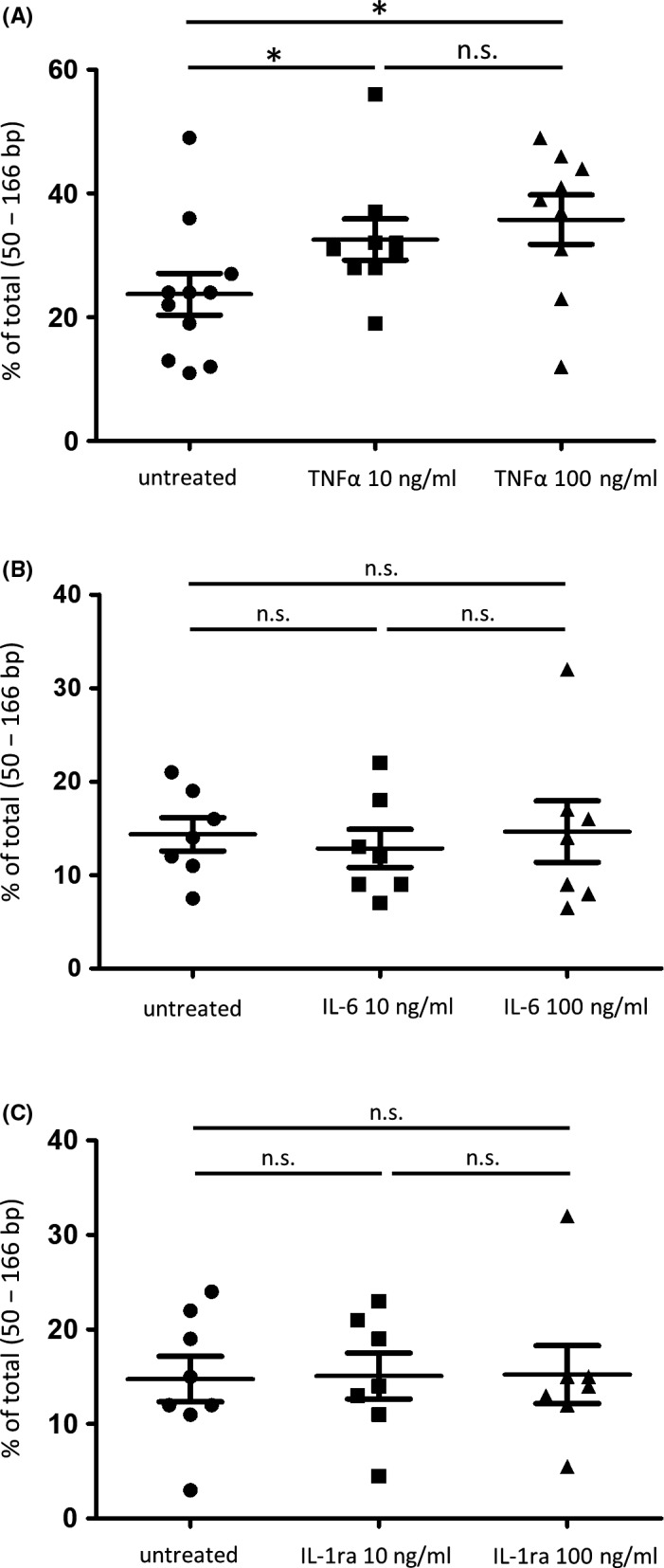
Measurement of the proportion of short fragment fraction in cell‐free DNA (cfDNA) derived from peripheral blood mononuclear cells [PBMCs]. Proportions of short fragment fraction of cfDNA in the culture supernatant of cytokine‐untreated PBMCs and TNFα‐treated PBMCs (10 ng/mL, 100 ng/mL). (*P < .05). A, Proportions of short fragment fraction of cfDNA in the culture supernatant of cytokine‐untreated PBMCs and interleukin‐6 (IL‐6)‐treated PBMCs (10 ng/mL, 100 ng/mL). B, Proportions of short fragment fraction of cfDNA in the culture supernatant of cytokine‐untreated PBMCs and IL‐1ra–treated PBMCs (10 ng/mL, 100 ng/mL). A comparison among the three groups was performed by Dunn's multiple comparison test, *P < .05
FIGURE 5.
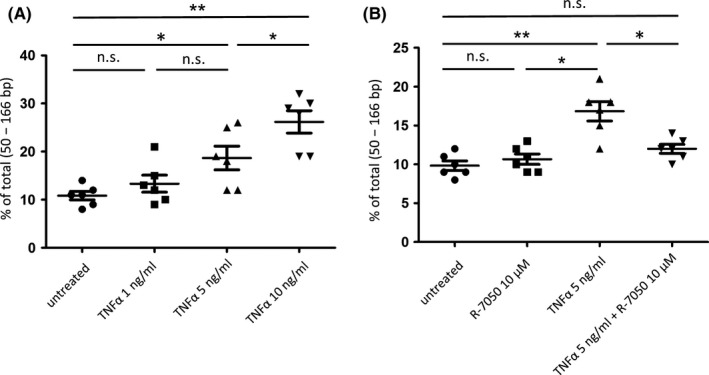
The fragmentation of cell‐free DNA (cfDNA) is a direct effect of tumor necrosis factor‐α (TNFα). A, Proportions of short fragment fraction of cfDNA in the culture supernatant of untreated PBMCs and TNFα‐treated PBMCs (1 ng/mL, 5 ng/mL, 10 ng/mL). (*P < .05, **P < .01). B, Proportions of short fragment fraction of cfDNA in the culture supernatant of untreated PBMCs, TNFα‐treated PBMCs (5 ng/mL), and TNFα inhibitor (R‐7050)‐treated PBMCs (10 μmol/L) (*P < .05, **P < .01)
We attempted to collect cfDNA from the culture supernatants of UC cell lines (T24, J82, 5637) to evaluate cancer cell–derived cfDNA, but we were unable to collect cfDNA of matching size to mononucleosome unit (Figure S4).
4. DISCUSSION
Currently, no reliable blood biomarkers for UTUC have been identified which are both minimally invasive and informative for diagnosis. Recently, cfDNA has been widely investigated as a biomarker in translational and clinical research as “liquid biopsies.” Although we reported that urinary ctDNA is useful for the diagnosis of UTUC, 13 a useful blood biomarker does not yet exist. In this study, we clarified that the fragment size of plasma cfDNA had moderate accuracy for diagnosing UTUC. We consider that the fragment size of plasma cfDNA could be an auxiliary tool for diagnosing UTUC, but it is not UTUC‐specific as CRP and NLR are. Although we did not find a shortened fragment size of plasma cfDNA in UBC patients, this phenomenon has already been reported in several cancer types, including renal cell carcinoma, lung cancer, and melanoma. 8 , 17 From these findings we suspect that the shortening fragment size of plasma cfDNA is not specific to only UTUC. This is the first study to show that the fragment size of plasma cfDNA could be an auxiliary tool for the diagnosis of UTUC patients.
Ge et al reported that copy number alteration (CNA) profiles of urinary cfDNA had high accuracy for detecting UTUC. 22 It has also been reported that the concentration of plasma cfDNA was more accurate than classical tumor markers, such as a carcinoembryonic antigen or carbohydrate antigen 19‐9, 72‐4, and 50 in diagnosing gastric cancer. 23 Our study also found a significantly higher concentration of plasma cfDNA in UTUC patients than in healthy controls. However, there was no correlation between the pathological stage of the tumor and the concentration of plasma cfDNA. In this study, quantitative real‐time PCR was used to measure the concentration of plasma cfDNA, but further research is needed using the latest technologies, such as digital PCR.
Although it has been reported that cfDNA is released into the blood circulation by apoptosis, necrosis, and secretion, the fragment size of cfDNA in plasma is centered around the mononucleosome size, and cfDNA is thought to be mainly derived from apoptosis. 7 , 24 Several reports have shown that cfDNA with cancer‐specific mutations (ctDNA) are shorter than cfDNA without the mutation. 9 , 12 , 24 These results indicate that an increase in ctDNA may be responsible for the increased fragmentation of plasma cfDNA in cancer patients. However, most of the cfDNA in circulating blood is thought to be derived from hematopoietic cells, and in this study we clarified that this hematopoietic cell–derived cfDNA was fragmented by systemic inflammation induced by TNFα.
In the fragmentation of cfDNA, caspase‐dependent digestion has been reported to play an important role. 25 Caspases are activated during apoptosis via exogenous (death receptor) or endogenous (mitochondrial) pathways. 26 , 27 TNFα has been reported to induce apoptosis via TNFR1, one of the death receptors. 28 The results of this study were attributed to the fact that of the three cytokines, only TNFα has the function to activate the death receptor pathway. Although this experiment does not fully replicate the immune response in vivo, it is a novel finding that TNFα, an inflammatory cytokine, promotes fragmentation of hematopoietic cell–derived cfDNA. In this study, only three cytokines were investigated, so we will be looking at more types of cytokines in the future.
There are some apparent limitations in this study. First, the number of cases is small. Further investigations are needed to validate our results in larger numbers of patients by multi‐institutional studies. Second, we proved that systemic inflammation affects the fragmentation of cfDNA, using PBMCs of healthy controls, which may not reflect the entire cfDNA landscape in cancer patients. Third, we consider it necessary to validate the fragment size of plasma cfDNA in patients with noncancer inflammatory diseases to distinguish it from existing inflammatory markers such as CRP and NLR. It is also necessary to validate whether cellular stresses other than inflammation, such as chemotherapy, radiation, hypoxia, and oxidative stress, also cause the fragmentation of plasma cfDNA.
In conclusion, the results of this study demonstrate the usefulness of plasma cfDNA as a new blood biomarker for UTUC patients. We show that cancer‐related systemic inflammation, particularly TNFα, contributes to the fragmentation of cfDNA from hematopoietic cells. The mechanism of plasma cfDNA fragmentation in cancer patients involves not only increased cancer cell–derived cfDNA, but also hematopoietic cell–derived cfDNA in an inflammatory environment.
DISCLOSURE
The authors have no conflicts of interest.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
ACKNOWLEDGMENTS
This study was supported by the JSPS KAKENHI grant (19K18558). We thank Mutsumi Tuchiya and Atsuko Yasumoto for their excellent technical support.
Nakano K, Yamamoto Y, Yamamichi G, et al. Fragmentation of cell‐free DNA is induced by upper‐tract urothelial carcinoma–associated systemic inflammation. Cancer Sci. 2021;112:168–177. 10.1111/cas.14679
REFERENCES
- 1. Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;2015:68. [DOI] [PubMed] [Google Scholar]
- 2. Margulis V, Shariat SF, Martin SF, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. 2009;115:1224‐1233. [DOI] [PubMed] [Google Scholar]
- 3. Fujita K, Uemura M, Yamamoto Y, et al. Preoperative risk stratification for cancer‐specific survival of patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int J Clin Oncol. 2015;20:156‐163. [DOI] [PubMed] [Google Scholar]
- 4. Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100‐114. [DOI] [PubMed] [Google Scholar]
- 5. Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell‐free DNA comprises an in vivo nucleosome footprint that informs its tissues‐of‐origin. Cell. 2016;164:57‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ulz P, Thallinger GG, Auer M, et al. Inferring expressed genes by whole‐genome sequencing of plasma DNA. Nat Genet. 2016;48:1273–1278. [DOI] [PubMed] [Google Scholar]
- 7. Wan JCM, Massie C, Garcia‐Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223‐238. [DOI] [PubMed] [Google Scholar]
- 8. Underhill HR, Kitzman JO, Hellwig S, et al. Fragment length of circulating tumor DNA. PLoS Genet. 2016;12:e1006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mouliere F, Chandrananda D, Piskorz AM, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. 2018;10:eaat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cristiano S, Leal A, Phallen J, et al. Genome‐wide cell‐free DNA fragmentation in patients with cancer. Nature. 2019;570:385‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bedin C, Enzo MV, Del Bianco P, Pucciarelli S, Nitti D, Agostini M. Diagnostic and prognostic role of cell‐free DNA testing for colorectal cancer patients. Int J Cancer. 2017;140(8):1888‐1898. [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto Y, Uemura M, Fujita M, et al. Clinical significance of the mutational landscape and fragmentation of circulating tumor DNA in renal cell carcinoma. Cancer Sci. 2019;110:617‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi Y, Fujita K, Matsuzaki K, et al. Diagnostic potential of TERT promoter and FGFR3 mutations in urinary cell‐free DNA in upper tract urothelial carcinoma. Cancer Sci. 2019;110:1771‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. Journal of immunology research. 2014;2014:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221‐233. [DOI] [PubMed] [Google Scholar]
- 16. American Joint Committee on Cancer . AJCC Cancer Staging Manual, 8th edn New York, NY: Springer Publishing; 2016. [Google Scholar]
- 17. Yamamoto Y, Uemura M, Nakano K, et al. Increased level and fragmentation of plasma circulating cell‐free DNA are diagnostic and prognostic markers for renal cell carcinoma. Oncotarget. 2018;9:20467‐20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellinger J, Wittkamp V, Albers P, et al. Cell‐free circulating DNA: diagnostic value in patients with testicular germ cell cancer. J Urol. 2009;181:363‐371. [DOI] [PubMed] [Google Scholar]
- 19. Inamoto T, Matsuyama H, Sakano S, et al. The systemic inflammation‐based glasgow prognostic score as a powerful prognostic factor in patients with upper tract urothelial carcinoma. Oncotarget. 2017;8:113248‐113257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumari N, Agrawal U, Mishra AK, et al. Predictive Role of Serum and Urinary Cytokines in Invasion and Recurrence of Bladder Cancer. Tumor Biol. 2017;39:1010428317697552. [DOI] [PubMed] [Google Scholar]
- 21. Kishimoto N, Takao T, Kuribayashi S, et al. The neutrophil‐to‐lymphocyte ratio as a predictor of intravesical recurrence in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Int J Clin Oncol. 2017;22:153‐158. [DOI] [PubMed] [Google Scholar]
- 22. Ge G, Peng D, Guan B, et al. Urothelial carcinoma detection based on copy number profiles of urinary cell‐free DNA by shallow whole‐genome sequencing. Clin Chem. 2020;66:188‐198. [DOI] [PubMed] [Google Scholar]
- 23. Qian C, Ju S, Qi J, et al. Alu‐based Cell‐Free DNA: A novel biomarker for screening of gastric cancer. Oncotarget. 2016;8:54037‐54045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang P, Chan CW, Chan KC, et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci USA. 2015;112:E1317‐E1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heitzer E, Auinger L, Speicher MR. Cell‐free DNA and apoptosis: how dead cells inform about the living. Trends Mol Med. 2020;26:519‐528. [DOI] [PubMed] [Google Scholar]
- 26. Ichim G. Tait SW. A fate worse than death: apoptosis as an oncogenic process. Nature Rev Cancer. 2016;16:539‐548. [DOI] [PubMed] [Google Scholar]
- 27. Green DR. The coming decade of cell death research: five riddles. Cell. 2019;177:1094‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aggarwal BB. Signalling pathways of the TNF superfamily: a double‐edged sword. Nat Rev Immunol. 2003;3:745‐756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4


