Abstract
Although nivolumab, a programmed cell death 1 (PD‐1) inhibitor, is a standard therapy for platinum‐refractory recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC), no definitive biomarkers have been reported thus far. This study aimed to select promising prognostic markers in nivolumab therapy and to create a novel prognostic scoring system. In this retrospective cohort study, we reviewed patients with R/M HNSCC who were treated with nivolumab from April 2017 to April 2019. We developed a prognostic score for immune checkpoint inhibitor (ICI) therapy that was weighed using hazard ratio–based scoring algorithms. Significant variables were selected from the multivariate Cox proportional hazard analyses on overall survival (OS). A total of 85 patients with HNSCC were analyzed in the present study. The relative eosinophil count (REC), the ratio of eosinophil increase (REI), and Eastern Cooperative Oncology Group Performance Status (ECOG PS) were selected as variables affecting the prognostic score. The patients were divided into four groups: very good (score = 0), good (score = 1), intermediate (score = 2), and poor (score = 3). The OS hazard ratios were 2.77, 10.18, and 33.21 for the good, intermediate, and poor risk groups compared with the very good risk group, respectively. The Eosinophil Prognostic Score is a novel prognostic score that is effective for predicting the prognosis of HNSCC patients treated with nivolumab. This score is more precise as it includes changes in biomarkers before and after the treatment.
Keywords: eosinophils, head and neck squamous cell carcinoma, immunotherapy, nivolumab, prognostic factors
For predicting the prognosis of head and neck squamous cell carcinoma treated with nivolumab, we created the Eosinophil Prognostic Score, which includes an Eastern Cooperative Oncology Group Performance Status, a relative eosinophil count, and a ratio of eosinophil increase. Our score demonstrated a significant dose‐response relationship with both overall survival and progression‐free survival. The Eosinophil Prognostic Score is effective for predicting the prognosis of nivolumab treatment.

Abbreviations
- BOR
best overall response
- BRAF
v‐raf murine sarcoma viral oncogene homolog B1
- CR
complete response
- DCR
disease control rate
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- GRIm‐Score
Gustave Roussy Immune Score
- ICI
immune checkpoint inhibitor
- IL
interleukin
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- R/M HNSCC
recurrent or metastatic head and neck squamous cell carcinoma
- REC
relative eosinophil count
- REI
ratio of eosinophil increase
- RR
response rate
1. INTRODUCTION
Recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) has a poor prognosis, particularly in platinum‐refractory tumors, which are tumors that progress after platinum‐based chemotherapy. 1 In recent years, immune checkpoint inhibitors (ICIs), particularly programmed cell death 1 (PD‐1) inhibitors, have been widely used for multiple tumor types. The Checkmate 141 phase III randomized trial showed that overall survival (OS) was significantly prolonged with nivolumab treatment compared with standard therapy in patients with platinum‐refractory R/M HNSCC (hazard ratio [HR] for death, 0.70; 97.73% confidence interval [CI], 0.51‐0.96; P = .01). 2
Unlike conventional cytotoxic anticancer drugs, ICIs can result in long‐term survival in some cases. However, the overall response rates (RRs) of ICIs are as low as 13%‐17%. 2 , 3 Therefore, prognostic and predictive biomarkers must be determined that can identify potential responders to ICI therapy. Immunotherapy‐specific prognostic markers are required rather than general prognostic markers for recurrent tumors because ICIs have different mechanisms than those of conventional cytotoxic anticancer drugs. Several indicators have been designated as immunotherapy‐specific biomarkers. For example, previous studies have investigated tumor mutation burden (TMB), 4 tumor‐infiltrating CD8+ T‐lymphocyte (TIL), 5 and programmed cell death ligand 1 (PD‐L1) expression on the tumor surface. 6 However, TMB has the disadvantage of having a cut‐off value that varies depending on the tumor type. 7 Moreover, the function of TILs may be suppressed by regulatory T‐lymphocytes, and PD‐L1 expression has the problem of heterogeneity of both intratumor and tumor sites. 8 Therefore, no definitive biomarker for immunotherapy has been found to date. Thus, there is a great need for prognostic biomarkers that can predict response and prognosis early during ICI treatment.
We have previously reported that the relative eosinophil count (REC) and Eastern Cooperative Oncology Group Performance Status (ECOG PS) at the initiation of nivolumab administration were prognostic factors in patients with R/M HNSCC who were treated with nivolumab. 9 The aims of the present study are to select promising prognostic markers in nivolumab therapy and create a novel prognostic score for ICI treatment.
2. MATERIALS AND METHODS
2.1. Patients and treatment
This retrospective study was approved by the institutional review board of Aichi Cancer Center Hospital (2020‐1‐019). Patients treated with nivolumab for R/M HNSCC from April 2017 to April 2019 at our institute were included in this study. We reviewed the electronic clinical records and extracted data on age, gender, ECOG PS, peripheral blood laboratory data, treatment history, and smoking status.
The patients received 3 mg/kg of nivolumab every two weeks from April 2017 to October 2018 and 240 mg/body every two weeks from November 2018. Tumor response was evaluated every 8‐12 weeks, according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, using computed tomography (CT) imaging.
2.2. Statistical analysis
The prognostic scoring system of the present study was developed as follows: (a) Promising prognostic markers were selected, including the two peripheral eosinophilic markers described below, and (b) a modeling scoring system using HR‐based scoring algorithms with the selected prognostic markers was applied. The primary outcome was OS, which was defined as the time from the first nivolumab administration to the date of death or last contact. The secondary outcomes were (a) progression‐free survival (PFS), which was defined as the time from the first nivolumab administration to the date of radiologic progressive disease (PD) or clinically unequivocal progression, and (b) the best overall response (BOR), which was defined as the best response from the start of nivolumab administration to PD. We used the Kaplan‐Meier method to plot the survival curves and the log‐rank test to compare them. Multivariable Cox proportional hazard models were performed to evaluate HRs and 95% CIs.
First, we analyzed two peripheral blood biomarkers related to eosinophils: the REC and the ratio of eosinophil increase (REI). The REC was defined as the highest rate of eosinophil count divided by white blood cell (WBC) count (%) between the initial and secondary administrations of nivolumab, and the REI was defined as the REC at two weeks after the initial nivolumab administration divided by the REC at the initial nivolumab administration (%). Other possible prognostic markers were evaluated using Cox proportional hazard models. We selected variables significantly associated with OS (P < .05). Next, each selected variable was weighted using the HR‐based scoring algorithms, 10 and we created a novel prognostic score for patients with R/M HNSCC who were treated with ICIs. We used the area under the receiver operating characteristic (ROC) curve to determine the cut‐off values of the continuous variables. Spearman’s rank correlation coefficient was used to analyze the correlation of each continuous variable. The trend of the BOR within score groups was ascertained using the Cochran‐Armitage trend test. Stratification analysis was performed according to the study period from April 2017 to April 2018 and from May 2018 to April 2019. The statistical comparisons were two‐sided, and statistical significance was defined as a P value of <0.05. All analyses were performed using the R version 1.6‐3 software program (R Foundation for Statistical Computing).
3. RESULTS
3.1. Patient characteristics
A total of 107 patients were treated with nivolumab during the study period. Twenty patients were excluded from the study, including seven with non‐SCC histology, five whose treatment response was not evaluated, and eight who lacked information on ECOG PS and blood cell count. Finally, 87 patients were included in the analysis. The patient characteristics are shown in Table 1. The oral cavity was the most frequent primary site, followed by the hypopharynx, oropharynx, and nasopharynx. Eighteen patients (20.7%) had a poor PS (ECOG PS = 2 or 3). Most patients (89.7%) had received previous radiation therapy.
Table 1.
Patient characteristics
| Characteristic | Total (n = 87) |
|---|---|
| No. (%) | |
| Age (y) | |
| <65 | 44 (50.6) |
| ≤65 | 43 (49.4) |
| Gender | |
| Male | 64 (73.6) |
| Female | 23 (26.4) |
| Primary site | |
| Oral cavity | 28 (32.2) |
| Nasopharynx | 14 (16.1) |
| Oropharynx | 13 (14.9) |
| Hypopharynx | 13 (14.9) |
| Larynx | 8 (9.2) |
| Other | 11 (12.6) |
| ECOG PS | |
| 0 | 23 (26.4) |
| 1 | 46 (52.9) |
| 2 | 13 (14.9) |
| 3 | 5 (5.8) |
| Chemotherapy line | |
| 1 | 24 (27.6) |
| 2 | 49 (56.3) |
| ≤3 | 14 (16.1) |
| Radiation history | |
| Yes | 78 (89.7) |
| No | 9 (10.3) |
| Prior systemic therapy | |
| Platinum‐based | 59 (67.8) |
| Taxane‐based | 11 (12.6) |
| Cmab‐contained | 34 (39.1) |
| Other | 17 (19.5) |
Abbreviations: Cmab, cetuximab; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
3.2. Survival analysis and selection of variables
The median PFS and OS were 2.8 (95% CI 2.1‐5.2) and 13.2 (95% CI 8.8‐17.0) months, respectively. Regarding the BOR, complete response (CR), partial response (PR), stable disease (SD), and PD occurred in 6.9% (n = 6), 13.8% (n = 12), 25.3% (n = 22) and 54.0% (n = 47) of patients, respectively.
The results of the univariate and multivariate analyses for OS are shown in Table 2. ECOG PS ≥ 3 (HR 124.90, 95% CI 19.78‐788.20; P < .001), REC ≥ 0.015 (HR 0.39, 95% CI 0.19‐0.83; P = .01), and REI ≥ 15 (HR 0.39, 95% CI 0.19‐0.82; P = .01) were significantly associated with OS in the multivariate analysis, whereas C‐reactive protein (CRP), Alb, and neutrophil‐to‐lymphocyte ratio (NLR) were significantly associated with OS only in the univariate analysis.
Table 2.
Univariate and multivariate analyses for overall survival (OS)
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (y) | ||||
| <65 | Reference | .49 | Reference | .60 |
| ≥65 | 0.81 (0.45‐1.47) | 1.19 (0.62‐2.26) | ||
| Gender | ||||
| Male | Reference | .17 | Reference | .50 |
| Female | 1.57 (0.82‐3.01) | 1.31 (0.60‐2.85) | ||
| ECOG PS | ||||
| 0 | Reference | Reference | ||
| 1 | 3.15 (1.29‐7.70) | .012 | 2.69 (1.01‐7.20) | .048 |
| 2 | 3.97 (1.36‐11.56) | .012 | 2.77 (0.81‐9.46) | .10 |
| 3 | 87.83 (20.16‐382.70) | <.001 | 124.90 (19.78‐788.20) | <.001 |
| Primary site | ||||
| Others | Reference | .77 | ||
| Oropharynx | 0.88 (0.37‐2.09) | |||
| Smoking status | ||||
| Never | Reference | .050 | ||
| Smoker | 0.54 (0.29‐1.00) | |||
| Albumin (mg/dL) | ||||
| <3.5 | Reference | .003 | Reference | .30 |
| ≥3.5 | 0.39 (0.21‐0.72) | 0.68 (0.33‐1.41) | ||
| CRP (mg/dL) | ||||
| <1.0 | Reference | .011 | Reference | .53 |
| ≥1.0 | 2.18 (1.20‐3.97) | 1.30 (0.58‐2.90) | ||
| LDH (IU/L) | ||||
| <240 | Reference | .30 | ||
| ≥240 | 1.47 (0.71‐3.05) | |||
| REC | ||||
| <0.015 | Reference | <.001 | Reference | .014 |
| ≥0.015 | 0.24 (0.13‐0.45) | 0.39 (0.19‐0.83) | ||
| REI (%) | ||||
| <15 | Reference | .002 | Reference | .013 |
| ≥15 | 0.38 (0.21‐0.70) | 0.39 (0.19‐0.82) | ||
| NLR | ||||
| <5 | Reference | .008 | Reference | .49 |
| ≥5 | 2.24 (1.23‐4.09) | 1.30 (0.62‐2.73) | ||
| Chemotherapy line | ||||
| 1‐2 | Reference | .03 | Reference | .13 |
| ≥3 | 2.12 (1.06‐4.24) | 1.87 (0.83‐4.22) | ||
| Cmab history | ||||
| Reference | .63 | |||
| 1.16 (0.64‐2.08) | ||||
Abbreviations: CI, confidence interval; Cmab, cetuximab; CRP, C‐reactive protein; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; LDH, lactate dehydrogenase; NLR, neutrophil to lymphocyte ratio; REC, relative eosinophil count; REI, ratio of eosinophil increase.
3.3. Eosinophil prognostic score and treatment outcomes
We selected three variables according to the multivariate analysis results: ECOG PS, REC, and REI. No significant correlation was found between the two variables associated with eosinophils (REC and REI, correlation coefficient = .17; P = .114) (Figure 1). These variables were then weighted using the HR‐based scoring algorithms 10 and divided into four prognostic groups: very good (score = 0), good (score = 1), intermediate (score = 2), and poor (score = 3) (Table 3). This score was named the Eosinophil Prognostic Score. The OS and PFS of the prognostic groups differed significantly. When assessing OS, the patients with poor, intermediate, and good prognoses showed significantly higher HRs for death (poor: 33.21 [95% CI; 6.83‐161.60], moderate: 10.18 [95% CI; 2.34‐44.34], good: 2.77 [95% CI; 0.63‐12.13]) compared with the very good group, respectively (trend P < .001, Figure 2B). A similar trend was observed for PFS (trend P < .001; Figure 2A). A significant dose‐response relationship between the Eosinophil Prognostic Score and survival was observed for both OS and PFS (trend P < .001). When stratification was performed according to the study period, baseline patient characteristics between two cohorts were similar (Table S1). Moreover, similar trends were observed, without any significant difference (Table S2).
Figure 1.
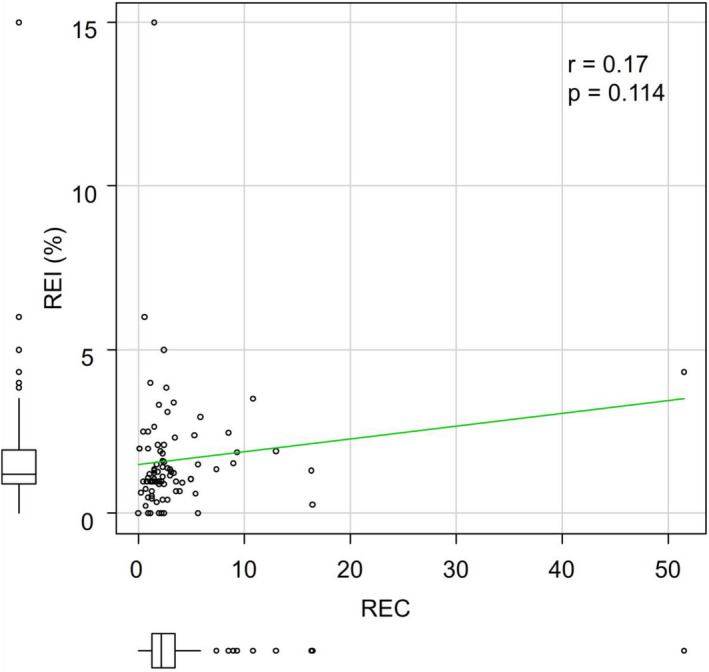
Spearman’s rank correlation coefficient (r) and P‐value (p) between relative eosinophil count (REC) and ratio of eosinophil increase (REI)
Table 3.
Eosinophil prognostic score
| Variable | Score |
|---|---|
| ECOG PS | |
| 0 | 0 |
| 1‐2 | 1 |
| 3‐4 | 2 |
| REC | |
| ≥1.5 | 0 |
| <1.5 | 1 |
| REI (%) | |
| ≥15 | 0 |
| <15 | 1 |
Prognostic groups(score): very good = 0; good = 1; intermediate = 2; poor = 3.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; REC, relative eosinophil count; REI, ratio of eosinophil increase.
Figure 2.
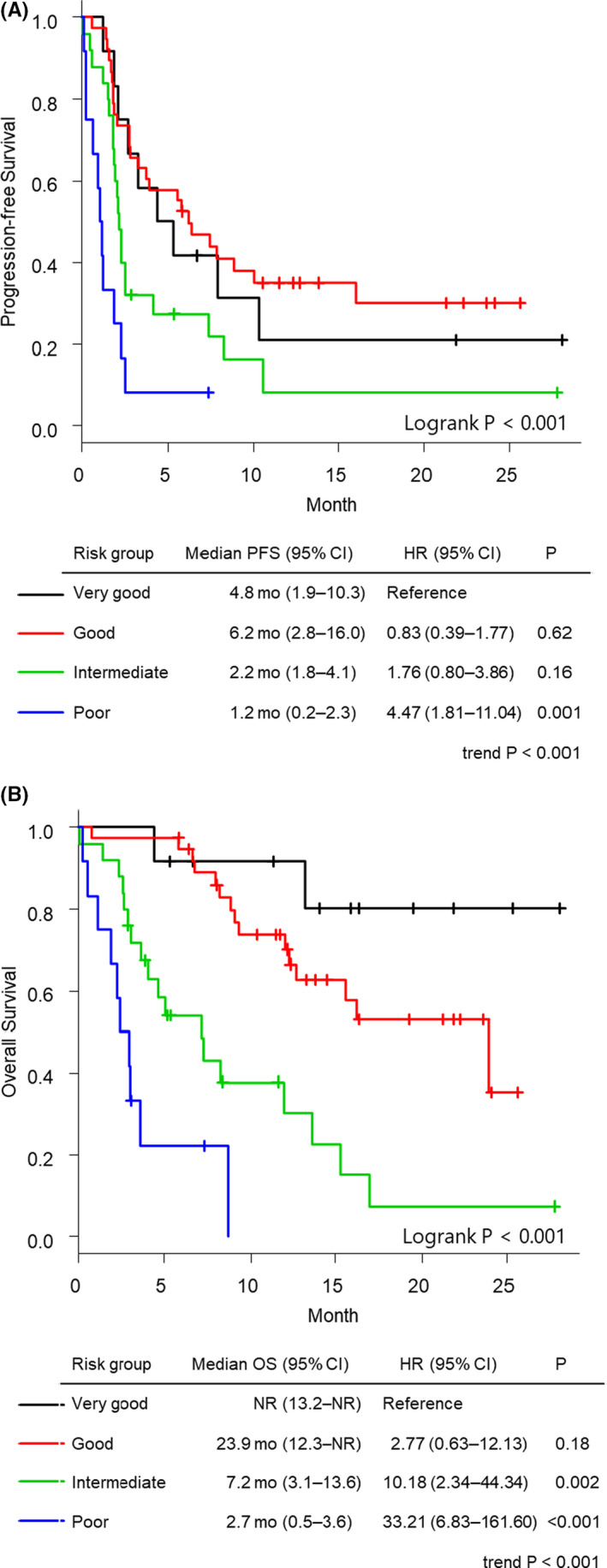
Kaplan‐Meier curves of overall survival (A) and progression‐free survival (B) of patients with head and neck squamous cell carcinoma treated with nivolumab according to the Eosinophil Prognostic Score
The BOR according to the Eosinophil Prognostic Score is shown in Figure 3. The disease control rates (DCRs: CR + PR + SD) of the poor, intermediate, good, and very good groups were 16.7%, 32.0%, 57.9%, and 66.7%, respectively (Cochran‐Armitage trend test P = .002). The RRs (CR + PR) of the poor, intermediate, good, and very good groups were 8.3%, 12.0%, 26.3%, and 33.3%, respectively (Cochran‐Armitage trend test P = .048).
Figure 3.
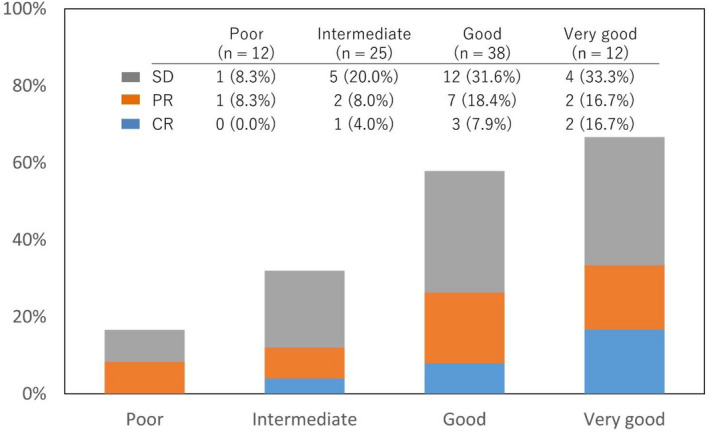
Best overall response according to Eosinophil Prognostic Score. Cochran‐Armitage trend test showed an increasing trend in the proportions of disease control rates (P = .002) and response rates (P = .048). CR, complete response; PR, partial response; SD, stable disease
4. DISCUSSION
We stratified the prognosis of HNSCC patients who were treated with nivolumab based on our prognostic score, which combines the ECOG PS, REC, and REI after the first nivolumab administration. To the best of our knowledge, this is the first report of a prognostic score of ICI efficacy for head and neck cancer. In addition, no previous studies have been found that established a scoring system using the eosinophil ratio and eosinophil increase.
There have been several reports on prognostic scores of ICI treatments for other types of cancers. In non–small cell lung cancer (NSCLC) patients treated with anti‐PD‐1/PD‐L1 antibodies, the ESPILoN score, which combines smoking history, liver metastasis, lactate dehydrogenase (LDH), and the NLR, was significantly correlated with PFS and OS. 11 In recurrent/metastatic renal cell carcinoma treated with anti‐PD‐1 antibodies, due to the Emory risk scoring system, which includes the monocyte‐to‐lymphocyte ratio (MLR), body mass index (BMI), and number of metastatic sites, patients in the very poor risk group had a significantly worse PFS and OS. 12 The Gustave Roussy Immune Score (GRIm‐Score) uses LDH, serum albumin, and the NLR and is reported to be significantly correlated with OS in patients who are administered anti‐PD‐1/PD‐L1 antibodies for NSCLC. 13 The RHM score is created by retrospectively extracting negative prognostic factors. 14 The combined scores of LDH, serum albumin, and the number of metastatic sites were significantly correlated with PFS in NSCLC patients treated with anti‐PD‐1/PD‐L1 antibodies. Although some reports include indicators of peripheral blood cell count, e.g., NLR and MLR, no reports have evaluated indicators related to eosinophils.
In a previous article, we reported that low REC was an independent poor prognostic factor for both PFS and OS in patients with R/M HNSCC treated with nivolumab. 9 This result was correlated with other previous reports on other types of cancer treated with ICIs, 15 , 16 , 17 , 18 , 19 specifically colon, esophageal, penile, and prostate cancers. Eosinophils directly eradicate tumors by degranulating cytotoxic proteins, e.g., major basic protein (MBP). 20 Moreover, eosinophils indirectly lead to tumor elimination by releasing chemoattractants, which induce the migration of tumor‐specific CD8+ T‐cells to the tumor. 21 In the present study, eosinophil infiltration at the tumor site was not investigated. However, it has been reported that in patients receiving ICI treatment, the number of peripheral blood eosinophils is directly proportional to the number of eosinophils infiltrating the primary skin tumor. 22 Therefore, we assume that the high number of peripheral blood eosinophils reflects the high number of tumor‐infiltrating eosinophils.
Eosinophil recruitment at tumor sites is mediated by several chemokines produced by tumor cells. Tissue expression of eotaxin (CCL11), which is secreted by eosinophils, was correlated with the increased recruitment of eosinophils in Hodgkin lymphoma. 23 In oral SCC, eotaxin is mainly secreted by eosinophils, which suggests the existence of an autocrine and/or paracrine pathway to maintain tissue eosinophilia. 24 Additionally, damage‐associated molecular pattern (DAMP) molecules released by tumor necrotic tissue induces eosinophil migration. 17 , 25 High‐mobility group box 1 (HMGB1) is one of these molecules. HMGB1 released from tumor necrotic tissues recruits eosinophils through a receptor for advanced glycation end products (RAGE) expressed on eosinophils and induces degranulation. 26 Thus, a high eosinophil count reflects an increase of tumor antigens due to tumor necrosis and collapse. The increase of tumor antigens could lead to a high treatment response to immunotherapy. DAMPs also act as danger signals, alerting the immune system by binding to their receptors on immune cells. DAMP‐based danger signals directly activate innate immune cells including dendritic cells, macrophages, neutrophils, and natural killer cells 27 ; cause functional maturation of dendritic cells or neutrophils 28 ; and facilitate proper tumor‐associated antigen processing and presentation. 29
The results of the present study showed that an eosinophil increase after nivolumab administration is also related to a better prognosis. We speculate that early eosinophil increase indicates the reactivity of immune function to ICIs and/or the progression of tumor lysis due to ICIs. Multiple studies have shown a correlation between eosinophil increase during ICI treatment and longer OS. Delyon et al. reported that an increase in the eosinophil count > 100/mm3 between the first and second ipilimumab administrations was correlated with an improved OS in unresectable stage III or IV melanoma (median OS of 11.3 vs 6.8 months; P = .012). 30 Gebhardt et al 31 observed an early significant increase in eosinophil count in the peripheral blood after the first infusion of ipilimumab in responding patients (HR 23.2; P = .017). Although increased eosinophil concentrations during ipilimumab therapy were correlated with an improved OS in metastatic melanoma patients, no correlation with OS was found for treatment with vemurafenib, a BRAF (v‐raf murine sarcoma viral oncogene homolog B1) inhibitor. 32 This suggests that an increase in eosinophils is a specific indicator of ICI treatments.
Eosinophilia is also observed during treatment with other immunotherapies. Interleukin‐2 (IL‐2) and IL‐4 administrations for cancer patients were reportedly related to eosinophil degranulation. 33 , 34 Eosinophil degranulation on bladder cancer cells was observed after IL‐2 administration. 33 Similarly, the administration of IL‐4 to cancer patients induced eosinophil degranulation based on increased levels of MBP in serum and urine. 34 Hence, our prognostic score may be used for immunotherapies other than ICI treatments.
Nivolumab is the standard therapy for platinum‐refractory R/M HNSCC. However, the low RR to nivolumab therapy encouraged us to develop an easy‐to‐use scoring system that can accurately estimate the efficacy of nivolumab therapy. Although the exact mechanism of the effects of eosinophils during ICI treatment remain unclear, the Eosinophil Prognostic Score, which uses the REC and REI, appears beneficial for identifying patients who respond to ICI treatment. Some authors have reported a high RR to salvage chemotherapy after ICI failures in head and neck cancer, NSCLC, and urothelial cancer. 35 , 36 , 37 Therefore, it is important to sequentially lead to the next regimen at the appropriate time. From that perspective, including the assessment of early changes in the eosinophil rate after the start of ICIs in the Eosinophil Prognostic Score contributes to switching to promising sequential chemotherapy from ICIs when the tumor has not responded to ICI administration.
There are some limitations to our study. First, this was a single‐institution retrospective study with a relatively small sample size and a short follow‐up period. Second, some background patient data were unknown (e.g., comorbidities). Third, IL‐5, which is reportedly associated with increased eosinophil count, was not investigated in this study. Fourth, validation in another separate cohort was not available. However, when stratified according to the study period, a similar trend was observed across two cohorts, suggesting that validation might be obtainable. To replicate our findings, future large‐scale, multicenter studies are warranted.
We have created a novel prognostic score composed of the eosinophil count, eosinophil increase, and ECOG PS for nivolumab treatment. The Eosinophil Prognostic Score may be useful for determining whether nivolumab treatment should be continued or changed to another chemotherapy regimen early in the nivolumab administration period.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
Table S1
Table S2
Nishikawa D, Suzuki H, Beppu S, et al. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 2021;112:339–346. 10.1111/cas.14706
REFERENCES
- 1. Saloura V, Cohen EEW, Licitra L, et al. An open‐label single‐arm, phase II trial of zalutumumab, a human monoclonal anti‐EGFR antibody, in patients with platinum‐refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2014;73:1227–1239. [DOI] [PubMed] [Google Scholar]
- 2. Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): a randomised, open‐label, phase 3 study. Lancet. 2019;394:1915–1928. [DOI] [PubMed] [Google Scholar]
- 4. Samstein RM, Lee C‐H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tumeh PC, Harview CL, Yearley JH, et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 7. Alexandrov LB, Nik‐Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haragan A, Field JK, Davies MPA, Escriu C, Gruver A, Gosney JR. Heterogeneity of PD‐L1 expression in non‐small cell lung cancer: Implications for specimen sampling in predicting treatment response. Lung Cancer. 2019;134:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishikawa D, Suzuki H, Koide Y, et al. Prognostic markers in head and neck cancer patients treated with nivolumab. Cancers. 2018;10(12):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. [DOI] [PubMed] [Google Scholar]
- 11. Prelaj A, Ferrara R, Rebuzzi SE, et al. EPSILoN: a prognostic score for immunotherapy in advanced non‐small‐cell lung cancer: a validation cohort. Cancers. 2019;11:1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martini DJ, Liu Y, Shabto JM, et al. Novel risk scoring system for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Oncologist. 2020;25:e484–e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bigot F, Castanon E, Baldini C, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm‐Score). Eur J Cancer. 2017;84:212–218. [DOI] [PubMed] [Google Scholar]
- 14. Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandez‐Acenero MJ, Galindo‐Gallego M, Sanz J, Aljama A. Prognostic influence of tumor‐associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544–1548. [PubMed] [Google Scholar]
- 16. Ishibashi S, Ohashi Y, Suzuki T, et al. Tumor‐associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26:1419–1424. [PubMed] [Google Scholar]
- 17. Costello R, O'Callaghan T, Sebahoun G. [Eosinophils and antitumour response]. Rev Med Interne. 2005;26:479–484. [DOI] [PubMed] [Google Scholar]
- 18. Ono Y, Ozawa M, Tamura Y, et al. Tumor‐associated tissue eosinophilia of penile cancer. Int J Urol. 2002;9:82–87. [DOI] [PubMed] [Google Scholar]
- 19. Luna‐More S, Florez P, Ayala A, Diaz F, Santos A. Neutral and acid mucins and eosinophil and argyrophil crystalloids in carcinoma and atypical adenomatous hyperplasia of the prostate. Pathol Res Pract. 1997;193:291–298. [DOI] [PubMed] [Google Scholar]
- 20. Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1–8. [DOI] [PubMed] [Google Scholar]
- 21. Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–617. [DOI] [PubMed] [Google Scholar]
- 22. Simon SCS, Hu X, Panten J, et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology. 2020;9:1727116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teruya‐Feldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin's disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood. 1999;93:2463–2470. [PubMed] [Google Scholar]
- 24. Lorena SC, Oliveira DT, Dorta RG, Landman G, Kowalski LP. Eotaxin expression in oral squamous cell carcinomas with and without tumour associated tissue eosinophilia. Oral Dis. 2003;9:279–283. [DOI] [PubMed] [Google Scholar]
- 25. Stenfeldt AL, Wenneras C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lotfi R, Herzog GI, DeMarco RA, et al. Eosinophils oxidize damage‐associated molecular pattern molecules derived from stressed cells. J Immunol. 2009;183:5023–5031. [DOI] [PubMed] [Google Scholar]
- 27. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. [DOI] [PubMed] [Google Scholar]
- 28. Garg AD, Vandenberk L, Fang S, et al. Pathogen response‐like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil‐mediated residual cell killing. Cell Death Differ. 2017;24:832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll‐like receptor 4‐dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. [DOI] [PubMed] [Google Scholar]
- 30. Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–1703. [DOI] [PubMed] [Google Scholar]
- 31. Gebhardt C, Sevko A, Jiang H, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. 2015;21:5453–5459. [DOI] [PubMed] [Google Scholar]
- 32. Lang BM, Peveling‐Oberhag A, Faidt D, et al. Long‐term survival with modern therapeutic agents against metastatic melanoma‐vemurafenib and ipilimumab in a daily life setting. Med Oncol. 2018;35:24. [DOI] [PubMed] [Google Scholar]
- 33. Huland E, Huland H. Tumor‐associated eosinophilia in interleukin‐2‐treated patients: evidence of toxic eosinophil degranulation on bladder cancer cells. J Cancer Res Clin Oncol. 1992;118:463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sosman JA, Bartemes K, Offord KP, et al. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin‐4: effects of interleukin‐4 alone and following interleukin‐2 administration. Clin Cancer Res. 1995;1:805–812. [PubMed] [Google Scholar]
- 35. Saleh K, Daste A, Martin N, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019;121:123–129. [DOI] [PubMed] [Google Scholar]
- 36. Schvartsman G, Peng SA, Bis G, et al. Response rates to single‐agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non‐small cell lung cancer. Lung Cancer. 2017;112:90–95. [DOI] [PubMed] [Google Scholar]
- 37. Szabados B, van Dijk N, Tang YZ, et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol. 2018;73:149–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


