Abstract
The turnover of intestinal epithelial cells (IECs) is relatively rapid (3‐5 days in mouse and human), and this short existence and other aspects of the homeostasis of IECs are tightly regulated by various signaling pathways including Wnt‐β‐catenin signaling. Dysregulation of IEC homeostasis likely contributes to the development of intestinal inflammation and intestinal cancer. The roles of receptor protein tyrosine kinases and their downstream signaling molecules such as Src family kinases, Ras, and mTOR in homeostatic regulation of IEC turnover have recently been evaluated. These signaling pathways have been found to promote not only the proliferation of IECs but also the differentiation of progenitor cells into secretory cell types such as goblet cells. Of note, signaling by Src family kinases, Ras, and mTOR has been shown to oppose the Wnt‐β‐catenin signaling pathway and thereby to limit the number of Lgr5+ intestinal stem cells or of Paneth cells. Such cross‐talk of signaling pathways is important not only for proper regulation of IEC homeostasis but for the development of intestinal tumors and potentially for anticancer therapy.
Keywords: Intestinal epithelial cell, mTORC1, Ras, Src, Wnt
The roles of Src family kinases (SFKs), Ras, and mTORC1 in homeostatic regulation of intestinal epithelial cells (IECs) have recently been evaluated, and they promote the proliferation and differentiation of IECs. Of note, SFKs, Ras, and mTORC1 oppose the Wnt‐β‐catenin signaling pathway, particularly in the intestinal crypt. Such cross‐talk of signaling pathways is thus important for regulation of IEC homeostasis but also for intestinal tumorigenesis and anticancer therapy.
1. INTRODUCTION
Intestinal epithelial cells (IECs) of the small and large intestine turn over rapidly and are renewed every 3‐5 days in both mouse and human. Intestinal epithelial cells are continuously regenerated from Lgr5+ intestinal stem cells (ISCs) that reside in a region of the epithelium near the base of intestinal crypts (Figure 1A). 1 , 2 These ISCs generate proliferating progeny, known as transient amplifying (TA) cells, that eventually differentiate into the various cell lineages of mature intestinal villi including absorptive enterocytes, mucin‐producing goblet cells, and antimicrobial peptide‐producing Paneth cells. Paneth cells in the crypt are also thought to be important for maintenance of ISCs by secreting Wnt ligands such as Wnt3. 3 The mature enterocytes then migrate up the crypt toward the tip of the villus and eventually die as a result of their expulsion from the luminal surface of the intestinal epithelium. 1 , 2 The elimination of older cells is thus balanced by the ongoing production of new IECs in each crypt, resulting in the rapid turnover of IECs.
FIGURE 1.
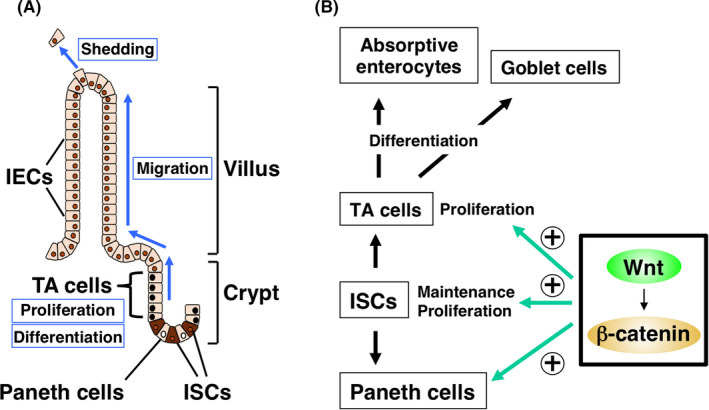
A, Rapid turnover of intestinal epithelial cells (IECs) in the intestinal epithelium. B, Role of Wnt‐β‐catenin signaling in the proliferation and maintenance of intestinal stem cells (ISCs) and transient amplifying (TA) cells in intestinal crypts
The proliferative activity of crypt cells, such as TA cells and ISCs, is thought to be a major determinant of the rapid turnover rate of mature IECs. However, aberrant activation of this proliferative activity likely contributes to tumorigenesis in the intestine. The Wnt‐β‐catenin signaling pathway is best characterized as a positive regulator of the proliferation of TA cells and ISCs (Figure 1B). 2 , 4 Indeed, deletion or mutation of the gene for Apc (adenomatous polyposis coli), a negative regulator of the Wnt‐β‐catenin signaling pathway, results in intestinal tumorigenesis in both mice and humans. 5 In addition, Wnt‐β‐catenin signaling plays an important role in the maintenance of ISCs as well as in the generation of Paneth cells (Figure 1B), which contribute to the nursing of ISCs, as mentioned above. 4 Epidermal growth factor (EGF), whose receptor is a protein tyrosine kinase (PTK), is also thought to serve as a major driver of proliferation of TA cells as well as IECs. 2 , 6 Moreover, signaling molecules such as Ras and mTOR that act downstream of the EGF receptor protein tyrosine kinase (RPTK) have been much studied with regard to their role in regulation of the proliferative activity of IECs. In this review, we will describe new insights into the functions of such signaling molecules that act downstream of RPTKs in the regulation of IEC homeostasis, including their cross‐talk with Wnt‐β‐catenin signaling and their relation to intestinal oncogenesis and its potential treatment.
2. SRC FAMILY KINASES
Src family kinases (SFKs)—such as Src, Fyn, and Yes—are nonreceptor‐type PTKs that play important roles in the regulation of cell proliferation, migration, and differentiation. 7 They are thought to interact with RPTKs such as the EGF receptor or with activated focal adhesion kinase in response to integrin ligation, resulting in the activation of downstream Ras‐MAPK and PI3K pathways (Figure 2A). Src family kinases are expressed predominantly in the crypt area of the intestinal epithelium 8 , 9 and are localized to lipid rafts, 10 , 11 which are thought to be enriched at the apical surface of crypt IECs. 9 Lipid rafts thus likely recruit SFKs to the apical surface of crypt IECs. Ablation of Src specifically in IECs of mice resulted in no apparent defect under the steady‐state condition, whereas that of Src, Fyn, and Yes induced apoptosis in IECs of the small intestine. 8 However, IEC‐specific ablation of Src did prevent the regeneration of crypts after the induction of DNA damage by γ‐irradiation. 8 Csk is a PTK that inhibits the activity of all SFKs by catalyzing phosphorylation of a COOH‐terminal regulatory tyrosine residue. 7 , 12 Indeed, ablation of Csk specifically in IECs of mice resulted in hyperactivation not only of Src but also of Fyn and Yes, and gave rise to intestinal and colonic epithelial hyperplasia. 9 It also increased the proliferative activity and turnover of IECs as well as the number of goblet cells in the intestine, and all of these effects were accompanied by activation of the small GTP‐binding protein Rac and the transcription factor Yes‐associated protein (YAP) in intestinal crypts (Figure 2B). 9 Indeed, YAP had previously been shown to be expressed predominantly in crypts of the small intestine. 13 Src family kinases are thus likely important not only for the proliferative activity and turnover of IECs but also for control of the differentiation and maturation of goblet cells.
FIGURE 2.
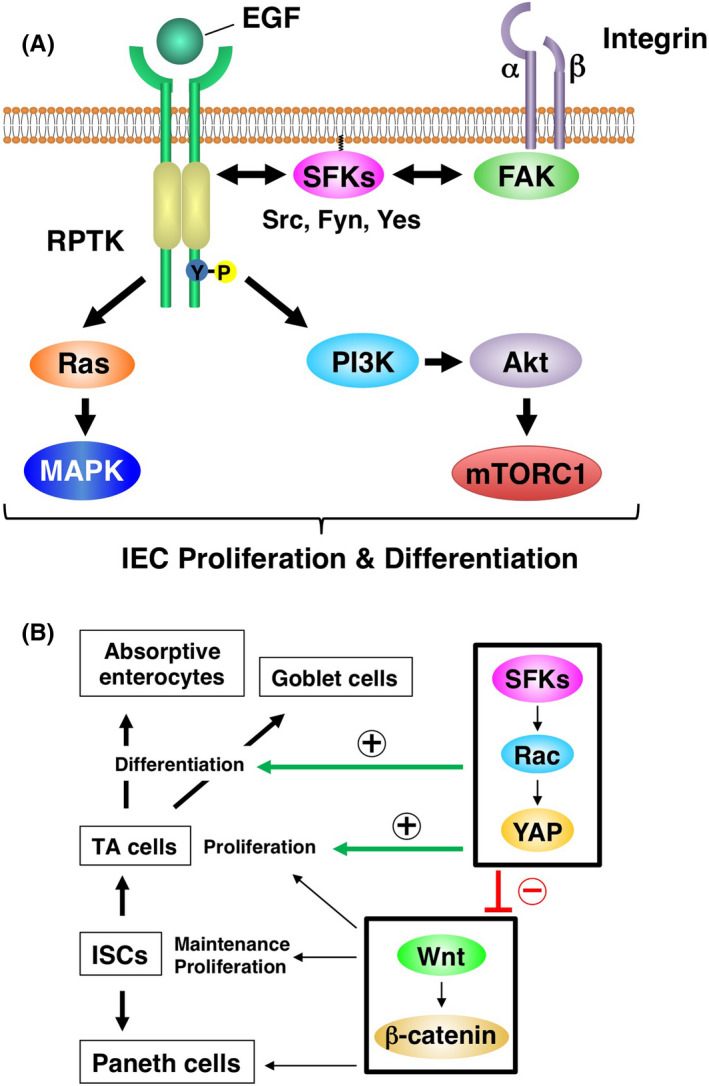
Signaling pathways downstream of growth factor receptors or integrins (A) as well as the role of Src family kinases (SFKs) (B) in the regulation of intestinal epithelial cell (IEC) proliferation and differentiation. EGF, epidermal growth factor; FAK, focal adhesion kinase; ISC, intestinal stem cell; RPTK, receptor protein tyrosine kinase; TA, transient amplifying; YAP, Yes‐associated protein.
In contrast with its effects on IECs, IEC‐specific ablation of Csk resulted in a reduction in the number of ISCs and of Paneth cells, and it attenuated the expression of Wnt target genes in the intestine. 9 Given that the Wnt‐β‐catenin signaling pathway is essential for the generation and maintenance of both ISCs and Paneth cells, 1 , 2 SFKs could negatively regulate Wnt‐β‐catenin signaling in the intestinal epithelium. Furthermore, given that YAP inhibits the Wnt‐β‐catenin signaling pathway, 14 , 15 , 16 SFKs likely target this pathway by upregulating YAP activity in crypt IECs (Figure 2B).
The importance of SFKs for promotion of IEC proliferation in the physiological condition suggests that aberrant activation of these kinases likely contributes to the development of colorectal cancer (CRC). Indeed, more than 80% of individuals with CRC have been found to overexpress Src in tumor tissue. 17 The Apc min/+ mouse is an experimental model of CRC in which Wnt‐β‐catenin signaling is markedly activated and in which ablation of Src was found to result in a pronounced attenuation of tumorigenesis and increase in survival of the mutant mice, 8 suggesting that Src is required for tumorigenesis in response to Apc loss. By contrast, it was also suggested that there are few activating mutations in Src in any type of cancer, although upregulation of Src protein and activity is frequently observed. 18 Moreover, although many inhibitors of Src have been developed and tested clinically, they have not received much attention with regard to their potential for CRC therapy. 17 Given that SFKs are thought to promote IEC proliferation in a YAP‐dependent manner, 9 , 19 YAP is another promising target for CRC therapy.
3. RAS
The small GTP‐binding protein Ras is a key signaling molecule that acts downstream of RPTKs, such as the EGF receptor, to promote the proliferation and differentiation of IECs (Figure 2A). Indeed, activation of Ras specifically in IECs has been shown to result in the development of hyperplasia throughout the intestinal epithelium of mice. 20 , 21 , 22 The IEC‐specific activation of Ras also increased the proliferative activity and turnover of IECs as well as the number of goblet cells, whereas it led to a marked reduction in the number of Paneth cells in crypts. 21 However, aberrant activation of Ras alone failed to induce the development of cancerous lesions in the mouse intestine. 21 , 23 The Ras family of proteins comprises K‐Ras, N‐Ras, and H‐Ras, 24 and the effect of IEC‐specific genetic ablation of all three family members has not been determined in mice. Src homology 2‐containing protein tyrosine phosphatase 2 (Shp2, also known as PTPN11) is a cytoplasmic protein tyrosine phosphatase that contains two tandem Src homology 2 domains. 25 , 26 Given that Shp2 is required for the activation of Ras by various growth factors and cytokines, ablation of Shp2 likely results in the efficient extinction of all Ras activity. 25 , 26 The IEC‐specific ablation of Shp2 was found to result in severe enterocolitis in mice. 27 , 28 , 29 In addition, the numbers of absorptive enterocytes and goblet cells were markedly reduced in such Shp2 mutant mice, and the development of intestinal organoids from isolated crypts of these animals was impaired. 27 , 28 , 29 These observations thus implicate the Ras‐MAPK signaling pathway in promotion both of IEC proliferation and of the differentiation of goblet cells and absorptive enterocytes (Figure 3). 28 , 29 In contrast, ablation of Shp2 in IECs resulted in a marked increase in the number of Paneth cells as well as of Lgr5+/Olfm4+ ISCs at the crypt base. 29 Indeed, the expression of Wnt target genes such as Myc and Cd44 was markedly increased in IECs by ablation of Shp2, suggesting that Ras activity downregulates Wnt signaling in these cells (Figure 3).
FIGURE 3.
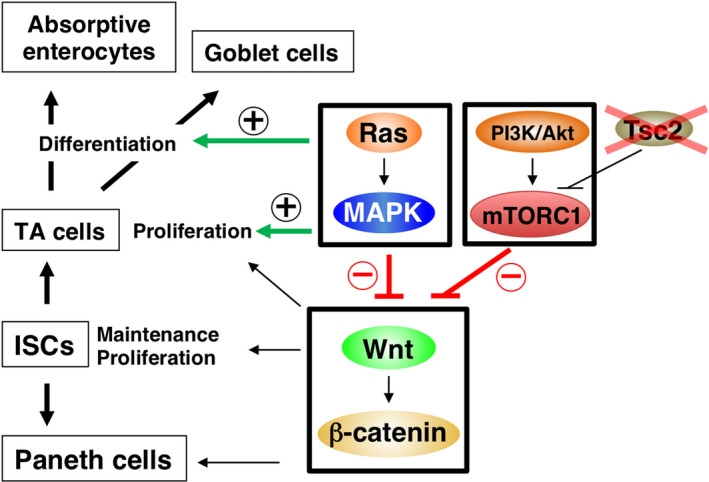
Role of Ras and mTORC1 in regulation of intestinal epithelial cell proliferation and differentiation. ISC, intestinal stem cell; TA, transient amplifying; Tsc2, tuberous sclerosis complex 2.
4. MAMMALIAN TARGET OF RAPAMYCIN
The serine‐threonine kinase mTOR forms two distinct multiprotein complexes known as mTOR complex 1 (mTORC1) and mTORC2. 30 The activity of mTORC1 is controlled by various upstream signals including growth factors, nutrients, and stress. The ligation of RPTKs by growth factors such as insulin or EGF induces the activation of PI3K and the downstream serine‐threonine kinase Akt. Akt activates the small GTP‐binding protein Rheb through phosphorylation and consequent inhibition of tuberous sclerosis complex 2 (Tsc2), a GTPase‐activating protein for Rheb, and Rheb then activates mTORC1. Such Rheb‐mediated activation of mTORC1 results in the phosphorylation of ribosomal protein S6 kinase and the translational repressor protein 4E‐BP1 and in consequent regulation of a variety of cellular processes such as protein synthesis, autophagy, and cell aging. 30 Activation of mTORC1 by IEC‐specific ablation of Tsc2 was found to promote the proliferative activity of IECs in the small intestine and colon of mice (Figure 3). 31 , 32 Conversely, ablation of mTOR in IECs reduced their proliferative activity. 33 As mentioned above, SFKs and Ras, both of which function downstream of growth factor receptors, promote not only the proliferation of IECs but also the generation and differentiation of secretory cells such as goblet cells (Figures 2 and 3). In contrast, activation of mTORC1 by Tsc2 ablation did not affect the number of Muc2+ mucus‐secreting cells in the intestine, whereas it disturbed the clustering of Paneth cells at the crypt base, 31 suggesting that mTORC1 activity is not essential for differentiation of either of these cell types from their progenitors. The number of Lgr5+ ISCs and the expression of Wnt target genes in the intestine were markedly reduced by activation of mTORC1. 32 , 34 Indeed, Tsc1/2 is required for the maintenance of ISCs in Drosophila. 35 The activity of mTORC1 was also shown to be important for downregulation of Wnt signaling in melanocytes. 36
Given the importance of mTORC1 activity for the proliferation of IECs, aberrant activation of mTORC1 likely participates in intestinal tumorigenesis. 37 , 38 Indeed, tumorigenesis driven by mutation of Apc in the mouse intestine was shown to require the activity of mTORC1. 39 , 40 Mutation of the mTOR gene appears to be infrequent in CRC, however, with aberrant activation of the mTOR pathway being instead attributable to mutation of genes in the upstream PI3K‐Akt signaling pathway. For instance, mutations in the kinase domain of the α catalytic subunit of PI3K (PIK3CA) tend to arise late in tumorigenesis and have been identified in 32% of CRC tumors. 41 Defects of the PTEN gene, which encodes a phosphatase for phosphatidylinositol 3,4,5‐trisphosphate that opposes PI3K activity, have also been identified in CRC. 42
5. CROSS‐TALK BETWEEN SFKs, Ras, AND mTOR SIGNALING PATHWAYS AND Wnt‐β‐CATENIN SIGNALING IN IECs
Activation of SFKs, Ras, and mTOR likely suppresses the Wnt‐β‐catenin signaling pathway in crypts of the intestine and thereby regulates ISCs as well as the differentiation of TA cells into mature IECs, such as absorptive enterocytes and goblet cells (Figure 4). However, the molecular mechanisms by which these signaling molecules inhibit the Wnt‐β‐catenin pathway remain unclear. The binding of Wnt ligands to the Frizzled‐LRP5/6 receptor complex inhibits the activity of glycogen synthase kinase 3β (Gsk3β), which mediates the phosphorylation of β‐catenin at serine 37 or serine 33 and thereby triggers its degradation by the ubiquitin‐proteasome system. 43 The nonphosphorylated form of β‐catenin thus accumulates, translocates to the nucleus, and acts as a transcriptional cofactor for T‐cell factor (Tcf), resulting in the transcription of Wnt‐β‐catenin target genes. 44 Activation of the Ras‐MEK‐MAPK pathway suppresses Wnt target gene expression in IECs by increasing the abundance of the ~50‐kDa (shorter) isoforms of Tcf4 (Tcf M/S), which are thought to be transcriptionally inactive and to inhibit activation of Wnt target genes. 29 The kinase Akt, which is an upstream activator of mTORC1, inhibits the activity of Gsk3β by mediating its phosphorylation at serine 9. 45 Feedback inhibition of Akt as a result of aberrant activation of mTORC1 in melanocytes was shown to reduce the level of Gsk3β phosphorylation at serine 9 and thereby to promote Gsk3β activation, resulting in suppression of Wnt target gene expression. 36 Indeed, phosphorylation of both Akt and Gsk3β was also attenuated by IEC‐specific activation of mTORC1 in IECs. 32 An increase in the activity of Gsk3β could therefore promote β‐catenin degradation and consequent downregulation of Wnt target gene expression. The activation of mTORC1 has also been shown to suppress the amount of the Wnt receptor Frizzled at the cell surface, resulting in inhibition of Wnt signaling. 34 Further investigation will be required to clarify the molecular mechanisms by which SFKs, Ras, and mTOR inhibit Wnt‐β‐catenin signaling in IECs.
FIGURE 4.
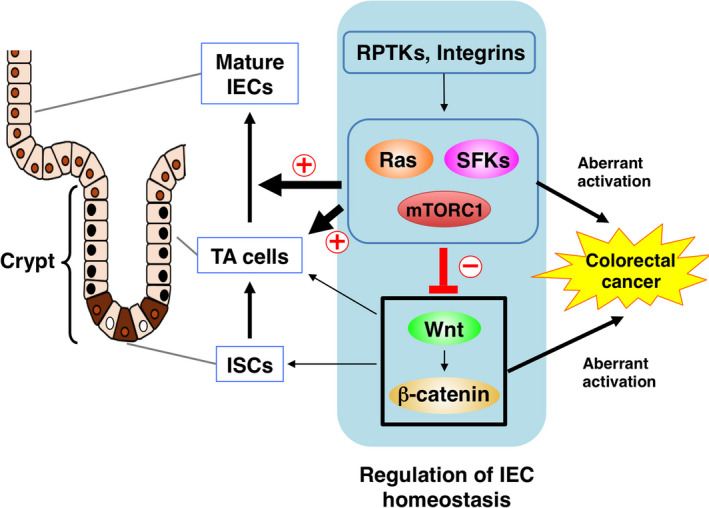
Opposing regulatory roles of Src family kinases (SFK), Ras, and mTORC1 signaling and of the Wnt‐β‐catenin signaling pathway in intestinal epithelial cell (IEC) homeostasis and their relation to intestinal tumorigenesis. ISC, intestinal stem cell; RPTK, receptor protein tyrosine kinase; TA, transient amplifying.
6. CONCLUSION
Activation or inactivation of SFKs, Ras, or mTORC1 by gene targeting in mice as well as studies of intestinal organoids have recently uncovered roles for these signaling molecules that act downstream of growth factor RPTKs or integrins in the regulation of ISC maintenance as well as of the proliferation and differentiation of IECs from their progenitors. These signaling molecules have also been implicated in the opposition of Wnt‐β‐catenin signaling associated with homeostatic regulation of normal IEC turnover, although the molecular basis for such counterregulation remains to be fully elucidated. Given that Wnt ligand such as Wnt3 is predominantly produced and secreted by Paneth cells, the gradient of Wnt concentration from the bottom to the upper region of the crypt is likely important for both maintenance of stemness of ISCs at the bottom and the promotion of TA or mature IEC proliferation at the upper region, respectively. Interestingly, inhibition of Wnt signaling promotes the activation of MAPK in the crypts, suggesting that the gradient of Wnt in the crypt likely regulates the activation of MAPK in the upper part of the crypt and promotion of TA cell proliferation. 46 Counterregulation by the Wnt‐β‐catenin pathway and the growth factor RPTK‐activated signaling pathways (such as Ras or PI3K signaling) could also provide a basis for the development of new treatment strategies for CRC. Simultaneous targeting of these two signaling pathways could thus result in more effective eradication of cancer derived from the intestinal epithelium (Figure 4).
CONFLICT OF INTEREST
T.M. has received research funding from the Mitsubishi Foundation and Yakult Bio‐Science Foundation. The other authors declare no conflict of interest.
ACKNOWLEDGMENTS
The work in the authors’ laboratory was supported by a Grant‐in‐Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (JSPS) grant number (18H04032), a research grant from Mitsubishi Foundation, and a research grant from Yakult Bio‐Science Foundation.
Matozaki T, Kotani T, Murata Y, Saito Y. Roles of Src family kinase, Ras, and mTOR signaling in intestinal epithelial homeostasis and tumorigenesis. Cancer Sci. 2021;112:16–21. 10.1111/cas.14702
Funding information
Japan Society for the Promotion of Science (grant number: 18H04032); Mitsubishi Foundation; Yakult Bio‐Science Foundation.
REFERENCES
- 1. Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19‐33. [DOI] [PubMed] [Google Scholar]
- 2. Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274‐284. [DOI] [PubMed] [Google Scholar]
- 3. Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877‐890. [DOI] [PubMed] [Google Scholar]
- 5. Fodde R, Smits R, Clevers H APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55‐67. [DOI] [PubMed] [Google Scholar]
- 6. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262‐265. [DOI] [PubMed] [Google Scholar]
- 7. Okada M. Regulation of the SRC family kinases by Csk. Int J Biol Sci. 2012;8:1385‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cordero JB, Ridgway RA, Valeri N, et al. c‐Src drives intestinal regeneration and transformation. EMBO J. 2014;33:1474‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imada S, Murata Y, Kotani T, et al. Role of Src family kinases in regulation of intestinal epithelial homeostasis. Mol Cell Biol. 2016;36:2811‐2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403‐411. [DOI] [PubMed] [Google Scholar]
- 11. Liang X, Nazarian A, Erdjument‐Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J Biol Chem. 2001;276:30987‐30994. [DOI] [PubMed] [Google Scholar]
- 12. Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein‐tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c‐src . Nature. 1991;351:69‐72. [DOI] [PubMed] [Google Scholar]
- 13. Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054‐2060. [DOI] [PubMed] [Google Scholar]
- 14. Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β‐catenin signalling. EMBO J. 2012;31:1109‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap‐dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715‐718. [DOI] [PubMed] [Google Scholar]
- 17. Chen J, Elfiky A, Han M, Chen C, Saif MW. The role of Src in colon cancer and its therapeutic implications. Clin Colorectal Cancer. 2014;13:5‐13. [DOI] [PubMed] [Google Scholar]
- 18. Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636‐5642. [DOI] [PubMed] [Google Scholar]
- 19. Taniguchi K, Wu LW, Grivennikov SI, et al. A gp130‐Src‐YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haigis KM, Kendall KR, Wang Y, et al. Differential effects of oncogenic K‐Ras and N‐Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng Y, Bommer GT, Zhao J, et al. Mutant Kras promotes hyperplasia and alters differentiation in the colon epithelium but does not expand the presumptive stem cell pool. Gastroenterology. 2011;141:1003‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennecke M, Kriegl L, Bajbouj M, et al. Ink4a/Arf and oncogene‐induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell. 2010;18:135‐146. [DOI] [PubMed] [Google Scholar]
- 23. Trobridge P, Knoblaugh S, Washington MK, et al. TGF‐β receptor inactivation and mutant Kras induce intestinal neoplasms in mice via a β‐catenin‐independent pathway. Gastroenterology. 2009;136(5):1680‐1688.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takai Y, Sasaki T, Matozaki T. Small GTP‐binding proteins. Physiol Rev. 2001;81:153‐208. [DOI] [PubMed] [Google Scholar]
- 25. Matozaki T, Murata Y, Saito Y, Okazawa H, Ohnishi H. Protein tyrosine phosphatase SHP‐2: a proto‐oncogene product that promotes Ras activation. Cancer Sci. 2009;100:1786‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain‐containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284‐293. [DOI] [PubMed] [Google Scholar]
- 27. Coulombe G, Leblanc C, Cagnol S, et al. Epithelial tyrosine phosphatase SHP‐2 protects against intestinal inflammation in mice. Mol Cell Biol. 2013;33:2275‐2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamashita H, Kotani T, Park JH, et al. Role of the protein tyrosine phosphatase Shp2 in homeostasis of the intestinal epithelium. PLoS One. 2014;9:e92904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heuberger J, Kosel F, Qi J, Grossmann KS, Rajewsky K, Birchmeier W. Shp2/MAPK signaling controls goblet/paneth cell fate decisions in the intestine. Proc Natl Acad Sci USA. 2014;111:3472‐3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Setiawan J, Kotani T, Konno T, et al. Regulation of small intestinal epithelial homeostasis by Tsc2‐mTORC1 signaling. Kobe J Med Sci. 2019;64:E200‐E209. [PMC free article] [PubMed] [Google Scholar]
- 32. Kotani T, Setiawan J, Konno T, et al. Regulation of colonic epithelial cell homeostasis by mTORC1. Sci Rep. 2020;10(1):13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sampson LL, Davis AK, Grogg MW, Zheng Y. mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB J. 2016;30:1263‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng H, Lu B, Zamponi R, et al. mTORC1 signaling suppresses Wnt/β‐catenin signaling through DVL‐dependent regulation of Wnt receptor FZD level. Proc Natl Acad Sci USA. 2018;115:E10362‐E10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quan Z, Sun P, Lin G, Xi R. TSC1/2 regulates intestinal stem cell maintenance and lineage differentiation through Rheb‐TORC1‐S6K but independently of nutritional status or Notch regulation. J Cell Sci. 2013;126:3884‐3892. [DOI] [PubMed] [Google Scholar]
- 36. Cao J, Tyburczy ME, Moss J, Darling TN, Widlund HR, Kwiatkowski DJ. Tuberous sclerosis complex inactivation disrupts melanogenesis via mTORC1 activation. J Clin Invest. 2017;127:349‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Francipane MG, Lagasse E. mTOR pathway in colorectal cancer: an update. Oncotarget. 2014;5:49‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prossomariti A, Piazzi G, Alquati C, Ricciardiello L. Are Wnt/β‐Catenin and PI3K/AKT/mTORC1 distinct pathways in colorectal cancer? Cell Mol Gastroenterol Hepatol. 2020;10:491‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faller WJ, Jackson TJ, Knight JR, et al. mTORC1‐mediated translational elongation limits intestinal tumour initiation and growth. Nature. 2015;517:497‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in Apc Δ716 mice. Proc Natl Acad Sci USA. 2008;105:13544‐13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. [DOI] [PubMed] [Google Scholar]
- 42. Zhou XP, Loukola A, Salovaara R, et al. PTEN mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am J Pathol. 2002;161:439‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu C, Li Y, Semenov M, et al. Control of β‐catenin phosphorylation/degradation by a dual‐kinase mechanism. Cell. 2002;108:837‐847. [DOI] [PubMed] [Google Scholar]
- 44. Nusse R, Clevers H. Wnt/β‐catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985‐999. [DOI] [PubMed] [Google Scholar]
- 45. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase‐3 by insulin mediated by protein kinase B. Nature. 1995;378:785‐789. [DOI] [PubMed] [Google Scholar]
- 46. Kabiri Z, Greicius G, Zaribafzadeh H, Hemmerich A, Counter CM, Virshup DM. Wnt signaling suppresses MAPK‐driven proliferation of intestinal stem cells. J Clin Invest. 2018;128:3806‐3812. [DOI] [PMC free article] [PubMed] [Google Scholar]


