Abstract
Abstract
COVID-19 is a disease caused by SARS-CoV-2 capable of causing mild to severe infections in humans. Since its first appearance in China in December 2019, the pandemic has spread rapidly throughout the world. Despite considerable efforts made to contain the disease, the virus has continued its prevalence in many countries with varying degrees of clinical manifestations. To contain this pandemic, collaborative approach involving accurate diagnosis, epidemiology, surveillance, and prophylaxis is essential. However, proper diagnosis using rapid technologies plays a crucial role. With increasing incidence of COVID-19 cases, the accurate and early detection of the SARS-CoV-2 is need of the hour for effective prevention and management of COVID-19 cases as well as to curb its spread. RT-qPCR assay is considered to be the gold standard for the early detection of virus, but this protocol has limited application to use as bedside test because of its technical complexity. To address these challenges, several POC assays have been developed to facilitate the COVID-19 diagnosis outside the centralized testing laboratories as well to accelerate the clinical decision making with a least turnaround time. Hence, in this report, we review different nucleic acid-based and serological techniques available for the diagnosis and effective prevention of COVID-19.
Key points
• Provides comprehensive information on the different diagnostic tools available for COVID-19
• Nucleic acid based tests or antigen detection tests are used for diagnostic purpose
• Accurate diagnosis is essential for the efficient management of COVID-19
Keywords: COVID-19, SARS-CoV-2, RT-qPCR, RT-LAMP, Diagnosis, Serology
Introduction
The outbreak of Coronavirus Disease 2019 also known as COVID-19, due to novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first detected in China on 31 December 2019 (Wang et al. 2020a). Within a short span of time, SARS-CoV-2 has raced around the globe, and on 30th January 2020, the World Health Organization (WHO) officially declared the COVID-19 epidemic as a public health emergency of international concern (Karunasagar and Karunasagar 2020). The number of infected cases and deaths due to COVID-19 is rising alarmingly and on 22nd July 2020, there are more than 1,47,65,256 confirmed cases with over 6,12,054 deaths across 200 countries(mortality rate approximately 3.7%) (WHO 2020).
Coronaviruses are enveloped, positive single-stranded RNA genome (26 to 32 kb) viruses belonging to the Coronaviridae family in the Nidovirales order (Su et al. 2016). Till date, there are four genera, i.e., alpha (α), beta (β), gamma (γ), and delta (δ), of the virus that have been recognized (Perlman and Netland 2009). However, the novel SARS-CoV-2 belongs to the genera of β-coronavirus with a RNA genome size of 29.9 kb (Wu et al. 2020). SARS-CoV-2 shows 88% nucleotide sequence identity to the two bat-derived SARS-like coronaviruses (bat-SL-CoVZC45 and bat-SL-CoVZXC2) and about 79% similarity to the SARS-CoV and 50% to the MERS-CoV (Lu et al. 2020). There are growing numbers of reports that indicate that the genome of SARS-CoV2 has undergone evolutionary changes and diversification during the geographic dissemination process. The pan-genomic analysis of global SARS-CoV-2 isolates has revealed the identification of several genomic regions with increased genetic variation, and distinct mutation pattern (Korber et al. 2020; Kumar et al. 2020). The genome characterization of Indian SARS-CoV-2 by our group showed genetic variation in the SARS-CoV-2 circulating in India, which is extensively dominated by G614 genotype with a strong correlation to CFR of COVID-19 posing enormous challenge for the effective prevention and management of COVID-19 cases in India (Kumar et al. 2020). According to the recent evidence, it is observed that SARS-CoV-2 virus is primarily transmitted between humans by inhalation or contact with infected droplets with the incubation period ranging from 2 to 14 days (Lin et al. 2020; Liu et al. 2020; Rohit et al. 2020). SARS-CoV-2 infection has a broad range of clinical manifestations varying from asymptomatic to symptomatic including respiratory symptoms, fever, shortness of breath, cough, dyspnea, and viral pneumonia and in severe cases, pneumonia, severe acute respiratory syndrome, heart failure, renal failure, and even death (Huang et al. 2020a). However, the main cause of death related to COVID-19 is respiratory failure, followed by septic shock, renal failure, and hemorrhage and heart failure.This review provides a unique up-to-date and comprehensive overview on the performance of different nucleic acid-based and serological techniques currently available for the diagnosis of COVID-19.The information presented in this review is hoped to help physicians and clinical microbiologists select a suitable technique for COVID-19 diagnosis and clinical management.
Diagnosis of COVID-19
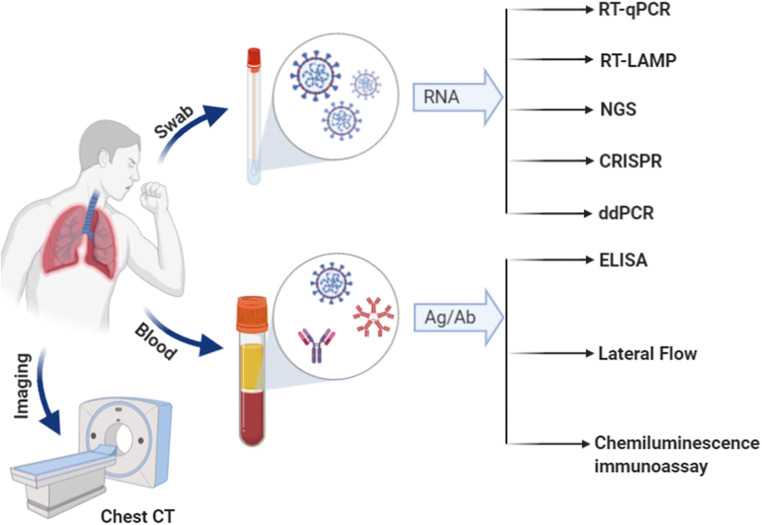
The clinical manifestation of novel SARS-CoV-2 (or COVID-19) is highly variable from individual to individual, with asymptomatic to acute respiratory distress syndrome and multi organ failure. Hence, the accurate diagnosis of COVID-19 is challenging. The routine clinical diagnosis of COVID-19 is primarily based on epidemiological history, clinical manifestations, and confirmed by a variety of laboratory detection methods, including computed tomography (CT) scan, nucleic acid amplification test amplification test (NAAT), and serological techniques (Corman et al. 2020; Wan et al. 2020). A graphical abstract depicting the various analytical methods available for the diagnosis of COVID-19 is mentioned in Fig. 1 and their technical details are discussed here below. For early screening or diagnosis of SARS-CoV-2 infection, specimens such as nasopharyngeal and/or oropharyngeal swab, bronchoalveolar lavage fluid, sputum, bronchial aspirate, or blood are generally recommended (Chan et al. 2004; Kim et al. 2011; Zou et al. 2020).
Fig. 1.
Schematic representation of various analytical methods available for SARS-CoV-2 detection
There are many viruses’ especially severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) that also causes upper and lower respiratory tract infections and shows overlapping clinical symptoms; therefore, it is difficult to differentiate SARS-CoV-2 infections from other viruses causing respiratory infections. Hence, laboratory examination is very essential along with clinical and epidemiological assessments for accurate and rapid diagnosis of the causative agent. This is also known to improve quarantine efficacy.
Computed tomography
The chest computed tomography (CT) is currently one of the first live imaging techniques to detect pneumonia-related illnesses. It has been widely used earlier for the detection of lung abnormalities in SARS and MERS and found to be more sensitive than X-rays (Memish et al. 2014; Rao et al. 2003). Recently, the technique has also been utilized for the diagnosis of COVID-19 in hospitals. However, the technique has its own limitations. For instance, in a retrospective study conducted in Hong Kong on 64 patients, chest radiography showed a sensitivity of 69% when compared to 91% in RT-PCR. Among the RT-PCR positive cases, 20% did not show any lung abnormalities on chest radiograph (Wong et al. 2019). Conversely, in another study, 75% of the RT-PCR negative cases showed chest CT findings with 48% likely to be positive for COVID19 (Ai et al. 2020). In addition, chest computed tomography alone could lead to false positive results since it can overlap with other infections such as influenza, SARS, and MERS. Considering all these points, most of the health commissions have recently eliminated chest CT scanning as a criterion for the diagnosis of suspected cases of COVID-19. However, these ambiguities in the diagnosis can be effectively overcome by using a combination of both chest CT scanning and RT-PCR techniques. In addition, chest CT imaging could also become a useful tool in monitoring COVID-19 progression and therapeutic effect in clinical settings.
Nucleic acid amplification test
Nucleic acid amplification tests (NAAT) are the most sensitive assays and generally preferred test to detect early viral infections because viraemia is usually seen early in the course of a disease. The different types of NAAT assays, such as reverse transcriptase real-time PCR (RT-qPCR), loop-mediated isothermal amplification-based assay (RT-LAMP), microarray, and high-throughput sequencing have been developed for the rapid and accurate diagnosis of COVID-19. However, NAAT demands high quality of SARS-CoV-2 RNA.
Reverse transcriptase real-time polymerase chain reaction
Probe-based RT-qPCR has been considered to be the gold standard method for SARS-CoV-2 detection and currently one of the most widely used test in many countries for screening the populations as recommended by WHO and CDC (Chu et al. 2020; Corman et al. 2020; Loeffelholz and Tang 2020). After the first outbreak, several RT-qPCR assays have been deployed for the detection of SARS-CoV-2 from clinical samples. RT-qPCR assay were developed targeting different genes such as RNA dependent RNA polymerase(RdRp) gene, nucleocapsid (N) gene, envelope (E) gene, spike (S) gene, and ORF1b or ORF8 regions of the SARS-CoV-2 genome (Table 1) (Chan et al. 2020; Corman et al. 2020; Konrad et al. 2020; Reusken et al. 2020). The WHO recommends RT-qPCR-based assay targeting E gene for screening of SARS-CoV-2 followed by a confirmatory test targeting the RdRp gene (Corman et al. 2020). Whereas CDC advocated RT-qPCR assay was based on two nucleocapsid protein genes (N1, N2) (Holshue et al. 2020).
Table 1.
List of nucleic acid-based diagnostic assays currently available for the detection of SARS-CoV-2
| Sl. No | Name of the diagnostic assay | Test principle | Name of company | Sensitivity | Gene of target | Turnaround time |
|---|---|---|---|---|---|---|
| 1 | TaqMan 2019-nCoV Control Kit v1 | Real-time RT-PCR | ABI (Applied bio-systems), United States | NA | ORF1ab, S protein, and N protein | NA |
| 2 | A*STAR FORTITUDE KIT 2.0 | Real-time RT-PCR | Accelerate Technologies Pte Ltd (DxD Hub), Singapore | 1×103 copies/ml | Viral RNA | NA |
| 3 | BioFire COVID-19 test | Multiplex real-time RT-PCR | BioFire Defense, LLC | NA | ORF1ab and ORF8 | Results in ∼ 45 min |
| 4 | LyteStar 2019-nCoV RT PCR Kit 1.0 | Real-time RT-PCR | ADT India Ltd, New Delhi, India | NA | E and RdRP genes | NA |
| 5 | RealStar SARS-CoV-2 RT-PCR kit 1.0 | Real-time RT-PCR | Altona Diagnostics, Germany | NA | E and S gene | NA |
| 6 | SARS-CoV-2 assay | Real-time RT-PCR | Diagnostic Molecular Laboratory−Northwestern Medicine | NA | N1 and RdRP genes | Results in < 1 h without manual RNA extraction |
| 7 | ANGPCR 2019-nCoV | Real-time RT-PCR | Angstrom Biotech Pvt. Ltd, Rajasthan | 500 copies / mL | ORF 1ab and N gene | NA |
| 8 | CDC 2019- Novel Coronavirus Real-Time RT-PCR Diagnostic Panel | Real-time RT-PCR | CDC-US | 10 copies/ul | N gene | NA |
| 9 | Simplexa COVID-19 Direct | Real-time RT-PCR | DiaSorin Molecular LLC | 500 copies/mL | OFR1ab and S gene | Results in ∼1 h with no RNA extraction |
| 11 | Real Time Fluorescent RT-PCR Kit for detecting 2019-nCoV | Real-time RT-PCR | BGI Genomics, China | 100 copies/mL | ORF1ab gene | Results in 3 h |
| 13 | Xpert Xpress SARS-CoV-2 | Real-time RT-PCR | Cepheid, United States | 250 copies/mL | N2 and E genes | Results in ∼ 45 min with < 1 min of hands on time |
| 14 | SARAGENETM Corona Virus (2019 NCV) Test Kit | Real-time RT-PCR | CoSara Diagnostics Pvt. Limited, Ahmedabad, India | NA | NA | NA |
| 15 | ePlex SARS-CoV-2 test | Cartridge Based Technology | GenMark Diagnostics, Inc. | 1 x 105 copies/mL | RNA | < 2 min hands-on time and results in ∼ 2 h |
| 16 | Daan Gene Co. Ltd | Real-time RT-PCR | Daan Gene Co. Ltd., China | 500 copies/mL | ORFlab and N genes | NA |
| 18 | One-Step WRTaqman qRT-PCR | Real-time RT-PCR | GCC Biotech Pvt. Ltd, 24 Parganas, West Bengal, India | 10 copies | NA | NA |
| 19 | NeoPlex COVID-19 detection kit | Real-time RT-PCR | GeneMatrix, South Korea | 20 copies/μL | RdRp and N gene | NA |
| 20 | Panther Fusion SARS-CoV-2 assay (Panther Fusion System) | Real-time RT-PCR | PCR Hologic Inc. | 1x10-2 TCID50/mL | ORF1ab regions 1 and 2 | Each Panther Fusion system can provide results in < 3 h and process up to 1150 coronavirus tests in 24-h period |
| 24 | Helini Coronavirus [COVID-19] Real time-PCR Kit | Real-time RT-PCR | Helini Biomolecules, Chennai, India | 10copies/μL | Viral RNA S gene E gene | NA |
| 26 | COVID-19 RT-PCR test | Real-time RT-PCR | LabCorp Laboratory Corporation of America | 6.25 copies/μL for NP swabs and 12.5 copies/μL for BAL | N gene | Results in 2−4 days |
| 27 | Protect COVID-19 RT-qPCR Kit | Real-time RT-PCR | JN Medsys Pte Ltd, Singapore | 10 copies/reaction | N1, N2, N3 genes | Result in < 2 h |
| 28 | ARIES SARS-CoV-2 assay | Real-time RT-PCR | Luminex Corporation | 300 copies/target | ORF1ab and N gene | Minimal hands-on time and an automated workflow delivers results in 2 h |
| 30 | Power Check 2019 nCoV Real Time PCR Kit | Real-time RT-PCR | Kogene Biotech, Seoul, Korea | 10-fold serial dilutions | E and RdRp genes | NA |
| 31 | Covid 19 Probe-free Real Time PCR Diagnostic Kit | Real-time RT-PCR | Indian Institute of Technology, Delhi, India | NA | NA | NA |
| 33 | LabGun Real Time PCR Kit | Real-time RT-PCR | LabGenomics, South Korea | 20 genomic RNA copies/μL | RdRp and E genes | NA |
| 34 | COVID-19 RT-PCR kit | Real-time RT-PCR | Medsource Ozone Biomedicals, Faridabad, India | 20 copies/μL | ORF1ab and N genes | NA |
| 35 | Meril COVID-19 One-step RT-PCR Kit | Real-time RT-PCR | Meril Diagnostics, Vapi, Gujarat, India | < 5 RNA copies/reaction | ORF 1ab and nucleoprotein N genes | Result in 65 min |
| 36 | Patho Detect | Real-time RT-PCR | Mylab Discovery Solutions, Pune, India | 100% | Viral RNA | Results within 2.5 h |
| 37 | LYRA SARS-CoV-2 assay. | Real-time RT-PCR | Diagnostic Hybrids, Inc Quidel Corporation | 3.40e+4 copies /mL | ORF1ab | Results in < 75 min after extraction |
| 38 | STANDARD M nCoV RT detection kit | Real-Time RT-PCR | SD BIOSENSOR | 0.5 copies/μL | E and RdRP genes | Results within 90 min |
| 40 | Gene Finder COVID-19 | OSANG Health Care, South Korea | NA | NA | NA | |
| 41 | Viracor SARS-CoV-2 assay | Real-time RT-PCR | Viracor Eurofins Clinical Diagnostics | NA | N gene | Results the same day, 12–18 h from receipt of specimen |
| 42 | MiRXES FORTITUDE KIT 2.0 | Real-time RT-PCR | MiRXES Pte Ltd. | NA | Viral RNA genes less prone to mutation | Results in 90 min, produces 100,000 test kits/week |
| 43 | Q-line Molecular Coronavirus (COVID-19) RT-PCR kit | Real-time RT-PCR | POCT Services Pvt. Limited, Lucknow, India | NA | NA | NA |
| 44 | Z-Path Covid-19C (Genesig) | Real-time RT-PCR | Primer Design, UK | 0.58 copies/μl | Viral RNA | NA |
| 45 | Allplex 2019-nCoV assay | Multiplex real-time RT-PCR | Seegene | E gene-4,167Copies/mL N gene-1250 copies/mL RdRP gene-4167 copies/mL | E, N, and RdRP genes | Results in < 2 h after extraction |
| 47 | Novel Coronavirus(2019-nCoV) Nucleic Acid Diagnostic Kit (PCR Fluorescence Probing) | Real-time RT-PCR | Sansure Biotech Inc., Changsha, China | 200 copies/mL | ORF1ab gene and N gene | Results in 30 min |
| 48 | cobas SARS-CoV-2 | Real-time RT-PCR | Roche Molecular Systems, Inc. | 0.003 TCID50/mL | Viral RNA | Results in 3.5 h, instruments can process up to 384 results (cobas 6800 System) and 1056 results (cobas 8800 System) in 8 h |
| 51 | VitaPCR SARS-CoV-2 assay | Real-time PCR | Credo Diagnostics Biomedical Pte Ltd. | NA | Viral RNA | Results in 20 min with 1 min of hands-on time |
| 52 | QIAstat-Dx Respiratory SARS-CoV-2 panel | Multiplex real-time RT-PCR | Qiagen GmbH | 400 copies/mL | E and RdRP genes | Results in ≈1 h, by differentiating novel coronavirus from 21 other bacterial and viral respiratory pathogens |
| 55 | TaqPath COVID-19 combo kit |
Multiplex real-time RT-PCR |
Rutgers Clinical Genomics Laboratory ThermoFisher-Applied Biosystems | 200 copies/mL | ORF1b and N and S Genes |
- 94 specimens in under 3 h - 382 specimens in under 6.5 h |
| 56 | TRUPCR SARS-CoV-2 RT-qPCR Kit (V-3.2) (Single Tube Multiplex format) | Real-time RT-PCR | 3B Black Bio Biotech India Ltd. | NA | E, RdRP and N genes | NA |
| 57 | Accula SARS-CoV-2 test |
PCR and lateral flow technologies |
Mesa Biotech Inc | 200 copies/reaction | N gene | Results in 30 min, the palm-sized device can be used in physician office or patients’ home |
| 58 | iAMP COVID-19 detection kit | Real-time RT isothermal amplification test | Atila BioSystems, Inc. D11 | 4 copies/μL | ORF1ab and/ or N gene | Results < 1.5 h |
| 59 | ID NOW COVID-19 | Isothermal nucleic acid amplification technology | Abbott Diagnostics Scarborough, Inc. | 125 copies /mL | RdRP gene | Positive results < 5 min and negative results in 13 min |
| 60 | CRISPR-based tests for SARS-CoV-2 | CRISPR-based lateral flow assay isothermal amplificatio | Cepheid Sherlock Biosciences |
4.5 copies/μL–ORF1ab 0.9 copies/ μL–N gene |
viral RNA | Results in < 1 h |
| 61 | SARS-CoV-2 DETECTR | CRISPR-based lateral flow assay isothermal amplification | Mammoth Biosciences | 10 copies/μL | E and N genes | CRISPR Cas12a-based lateral flow assay results in 30–40 min |
CRISPR clustered regularly interspaced short palindromic repeats, E envelope protein, RdRP RNA dependent RNA polymerase, N nucleocapsid phosphoprotein, S spike protein, NA not analyzed in the literature, RT reverse transcription
In addition, inclusion of two or more genes such as E, RdRp, and ORF-1b-nsp14 in the RT-qPCR reaction could lead to enhanced identification of true positives. However, testing for more SARS-CoV-2 genes for confirmatory results would be laborious and time consuming because of continuous rise in the suspected cases throughout the world. This can be easily avoided by targeting highly specific region of the virus genome that could even detect the virus at a very low concentration. A recent report by Alagarasu et al. revealed a better performance of ORF-1b-nsp14-based assay when compared to RdRp-based assay (Alagarasu et al. 2020). Similarly, it is also reported that a modified RdRp-helicase-based qPCR assay was highly successful in detecting 35% more positive cases of SARS-CoV-2 when compared to RdRp-based assay (Chan et al. 2020). Some studies have also suggested that the lower sensitivity of RdRp-based assay might be due a degenerate base present at the 12th position of reverse primer (Lim et al. 2020; Vogel et al. 2020). The test protocol of all the above-mentioned nucleic acid-based techniques is complex and expensive which demands high-end experimental instruments, testing reagents, and skilled research personnel; hence, it cannot be deployed as point-of-care diagnostic or bedside test in resource-limited settings. Moreover, the tests typically take 4–6 h to complete, but the logistical requirement to ship clinical samples takes turnaround time more than 24 h that delays reporting. In addition, RT-qPCR result highly depends on the quality of viral RNA and in some cases the test cases test needs to be repeated 2 to 3 times for further confirmation. The Limit of detection of most assays is between 3.4 to 4.5 log10 copies/mL (LeBlanc et al. 2020). Though most tests use two gene targets, positivity in gene is considered adequate and this has been incorporated into some national case definitions eg in Canada (http://health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_case_definition.pdf). Test results depend on sample and highest detection rates were reported from brocheoalveolar lavage fluid, sputum, and nasal swabs (Wang et al. 2020c). Though, there are some shortcomings, use of RT-qPCR for the diagnosis of COVID-19 is still considered as the gold standard.
Reverse transcription loop-mediated isothermal amplification
Loop-mediated isothermal amplification (LAMP) is a PCR-based nucleic acid amplification, which has the ability to specifically amplify the target sequence very efficiently, rapidly under isothermal conditions. The method relies on the use of four-six different primers which recognize specific four or six regions on the target gene and Bst DNA polymerase that elongates the chain at constant temperature by using strand displacement mechanism. Amplification by this method can occur in a conventional water bath/heating block, and the amplified product can be visually identified by adding a fluorescent dye. Since SARS-CoV-2 is an RNA virus, a reverse transcription step is required (RT-LAMP). After the outbreak, several RT-LAMP assays have been developed and validated for point-of-care diagnosis of COVID-19 (Broughton et al. 2020; El-Tholoth et al. 2020; Huang et al. 2020b; Lamb et al. 2020; Park et al. 2020; Weihua et al. 2020; Yan et al. 2020; Yu et al. 2020). Park et al. have developed RT-LAMP for the detection of SARS-CoV-2 targeting the Nsp3 region of the virus. The technique could detect as low as 100 copies per reaction of SARS-CoV-2 RNA (Park et al. 2020). However, another research group in Japan evaluated a commercially available RT-LAMP (Loopamp® 2019-SARS-CoV-2 Detection Reagent Kit; http://loopamp.eiken.co.jp/), that showed a high sensitivity with detection limit of 1.0 × 101 copies/μL within 35 min. In addition, RT-LAMP-based method-iLACO (isothermal LAMP based method for COVID-19) targeting ORF1ab gene using 6 primers developed by Yu et al. (2020) was found to detect SARS-CoV-2 as low as 10 copies per reaction (Yu et al. 2020). Similarly, a combination of RT-LAMP with clustered regularly interspaced short palindromic repeats (CRISPR)-based DETECTOR technology was also developed for the rapid detection (30–40 min) of SARS-CoV-2 in clinical samples with the limit of detection of 10 copies per microliter (Broughton et al. 2020). In spite of the development of many RT-LAMP-based molecular techniques, very few have been commercialized due to cross reactivity and lack of sensitivity in the assays (Zhang et al. 2020b).
Nevertheless, the technique like RT-LAMP does not require skilled personnel or high-end equipments. However, it is important to look for multiple targets of SARS-CoV-2 for the optimum utilization of the technique. Since the accuracy of RT-LAMP will also be affected by the mutations at the primer binding region of the virus, it is necessary to avoid these mutation sites while designing the primers to increase the rate of detection.
CRISPR-based diagnosis
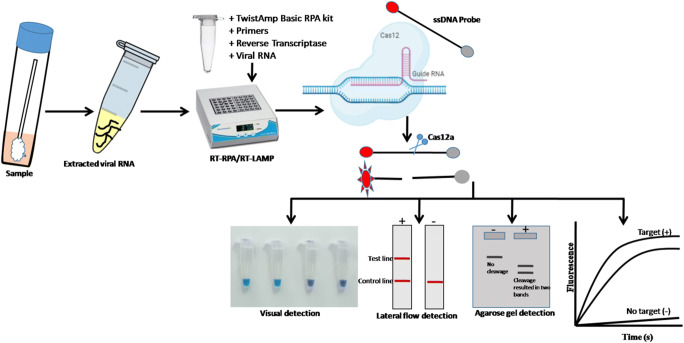
Clustered regularly interspaced short palindromic repeats has gained popularity with in the scientific community as a genome editing tool, but now slowly gaining their potential in diagnostic applications (Chertow 2018; Li et al. 2019). CRISPR requires guide RNA which binds to target complementary sequence and nuclease enzyme cleaves at the precise site. CRISPR components are used for biosensing nucleic acids from different pathogens including bacteria and viruses. In case of viral nucleic acid detection, a small RNA fragment called guide RNA (gRNA), which will in turn bind to the target segment of viral gene. Then, special CRISPR-associated nucleases such as cas9, cas12, or cas13 will be used for cutting the target molecule (Fig. 2).
Fig. 2.
Schematic representation of CRISPR-Cas12a based SARS-CoV-2 detection assay
Many researchers have attempted to use the CRISPR-based detection system for detection of SARS-CoV-2. For instance, a study by Zhang et al. (2020b) used CRISPR-based detection system (SHERLOCK) (Specific High sensitivity Enzymatic Reporter unLOCKing) with isothermal recombinase polymerase amplification (RPA) could detect single molecule per milliliter within 1 h (Zhang et al. 2020a). In this technique, cas13 was used for the detection of amplified products of S and Orf1ab gene of SARS-CoV-2. This assay developed by Sherlock Biosciences became the first FDA-approved CRISPR technology on the market. Another CRISPR diagnostic company, Mammoth Biosciences, used combination of RT-LAMP with CRISPR-cas12-based technique, which could detect 10–100 copies of viral RNA per microliter in 40 min. Till date, this the fastest test developed by Mammoth Biosciences for the detection of SARS-CoV-2 (Broughton et al. 2020). This technique targets the envelope gene of SARS-CoV-2 and results can be analyzed by fluorescence or/through lateral flow method. Further, Ding et al. (2020) developed a rapid, ultrasensitive all in one dual CRISPR/Cas12a (AIOD-CRISPR) assay, which utilizes dual crRNAs targeting two regions of the viral N gene (Ding et al. 2020) with limit of detection of 4.6–11 copies/microliter. Further, a recently developed bed side assay FELUDA (field-deployable nucleobase detection and identification using FnCas9) utilizes FnCas9 could detect as low as 110 femtomolar nucleic acid of the virus (Azhar et al. 2020). Even though all these advanced molecular diagnostic methods have shown promising results, it is important to carefully validate these tools for its efficient field application.
Cartridge-based nucleic acid amplification test and TrueNAT
Cartridge-based nucleic acid amplification test (CBNAAT) is a technique which uses the GeneXpert technology for the diagnosis of tuberculosis (TB). This cartridge-based nucleic acid amplification is a fully automated amplification system, which utilizes real-time PCR. However, due to abrupt raise in the COVID-19 cases, Indian Council of Medical Research (ICMR) has approved the use of CBNAAT to detect COVID-19 cases (https://www.icmr.gov.in/pdf/covid/labs/Cepheid_Xpert_Xpress_SARS-CoV2_advisory_v2.pdf). This technique targets E gene and N2 gene of the SARS-CoV-2 for screening and confirmation, respectively. Another nucleic acid-based test called TrueNAT targeting E gene for screening and RdRp gene for confirmation of COVID-19 cases has also been approved by ICMR. This technology mainly uses chip-based tools and takes up to 1 h for the test.
In addition to all the above molecular techniques, the next-generation sequencing of clinical specimen from the COVID-19 infected patients would allow rapid identification of SARS-CoV-2 and other pathogens contributing secondary/co infections that otherwise known to enhance the severity of SARS-CoV-2 symptoms. Metagenomic approach would help not only in pathogen detection but also provides genetic information, which further led to the better understanding of viral evolution, molecular epidemiology, and contact tracing. In addition, genetic sequencing allows us to assess the rate of genetic mutations of SARS-CoV-2; this information is very useful in determining the antiviral and vaccine efficacy.
The Illumina COVIDSeq is an amplicon-based NGS-based detection platform approved by US Food and Drug Administration (US-FDA) for the qualitative detection of SARS-CoV-2 from respiratory specimens collected from the suspected COVID-19 patients. This detection method utilizes different sets primers and probes leveraged from ARTIC multiplex PCR protocol (Itokawa et al. 2020) combined with Illumina sequencing technology. The COVIDSeqtest accommodates up to 3072 samples in single run on a NOVASeq with a turnaround time of 12 h (https://www.illumina.com/products/by-type/clinical-research-products/covidseq.html). Similarly, the Thermo Fischer Scientific has launched Ion AmpliSeq SARS-CoV-2 research panel that facilitates analysis of SARS-CoV-2 genome and provides high throughput workflow for monitoring the viral evolution. This research panel consists of two pools of amplicons ranging from 125 to 275 bp targeting more than 99% of the SARS-CoV-2 genome (https://thermofisher.mediaroom.com/2020-05-06-Rapid-COVID-19-Genome-Sequencing-Aids-Outbreak-Investigations). This assay requires 1 ng of viral RNA.
Oxford nanotechnology has introduced long-read sequencing platform which has demonstrated substantial benefits of analytical innovations over the currently existing methods for the genome sequencing. Moore et al. has demonstrated the application of MinIon based amplicon and metagenomic sequencing to identify SARS-CoV-2 and other microbes associated with COVID-19 illness (Moore et al. 2020). The study of Wang et al. has reported nanopore target sequencing (NTS) to SARS-CoV-2 and other pathogens simultaneously from respiratory specimens within 10 h (Wang et al. 2020b). The developed method has shown considerably higher sensitivity of detecting 10 viral copies per mL of sample. As this technology is designed to amplify log read sequences, it is important to consider the limitation of this technology in detecting short fragments of SARS-CoV-2 genome from highly degraded samples (Wei et al. 2018; Wilson et al. 2019).
Recently, FDA approved SARS-CoV-2 Droplet Digital PCR (ddPCR) Kit developed by Bio-Rad Laboratories for the diagnosis of COVID-19. The developed assay detected as low as 0.260 to 0.351 copies/μL for genetic markers, N1 and N2.This assay was found to be highly successful in detecting virus in the early stage of infection wherein viral load is usually less. This further helps in resolving the problem of indeterminate test results (https://www.bio-rad.com/featured/en/sars-cov-2-covid-19-testing-solutions.html).
Serological assays
Detecting the antibodies against a virus in infected individuals is one of the most important diagnostic methods in disease surveillance. Though RT-qPCR is the most established technique in detecting the SARS-CoV-2 active cases, viral RNA becomes almost undetectable 14 days post-illness; besides, false-negative results may also arise due to improper handling of viral samples. These challenges warrant the development of simple test kits based on the detection of human antibodies generated in response to viral infection. The fundamental principle behind antibody-based immunodiagnostic is the detection of antibodies developed in response to viral infection (IgG and IgM) and/or, viral antigen through enzyme-linked immunosorbent assay (ELISA). Studies have shown that antigen-specific antibody could be detected in a patient after 3 to 6 days, and IgG could be detected at the later stages of an infection. The application of these tests has the ability to provide information on both active and past infections and can be ramped up to analyze thousands of samples at labs with resource-limited settings. Moreover, it can be deployed for the disease surveillance programs to gain a better understanding of the rate of infection among the community. Although the serological tests have the ability to provide information on both active and past infections, its efficiency in confirming SARS-CoV-2-specific antibodies response to capture past infections is well established (Lee et al. 2010; Wang et al. 2003). Studies conducted in China showed that virus-specific antibodies titer is significantly lower in the asymptomatic group compared to the symptomatic COVID-19 patients (Long et al. 2020). In symptomatic COVID-19 patients, the medium duration for detection of IgM and IgA antibodies was 5 days and IgG was detected in 14 days. The detection efficiency of IgM ELISA was higher than that of RT-qPCR after 5.5 days of symptom onset (Guo et al. 2020). Presence of IgM antibodies indicates recent exposure to viral infection, whereas IgG antibodies indicate previous exposure to SARS-CoV-2 viral infection. Thus the immunodiagnostic assays are also very critical to support the development of vaccines against COVID-19. This further helps in identifying extent of infection in people without active infection. Given the incredible demand for the rapid test for the diagnosis of COVID-19 infections, R&D firms around the world have launched many rapid diagnostics with varying degrees of sensitivity.
Enzyme-linked immunosorbent assay
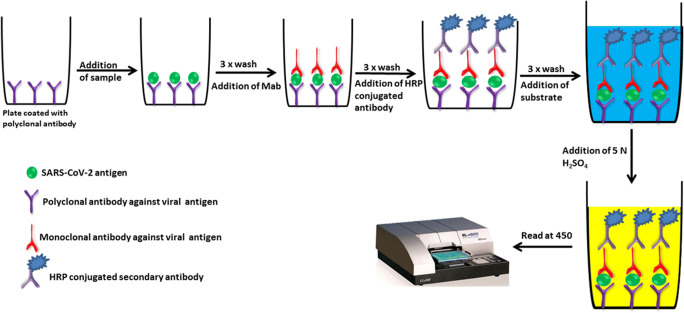
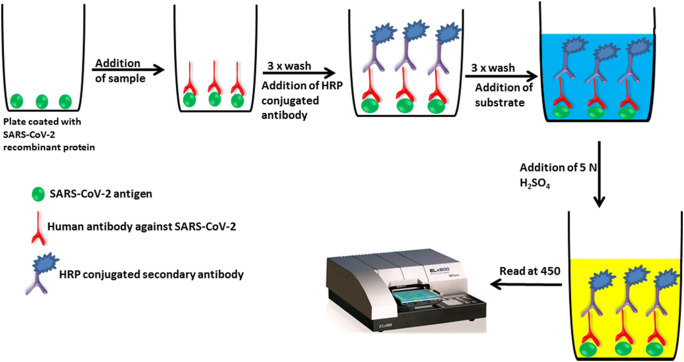
There exist several ELISA-based methods with high levels of reproducibility and enduring sensitivity which makes the test an excellent tool for the diagnosis of various infectious diseases. The test can be qualitative or quantitative, with the turnaround time of around 1–5 h. An overview of sandwich and indirect ELISA assay for the detection of SARS-CoV-2 antigens and human antibody against SARS-CoV-2 antigens are shown in Figs. 3 and 4. Recently, IgG and IgM-based ELISA kit (EDI™ Novel Coronavirus COVID-19 ELISA Kit) was developed by Epitope Diagnostics Inc for the detection of SARS-CoV-2 infection. EDI™ Novel Coronavirus COVID-19 IgM ELISA kit utilizes “IgM capture” method on microtiter plate-based ELISA for the qualitative measurement of the COVID-19 IgM antibody in the patient serum. In this assay, test samples are added to the microtiter plate, which was precoated with anti-human IgM-specific antibodies. Immunocomplex of “Anti-hIgM” antibody and COVID-19 IgM antibody will be detected by HRP labeled recombinant COVID-19 antigen. In the case of EDI™ Novel Coronavirus COVID-19 IgG ELISA Kit, the test utilizes ELISA plate coated with SARS-CoV-2 recombinant nucleocapsid protein to detect the presence of human IgG against SARS-CoV-2 in the test sample. Clinical validation of the EDI™ Novel Coronavirus COVID-19 ELISA Kits demonstrated high “true positivity” among the RT-PCR confirmed COVID-19 patients (Bundschuh et al. 2020). In India, the National Institute of Virology, Pune, in collaboration with Zydus Diagnostics, has developed an indigenous IgG-based ELISA (COVID KAVACH ELISA) for antibody detection for COVID-19. Preliminary validation of the COVID KAVACH ELISA is shown to have high sensitivity and specificity in detecting SARS-CoV-2 infection. In addition to the above-mentioned ELISA kits, there are few other ELISA kits available in the market for the diagnosis of SARS-CoV-2 infection by analyzing IgM and IgG antibodies (Table 2).
Fig. 3.
An overview of sandwich ELISA assay for the detection of SARS-CoV-2 antigens
Fig. 4.
An overview of indirect ELISA assay for the detection of human antibody against SARS-CoV-2 antigens
Table 2.
List of serological assays used for the detection of SARS-CoV-2 antigen or/antibodies to SARS-CoV-2 virus
| Sl. No | Name of the diagnostic assay | Test principle | Name of company | Sensitivity | Ig or antigen detection | Turnaround time |
|---|---|---|---|---|---|---|
| 1 | COVID Kavach ELISA IgG | ELISA | Zydus Cadila Healthcare Ltd., Ahmedabad, Gujarat, India | 92.37% | IgG | Testing 90 samples together in a single run of 2.5 h |
| 2 | Euroimmun Anti- SARS-COV-2 ELISA IgG | ELISA | Euroimmun US Inc., USA | 90% | IgG | NA |
| 3 | Erbalisa COVID-19 IgG ELISA | ELISA | Calbiotech Inc., USA | 98.3% | IgG | Results in 50 min |
| 4 | KT-1033 EDI Novel Coronavirus COVID-19 ELISA kit | ELISA | Epitope Diagnostics | 45% | IgG/IgM | |
| 5 | VITROS-Immunodiagnostics Products Anti-SARS-CoV-2 total reagent pack | ELISA | Ortho-Clinical Diagnostics | >8 days 100% | IgG/IgM | Cannot distinguish between IgG/IgM |
| 6 | DEIASL019/020 SARS-CoV-2 IgG ELISA kit | ELISA | Creative Diagnostics | NA | IgG/IgM | IgG specific for N protein |
| 7 | m2000 SARS-CoV-2 assay | Chemiluminescent microparticle immunoassay | Abbott Core Laboratory |
53.1% (day 7) 82.4% (day 10) 96.9% (day 14) 100% (> day 17) |
IgG | Runs up to 100–200 tests/h |
| 8 | IgG antibody test kit for Novel coronavirus 2019-nCoV | Magnetic particle-based chemiluminescence immunoassay | Bioscience (Chongqing) Diagnostic Technology Co., Ltd. | NA | IgG | NA |
| 9 | Standard Q COVID-19 Ag | Chromatographic immunoassay | SD Biosensor | 84.38% | Viral antigen | Results in 30 min |
| 10 | iFLASH-SARS-CoV-2-IgG/IgM | Immunoassay | Shenzhen Yhlo Biotech Company |
97.3%-IgG 86.1%-IgM |
IgG/IgM | |
| 11 | MAGLUMI IgG/IgM de 2019-nCoV (CLIA) | Chemiluminescence immunoassay | Snibe Diagnostic (China) |
100%-IgG-12 days 88%-IgM |
IgG/IgM | Results in 30 min |
| 12 | Diazyme DZ-Lite SARS-CoV-2 IgG/IgM test | Luminescent immunoassay | Diazyme Laboratories |
95.6%-IgG 89.9%-IgM |
IgG/IgM | NA |
| 13 | VivaDiag COVID-19 IgM/IgG rapid test | Lateral flow immunoassay | Everest Links Pte Ltd. | 18.4% | IgG/IgM | Results in 15 min |
| 14 | COVID-19 IgG/IgM LF | Lateral flow immunoassay | Advagen Biotech | NA | IgG/IgM | Results in 10 min |
| 15 | COVID-19 IgG/IgM Point of Care Rapid test | Lateral flow immunoassay | Aytu Biosciences/Orient Gene Biotech |
93%-(IgG) 69%-(IgM |
IgG/IgM | Results in 2–10 min |
| 16 | qSARS-CoV-2 IgG/IgM rapid test | Lateral flow immunoassay | Cellex Inc | NA | IgG/IgM | Results in 15–20 min, antibodies specific for N protein |
| 17 | COVID-19 IgM/IgG rapid test | Lateral flow immunoassay | BioMedomics | NA | IgG/IgM | Results in 15 min |
| 18 | One-Step COVID-2019 test | Lateral flow immunoassay | Celer Biotechnologia | NA | IgG/IgM | Results in 15 min |
| 19 | COVID-19 Ag Respi-Strip | Lateral flow immunoassay (dipstick) | Coris Bioconcept | NA | Viral antigen | Results in 15 min |
| 20 | DPP COVID-19 IgM/IgG system | Lateral flow immunoassay | Chembio Diagnostics | 95% | IgG/IgM | Results in 15 min |
| 21 | OnSite COVID-19 IgG/IgM rapid test | Lateral flow immunoassay | CTK Biotech Inc. (USA) | 97.1% | IgG/IgM | Results in 10 min |
| 22 | COVID-19 IgG/IgM rapid test cassette | Lateral flow immunoassay | Hangzhou Biotest Biotech Co. Ltd. | 100%-IgM 93.3%-IgG | IgG/IgM | Results in 15–20 min |
| 23 | SARS-CoV-2 rapid test | Lateral flow immunoassay | PharmACT | NA | IgG/IgM | Results in 20 min, N protein, S1 and S2 subunits used as antigens |
| 24 | Standard Q COVID-19 IgM/IgG Duo | Lateral flow immunoassay | SD Biosensor |
94.33%-> 7 days 99.1%-> 14 days |
IgG/IgM | Results in 10 min |
Point-of-care assay
Recent years have witnessed significant growth in the global market for point-of-care solution for infectious disease point-of-care (POC) test is performed at the patient’s bedside or near the site and has a rapid turnaround time which facilitates better disease diagnosis, monitoring, and change in the management of patient care (Kozel and Burnham-Marusich 2017). As a result of continuous development in the R&D sector, several POC testing platforms based on lateral flow assays, biosensors, microfluidic, bioanalytical platforms, and lab-on-a-chip technologies are available for the rapid detection of analytes near to the patient. Growing COVID-19 pandemic and the dearth of molecular testing capacity, as well as reagents around the world, demand the development of POC test for the rapid diagnosis of COVID-19, thereby aiding to establish infection control measures. In response to this, 233 POC assays are commercially available or in development for the diagnosis of COVID-19 worldwide (https://www.finddx.org/covid19/pipeline/?avance=all&type=Rapid+diagnostic+tests&testtarget=all&status=all§ion=immunoassays&action=default#diag_tab). These could detect SARS-CoV-2 antigen like spike protein or antibodies against viral antigens.
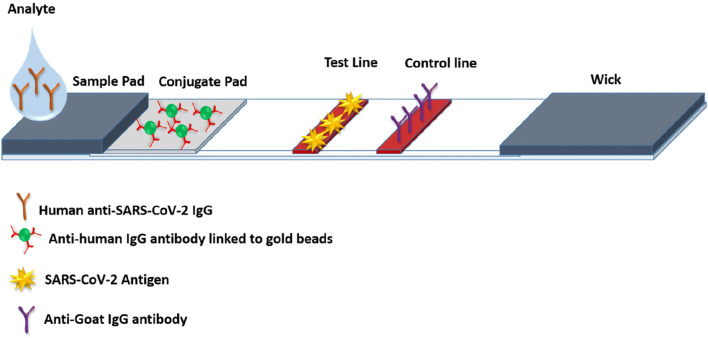
Lateral flow immunoassay
Lateral flow immunoassays are the handheld portable POC platform for the rapid detection of an analyte and being used in biomedical, veterinary, agriculture, and food industries. This assay works based on the principle of antigen-antibody reactions, where the sample to be analyzed is placed on a test device, and the results are displayed within 5–15 min (Fig. 5). The main advantage of Lateral flow immunoassays is that the ease of performing test outside of the clinical laboratory, which make the assays the superior without burdening the capacity of the laboratories. In response to public health emergency due to COVID-19, researchers around the world put an effort to develop lateral flow immunoassays to detect antibodies to SARS-CoV-2 or viral antigens (https://www.medrxiv.org/content/medrxiv/early/2020/05/07/2020.04.15.20066407.full.pdf). Various types of lateral flow immunoassays for the detection of COVID-19 and their features are given in Table 2. A comparative evaluation of the three different lateral flow immunoassays for the detection of COVID-19 showed an overall clinical sensitivity of 70% without any significant differences between the three different assays (Montesinos et al. 2020). Mertens and colleagues at CorisBioConcep, Belgium, have developed a lateral flow immunoassay (COVID-19 Ag Respi-Strip) for the rapid detection of SARS-CoV-2 antigen from nasopharyngeal specimens (Mertens et al. 2020). This is the only available POC assay which targets the highly conserved nucleoprotein region of SARS-CoV-2 and capable of detecting the antigens in 15 min with an overall sensitivity and specificity of 57.6 and 99.5%, respectively.
Fig. 5.
Lateral flow immunoassay for the detection of human anti-SARS-CoV-2 IgM or IgG antibody
Chemiluminescence immunoassay
Over the past few years, chemiluminescence immunoassay (CLIA) has gained increasing attention as a rapid and sensitive POC test in different fields, including clinical diagnosis. The detection of the analyte is based on the reaction wherein enzymes used for the immunochemical reaction converts the chemiluminescence substrate to a reaction product, which emits a photon of light instead of color development (Chen et al. 2012). Based on this principle, few CLIAs are available for the detection of serum immunoglobulin IgG and IgM against SARS-CoV-2 (Cai et al. 2020; Infantino et al. 2020; Wan et al. 2020). The performance of four different chemiluminescence immunoassay systems for the detection of COVID-19 showed varying degrees of diagnostic accuracy, thereby suggesting the necessity of performance evaluation diagnostic test before actual use (Wan et al. 2020).
Conclusion
Currently, a range of nucleic acid-based and antigen/antibody based tests and available for detection of SARS-CoV-2 infection. While nucleic acid-based tests or antigen detection tests are used for diagnostic purpose, antibody detection tests may be used for assessment of exposure to the virus or for sero-surveillance of populations. Nucleic acid-based tests and antigen/antibody detection tests vary widely in sensitivity. Most nucleic acid-based tests depend upon use of two gene targets, but in some countries, single target detection is considered adequate. Even the results of a nucleic acid-based test depend on the sample used with highest rates of detection obtained in broncheoalveolar lavage, sputum, and nasal swabs. But this might depend on the stage of infection. In asymptomatic and pre-symptomatic individuals, nasal swabs or sputum is generally used. Currently, RT-qPCR remains the frontline and gold standard technique for the detection of SARS-CoV-2 infection. However, due to the limited capacity of laboratory-based molecular testing and high turnaround time, we propose that newer rapid point-of-care technologies such as RT-LAMP and other isothermal amplification techniques may serve as an alternative detection modality for the screening of SARS-CoV-2 infection in highly populated countries including India. Chip-based (nucleic acid-based) tests have been validated for performance and are being widely used in India. Antigen detection tests have lower sensitivity compared to nucleic acid-based tests and negative results need to be reconfirmed by RT-PCR or other nucleic acid-based tests. However, point-of-care tests are still under development and following validation, these point-of-care tests could become available in the near future.
Acknowledgments
This work was supported by Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India (CVD/2020/000150) and the authors are grateful to Nitte (Deemed to be University) for providing the necessary research facilities to carry out this work.
Author’s contribution
PRconceived and designed the review. PR, BKK, and VKD wrote the manuscript. IK and IK review and editing. All authors read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Praveen Rai, Ballamoole Krishna Kumar and Vijaya Kumar Deekshit contributed equally to this work.
Contributor Information
Praveen Rai, Email: raiprav@nitte.edu.in.
Iddya Karunasagar, Email: iddya.karunasagar@nitte.edu.in.
References
- Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 Cases. Radiology. 2020;296:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagarasu K, Choudhary ML, Lole KS, Abraham P, Potdar V. Evaluation of RdRp & ORF-1b-nsp14-based real-time RT-PCR assays for confirmation of SARS-CoV-2 infection: An observational study. Indian J Med Res. 2020;151(5):483–485. doi: 10.4103/ijmr.IJMR_1256_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Phutela R, Ansari AH, Sinha D, Sharma N, Kumar M, Aich M, Sharma S, Rauthan R, Singhal K, Lad H, Patra PK, Makharia G, Chandak GR, Chakraborty D, Souvik M (2020) Rapid, field-deployable nucleobase detection and identification using FnCas 9. bioRxiv 10.1101/2020.04.07.028167
- Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, Miao X, Streithorst JA, Granados A, Sotomayor-Gonzalez A, Zorn K, Gopez A, Hsu E, Gu W, Miller S, Pan CY, Guevara H, Wadford DA, Chen JS, Chiu CY. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundschuh C, Egger M, Wiesinger K, Gabriel C, Clodi M, Mueller T, Dieplinger B. Evaluation of the EDI enzyme linked immunosorbent assays for the detection of SARS-CoV-2 IgM and IgG antibodies in human plasma. Clin Chim Acta. 2020;509:79–82. doi: 10.1016/j.cca.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XF, Chen J, Li Hu J, Long QX, Deng HJ, Liu P, Fan K, Liao P, Liu BZ, Wu GC, Chen YK, Li ZJ, Wang K, Zhang XL, Tian WG, Xiang JL, Du HX, Wang J, Hu Y, Tang N, Lin Y, Ren JH, Huang LY, Wei J, Gan CY, Chen YM, Gao QZ, Chen AM, He CL, Wang DX, Hu P, Zhou FC, Huang AL, Wang DQ. A peptide-based magnetic chemiluminescence enzyme immunoassay for serological diagnosis of Coronavirus Disease 2019. J Infect Dis. 2020;222(2):189–193. doi: 10.1093/infdis/jiaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, Fung AY, Ng AC, Zou Z, Tsoi HW, Choi GK, Tam AR, Cheng VC, Chan KH, Tsang OT, Yuen KY (2020) Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol 58(5). 10.1128/JCM.00310-20 [DOI] [PMC free article] [PubMed]
- Chan PK, To WK, Ng KC, Lam RK, Ng TK, Chan RC, Wu A, Yu WC, Lee N, Hui DS, Lai ST, Hon EK, Li CK, Sung JJ, Tam JS. Laboratory diagnosis of SARS. Emerg Infect Dis. 2004;10(5):825–831. doi: 10.3201/eid1005.030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Spiering AJ, Karthikeyan S, Peters GW, Meijer EW, Sijbesma RP. Mechanically induced chemiluminescence from polymers incorporating a 1,2-dioxetane unit in the main chain. Nat Chem. 2012;4(7):559–562. doi: 10.1038/nchem.1358. [DOI] [PubMed] [Google Scholar]
- Chertow DS. Next-generation diagnostics with CRISPR. Science. 2018;360(6387):381–382. doi: 10.1126/science.aat4982. [DOI] [PubMed] [Google Scholar]
- Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, Ng DYM, Wan CKC, Yang P, Wang Q, Peiris M, Poon LLM. Molecular diagnosis of a novel coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brunink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Yin K, Li Z, Liu C (2020) All-in-one dual CRISPR-Cas12a (AIOD-CRISPR) assay: a case for rapid, ultrasensitive and visual detection of novel coronavirus SARS-CoV-2 and HIV virus. bioRxiv. 10.1101/2020.03.19.998724
- El-Tholoth M, Bau HH, Song J (2020) A single and two-stage, closed-tube, molecular test for the 2019 novel coronavirus (COVID-19) at home, clinic, and points of entry. ChemRxiv. 10.26434/chemrxiv.11860137.v1
- Guo L, Ren L, Yang S, Xiao M, Chang, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L, Han L, Dang S, Xu Y, Yang Q, Xu S, Zhu H, Xu Y, Jin Q, Sharma L, Wang L, Wang J. Profiling early humoral response to diagnose Novel Coronavirus Disease (COVID-19) Clin Infect Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK, Washington State -nCo VCIT First case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WE, Lim B, Hsu CC, Xiong D, Wu W, Yu Y, Jia H, Wang Y, Zeng Y, Ji M, Chang H, Zhang X, Wang H, Cui Z. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol. 2020;13(4):950–961. doi: 10.1111/1751-7915.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino M, Grossi V, Lari B, Bambi R, Perri A, Manneschi M, Terenzi G, Liotti I, Ciotta G, Taddei C, Benucci M, Casprini P, Veneziani F, Fabbri S, Pompetti A, Manfredi M. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: an Italian experience. J Med Virol. 2020;92:1671–1675. doi: 10.1002/jmv.25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokawa K, Sekizuka T, Hashino M, Tanaka R, Kuroda M (2020) A proposal of alternative primers for the ARTIC Network's multiplex PCR to improve coverage of SARS-CoV-2 genome sequencing. BioRxiv. 10.1101/2020.03.10.985150
- Karunasagar I, Karunasagar I. Ongoing COVID-19 global crisis and scientific challenges. J Health Allied Sci NU. 2020;10(1):1–2. [Google Scholar]
- Kim C, Ahmed JA, Eidex RB, Nyoka R, Waiboci LW, Erdman D, Tepo A, Mahamud AS, Kabura W, Nguhi M, Muthoka P, Burton W, Breiman RF, Njenga MK, Katz MA. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS One. 2011;6(6):e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad R, Eberle U, Dangel A, Treis B, Berger A, Bengs K, Fingerle V, Liebl B, Ackermann N, Sing A (2020) Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020. Euro Surveill 25(9). 10.2807/1560-7917.ES.2020.25.9.2000173 [DOI] [PMC free article] [PubMed]
- Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Hengartner N, Giorgi EE, Bhattacharya T, Foley B, Hastie KM. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Burnham-Marusich AR. Point-of-care testing for infectious diseases: past, present, and future. J Clin Microbiol. 2017;55(8):2313–2320. doi: 10.1128/JCM.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BK, Venkatraja B, Prithvisagar KS, Rai P, Rohit A, Hegde MN, Karunasagar I, Karunasagar I (2020) Mutational analysis unveils the temporal and spatial distribution of G614 genotype of SARS-CoV-2 in different Indian states and its association with case fatality rate of COVID-19. bioRxiv
- Lamb LE, Bartolone SN, Ward E, Chancellor MB. Rapid detection of novel coronavirus/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by reverse transcription-loop-mediated isothermal amplification. PLoS One. 2020;15(6):e0234682. doi: 10.1371/journal.pone.0234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc JJ, Gubbay JB, Li Y, Needle R, Arneson SR, Marcino D, Charest H, Desnoyers G, Dust K, Fattouh R, Garceau R, German G, Hatchette TF, Kozak RA, Krajden M, Kuschak T, Lang ALS, Levett P, Mazzulli T, Mc Donald R, Mubareka S, Prystajecky N, Rutherford C, Smieja M, Yu Y, Zahariadis G, Zelyas N, Bastien N, Group C-PDITotCPHLNRVW Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J Clin Virol. 2020;128:104433. doi: 10.1016/j.jcv.2020.104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Lee BH, Seok SH, Baek MW, Lee HY, Kim DJ, Na YR, Noh KJ, Park SH, Kumar DN, Kariwa H, Nakauchi M, Heo SJ, Park JH. Production of specific antibodies against SARS-coronavirus nucleocapsid protein without cross reactivity with human coronaviruses 229E and OC43. J Vet Sci. 2010;11(2):165–167. doi: 10.4142/jvs.2010.11.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li S, Wang J, Liu G. CRISPR/Cas systems towards next-generation biosensing. trends. Biotechnol. 2019;37(7):730–743. doi: 10.1016/j.tibtech.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Lim J, Kim CM, Lee KH, Seo JW, Yun NR, Lee YM, et. al (2020) Insufficient sensitivity of RNA dependent RNA polymerase gene of SARS-CoV-2 viral genome as confirmatory test using Korean COVID-19 cases. Preprints.org. 10.20944/preprints202002.0424.v1
- Lin Q, Zhao S, Gao D, Lou Y, Yang S, Musa SS, Wang MH, Cai Y, Wang W, Yang L, He D. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int J Infect Dis. 2020;93:211–216. doi: 10.1016/j.ijid.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chen J, Huang AL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Al-Tawfiq JA, Assiri A, AlRabiah FA, Al Hajjar S, Albarrak A, Flemban H, Alhakeem RF, Makhdoom HQ, Alsubaie S, Al-Rabeeah AA. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33(9):904–906. doi: 10.1097/INF.0000000000000325. [DOI] [PubMed] [Google Scholar]
- Mertens P, De Vos N, Martiny D, Jassoy C, Mirazimi A, Cuypers L, Van den Wijngaert S, Monteil V, Melin P, Stoffels K, Yin N, Mileto D, Delaunoy S, Magein H, Lagrou K, Bouzet J, Serrano G, Wautier M, Leclipteux T, Van Ranst M, Vandenberg O, Group L-US-C-WD Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front Med (Lausanne) 2020;7:225. doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos I, Gruson D, Kabamba B, Dahma H, Van den Wijngaert S, Reza S, Carbone V, Vandenberg O, Gulbis B, Wolff F, Rodriguez-Villalobos H. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J Clin Virol. 2020;128:104413. doi: 10.1016/j.jcv.2020.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SC, Penrice-Randal R, Alruwaili M, Dong X, Pullan ST, Carter D, Bewley K, Zhao Q, Sun Y, Hartley C, Zhou EM (2020) Amplicon based MinION sequencing of SARS-CoV-2 and metagenomic characterisation of nasopharyngeal swabs from patients with COVID-19. medRxiv
- Park GS, Ku K, Baek SH, Kim SJ, Kim SI, Kim BT, Maeng JS. Development of reverse transcription loop-mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J Mol Diagn. 2020;22(6):729–735. doi: 10.1016/j.jmoldx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TN, Paul N, Chung T, Mazzulli T, Walmsley S, Boylan CE, Provost Y, Herman SJ, Weisbrod GL, Roberts HC. Value of CT in assessing probable severe acute respiratory syndrome. AJR Am J Roentgenol. 2003;181(2):317–319. doi: 10.2214/ajr.181.2.1810317. [DOI] [PubMed] [Google Scholar]
- Reusken C, Broberg EK, Haagmans B, Meijer A, Corman VM, Papa A, Charrel R, Drosten C, Koopmans M, Leitmeyer K, On Behalf Of E-L. Erli N. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Euro Surveill. 2020;25(6):2000082. doi: 10.2807/1560-7917.ES.2020.25.6.2000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohit A, Rajasekaran S, Karunasagar I, Karunasagar I. Fate of respiratory droplets in tropical vs temperate environments and implications for SARS-CoV-2 transmission. Med Hypotheses. 2020;144:109958. doi: 10.1016/j.mehy.2020.109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CBF, Brito AF, Wyllie AL, Fauver JR, Ott IM, Kalinich CC et al (2020) Analytical sensitivity and efficiency comparisons of SARS-COV-2 qRT-PCR primer probe sets. medRxiv. 10.1101/2020.03.30.20048108 [DOI] [PMC free article] [PubMed]
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127–e00120. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Liu JH, Ouyang XL, Yu Y, Ma SX, Li XJ, Lu LC, Tian YP, Liu HY, Xu HM, Yao W. Detection of the anti-SARS-coronavirus specific antibody levels in 156 SARS patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003;11(5):441–443. [PubMed] [Google Scholar]
- Wang MH, Fu A, Hu B, Tong Y, Liu R, Gu J, Liu J, Jiang W, Shen G, Zhao W, Men D (2020b) Nanopore target sequencing for accurate and comprehensive detection of SARS-CoV-2 and other respiratory viruses. medRxiv [DOI] [PMC free article] [PubMed]
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (2020c) Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed]
- Wei S, Weiss ZR, Williams Z. Rapid multiplex small dna sequencing on the MinION nanopore sequencing platform. G3 (Bethesda) 2018;8(5):1649–1657. doi: 10.1534/g3.118.200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Y, Xiaofei D, Qingxi W, Mingjie X, Qianqian Z, Yunying Z, Huailong Z, Li W, Yihui X, Jun W, Shuyi H, Min W, Fengyan P, Yunshan W (2020) Rapid detection of SARS-CoV-2 using reverse transcription RT-LAMP method. medRxiv
- WHO (2020) Coronavirus disease 2019 (COVID-19) Situation report -184 2020. World Health Organization Accessed July 23, 2020.
- Wilson BD, Eisenstein M, Soh HT. High-fidelity nanopore sequencing of ultra-short DNA targets. Anal Chem. 2019;91(10):6783–6789. doi: 10.1021/acs.analchem.9b00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, Lui MM, Lee JCY, Chiu KW, Chung T, Lee EYP, Wan EYF, Hung FNI, Lam TPW, Kuo M, Ng MY. Frequency and Distribution of Chest Radiographic Findings in COVID-19 Positive Patients. Radiology. 2019;296(2):E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Cui J, Huang L, Du B, Chen L, Xue G, Li S, Zhang W, Zhao L, Sun Y, Yao H, Li N, Zhao H, Feng Y, Liu S, Zhang Q, Liu D, Yuan J. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;26(6):773–779. doi: 10.1016/j.cmi.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wu S, Hao X, Dong X, Mao L, Pelechano V, Chen WH, Yin X. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin Chem. 2020;66:975–977. doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Abudayyeh OO, Gootenberg JS (2020a) A protocol for detection of COVID-19 using CRISPR diagnostics. Broad Institute webpage. https://www.broadinstitute.org/files/publications/special/ COVID-19%20detection%20(updated).pdf
- Zhang Y, Odiwuor N, Xiong J, Sun L, Nyaruaba RO, Wei H, Tanner NA (2020b) Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. MedRxiv
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]