Abstract
CD24, a heavily glycosylated glycosylphosphatidylinositol–anchored surface protein, inhibits phagocytosis as potently as CD47. The relationship between such anti‐phagocytic factors and the immune response with immune–checkpoint inhibitors (ICI) remains unexplored. We evaluated CD24 and CD47 tumor proportion scores (TPS) in 68 of the 106 patients with advanced non–small‐cell lung cancer who participated in a prospective observational study of ICI treatment. We also explored the impact of CD24 TPS and CD47 TPS on ICI efficacy and serum cytokine changes. CD24 positivity (TPS ≥ 1) was negatively associated with progression–free survival (PFS) of ICI when PD‐L1 TPS was < 50 (median PFS; 37 vs 127 d, P = .033), but there was no association when PD‐L1 TPS was ≥ 50 (median PFS; 494 vs 144 d, P = .168). CD24 positivity was also related to significantly higher increase of CCL2 from baseline to 4‐6 wk later, and such increase was notably observed only when PD‐L1 TPS < 50 (P = .0004). CCL2 increase after ICI initiation was negatively predictive for survival after initiation of ICI (median survival time; not reached vs 233 d; P = .028). CD47 TPS high (≥60) significantly suppressed the increase in vascular endothelial growth factor (VEGF)‐A, D and PDGF‐AB/BB after ICI initiation. There was no association, however, between CD47 tumor expression and the efficacy of ICI. In conclusion, CD24, not CD47, is a candidate negative predictive marker of ICI in advanced, non–small‐cell lung cancer with PD‐L1 TPS < 50. Tumor expression of both CD24 and CD47 was associated with changes in factors related to monocytes and angiogenesis after ICI initiation (UMIN000024414).
Keywords: CCL2, CD24, CD47, lung cancer, PD‐L1
We evaluated tumor proportion score (TPS) of CD24 and CD47 in 68 patients with advanced, non–small‐cell lung cancer who participated in a prospective observational study for treatment with ICI, and we also explored the impact of CD24 TPS and CD47 TPS on the efficacy of ICI and serum cytokine alterations. CD24 positivity (TPS ≥ 1) was negatively associated with progression–free survival of ICI when PD‐L1 TPS < 50 (median PFS; 37 vs 127 d, P = .033), but there was no association between CD47 tumor expression and the efficacy of ICI. CD24 positivity was also related to significantly higher increases in CCL2 from baseline to 4‐6 wk later, and such increase was notably observed only when PD‐L1 TPS was < 50 (P = .0004).
1. INTRODUCTION
Immune–checkpoint inhibitors (ICI) are currently indispensable in the treatment of advanced non–small‐cell lung cancer (NSCLC), but the effect can vary greatly from patient to patient. Using predictive markers, the selection of patients in whom ICI is suggested to be effective is important. Since KEYNOTE 010, the KEYNOTE 024 trial revealed that the effectiveness of pembrolizumab monotherapy could greatly differ depending on whether or not the PD‐L1 tumor proportion score (TPS) was ≥50%. 1 , 2 Most patients with advanced NSCLC are examined for PD‐L1 TPS to determine treatment strategy in clinical practice. PD‐L1 expression in tumors is induced by IFN‐γ released by tumor–infiltrating lymphocytes, so high PD‐L1 (TPS ≥ 50) indicates the presence of lymphocytes primed against tumors and release of IFN‐γ. 3 , 4 , 5 However, reported response rate of ICI monotherapy in NSCLC is still c. 15% in patients with PD‐L1 TPS < 50, 6 and 20% and 45% in non‐selected and PD‐L1 TPS ≥ 50 NSCLC, respectively. 1 , 7 , 8 , 9 Further investigations for additional predictive markers are highly anticipated.
To induce antitumor immunity, antigen‐presenting cells (APCs) phagocytose tumor cells and process antigens to load peptides onto major histocompatibility complex (MHC) class I or MHC class II molecules, which leads to the priming of tumor–specific lymphocytes. 10 The combination of PD‐1 inhibitors with inhibitors of CD47, a potent inhibitor of phagocytosis, has been reported to enhance antitumor efficacy, and APCs infiltration around the tumor has shown a significant impact on manifestation of ICI efficacy, 11 , 12 suggesting the importance of such a mechanism that begins with phagocytosis.
CD24, a heavily glycosylated glycosylphosphatidylinositol–anchored surface protein, was recently reported to inhibit phagocytosis by binding to sialic acid–binding Ig‐like lectin 10 (Siglec‐10) on macrophages. 13 CD47 has a so‐called “don't eat me signal” through its interaction with signal regulatory protein alpha (SIRPα), which is a transmembrane glycoprotein expressed on normal cells and myeloid cells including macrophages and dendritic cells. 14 , 15 CD24 and CD47 each enhance phagocytosis by inhibiting their respective signals and, furthermore, inhibition of CD24 and CD47 simultaneously enhances phagocytosis in an additive manner, suggesting that CD24 and CD47 are independent of each other. 13 , 16 CD24 has been reported to be associated with a poor prognosis in several cancers, including NSCLC, breast, and ovarian cancer. 17 , 18 , 19 CD47 promotes tumor invasion and metastasis, 20 and is also indicated to be associated with poor prognosis in some types of neoplasms. 21 , 22 The impact on efficacy of ICI is not well understood, but they inhibit initiation of immune induction, so expressions of CD24 and CD47 in tumors are expected to play crucial roles, specifically in tumors with low expression of PD‐L1.
By binding to PD‐L1, PD‐1 generates an inhibitory signal that suppresses the function of PD‐1‐expressing cells. PD‐1 is expressed not only on lymphocytes, but also on macrophages and monocytes, and inhibition of PD‐1/L1 axis on such cells enhances phagocytosis. 23 Tumor–associated macrophages have been shown to produce various cytokines including VEGF, PDFG, and CCL2, 24 , 25 , 26 which are angiogenic and macrophage/monocyte recruiting factors, and tumor expression of CD24 and CD47 is expected to affect cytokine alterations after ICI initiation through inhibition of phagocytosis. We conducted a prospective observational study of patients with advanced NSCLC treated with ICI monotherapy, and collected serum samples for evaluation of cytokine levels before and after ICI administration. 27 , 28 In the current study, we evaluated CD24 and CD47 tumor expression using tissues from patients enrolled in the observational study, and investigated the relationship between the expression, alterations of cytokine serum level, and ICI efficacy.
2. MATERIALS AND METHODS
2.1. Patients and materials
This is a post–hoc analysis of a prospective biomarker study that enrolled 106 patients with advanced NSCLC who provided written informed consent and were treated with ICI monotherapy in our department between December 2015 and September 2018 (UMIN000024414). We investigated 68 patients with preserved evaluable tissue samples taken before the start of ICI treatment. Details of the prospective biomarker study were previously reported with results from the initial analysis. 27 , 28 Briefly, the study collected peripheral blood just before ICI treatment and again in weeks 4‐6 and later. Administration of ICI and evaluation of efficacy and toxicity were determined by each investigator. Efficacy was evaluated through radiological examination every 6‐8 wk in accordance with RECIST v.1.1. Progression–free survival (PFS) was defined as the period from the day of ICI initiation to the time of progression or death from any cause. Survival time was defined as the period from the day of ICI initiation to the latest visit or to death. This study was approved by the Wakayama Medical University Institutional Review Board (# 2654).
2.2. Immunohistochemistry staining and evaluation
CD24, CD47, and PD‐L1 expression in tumors were stained by immunohistochemistry using formalin‐fixed paraffin‐embedded tumor tissue specimens. The BenchMark XT used in the immunostaining performed deparaffinization of tissue sections at 75°C using EZ Prep buffer (Ventana Medical System). Antigen retrieval was achieved in Tris‐EDTA base buffer, pH 8.5 (Cell Conditioning 1) for 60 min at 95°C. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 min. The primary CD24 antibody (ab31622; Abcam plc) and CD47 antibody (ab175388; Abcam) were diluted 1:100 and 1:1500 and incubated at 37°C for 60 min. The immunohistochemical reaction was visualized using the Ultraview Universal Diaminobenzidine (DAB) IHC Detection Kit in accordance with the manufacturer's protocol. Patients with known reactivity to antibodies were included as positive controls, and negative controls were obtained by omitting the primary antibody.
CD24, CD47, and PD‐L1 expression in tumor cells were judged to be positive if membranous staining was present and evaluated by TPS, which is the percentage of viable tumor cells showing partial or complete membrane staining. All pathological evaluation was made independently by 2 pathologists (YK and JF) and results were determined by consensus. Both clinical and pathological data were masked at the time of scoring.
2.3. Serum protein analysis
Using the collected peripheral blood, serum proteins were quantified using Luminex 200 analyzer (Luminex) with Milliplex MAP system (Millipore). Assays were performed with human cytokine/chemokine panel 1, human angiogenesis/growth factor panel 1 and a multi‐species TGF‐β panel in accordance with the manufacturer's instructions. Standards or serum samples were mixed with antibody‐bound, chemically dyed beads, and incubated overnight at 4°C. Beads were washed and then incubated with the biotinylated detection antibody for 1 h at room temperature. After washing, the beads were incubated with phycoerythrin–labeled streptavidin for 30 min at room temperature and the median fluorescent intensities were quantified. All samples were measured in duplicate.
2.4. Statistical analysis
Clinical backgrounds of the enrolled patients were compared between high (TPS ≥ 50) and low (<50) PD‐L1 by the χ2 test and the Mann‐Whitney test. PFS with ICI and survival time after ICI initiation were calculated by Kaplan‐Meier method. PFS and survival time were compared by CD24 TPS (≥1 vs <1) and CD47 TPS (≥60 vs <60) using log‐rank test. Comparison was also made in high and low PD‐L1 groups in the same manner. Correlation between CD24 TPS and CD47 TPS was analyzed using Spearman rank correlation coefficient.
Regarding serum protein analysis, 57 proteins measured in the prospective observational studies (Supporting Information Table S1) were compared for baseline (pre‐dose) values and the fold change. This was calculated by dividing each value 4‐6 wk later by those at baseline, between CD24 TPS positive (TPS ≥ 1) vs negative (TPS < 1) patients, and high (TPS ≥ 60) vs low (<60) CD47 TPS patients using the Mann‐Whitney test. For proteins with significant differences in the fold change, we made further comparison of difference between high and low PD‐L1. To evaluate the effect of the fold change in serum protein concentration on survival time, we divided the enrolled patients into high and low by median of the fold change for comparison.
Statistical analyses were conducted with JMP version 14.0 software (SAS Inc). In all analyses, P values were two‐sided, and P < .05 was considered to be statistically significant.
3. RESULTS
Clinical backgrounds of the 68 enrolled patients are shown in Table 1. Median age was 70 y (range: 31‐91) and 52 patients were East Cooperative Oncology Group performance status (PS) 0‐1 (76%). All of the administered ICI were PD‐1/L1 inhibitors and there were 26 patients with pembrolizumab, 27 with nivolumab, and 15 with atezolizumab. The 26, 27, and 15 patients received ICI at 1st, 2nd, and 3rd, or later lines, respectively. PD‐L1 was high in 27 patients (40%). In the high PD‐L1 group, 22 patients (81%) received ICI as the first treatment, while 4 patients (10%) received it as the first treatment in low PD‐L1 group (P > .001). Not surprisingly, pembrolizumab was frequently chosen in the high PD‐L1 group (89%) while in the low PD‐L1 group, nivolumab, pembrolizumab, and atezolizumab were chosen in 59%, 22%, and 20% of the patients, respectively. We saw no other clear differences between the high and low PD‐L1 groups.
TABLE 1.
Clinical characteristics
| All (n = 68) | PD‐L1 high (n = 27) | PD‐L1 low (n = 41) | P value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age (y), median (range) | 70 (31, 91) | 70 (55, 91) | 71 (31, 85) | .405 |
| Gender | ||||
| Male | 51 (75) | 22 (85) | 29 (71) | .310 |
| Female | 17 (25) | 5 (19) | 12 (29) | |
| Smoking status, n (%) | ||||
| Never | 11 (16) | 2 (7) | 9 (22) | .602 |
| Ex or current | 57 (84) | 25 (93) | 32 (78) | |
| PS | ||||
| 0‐1 | 52 (76) | 23 (85) | 29 (71) | .160 |
| 2 | 16 (24) | 4 (15) | 12 (29) | |
| Histology | ||||
| Squamous cell | 24 (35) | 8 (30) | 16 (39) | .682 |
| Adenocarcinoma | 38 (56) | 16 (59) | 22 (54) | |
| Others | 6 (9) | 3 (11) | 3 (7) | |
| Previous chemotherapy | ||||
| 0 | 26 (38) | 22 (81) | 4 (10) | <.001* |
| 1 | 27 (40) | 3 (11) | 24 (59) | |
| >2 | 15 (22) | 2 (7) | 13 (32) | |
| Treatment | ||||
| Nivolumab | 26 (38) | 2 (7) | 24 (59) | <.001* |
| Pembrolizumab | 33 (49) | 24 (89) | 9 (22) | |
| Atezolizumab | 9 (13) | 1 (4) | 8 (20) | |
| Stage | ||||
| III | 11 (16) | 3 (11) | 8 (20) | .333 |
| IV | 38 (56) | 18 (67) | 20 (49) | |
| Recurrence | 19 (28) | 6 (22) | 13 (32) | |
| PD‐L1 tumor expression | ||||
| High (≥50%) | 27 (40) | 27 (100) | 0 | |
| Low (<50%) | 41 (60) | 0 | 41 (100) | |
| Gene alterations | ||||
| WT | 39 (57) | 18 (67) | 21 (51) | .116 |
| EGFR | 5 (7) | 0 | 5 (12) | |
| ALK | 2 (3) | 1 (4) | 1 (2) | |
| NE | 22 (32) | 8 (30) | 14 (34) | |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; NE, not examined; PD‐L1, programmed death–ligand 1; PS, East Cooperative Oncology Group performance status; WT, wild type.
P < .05.
Representative images and TPS of CD24 and CD47 are shown in Figure S1 and Figure 1A. The median CD47 TPS was 60% (0‐100). Regarding CD24, more than half of enrollments expressed only < 1% and patients of CD24 TPS ≥ 1 and ≥ 10 were 27 and 12 patients, respectively. There were no differences in CD24 TPS or CD47 TPS between high and low PD‐L1 groups (P = .556, .098) (Figure 1B). There was low but significant correlation between CD24 TPS and CD47 TPS (r = .298, P = .014) (Figure 1C).
FIGURE 1.
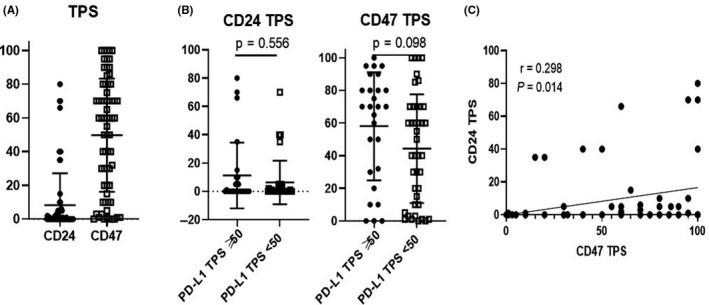
A, CD24 TPS and CD47 TPS. B, There was no significant difference between high (TPS ≥ 50) and low (TPS < 50) PD‐L1 in CD24 TPS and CD47 TPS. C, TPS of CD24 and CD47 was mildly correlated
Patients were then divided into 2 groups according to CD47 TPS by the median, 60, and CD24 TPS by 1, respectively, and compared for PFS with ICI. There were no statistically significant differences between CD24 positive and negative (TPS ≥ 1, TPS < 1, median PFS 64 and 130 d, P = .924), or CD47 high and low (TPS ≥ 60, TPS < 60, median PFS 113 and 86 d, P = .213) (Figure 2A,D). However, the PFS curves of CD24 positive and negative intersected, and we further analyzed whether PD‐L1 affects the impact of CD24 and CD47 TPS on PFS.
FIGURE 2.
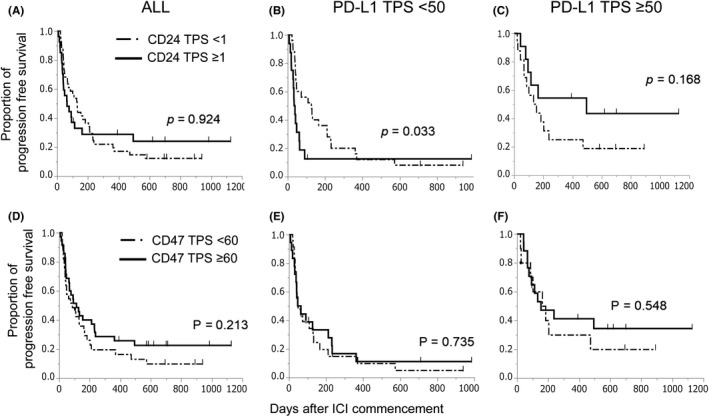
A‐C, PFS curves of CD24 TPS ≥ 1 and < 1 for ICI treatment. A, All patients. B, Patients with PD‐L1 TPS < 50. C, Patients with PD‐L1 TPS ≥ 50. D‐F, PFS curves of CD47 TPS ≥ 60 and < 60 for ICI treatment. D, All patients. E, Patients with PD‐L1 TPS < 50. F, Patients with PD‐L1 ≥ 50
The CD24–positive patients showed significantly shorter PFS when PD‐L1 was low (median PFS; 37 [95% confident interval: 20‐62] vs 127 d [44‐212], P = .033), while patients with CD24 positive tumors showed tendency of longer PFS compared with patients with CD24 negative when PD‐L1 was high (494 [77 to not reached] vs 144 d [67‐238], P = .168) (Figure 2B,C).
Survival time from initiation of ICI treatment also showed similar tendency when PD‐L1 was low (median survival time; 120 vs 428 d, P = .172), while survival curves were similar in all patients or patients when PD‐L1 was high (232 vs 428 d, P = .704; 482 vs 833 d, P = .562) (Figure 3A‐C). Response rates of CD24 positivity and negativity in the low PD‐L1 group were 7% and 20%, which were 55% and 47% in the high PD‐L1 group, respectively (P = .229, .691). CD47 TPS did not affect survival time after initiation of ICI either (P = .861) (Figure 3D). Moreover, the impact of CD47 TPS was not different between high and low PD‐L1 on either PFS or survival time (Figures 2E,F and 3E,F). CD24 TPS, not CD47, was indicated to be a candidate negative predictor for ICI treatment in tumors with low PD‐L1.
FIGURE 3.
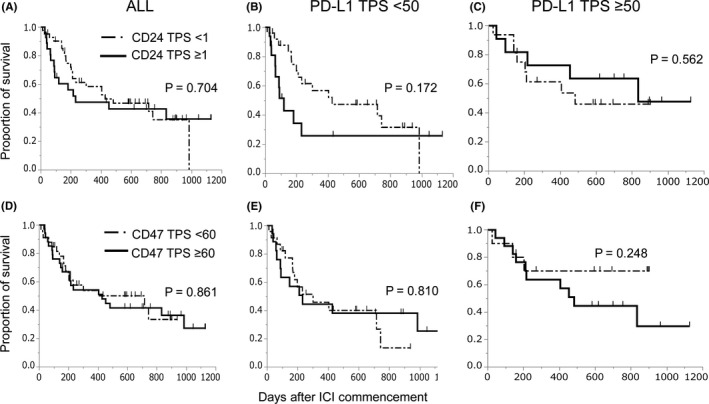
A‐C, Survival curves of CD24 TPS ≥ 1 and < 1 after ICI initiation. A, All patients. B, Patients with PD‐L1 TPS < 50. C, Patients with PD‐L1 ≥ 50. D‐F, Survival curves of CD47 TPS ≥ 60 and < 60 after ICI initiation. D, All patients. E, Patients with PD‐L1 TPS < 50. F, Patients with PD‐L1 ≥ 50
Regarding serum protein concentration analysis, we analyzed the fold change (ratio) of serum protein concentration from baseline to 4‐6 wk after ICI initiation. Notably, CD24 positive tumors significantly augmented CCL2 increase, while CD47 high significantly suppressed VEGF‐A, VEGF‐D, and PDGF‐AB/BB after ICI initiation compared with CD47 low (Figure 4A). Furthermore, we also analyzed the impact of PD‐L1 expression on the effect of CD24 TPS in changes of CCL2. Interestingly, significant augmentation of CCL2 increase in CD24–positive patients was observed only when PD‐L1 was low (P = .0004) (Figure 4B). Regarding CD47, the suppressive effect of CD47 on VEGF‐D and PDGF‐AB/BB was also seen when PD‐L1 was low (P = .041, 0.030, respectively) while no impact of PD‐L1 was seen in VEGF‐A (Figure S2).
FIGURE 4.
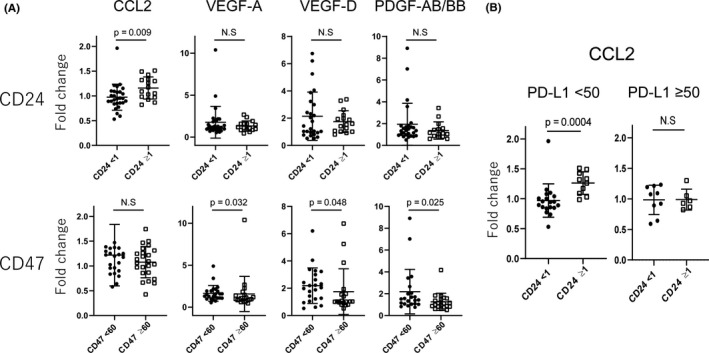
A, Fold changes of CCL2, VEGF‐A, ‐D, and PDGF‐AB/BB from baseline (pre–ICI initiation) to 4‐6 wk later after ICI initiation. CCL2 significantly increased in CD24 TPS ≥ 1 compared with CD24 TPS < 1 while increase of VEGF‐A, ‐D, and PDGF‐AB/BB were suppressed in CD47 TPS ≥ 60 compared with CD47 TPS < 60. B, Significant increase of CCL2 was observed only in patients with PD‐L1 TPS < 50
CCL2 increase after ICI initiation was suggested to be at least partially related to the negative impact of CD24 on ICI efficacy, so we explored the impact of CCL2 fold changes on survival time after ICI initiation. Comparing the survival curves by dividing enrolled patients by the median of CCL2 fold change (1.00), the survival time was significantly better in patients with CCL2 fold change < 1.00 (decrease) compared with in patients with CCL2 fold change ≥ 1.00 (increase) (median survival time; not reached [95% confident interval: 428‐not reached] vs 233 d [148‐17], P = .028) (Figure S3A). PD‐L1 TPS did not affect the impact of CCL2 alteration on survival (Figure S3B). Alterations of VEGF‐A, VEGF‐D, or PDGF‐AB/BB after ICI initiation did not impact on survival time after ICI initiation (P = .818, .592, .528, respectively) (Figure S4A‐C).
The effect of CD24 tumor expression was further analyzed by subdividing the PD‐L1 TPS < 50 group into PD‐L1 TPS < 1 and 1‐49 groups. Greater increase of CCL2 after ICI initiation and shorter survival tendency in the CD24–positive group were observed similarly in both PD‐L1 TPS < 1 and 1‐49 groups (Figure S5). PFS was significantly shorter in CD24–positive patients when PD‐L1 TPS was < 1, but there was no obvious overall difference between PD‐L1 TPS < 1 and 1‐49 (Figure S6A,B).
4. DISCUSSION
In this study, CD24 expression (TPS ≥ 1) was associated with shorter PFS of ICI when PD‐L1 TPS < 50, whereas CD47 did not affect ICI efficacy, regardless of PD‐L1 TPS. Serum CCL2 increased after the initiation of ICI, but this was only seen in patients of CD24 positivity and PD‐L1 TPS < 50.
PD‐L1 expression in tumors is induced by IFN‐γ from tumor–infiltrating lymphocytes. 29 In the patient of PD‐L1 TPS < 50, antitumor immunity is not considered to be well established. The priming of lymphocytes by tumor antigen presentation through phagocytosis should have a crucial role for the antitumor effect of ICI. It is not therefore surprising that an anti‐phagocytic signal, CD24, was associated with poor efficacy of ICI in the patients with PD‐L1 TPS < 50. Notably, the median PFS was only 37 d in CD24–positive/low PD‐L1 patients, while it was 127 d in CD24–negative, low PD‐L1 patients. CD24 positivity was seen in 39% (16 out of 41) of low PD‐L1 patients and 40% (27 out of 68) of all ICI–treated patients. As a negative predictive marker for ICI, it was reported that PFS of ICI combined with chemotherapy was 4.8 mo in patients with STK11 mutation, while it was 6.9 mo in patients without STK11 mutation (P = .0012), and the positive rate of STK11 mutation was 30% (102 out of 337 patients). 30 Very recently, however, PFS of ICI monotherapy was reported to be similar between STK11 mutated and non‐mutated patients. 31 Considering this together with the easiness of evaluation, CD24 TPS ≥ 1 combined with PD‐L1 TPS < 50 could be a future negative predictive marker of ICI, although further evaluation with more patients is necessary.
In contrast with PD‐L1 TPS < 50, there was a trend for better PFS in the CD24–positive patients with PD‐L1 TPS ≥ 50. Antitumor immunity is probably already induced in patients with PD‐L1 TPS ≥ 50, and immune induction by phagocytosis and lymphocyte priming are not considered essential for the efficacy of ICI. Furthermore, CD24 also inhibits Toll‐like receptor 4 (TLR4) activation by damage‐associated molecular patterns (DAMPs) such as high mobility group box‐1 protein. 32 TLR4 activation of cancer cells promotes the proinflammatory cytokine secretion, 33 , 34 which recruit immune‐suppressive cells such as myeloid–derived suppressor cells, and the proliferation of cancer cells themselves. Cancer cell disruption by ICI treatment causes release of DAMPs, so CD24 on tumors may have enhanced the efficacy of ICI through the TLR4 signal inhibition of cancer cells by DAMPs when PD‐L1 TPS ≥ 50.
Avoidance of phagocytosis by CD47 has been reported to lead to the suppression of antigen presentation by APCs, resulting in suppression of the induction of antitumor immunity. 16 , 31 In the present study, CD47 expression did not influence the effect of ICI, but we showed for the first time that VEGF‐A, ‐D and PDGF‐AB/BB were less increased in CD47 TPS (≥60) tumors after ICI initiation. In addition to a role as a “don't eat me signal,” CD47 suppresses the vascular endothelial growth factor receptor 2 (VEGFR2) signal. 35 Angiogenic factors promote the production of VEGF themselves through autocrine signaling, which acts through VEGFR2 on the tumor surface. 32 , 33 It can therefore be explained by CD47 expression on the tumor inhibiting the production of VEGFs after ICI initiation. The occurrence of both inhibition of phagocytosis and suppression of production of VEGFs may explain that there was no impact on antitumor efficacy of ICI by CD47 tumor expression.
Unexpectedly, in current study, serum CCL2 increased in the CD24–positive, low PD‐L1 tumor patients after ICI initiation. Signaling pathways modulated by CCL2 and its receptor play important roles in monocyte–, macrophage–, and myeloid–derived suppressor cell recruitment to the tumor microenvironment. 36 , 37 , 38 The attracted macrophages or monocytes easily become immune‐suppressive phenotypes, such as M2 macrophage or myeloid–derived suppressor cells under the influence of the tumor microenvironment, and such immune‐suppressive cells inhibit the antitumor effects of T cell. 39 CCL2 has been reported to be associated with a poor prognosis in breast, prostate, and colorectal cancers, 40 , 41 and this study is the first to show that increased CCL2 after ICI initiation was significantly associated with shorter survival after ICI initiation. However, the difference in survival between the CCL2‐increased and non‐increased groups was similar for PD‐L1 TPS < 1 and ≥ 50, and the association between the negative effect of CD24 tumor expression on ICI and CCL2 changes remains unclear.
The mechanism behind CD24 tumor expression enhancing CCL2 production after ICI initiation is not understood. CD24 is expressed not only in tumors, but also in macrophages and dendritic cells. CD24 on such cells also binds to DAMPs and it inhibits TLR4 signaling through an interaction with their own Siglec‐10, which subsequently suppresses production of CCL2. 32 Binding of CD24 on tumors to Siglec‐10 on immune cells may therefore have prevented the suppression of TLR4 signaling of immune cells by inhibiting the binding of CD24 on immune cells to Siglec‐10. Moreover, increased production of CCL2 was observed only in tumors with low PD‐L1, which may be because Siglec‐10 is also expressed on B and T cells. 42 , 43 , 44 It is possible that Siglec‐10 on tumor–infiltrating macrophages did not sufficiently bind to tumor CD24 because of the high lymphocyte infiltration in PD‐L1 high tumors. Our results show that CD24 and CD47 have very different effects on not only the antitumor effects of ICIs, but also on cytokine production after ICI administration. CD24 and CD47 should therefore be considered to be completely independent factors.
A limitation of this study is that we only evaluated patients with evaluable preserved tissue. Inevitably, it is expected that the number of patients in which specimens are easily obtained will increase, which may be a bias. In addition, the expression of CD24 and CD47 was divided into 2 groups based on the median value in the current study, which may not be the best means of division. Confirmation of the current results by evaluation with a larger number of patients in the future is therefore required.
In conclusion, CD24 TPS ≥ 1 can be a candidate negative predictive marker of ICI, and CD24 inhibition may be a promising therapeutic target in combination with ICI, especially when PD‐L1 TPS was < 50. Tumor expression of both CD24 and CD47 was associated with changes in factors related to monocytes and angiogenesis after ICI initiation. This study further highlights the variation in CCL2 after ICI administration and further investigation is warranted.
DISCLOSURE
Hiroaki Akamatsu received a research grant from Merck Sharp & Dohme and Chugai Pharmaceutical Co. Ltd., and received honoraria from Taiho Pharmaceutical Co. Yasuhiro Koh received a research grant from Merck Sharp & Dohme, Chugai Pharmaceutical Co., Bristol‐Myers Squibb, and Ono Pharmaceutical Co. Nobuyuki Yamamoto received a research grant from Merck Sharp & Dohme, Chugai Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. and honoraria from Merck Sharp & Dohme, Chugai Pharmaceutical Co. Ltd., AstraZeneca PLC, and Ono Pharmaceutical Co. The other authors declare no financial relationships. All authors had full access to all of the data in the study and agreed with the decision to submit for publication.
Supporting information
Fig S1‐S6
Table S1
ACKNOWLEDGMENTS
We acknowledge editing and proofreading by Benjamin Phillis at the Clinical Study Support Center at Wakayama Medical University.
Ozawa Y, Harutani Y, Oyanagi J, et al. CD24, not CD47, negatively impacts upon response to PD‐1/L1 inhibitors in non–small‐cell lung cancer with PD‐L1 tumor proportion score < 50. Cancer Sci. 2021;112:72–80. 10.1111/cas.14705
REFERENCES
- 1. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 3. Abiko K, Matsumura N, Hamanishi J, et al. IFN‐γ from lymphocytes induces PD‐L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellucci R, Martin A, Bommarito D, et al. Interferon‐γ‐induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD‐L1 expression. Oncoimmunology. 2015;4:e1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD‐1, PD‐L1, and PD‐L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706‐2716. [DOI] [PubMed] [Google Scholar]
- 6. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 7. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389:255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143‐156. [DOI] [PubMed] [Google Scholar]
- 11. Barry KC, Hsu J, Broz ML, et al. A natural killer‐dendritic cell axis defines checkpoint therapy‐responsive tumor microenvironments. Nat Med. 2018;24:1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garris CS, Arlauckas SP, Kohler RH, et al. Successful anti‐PD‐1 cancer immunotherapy requires T cell‐dendritic cell crosstalk involving the cytokines IFN‐γ and IL‐12. Immunity. 2018;49:1148‐1161.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec‐10 is a target for cancer immunotherapy. Nature. 2019;572:392‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science (New York, NY). 2000;288:2051‐2054. [DOI] [PubMed] [Google Scholar]
- 15. Tsai RK, Discher DE. Inhibition of "self" engulfment through deactivation of myosin‐II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47‐SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72‐80. [DOI] [PubMed] [Google Scholar]
- 17. Kristiansen G, Winzer KJ, Mayordomo E, et al. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9:4906‐4913. [PubMed] [Google Scholar]
- 18. Majores M, Schindler A, Fuchs A, et al. Membranous CD24 expression as detected by the monoclonal antibody SWA11 is a prognostic marker in non‐small cell lung cancer patients. BMC Clin Pathol. 2015;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarhriz V, Bandehpour M, Dastmalchi S, Ouladsahebmadarek E, Zarredar H, Eyvazi S. Overview of CD24 as a new molecular marker in ovarian cancer. J Cell Physiol. 2019;234:2134‐2142. [DOI] [PubMed] [Google Scholar]
- 20. Zhao H, Wang J, Kong X, et al. CD47 promotes tumor invasion and metastasis in non‐small cell lung cancer. Sci Rep. 2016;6:29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen J, Zheng DX, Yu XJ, et al. Macrophages induce CD47 upregulation via IL‐6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology. 2019;8:e1652540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kazama R, Miyoshi H, Takeuchi M, et al. Combination of CD47 and SIRPα constituting the "don't eat me signal" is a prognostic factor in diffuse large B‐cell lymphoma. Cancer Sci. 2020;111:2608–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordon SR, Maute RL, Dulken BW, et al. PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sylvestre M, Crane CA, Pun SH. Progress on modulating tumor‐associated macrophages with biomaterials. Adv Mater. 2020;32:e1902007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang W, Huang X, Carlos CJJ, Lu X. Research progress of tumor microenvironment and tumor‐associated macrophages. Clin Transl Oncol. 2020;22:2141–2152. [DOI] [PubMed] [Google Scholar]
- 26. Li D, Ji H, Niu X, et al. Tumor‐associated macrophages secrete CC‐chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020;111:47‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oyanagi J, Koh Y, Sato K, et al. Predictive value of serum protein levels in patients with advanced non‐small cell lung cancer treated with nivolumab. Lung Cancer. 2019;132:107‐113. [DOI] [PubMed] [Google Scholar]
- 28. Akamatsu H, Murakami E, Oyanagi J, et al. Immune‐related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non‐small cell lung cancer. Oncologist. 2020;25:e679‐e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skoulidis F, Arbour KC, Hellmann MD, et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non‐squamous non‐small cell lung cancer. J Clin Oncol Suppl. 2019;37:102. [Google Scholar]
- 31. Cho BC, Lopes G, Kowalski DM, Kasahara K, Wu Y‐L, Jr GC. Relationship Between STK11 and KEAP1 Mutational Status and Efficacy in KEYNOTE‐042: Pembrolizumab Monotherapy versus Platinum‐Based Chemotherapy as First‐Line Therapy for PD‐L1‐Positive Advanced NSCLC [abstract]. Proceedings of the 111th Annual Meeting of the American Association for Cancer Research Abstract nr CT084 2020.
- 32. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec‐10 selectively repress tissue damage‐induced immune responses. Science (New York, NY). 2009;323:1722‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang B, Zhao J, Li H, et al. Toll‐like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009‐5014. [DOI] [PubMed] [Google Scholar]
- 34. Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of toll‐like receptors in cancer: a double‐edged sword for defense and offense. Arch Pharm Res. 2012;35:1297‐1316. [DOI] [PubMed] [Google Scholar]
- 35. Kaur S, Roberts DD. CD47 applies the brakes to angiogenesis via vascular endothelial growth factor receptor‐2. Cell Cycle (Georgetown, Tex). 2011;10:10‐12. [DOI] [PubMed] [Google Scholar]
- 36. Arenberg DA, Keane MP, DiGiovine B, et al. Macrophage infiltration in human non‐small‐cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000;49:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steiner JL, Murphy EA. Importance of chemokine (CC‐motif) ligand 2 in breast cancer. Int J Biol Markers. 2012;27:179‐185. [DOI] [PubMed] [Google Scholar]
- 38. Rivas‐Fuentes S, Salgado‐Aguayo A, Pertuz Belloso S, Gorocica Rosete P, Alvarado‐Vásquez N, Aquino‐Jarquin G. Role of chemokines in non‐small cell lung cancer: angiogenesis and inflammation. J Cancer. 2015;6:938‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gschwandtner M, Derler R, Midwood KS. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front Immunol. 2019;10:2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu X, Kang Y. Chemokine (C‐C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284:29087‐29096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao L, Lim SY, Gordon‐Weeks AN, et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology (Baltimore, MD). 2013;57:829‐839. [DOI] [PubMed] [Google Scholar]
- 42. Bandala‐Sanchez E, Bediaga NG, Naselli G, Neale AM, Harrison LC. Siglec‐10 expression is up‐regulated in activated human CD4(+) T cells. Hum Immunol. 2020;81:101‐104. [DOI] [PubMed] [Google Scholar]
- 43. Toubai T, Rossi C, Oravecz‐Wilson K, et al. Siglec‐G represses DAMP‐mediated effects on T cells. JCI Insight. 2017;2:e92293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Macauley MS, Crocker PR, Paulson JC. Siglec‐mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S6
Table S1


