Abstract
N‐myc downstream‐regulated gene 2 (NDRG2) is a candidate tumor suppressor in various cancers, including adult T‐cell leukemia/lymphoma (ATLL). NDRG2, as a stress‐responsive protein, is induced by several stress‐related signaling pathways and NDRG2 negatively regulates various signal transduction pathways. Although it has not been found to function alone, NDRG2 binds serine/threonine protein phosphatase 2A (PP2A), generating a complex that is involved in the regulation of various target proteins. The main function of NDRG2 is to maintain cell homeostasis by suppressing stress‐induced signal transduction; however, in cancer, genomic deletions and/or promoter methylation may inhibit the expression of NDRG2, resulting in enhanced tumor development through overactivated signal transduction pathways. A wide variety of tumors develop in Ndrg2‐deficient mice, including T‐cell lymphoma, liver, lung and other tumors, the characteristics of which are similar to those in Pten‐deficient mice. In particular, PTEN is a target molecule of the NDRG2/PP2A complex, which enhances PTEN phosphatase activity by dephosphorylating residues in the PTEN C‐terminal region. In ATLL cells, loss of NDRG2 expression leads to the failed recruitment of PP2A to PTEN, resulting in the inactivation of PTEN phosphatase with phosphorylation, ultimately leading to the activation of PI3K/AKT. Thus, NDRG2, as a PP2A adaptor, regulates the global phosphorylation of important signaling molecules. Moreover, the downregulation of NDRG2 expression by long‐term stress‐induced methylation is directly correlated with the development of ATLL and other cancers. Thus, NDRG2 might be important for the development of stress‐induced leukemia and other cancers and has become an important target for novel molecular therapies.
Keywords: ATLL, HSP90, NDRG2, PP2A, PTEN
In tumor cells, the loss of NDRG2 expression induces constitutive phosphorylation of PTEN C‐terminal, which maintains PTEN in an inactive closed conformation and leads to constitutive AKT activation.
Abbreviations
- 4‐NQO
4‐Nitroquinoline‐1‐oxide
- AKT
AKT serine/threonine kinase 1
- ATLL
adult T‐cell leukemia/lymphoma
- CIP2A
cellular inhibitor of PP2A
- EMT
epithelial‐to‐mesenchymal transition
- ER
endoplasmic reticulum
- ERR‐α
estrogen‐related receptor‐alpha
- EZH2
enhancer of zeste 2 polycomb repressive complex 2 subunit
- HIF‐1α
hypoxia‐inducible factor 1‐alpha
- HSP90
heat shock protein 90
- IKK
IκB kinase
- IRF3
interferon regulatory factor 3
- IκB
inhibitor of NF‐κB
- NDRG2
N‐myc downstream‐regulated gene 2
- NEMO
NF‐κB essential modulator
- NF‐κB
nuclear factor kappa B
- NIK
NF‐κB‐inducing kinase
- OSCC
oral squamous cell carcinoma
- PIP3
phosphatidylinositol 3,4,5‐trisphosphate
- PP2A
protein phosphatase 2A
- PRMT5
protein arginine methyltransferase 5
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- RACK1
receptor for activated C kinase 1
- SET
SET nuclear proto‐oncogene
- SNAIL
SNAIL family of transcriptional repressors
- T‐ALL
T‐cell acute lymphoblastic leukemia
- TWIST
twist family bHLH transcription factors
1. INTRODUCTION
N‐myc downstream‐regulated gene 1 (NDRG1), a member of the NDRG family, was identified as a stress stimulus‐induced gene 1 that is downregulated by N‐myc gene expression. 2 Four family members, namely, NDRG1, NDRG2, NDRG3, and NDRG4, are found in mammals, and they have 57%‐65% identical amino acid sequences. 3 NDRG proteins have a common NDR domain in the middle of the protein with an alpha (α)/beta (β) hydrolase‐like region; however, no NDRG family member plays a hydrolase role or has an enzymatic function. 4 Expression of NDRG2 is induced by several stress stimuli, such as hypoxia, DNA damage or endoplasmic reticulum stress (ERS) and other pathological conditions, and are considered tumor suppressor genes. 5 We isolated NDRG2 as a candidate tumor suppressor gene based on a the genome analysis of ATLL. Moreover, downregulated NDRG2 expression was reported to be a candidate tumor suppressor in various types of human cancers and, in our study, the development of a wide‐variety of tumors, including T‐cell lymphoma, was observed in Ndrg2‐deficient mice. 6 Therefore, in this review, we summarize the elucidation of the physiological function of NDRG2, particularly the possible role of downregulated NDRG2 expression in ATLL and other cancers, and the possible value of NDRG2 as a tumor suppressor. Future directions of research are discussed.
2. WHAT ROLE DOES NDRG2 PLAY IN NORMAL CELLS?
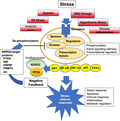
NDRG2 was found to be a stress‐responsive gene that is transcriptionally activated by several types of cellular stress stimuli. 7 , 8 , 9 NDRG2 mRNA increases in response to glucocorticoid stimulation in astrocytes, 10 to hypoxia through the activation of HIF‐1α, 9 DNA damage through p53 activation, 7 and to other kinds of stresses (Figure 1). After NDRG2 expression is increased by stress, NDRG2 suppresses cell proliferation, induces cell death, and suppresses protein synthesis and other metabolic processes. However, the actual function of NDRG2 in suppressing signaling systems has remained unclear.
Figure 1.

N‐myc downstream‐regulated gene 2 (NDRG2) is a key regulator of cellular stress responses. NDRG2 maintains cellular homeostasis by regulating multiple biological processes in response to genotoxic stress that involves p53, 7 hypoxic stress mediated by hypoxia‐inducible factor 1‐alpha (HIF‐1α), 9 hyperthermia mediated by AKT signaling, 56 inflammation mediated by the NF‐κB signaling pathway, 33 and anabolic stress mediated by estrogen‐related receptor‐alpha (ERR‐α). 57 Under stress conditions, cells may activate signaling pathways such as AKT or gene transcription to promote stress‐defense responses including apoptosis, the immune response, inflammation, or metabolic regulation, and these cellular responses can be terminated by the dephosphorylation of important signaling molecules such as phosphatase and tensin homolog deleted on chromosome 10 (PTEN), NF‐κB‐inducing kinase (NIK) or others by the NDRG2‐protein phosphatase 2A (PP2A) complex as a negative feedback loop
To elucidate the function of NDRG2, we carried out a comprehensive analysis of NDRG2‐binding proteins. Among these binding proteins, we found PP2A, which is a major serine/threonine protein phosphatase. PP2A is known to be involved in the regulation of a wide range of signaling systems through its phosphatase activity. 11 PP2A itself is thought to be a tumor suppressor and has been shown to be downregulated in many cancers, and PP2A inhibitors such as SET and CIP2A have been identified. 12 , 13 In addition to PP2A inhibitors, PP2A is affected by RACK1 adaptor (scaffold) protein, which recruits PP2A and deactivates transcription factor called IRF3 via the inhibition of type I interferon signaling. 14 Similar to RACK1, we found that NDRG2, a scaffold protein, is able to bind PP2A, which results in PP2A dephosphorylation of a group of signaling proteins, such as PTEN, NIK, PRMT5 and HSP90 (Figure 1), which cannot directly bind to PP2A. 6 , 15 , 16 PTEN can be dephosphorylated by PP2A only through NDRG2‐mediated binding to PTEN. 6 The enzymatic activity of PTEN as a phosphatase is regulated by its phosphorylation at the C‐terminus (Ser380, Thr382, and Thr383, the STT cluster), and when PTEN is phosphorylated at the PTEN‐STT cluster, it is uncoupled from the cell membrane and loses its enzyme activity. 17 PTEN that has been dephosphorylated by the NDRG2/PP2A complex is activated and binds to the cell membrane to dephosphorylate PIP3 (Figure 2).
Figure 2.

Regulation of PI3K/AKT signaling by N‐myc downstream‐regulated gene 2 (NDRG2)/protein phosphatase 2A (PP2A) complex. In normal cells, NDRG2 recruits PP2A to phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which induces the dephosphorylation of PTEN at Ser380/Thr382/Thr383 (PTEN‐STT cluster). This dephosphorylation of the PTEN‐STT cluster leads to the open conformation of the C‐tail that allows PTEN to associate with the cell membrane and the C2 domain can bind phosphatidylserine (PS) inside the cell membrane and the subsequent suppression of PI3K/AKT signaling via dephosphorylation of phosphatidylinositol 3,4,5‐trisphosphate (PIP3). In tumor cells, the loss of NDRG2 expression induces constitutive phosphorylation of the PTEN‐STT cluster, which prevents binding of PTEN to the cell membrane by the closed conformation, leading to constitutive AKT activation via inactivation of the phosphatase activity of PTEN. 6 This figure has been adapted from reference 6 with permission
In cell survival, various types of stress stimuli, such as inflammation, proliferative stimuli, and metabolic activation by insulin, activate several signaling systems, including the PI3K/AKT signaling pathway. 18 , 19 , 20 Eventually, the activated signaling systems return to their unstimulated state. The expression of NDRG2 is upregulated under stress conditions 7 , 8 , 9 and forms a complex with PP2A that binds to PTEN and dephosphorylates the PTEN‐STT cluster. 6 The active form of PTEN dephosphorylates PIP3, resulting in the suppression of PI3K/AKT signaling. Thus, NDRG2 is upregulated by various stresses and consequently triggers a negative feedback mechanism by suppressing the stress‐activated signaling pathway to return the cells to their normal state (Figure 1). In addition to that of the PI3K/AKT signaling pathway, the kinase activity of NIK in the non‐canonical NF‐κB pathway is regulated by phosphorylation via the NDRG2/PP2A complex. 15 Moreover, the enzyme activity of arginine methyltransferase of PRMT5 and the activation status of HSP90 are regulated by the NDRG2/PP2A complex. 16
Thus, the stress‐activated signaling system upregulates NDRG2 expression, and NDRG2 forms a complex with PP2A, which binds to various signaling proteins. The NDRG2/PP2A complex regulates enzymatic activity via dephosphorylation, resulting in the suppression of the signaling pathways in a negative feedback mechanism. This suppressive signaling pathway triggered by the NDRG2/PP2A complex might lead cells to resume their conventional inactivate state.
3. ROLES OF NDRG2 IN CANCER
3.1. Downregulation of NDRG2 expression in ATLL and other cancers
Chromosome 14q11 is one of the most common chromosomal breakpoint regions in ATLL. 21 In patients with chromosomal deletions at 14q11, breakpoints are most often found adjacent to the T cell receptor alpha‐delta chain locus (TCRα/δ), and a recurrent 0.9‐Mb interstitial deletion was identified in a region that includes part of the TCRα/δ locus. 6 Among the genes that map within this deleted region common to ATLL, NDRG2 is consistently downregulated in ATLL cells in patients with acute ATLL. 6 Furthermore, no somatic mutations were observed in the NDRG2 gene in samples obtained from 34 patients with primary ATLL. Epigenetic modifications, including DNA methylation and histone deacetylation, occur at the NDRG2 locus in ATLL. 6 We isolated NDRG2 because it is a 14q11 tumor suppressor candidate commonly inactivated by genomic deletion and epigenetic silencing in ATLL. Increasing evidence has shown that NDRG2 is downregulated in ATLL and in other types of cancers, such as liver cancer, gastric cancer, and OSCC, through methylation of the NDRG2 gene promoter. 22 , 23 , 24 , 25 , 26 , 27 Moreover, cancer cells with restored NDRG2 expression show suppressed cell proliferation or metastasis; however, the mechanism by which NDRG2 expression is downregulated in cancer cells is still unknown. 28 , 29 , 30 , 31 , 32
The transcriptional regulation of NDRG2 via epigenetic promoter methylation was investigated in ATLL cells associated with HTLV‐1 infection. 33 In CD4+ T cells, NDRG2 expression was induced by HTLV‐1/Tax, 33 an oncogenic viral protein of HTLV‐1. 34 In the early stage of HTLV‐1 infection, the Tax‐inducible NF‐κB signaling pathway upregulates NDRG2 expression, thereby suppressing the cancer‐promoting effect of Tax in HTLV‐1+ T cells. In addition, Tax‐inducible NF‐κB enhances the expression of the polycomb gene (enhancer of zeste 2 polycomb repressive complex 2 subunit [EZH2]), and enhanced expression of EZH2 induces triple methylation of arginine 27 of histone H3 (H3K27me3) and DNA methylation at the NDRG2 promoter. These modifications result in the downregulation of NDRG2 expression. 33 Finally, H3K27me3 enhancement by EZH2 was observed in most ATLL cells. 35 This result indicated that the specific epigenetic abnormality of ATLL cells is caused by EZH2 and is involved in the development of ATLL. Therefore, epigenesis‐modification factors, including EZH2, are involved in NDRG2 expression and are important therapeutic targets.
3.2. Activation of the PI3K/AKT/NF‐κB signaling pathways by NDRG2 downregulation in cancer cells
PTEN is a lipid phosphatase that dephosphorylates PIP3 to generate PI(4,5)P2, which negatively regulates the PI3K/AKT pathway, thereby exerting tumor suppressor activity. 36 , 37 , 38 PTEN is one of the most commonly mutated genes in cancers. 39 However, the activation of the PI3K/AKT signaling pathway is likely to occur in different types of cancers regardless of whether PTEN is mutated. 40 Indeed, no mutations or genomic deletions were observed within the coding regions of PTEN in leukemic cells for 34 patients with ATLL and in 47 of 50 cancer cell lines, including lung cancer, liver cancer, and OSCC cells. 6 In contrast, under certain conditions, PTEN is subjected to phosphorylation at the C‐terminus at the PTEN‐STT cluster. This phosphorylation downregulates PTEN phosphatase activity 17 , 41 , 42 because PTEN is inactive, in a closed conformation in the cytoplasm after detaching from the cell membrane. In contrast, dephosphorylation causes PTEN to acquire an open conformation, which causes it to localize preferentially to the cell membrane where this active form can PTEN phosphatase antagonize PI3K/AKT signaling. 17 PTEN inactivation via increased phosphorylation of the PTEN‐STT cluster is observed in several malignancies, including T‐ALL. 43 , 44 Moreover, a high phosphorylation level was observed in the primary ATLL cells and ATLL cell lines. Moreover, the forced expression of a PTEN‐STT cluster mutant in ATLL‐related cell lines inhibited AKT phosphorylation and cell growth. 6 Thus, phosphorylation of the PTEN‐STT cluster plays an important role in the activation of PI3K‐AKT in ATLL. Intriguingly, the phosphorylation of the PTEN‐STT cluster was significantly decreased in ATLL cell lines upon transfection of an NDRG2 expression vector, which suppressed PI3K/AKT activation. 6 PTEN dephosphorylation is induced by NDRG2 expression. Hence, NDRG2 is considered a PTEN‐associated protein that recruits PP2A as an adaptor protein to facilitate the dephosphorylation of the PTEN‐STT cluster (Figure 2). 6 Moreover, Ndrg2‐deficient mice had high levels of the Pten‐STT cluster and Akt phosphorylation in various tissues. This finding supports the notion that tumor formation might be correlated with the activation of the PI3K/AKT signaling pathway through abrogation of phosphatase activity upon PTEN phosphorylation. Homozygous Pten‐deficient mice show an embryonically lethal phenotype; however, heterozygous Pten‐deficient mice develop different types of tumors with high penetration, such as T‐cell lymphoma and endometrial, prostate, and thyroid tumors. 45 T‐cell lymphomas are mainly observed in Pten‐ and Ndrg2‐deficient mice. 6 , 45 Thus, the phenotypes of heterozygous Pten‐deficient mice and homozygous and heterozygous Ndrg2‐deficient mice are quite similar in terms of cancer development, indicating that tumor formation may be dependent on Pten inactivation in Ndrg2‐deficient mice. Thus, in ATLL cells and other types of tumor cells without genetic alterations to the PI3K/AKT pathway, suppression of NDRG2 transcription disrupts the negative regulation of PI3K/AKT signaling by sustained phosphorylation of the PTEN‐STT cluster during tumor development.
The hyperphosphorylation of PTEN caused by the loss of NDRG2 function leads to activation of not only the PI3K/AKT signaling pathway but also to the NF‐κB canonical pathway. In the canonical NF‐κB pathway, various point mutations are involved in the activation of cytokine signaling in ATLL cells, 46 with the inactivation of p47, which is a negative regulator. 47 Further, NF‐κB signaling is activated by Tax protein associated with HTLV‐1 infection. 48 In addition to PTEN, NIK, which is an important factor in the non‐canonical NF‐κB pathway, is considered a target protein for dephosphorylation via the recruitment of the NDRG2/PP2A complex (Figure 3). 15 In ATLL cells, NIK is highly expressed when the expression of miR‐31 is reduced. 49 Moreover, overexpressed NIK is highly phosphorylated at Thr559 because of the loss of NDRG2 expression. 15 Phosphorylated NIK maintains its kinase activity, stimulating p100 proteolysis to generate p52, which leads to the nuclear translocation of the RelB/p52 complex. 15 In normal cells, NDRG2 expression induces the suppression of AKT by the dephosphorylation of PTEN, which in turn induces the suppression of the canonical NF‐κB signaling pathway by decreasing IKK activation. NIK is maintained at low levels. In ATLL cells, the loss of NDRG2 promotes the phosphorylation of PTEN and NIK, resulting in the activation of canonical and non‐canonical NF‐κB pathways. 15
Figure 3.
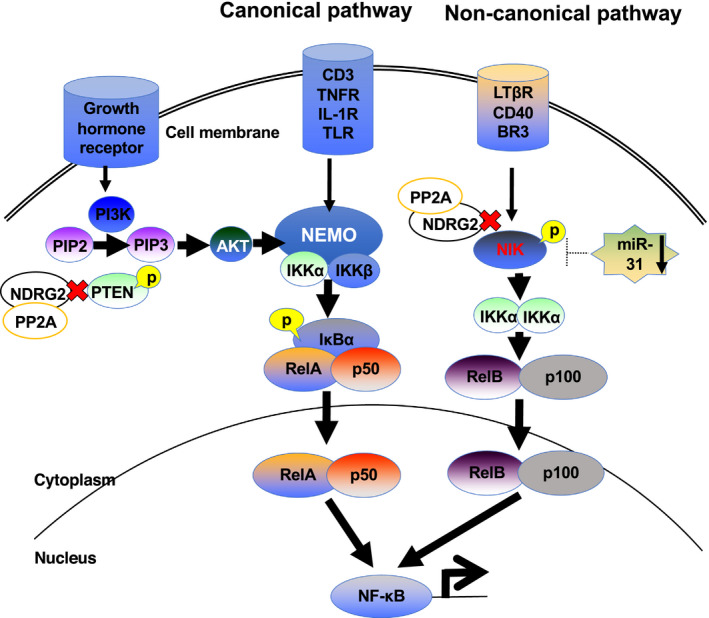
Regulation of nuclear factor kappa B (NF‐κB) signaling by N‐myc downstream‐regulated gene 2 (NDRG2)/protein phosphatase 2A (PP2A) complex. A central signaling component of the non‐canonical pathway is NF‐κB‐inducing kinase (NIK) and the NDRG2/PP2A complex regulating the kinase activity of NIK by dephosphorylation reaction. Homeostatic activation of the NF‐κB signaling system is known to be important in the development of adult T‐cell leukemia/lymphoma (ATLL). The low expression of miR‐31 in ATLL cells, which is involved in the repression of NIK expression, leads to increase in NIK protein. In addition to this phenomenon, NIK is highly phosphorylated with high kinase activity due to low expression of NDRG2, and dysregulation of NDRG2/PP2A complex was found to be an important factor in activating the non‐canonical NF‐κB pathway. Moreover, the downregulation of NDRG2 expression also induces activation of the canonical NF‐κB pathway through activation of the IκB kinase (IKK) complex by constitutive activation of AKT, which is derived from the phosphorylation of PTEN caused by downregulation of NDRG2 in ATLL cells. 15 Therefore, NDRG2 is one of the important regulatory factors of the NF‐κB pathway. This figure has been adapted from reference 15 with permission
Therefore, NDRG2 is an important repressor of the PI3K/AKT and NF‐κB signaling pathways, which have important functions in cell proliferation. Moreover, the inactivation of NDRG2 may be essential for the development of cancers, including leukemia.
3.3. Activation of HSP90 by NDRG2 downregulation in cancer cells
PRMT5 belongs to the arginine methyltransferase family, which has several functions in oncology, including transcriptional regulation in the nucleus upon arginine methylation of histones and several signal transduction proteins in the cytoplasm. 50 PRMT5 expression did not change in normal CD4+ T cells or samples obtained from patients with ATLL. 16 In NDRG2‐expressing normal cells, PRMT5 was observed in the cytoplasm and nucleus, and histones H4 and H3, which are the specific substrates of PRMT5, were arginine methylated. 16 However, in NDRG2‐deficient ATLL cells, PRMT5 was localized in the cytoplasm, and the arginine methylation of histones was lost, indicating that PRMT5 is involved in the development of ATLL. 16 NDRG2 deficiency leads to constitutively phosphorylated Ser335 of PRMT5, which is involved in the cytoplasmic localization and enhancement of PRMT5 enzyme activity. 16 Therefore, we comprehensively examined NDRG2‐binding proteins via immunoprecipitation‐mass spectrometry analysis. Results showed that NDRG2 specifically binds to PRMT5 and the chaperone HSP90 in the cytoplasm of ATLL cells. 16 Moreover, the NDRG2/PP2A complex binds to PRMT5 and regulates its enzymatic function by regulating its phosphorylation status. Further, in ATLL cells, PRMT5 was mainly found in the cytoplasm, where it maintains a hyperphosphorylated form and continuous enzymatic activity (Figure 4). 16 Moreover, HSP90 is considered a substrate for PRMT5. 16 HSP90 is a molecular chaperone that contributes to the stability and enhanced activity of AKT, NEMO, and other important signaling molecules, which are referred to as client proteins and are required for the survival and proliferation of tumor cells. 15 , 51 Once HSP90 is inactivated in tumor cells, the client proteins are degraded, inducing cell death by the inhibited chaperone activity. Post‐translational modifications, such as phosphorylation and acetylation, regulate the chaperone activity of HSP90. 52 We first identified arginine methylation of HSP90A at R345 and R386, which enhances chaperone activity and stabilizes client proteins through PRMT5 in ATLL cells. Therefore, it is proposed that in normal cells, PRMT5 dephosphorylated by the NDRG2/PPA2 complex localizes to the nucleus, leading to the arginine methylation of histones (Figure 4A). In ATLL cells, the loss of NDRG2 induces the phosphorylation of PRMT5 at Ser335, which induces the binding of HSP90A with PRMT5 in the cytoplasm. Methylation of HSP90A arginine by PRMT5 is associated with the stability of client proteins and tumor development (Figure 4B). When the expression of PRMT5 was downregulated in ATLL cells, the chaperone activity decreased with the suppression of HSP90A arginine methylation. This decrease in methylation resulted in the degradation of client proteins and the induction of apoptosis (Figure 4). 16 This effect was not observed in NDRG2‐expressing normal cells. Hence, NDRG2 and PRMT5/HSP90 have a synthetic lethal relationship and are useful therapeutic targets. 16
Figure 4.
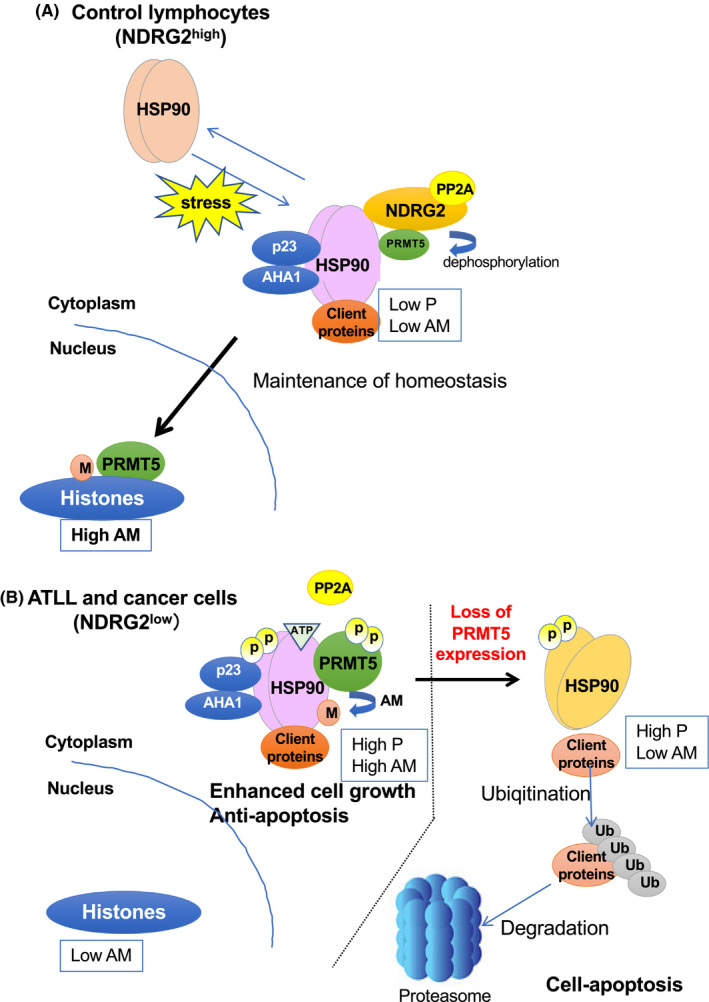
Regulation of heat shock protein 90 (HSP90A) function by arginine methylation of protein arginine methyltransferase 5 (PRMT5) via the N‐myc downstream‐regulated gene 2 (NDRG2)/protein phosphatase 2A (PP2A) complex (NDRG2/PP2A). Heat shock protein (HSP) 90, an ATP‐dependent molecular chaperone, is essential for oncogenic transformation through supporting a wide variety of activated oncoproteins, kinases, transcription factors and others. Although the activity of HSP90 is regulated by many protein modifications such as phosphorylation and acetylation, we first reported a mechanism for the activity regulation of HSP90 by arginine methylation through PRMT5. Moreover, the NDRG2/PP2A complex regulates the arginine methyltransferase activity of PRMT5 by its phosphorylation status. (A) In normal cells, the NDRG2/PP2A complex dephosphorylates PRMT5 and unphosphorylated PRMT5 is mainly localized to the nucleus and leads to arginine methylation of histones; however, cytoplasmic PRMT5 cannot catalyze the arginine residues in HSP90. 16 (B) In adult T‐cell leukemia/lymphoma (ATLL) or other cancer cells with low expression of NDRG2, PRMT5 is mainly localized in the cytoplasm and is highly phosphorylated by the downregulation of NDRG2, and induces the methylation of HSP90A arginine residues, leading to the enhancement of its chaperone activity (AHA1 and p23) and stabilization of client proteins, including AKT, NF‐κB essential modulator (NEMO) or others, contributing to enhanced cell growth and anti‐apoptosis. In the right half of the figure, knocking down PRMT5 expression in NDRG2‐deficient tumors induces cell apoptosis via the reduction of HSP90A function and the degradation of client proteins via activation of the ubiquitin‐proteasome pathway, suggesting that enzymatic inhibition of PRMT5 is a potential therapeutic target because it causes synthetic lethality in NDRG2‐deficient cancer or ATLL cells. 16 This figure has been adapted from reference 16 with permission
3.4. Enhanced metastasis of cancer cells upon NDRG2 downregulation and upregulation of the epithelial‐to‐mesenchymal transition
Several studies have shown that NDRG2 is associated with cancer metastasis and that the loss of NDRG2 expression enhanced EMT in colorectal, gallbladder, and breast cancer cells. 53 , 54 , 55 In a previous study, PTEN expression was maintained, and no somatic mutations were found in OSCC cells. 6 Specifically, the expression of NDRG2 was downregulated in most patients with OSCC due to enhanced promoter methylation, which resulted in enhanced PI3K/AKT signaling. 6 , 26 Moreover, the downregulation of NDRG2 expression was significantly correlated with high lymph node metastasis in OSCC. 27 These results indicated that low levels of NDRG2 expression may contribute to enhanced tumor development with cervical metastasis of OSCC. 27 Therefore, we developed an OSCC model in which 4‐NQO, which is a tobacco surrogate, was used to treat Ndrg2‐deficient mice. 27 In this model, both the number and size of the OSCC tumors increased significantly with cervical lymph node metastasis in Ndrg2‐deficient mice. The 4‐NQO treatment of human OSCC cell lines with low NDRG2 expression induced EMT via the activation of NF‐κB signaling. This signaling resulted in the downregulation of E‐cadherin through activation of the TWIST family bHLH transcription factors (TWIST) and SNAIL family of transcriptional repressors (SNAIL) (Figure 5). 27 In contrast, the ectopic expression of NDRG2 reversed the EMT phenotype and inhibited NF‐κB signaling by the suppression of PTEN‐STT and AKT‐Ser473 phosphorylation. 27 These results show the importance of NDRG2 expression, which is a critical determinant for the invasive and metastatic capacity of OSCC. Moreover, they indicate that the constitutive activators of the NF‐κB pathway, such as heavy tobacco use, alcoholism, and chronic infection, might be important risk factors for OSCC development and metastasis. These effects may be dependent on enhanced methylation of the NDRG2 promoter, which is caused by chronic stresses.
Figure 5.
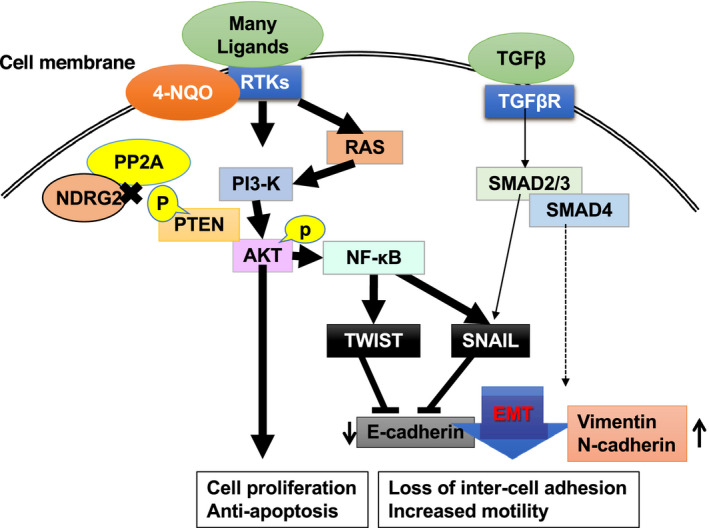
Enhancement of epithelial‐to‐mesenchymal transition (EMT) via the loss of N‐myc downstream‐regulated gene 2 (NDRG2) activity. Because loss of NDRG2 expression has been reported to promote cancer development and metastasis, we conducted animal studies in an oral cancer model using NDRG2‐deficient mice. 27 In Ndrg2‐deficient mice, the treatment of carcinogenic compound 4‐nitroquinoline‐1‐oxide (4‐NQO) induces development of oral cancer and cervical lymph node metastasis, which enhances the activation of the PI3K/AKT and nuclear factor kappa B (NF‐κB) signaling pathways via enhanced phosphorylation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) by downregulating NDRG2. Activation of the NF‐κB signaling pathway promotes EMT via the upregulation of twist family bHLH transcription factors (TWIST) and SNAIL family of transcriptional repressors (SNAIL) transcription, which induces metastasis of oral squamous cell carcinoma (OSCC) because intercell adhesion is lost and motility is increased. 27 Therefore, reduced NDRG2 expression contributes to cancer development and metastasis by regulation of EMT. This figure has been adapted from reference27 with permission
4. CONCLUSIONS
NDRG2 is not considered a tumor suppressor gene because its genome sequences lack point mutations. However, it regulates phosphorylation throughout the cell and is involved in the stress response. This conclusion is supported by the fact that Ndrg2‐deficient mice develop different types of cancers and several lifestyle‐related diseases. As cellular stress promotes genomic mutations and epigenetic modification alterations, defects in NDRG2 expression by genetic alteration or methylation may play a role in cancer development. Therefore, it is highly likely that the creation of a cellular state that can maintain NDRG2 function ultimately prevents cancer development. Thus, treatments including gene re‐expression are important. Studies in this direction should be conducted in the future.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENT
Japan Society for the Promotion of Science, (Grant/Award Number: ‘Grant‐in‐Aid for Scientific Research (B)/17H03581’, ‘Scientific Research (C)/18K07238’) Shinnihon Foundation of Advanced Medical Treatment Research, (Grant/Award Number:) Takeda Science Foundation, (Grant / Award Number:).’
Morishita K, Nakahata S, Ichikawa T. Pathophysiological significance of N‐myc downstream‐regulated gene 2 in cancer development through protein phosphatase 2A phosphorylation regulation. Cancer Sci. 2021;112:22–30. 10.1111/cas.14716
REFERENCES
- 1. Kokame K, Kato H, Miyata T. Homocysteine‐respondent genes in vascular endothelial cells identified by differential display analysis. GRP78/BiP and novel genes. J Biol Chem. 1996;271:29659‐29665. [DOI] [PubMed] [Google Scholar]
- 2. Shimono A, Okuda T, Kondoh H. N‐myc‐dependent repression of ndr1, a gene identified by direct subtraction of whole mouse embryo cDNAs between wild type and N‐myc mutant. Mech Dev. 1999;83:39‐52. [DOI] [PubMed] [Google Scholar]
- 3. Okuda T, Kokame K, Miyata T. Differential expression patterns of NDRG family proteins in the central nervous system. J Histochem Cytochem. 2008;56:175‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melotte V, Qu X, Ongenaert M, et al. The N‐myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24:4153‐4166. [DOI] [PubMed] [Google Scholar]
- 5. Yao L, Zhang J, Liu X. NDRG2: a Myc‐repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin (Shanghai). 2008;40:625‐635. [DOI] [PubMed] [Google Scholar]
- 6. Nakahata S, Ichikawa T, Maneesaay P, et al. Loss of NDRG2 expression activates PI3K‐AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat Commun. 2014;5:3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu N, Wang L, Li X, et al. N‐Myc downstream‐regulated gene 2 is involved in p53‐mediated apoptosis. Nucleic Acids Res. 2008;36:5335‐5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang M, Liu X, Wang Q, et al. NDRG2 acts as a PERK co‐factor to facilitate PERK branch and ERS‐induced cell death. FEBS Lett. 2017;591:3670‐3681. [DOI] [PubMed] [Google Scholar]
- 9. Wang L, Liu NA, Yao L, et al. NDRG2 is a new HIF‐1 target gene necessary for hypoxia‐induced apoptosis in A549 cells. Cell Physiol Biochem. 2008;21:239‐250. [DOI] [PubMed] [Google Scholar]
- 10. Takahashi K, Saitoh A, Yamada M, Iwai T, Inagaki M, Yamada M. Dexamethasone indirectly induces Ndrg2 expression in rat astrocytes. J Neurosci Res. 2012;90:160‐166. [DOI] [PubMed] [Google Scholar]
- 11. Lechward K, Awotunde OS, Swiatek W, Muszyńska G. Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim Pol. 2001;48:921‐933. [PubMed] [Google Scholar]
- 12. Meeusen B, Janssens V. Tumor suppressive protein phosphatases in human cancer: emerging targets for therapeutic intervention and tumor stratification. Int J Biochem Cell Biol. 2018;96:98‐134. [DOI] [PubMed] [Google Scholar]
- 13. O'Connor CM, Perl A, Leonard D, Sangodkar J, Narla G. Therapeutic targeting of PP2A. Int J Biochem Cell Biol. 2018;96:182‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long L, Deng Y, Yao F, et al. Recruitment of phosphatase PP2A by RACK1 adaptor protein deactivates transcription factor IRF3 and limits type I interferon signaling. Immunity. 2014;40:515‐529. [DOI] [PubMed] [Google Scholar]
- 15. Ichikawa T, Nakahata S, Fujii M, Iha H, Morishita K. Loss of NDRG2 enhanced activation of the NF‐κB pathway by PTEN and NIK phosphorylation for ATL and other cancer development. Sci Rep. 2015;5:12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ichikawa T, Shanab O, Nakahata S, et al. Novel PRMT5‐mediated arginine methylations of HSP90A are essential for maintenance of HSP90A function in NDRG2low ATL and various cancer cells. Biochim Biophys Acta Mol Cell Res. 2020;1867:118615. [DOI] [PubMed] [Google Scholar]
- 17. Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN. A phosphorylation‐dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA. 2009;106:480‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang F, Wang Y, Hemmings BA, Rüegg C, Xue G. PKB/Akt‐dependent regulation of inflammation in cancer. Semin Cancer Biol. 2018;48:62‐69. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins BD, Goncalves MD, Cantley LC. Insulin‐PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nat Rev Endocrinol. 2020;16:276‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11‐20. [DOI] [PubMed] [Google Scholar]
- 21. Hidaka T, Nakahata S, Hatakeyama K, et al. Down‐regulation of TCF8 is involved in the leukemogenesis of adult T‐cell leukemia/lymphoma. Blood. 2008;112(2):383‐393. [DOI] [PubMed] [Google Scholar]
- 22. Hu XL, Liu XP, Lin SX, et al. NDRG2 expression and mutation in human liver and pancreatic cancers. World J Gastroenterol. 2004;10:3518‐3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee DC, Kang YK, Kim WH, et al. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210‐4220. [DOI] [PubMed] [Google Scholar]
- 24. Choi S‐C, Yoon SR, Park YP, et al. Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fas‐mediated cell death. Exp Mol Med. 2007;39:705‐714. [DOI] [PubMed] [Google Scholar]
- 25. Chang X, Li Z, Ma J, et al. DNA methylation of NDRG2 in gastric cancer and its clinical significance. Dig Dis Sci. 2013;58:715‐723. [DOI] [PubMed] [Google Scholar]
- 26. Furuta H, Kondo Y, Nakahata S, Hamasaki M, Sakoda S, Morishita K. NDRG2 is a candidate tumor‐suppressor for oral squamous‐cell carcinoma. Biochem Biophys Res Commun. 2010;391:1785‐1791. [DOI] [PubMed] [Google Scholar]
- 27. Tamura T, Ichikawa T, Nakahata S, et al. Loss of NDRG2 expression confers oral squamous cell carcinoma with enhanced metastatic potential. Cancer Res. 2017;77:2363‐2374. [DOI] [PubMed] [Google Scholar]
- 28. Park Y, Shon S‐K, Kim A, et al. SOCS1 induced by NDRG2 expression negatively regulates STAT3 activation in breast cancer cells. Biochem Biophys Res Commun. 2007;363:361‐367. [DOI] [PubMed] [Google Scholar]
- 29. Kim YJ, Yoon SY, Kim JT, et al. NDRG2 expression decreases with tumor stages and regulates TCF/beta‐catenin signaling in human colon carcinoma. Carcinogenesis. 2009;30:598‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim A, Kim MJ, Yang Y, Kim JW, Yeom YI, Lim JS. Suppression of NF‐kappaB activity by NDRG2 expression attenuates the invasive potential of highly malignant tumor cells. Carcinogenesis. 2009;30:927‐936. [DOI] [PubMed] [Google Scholar]
- 31. Li R, Yu C, Jiang F, et al. Overexpression of N‐Myc downstream‐regulated gene 2 (NDRG2) regulates the proliferation and invasion of bladder cancer cells in vitro and in vivo. PLoS One. 2013;8:e76689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo Y, Ma JI, Wu L, et al. Hyperthermia‐induced NDRG2 upregulation inhibits the invasion of human hepatocellular carcinoma via suppressing ERK1/2 signaling pathway. PLoS One. 2013;8:e61079. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Ichikawa T, Nakahata S, Fujii M, Iha H, Shimoda K, Morishita K. The regulation of NDRG2 expression during ATLL development after HTLV‐1 infection. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2633‐2646. [DOI] [PubMed] [Google Scholar]
- 34. Mohanty S, Harhaj EW. Mechanisms of Oncogenesis by HTLV‐1 Tax. Pathogens. 2020;9:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fujikawa D, Nakagawa S, Hori M, et al. Polycomb‐dependent epigenetic landscape in adult T‐cell leukemia. Blood. 2016;127:1790‐1802. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943‐1947. [DOI] [PubMed] [Google Scholar]
- 37. Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356‐362. [DOI] [PubMed] [Google Scholar]
- 38. Papa A, Pandolfi PP. The PTEN⁻PI3K Axis in Cancer. Biomolecules. 2019;9:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922‐1932. [DOI] [PubMed] [Google Scholar]
- 40. Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184‐192. [DOI] [PubMed] [Google Scholar]
- 41. Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010‐5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vazquez F, Matsuoka S, Sellers WR, Yanagida T, Ueda M, Devreotes PN. Tumor suppressor PTEN acts through dynamic interaction with the plasma membrane. Proc Natl Acad Sci USA. 2006;103:3633‐3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762‐3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang Z, Xie C, Xu W, et al. Phosphorylation and inactivation of PTEN at residues Ser380/Thr382/383 induced by Helicobacter pylori promotes gastric epithelial cell survival through PI3K/Akt pathway. Oncotarget. 2015;6:31916‐31926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kishimoto H, Hamada K, Saunders M, et al. Physiological functions of Pten in mouse tissues. Cell Struct Funct. 2003;28:11‐21. [DOI] [PubMed] [Google Scholar]
- 46. Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304‐1315. [DOI] [PubMed] [Google Scholar]
- 47. Sarkar B, Nishikata I, Nakahata S, et al. Degradation of p47 by autophagy contributes to CADM1 overexpression in ATLL cells through the activation of NF‐κB. Sci Rep. 2019;9:3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peloponese JM, Yeung ML, Jeang KT. Modulation of nuclear factor‐kappaB by human T cell leukemia virus type 1 Tax protein: implications for oncogenesis and inflammation. Immunol Res. 2006;34:1‐12. [PubMed] [Google Scholar]
- 49. Yamagishi M, Nakano K, Miyake A, et al. Polycomb‐mediated loss of miR‐31 activates NIK‐dependent NF‐κB pathway in adult T cell leukemia and other cancers. Cancer Cell. 2012;21:121‐135. [DOI] [PubMed] [Google Scholar]
- 50. Xiao W, Chen X, Liu L, Shu Y, Zhang M, Zhong Y. Role of protein arginine methyltransferase 5 in human cancers. Biomed Pharmacother. 2019;114:108790. [DOI] [PubMed] [Google Scholar]
- 51. Haque A, Alam Q, Alam MZ, et al. Current understanding of HSP90 as a novel therapeutic target: an emerging approach for the treatment of cancer. Curr Pharm Des. 2016;22:2947‐2959. [DOI] [PubMed] [Google Scholar]
- 52. Backe SJ, Sager RA, Woodford MR, Makedon AM, Mollapour M. Post‐translational modifications of Hsp90 and translating the chaperone code. J Biol Chem. 2020;295:11099‐11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee DG, Lee S‐H, Kim J‐S, et al. Loss of NDRG2 promotes epithelial‐mesenchymal transition of gallbladder carcinoma cells through MMP‐19‐mediated Slug expression. J Hepatol. 2015;63:1429‐1439. [DOI] [PubMed] [Google Scholar]
- 54. Shen L, Qu X, Ma Y, et al. Tumor suppressor NDRG2 tips the balance of oncogenic TGF‐β via EMT inhibition in colorectal cancer. Oncogenesis. 2014;3:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim MJ, Lim J, Yang Y, Lee MS, Lim JS. N‐myc downstream‐regulated gene 2 (NDRG2) suppresses the epithelial‐mesenchymal transition (EMT) in breast cancer cells via STAT3/Snail signaling. Cancer Lett. 2014;354:33‐42. [DOI] [PubMed] [Google Scholar]
- 56. Tao Y, Guo Y, Liu W, et al. AKT inhibitor suppresses hyperthermia‐induced Ndrg2 phosphorylation in gastric cancer cells. Braz J Med Biol Res. 2013;46:394‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Foletta VC, Brown EL, Cho Y, Snow RJ, Kralli A, Russell AP. Ndrg2 is a PGC‐1α/ERRα target gene that controls protein synthesis and expression of contractile‐type genes in C2C12 myotubes. Biochim Biophys Acta. 2013;1833:3112‐3123. [DOI] [PubMed] [Google Scholar]


