Abstract
Objectives
A dysregulated inflammatory response against the dopamine‐2 receptor (D2R) has been implicated in movement and psychiatric disorders. D2R antibodies were previously reported in a subset of these patients; however, the role of T cells in these disorders remains unknown. Our objective was to identify and characterise pro‐inflammatory D2R‐specific T cells in movement and psychiatric disorders.
Methods
Blood from paediatric patients with movement and psychiatric disorders of suspected autoimmune and neurodevelopmental aetiology (n = 24) and controls (n = 16) was cultured in vitro with a human D2R peptide library, and D2R‐specific T cells were identified by flow cytometric quantification of CD4+CD25+CD134+ T cells. Cytokine secretion was analysed using a cytometric bead array and ELISA. HLA genotypes were examined in D2R‐specific T‐cell‐positive patients. D2R antibody seropositivity was determined using a flow cytometry live cell‐based assay.
Results
Three immunodominant regions of D2R, amino acid (aa)121–131, aa171–181 and aa396–416, specifically activated CD4+ T cells in 8/24 patients. Peptides corresponding to these regions were predicted to bind with high affinity to the HLA of the eight positive patients and had also elicited the secretion of pro‐inflammatory cytokines IL‐2, IFN‐ γ, TNF, IL‐6, IL‐17A and IL‐17F. All eight patients were seronegative for D2R antibodies.
Conclusion
Autoreactive D2R‐specific T cells and a pro‐inflammatory Th1 and Th17 cytokine profile characterise a subset of paediatric patients with movement and psychiatric disorders, further underpinning the theory of immune dysregulation in these disorders. These findings offer new perspectives into the neuroinflammatory mechanisms of movement and psychiatric disorders and can influence patient diagnosis and treatment.
Keywords: autoimmunity, autoimmune encephalitis, dopamine‐2 receptor antibodies, neurodevelopmental disorders, pro‐inflammatory T cells
In this study, we report of autoreactive dopamine‐2 receptor (D2R)‐specific T cells in a subset of paediatric movement and psychiatric disorders. These T cells recognised three immunodominant regions of D2R that were predicted to bind with high affinity to HLA class II molecules of patients, and T‐cell activation was associated with the secretion of pro‐inflammatory cytokines. These findings suggest a role for autoreactive T cells in the immune dysregulation of movement and psychiatric disorders.
Introduction
Recent years have seen a surge in evidence supporting autoimmunity as a cause of a subset of movement and psychiatric disorders. 1 , 2 , 3 , 4 , 5 , 6 , 7 This paradigm has been largely driven by the discovery of antibodies against various neuronal proteins that have guided differential diagnosis and treatment regimens and led to an improved prognosis. One such neuronal protein targeted by antibodies is the dopamine‐2 receptor (D2R). In the brain, D2R is expressed abundantly in the basal ganglia, limbic system and cerebral cortex. 8 Dopamine signalling in these neural networks primarily regulates voluntary action, motor control, learning and behavioural responses. 8 , 9 In the periphery, many immune cells also express dopamine receptors and may have immunomodulatory effects. 10 , 11 Given these roles, D2R has been implicated in movement and psychiatric disorders that have been associated with a dysregulated immune system.
Several studies have reported D2R antibodies in a subset of paediatric patients with movement and psychiatric disorders. These include disorders with a well‐recognised autoimmune aetiology, such as Sydenham's chorea and basal ganglia encephalitis, and also neuropsychiatric and neurodevelopmental disorders, such as first episode of psychosis, Tourette's syndrome and paediatric acute‐onset neuropsychiatric syndrome (PANS). 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 The precise mechanisms underlying autoimmunity in these disorders remain unclear, but it has been postulated that D2R antibodies may alter dopaminergic signalling by stimulating excess dopamine secretion, 20 inducing inhibitory D2R signalling 16 and triggering receptor internalisation. 19 Additionally, D2R antibodies can be useful in identifying patients who can be amenable to immunotherapy. 17 , 21 , 22 , 23 , 24 , 25
The field has focused predominantly on aberrant antibody responses and less is known about the autoreactive T‐cell response against D2R in movement and psychiatric disorders. Cellular immunity is a major compartment of the adaptive immune response, and in particular, CD4+ T helper cells are key in orchestrating the effects of other immune cells and molecules. The central nervous system (CNS) is an accessible site for T cells. In the steady state, they traverse the CNS and actively partake in immunosurveillance. In the case of a murine model of post‐infection associated autoimmunity, reactive T cells migrate from the periphery into the CNS and contribute to neuroinflammation. 26 , 27 , 28 Indeed, a dysregulated T‐cell compartment has been described in other neuroinflammatory disorders, most notably in multiple sclerosis (MS), 29 , 30 , 31 but also in neuromyelitis optica spectrum disorders (NMOSDs), 32 , 33 , 34 Rasmussen's encephalitis 35 , 36 and paraneoplastic syndromes. 37 , 38
In this study, we report on the presence of peripheral autoreactive T cells against D2R in paediatric patients with movement and psychiatric disorders. We identified three immunodominant regions of D2R, which induced T‐cell activation, were high‐affinity binders to HLA class II molecules and elicited pro‐inflammatory cytokine secretion. Notably, the presence of D2R‐specific T cells was not associated with D2R antibodies. Collectively, our findings inform the potential role of autoreactive T cells in movement and psychiatric disorders and provide new insights into the contribution of the immune system in neurodevelopmental disorders.
Results
T cells in patients with movement and psychiatric disorders recognised three immunodominant regions of D2R
D2R‐specific T cells in paediatric patients with movement and psychiatric disorders (n = 24) were identified by determining CD4+ T‐cell activation when whole blood was stimulated with 10 master pools of human D2R peptides. The co‐expression of CD25 and CD134 on CD4+ T cells was assessed as a proxy for T‐cell activation. 39 Compared to the control cohort, a 6.9‐ to 21.5‐fold higher frequency of activated D2R‐specific T cells was detected in 8/24 patients (33%) when stimulated with three master pools: pool 4 (n = 4; median: control = 0.025%, patients = 0.173%), pool 5 (n = 3; median: control = 0.005%, patients = 0.111%) and pool 10 (n = 4; median: control = 0.003%, patients = 0.029%; Figure 1a). D2R‐specific T cells were not detected in controls (0/16; Figure 1a). The immunodominant regions of D2R were restricted to master pools 4, 5 and 10 as CD4+ T‐cell activation was not observed in patients and controls in the remainder seven master pools (Supplementary figure 1). While most D2R‐specific T‐cell‐positive patients recognised one of the three immunodominant master pools (6/8, 75%), patient 2 (P2) recognised two master pools, pools 4 and 10, and P8 recognised all three master pools. The overall percentage of CD3+CD4+ T cells across all conditions tested did not vary significantly between patients (median = 44.65%; IQR = 34.08–47.30%) and controls (median = 40.35%; IQR = 38.87–45.19%; Mann–Whitney U‐tests, P = 0.86; Figure 1b). The percentage of T‐cell response against D2R in positive patients (median = 0.11%; IQR = 0.07–0.15%) was not significantly different to the percentage of T‐cell response against the recall antigen, TT, in all patients (median = 0.15%; IQR = 0.03–0.40%) and controls (median = 0.17%; IQR = 0.04–1.02%; Kruskal–Wallis tests, P = 0.46; Figure 1c). The eight D2R‐specific T‐cell‐positive patients were seronegative for D2R antibodies. 4/23 patients were seropositive for D2R antibodies but negative for D2R‐specific T cells (Supplementary figure 2). Patients with D2R antibodies presented with chronic Tourette's syndrome with obsessive–compulsive disorder (OCD) and basal ganglia encephalitis.
Figure 1.
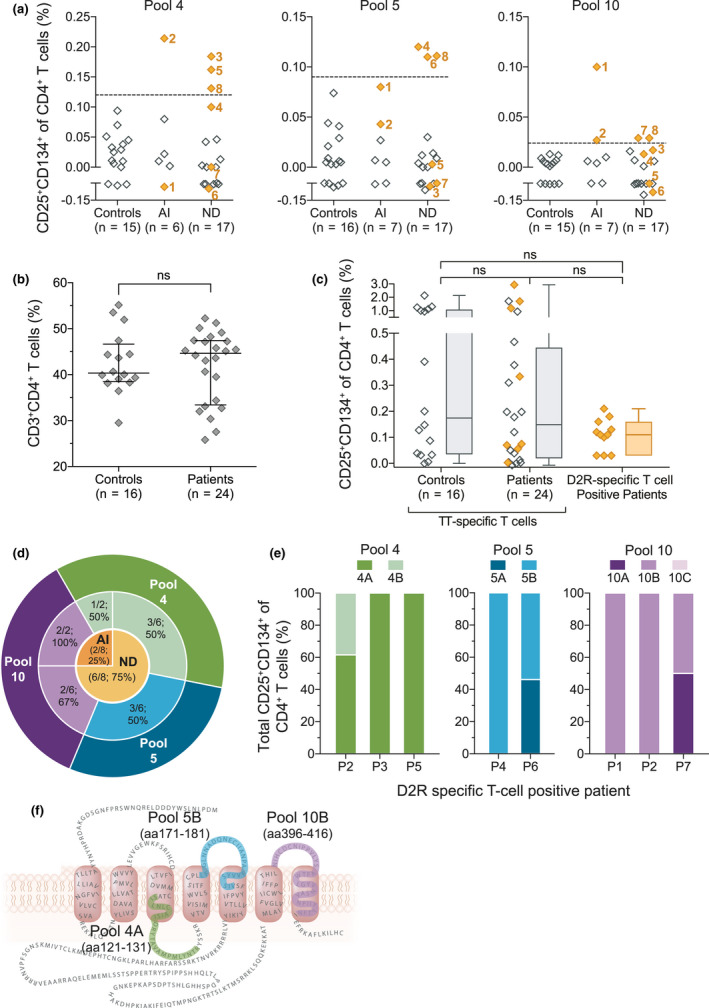
T cells in patients with movement and psychiatric disorders recognised dopamine‐2 receptor (D2R) at three distinct regions. D2R peptide pools that elicited T‐cell activation in patients with movement and psychiatric disorders of an autoimmune (AI) and neurodevelopmental (ND) aetiology were determined by assessing the co‐expression of CD25 and CD134 on CD4+ T cells using the CD25/CD134 assay. (a) Patient whole blood (n = 24) was stimulated with the 10 master pools of D2R peptides and eight patients (numbered orange diamonds) exhibited activation against at least one of three pools: pool 4, pool 5 and pool 10. Positive patients were defined as a frequency of CD25+CD134+CD4+ T cells above the threshold (dashed line; mean + 3SD of controls (n = 16)) for each peptide pool. Samples were tested once soon after their collection to preserve sample integrity. (b) The percentage of total CD3+CD4+ T cells in controls and patients was not significantly (ns) different (Mann–Whitney U‐tests, P = 0.86; error bars = mean ± SD). (c) Activation of antigen‐specific T cells in response to the recall antigen tetanus toxoid (TT) was tested concurrently to the activation of D2R‐specific T cells. The frequency of D2R‐specific T cells in patients was comparable to the frequency of TT‐specific T cells in controls, in patients with D2R‐specific T cells (orange diamonds) and in patients without D2R‐specific T cells (Kruskal–Wallis tests, P = 0.46; whiskers = minimum to maximum). (d) 6/8 D2R‐specific T‐cell‐positive patients had an ND aetiology, and 2/8 D2R‐specific T‐cell‐positive patients had an AI aetiology. (e) The peripheral blood mononuclear cells of 7/8 positive patients were re‐assessed against sub‐pool peptide of pools 4, 5 and 10. Due to sample limitation, samples were tested once, while ensuring all relevant sub‐pools were tested. Of the total activated D2R‐specific T cells, there was preferential activation by pool 4A (3/3), pool 5B (2/2) and pool 10B (2/3). (f) These three immunogenic sub‐pools corresponded to three discrete regions of thehuman D2R (green = pool 4A; blue = pool 5B; purple = pool 10B).
D2R‐specific T cells were detected in 35% (6/17) of patients with neurodevelopmental aetiology and in 29% (2/7) of patients with suspected autoimmunity, and thus, most of the eight patients with D2R‐specific T cells had a neurodevelopmental aetiology (6/8; 75%; Table 1 and Figure 1d). The two patients with suspected autoimmunity were previously healthy but had acute‐onset chorea and were diagnosed with Sydenham's chorea, a disorder of presumed post‐streptococcal autoimmune aetiology (Table 1). On the other hand, in D2R‐specific T‐cell‐positive patients with neurodevelopmental origins, Tourette's syndrome was the dominant manifestation (6/6) and most co‐presented with obsessive–compulsive disorder (OCD, 5/6; Table 1). Their disease course was chronic, and disease duration ranged from 2 to 12 years before sampling (median = 6.5 years; Table 1). Master pools 4 and 10 induced a T‐cell response in both clinical groups; however, master pool 5 was exclusively recognised by patients with a neurodevelopmental aetiology (Figure 1d). There were no discernible clinical differences in patients who were positive or negative for D2R‐specific T‐cell activation.
Table 1.
Clinical data of dopamine‐2 receptor‐specific T‐cell‐positive patients
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Gender | Male | Male | Female | Female | Female | Male | Male | Male |
| Disease duration at sampling (years) | 0.17 | 0.04 | 11 | 2 | 12 | 5 | 2 | 8 |
| Aetiology | Autoimmune | Autoimmune | ND | ND | ND | ND | ND | ND |
| Infection‐provoked onset and/or worsening | Yes | Yes | Yes | Yes | No | No | Yes | No |
| TS | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| OCD | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Other features | Chorea | Chorea | Anorexia | Autism, ADHD, ODD | Autism, depression | ‐ | ‐ | Anxiety |
| Disease course at time of sampling | Acute | Acute | Chronic | Chronic | Chronic | Chronic | Chronic | Chronic |
ADHD, attention‐deficit/hyperactivity disorder; ND, neurodevelopmental; OCD, obsessive–compulsive disorder; ODD, oppositional defiant disorder; TS, Tourette's syndrome
Having identified the putative regions of D2R that elicit CD4+ T‐cell activation, the immunodominant regions were further delineated by deconvoluting the master pools 4, 5 and 10 (Supplementary tables 1 and 2). These D2R sub‐pools were cultured with the peripheral blood mononuclear cells (PBMCs) of 7/8 D2R‐specific T‐cell‐positive patients to determine the relative activation of CD4+CD25+CD134+ T cells by each sub‐pool. P2, P3 and P5, who were previously shown to recognise master pool 4, demonstrated a preferential activation to sub‐pool 4A (P2: 61.5%, P3: 100% and P5: 100%) over pool 4B (Figure 1e). Master pool 5 induced CD4+ T‐cell activation in P4 and P6, and in particular, sub‐pool 5B (P4: 100%, P6: 53.9%) elicited a greater proportion of T‐cell activation than sub‐pool 5A (P4: 0.00%, P6: 46.1%; Figure 1e). T cells in P1, P2 and P7 were activated in response to master pool 10. Sub‐pool 10B was the only pool that T cells in P1 and P2 recognised (100%), while in P7, sub‐pool 10A and 10B equally stimulated T‐cell activation (50%; Figure 1e). Sub‐pool 10C was not tested in P7. Overall, a greater activation response to sub‐pools 4A, 5B and 10B suggested that they encompassed the T‐cell immunodominant regions on D2R. These three regions corresponded to amino acid (aa)121–131, aa171–181 and aa396–416, respectively, and are localised to the second intracellular loop, second and third extracellular loops and the seventh transmembrane domain (Figure 1f).
Presentation of immunodominant D2R peptides was restricted by HLA‐D molecules
CD4+ T‐cell recognition and activation by an antigen require a robust binding of the peptide to the HLA class II molecule, HLA‐D, on antigen‐presenting cells. Using the immune epitope database (IEDB), we predicted the likelihood of the D2R peptides within the immunodominant pools to bind to the HLA‐D genotype of D2R‐specific T‐cell‐positive patients (n = 8; Supplementary table 3). Clusters of 15‐mer peptides across the D2R protein sequence had prediction ranks in the top 10 percentile, indicating a high binding affinity to HLA‐D, and these clusters included peptides from the immunodominant pools which elicited T‐cell activation in vitro (Figure 2). The majority of peptides of sub‐pool 4A and sub‐pool 10B sequence bound with high affinity (median percentile rank = 1.55 and 2.6, respectively) to HLA‐D. Peptides of sub‐pool 5B sequence were also predicted to be high binders, but to a lesser extent (median percentile rank = 4). In P8, T‐cell activation was elicited by master pools 4, 5 and 10 (Figure 1a), but the precise sub‐pool that induced activation within the respective master pool could not be determined in vitro. Nevertheless, peptides of sub‐pools 4A, 5B and 10B were predicted to bind with high affinity to this patient's HLA genotype (Figure 2). Computational predictions of T‐cell epitopes agreed with our findings from in vitro studies, further supporting these three regions as the likely T‐cell immunodominant regions of D2R.
Figure 2.
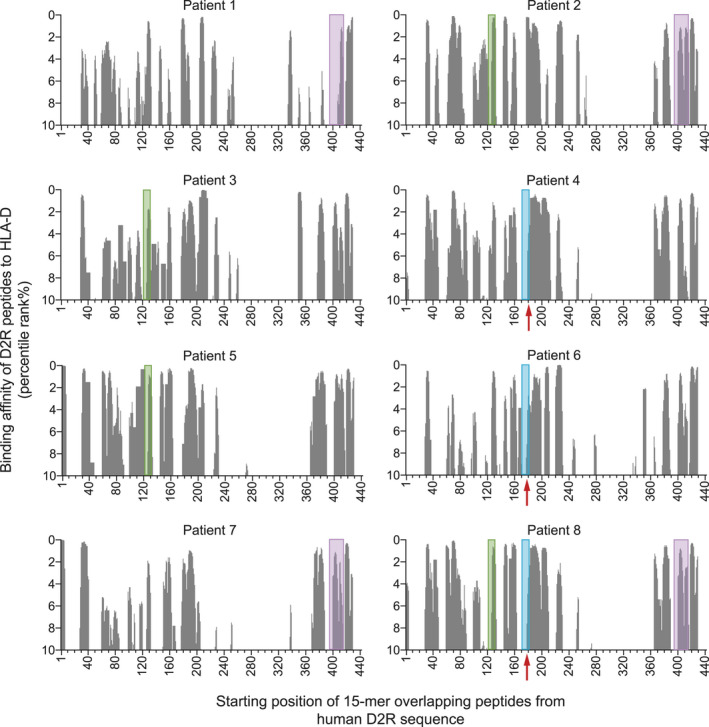
Immunodominant dopamine‐2 receptor (D2R) peptides identified in vitro were high‐affinity binders of HLA‐D molecules. In D2R‐specific T‐cell‐positive patients (n = 8), the likelihood of HLA‐DRB1, HLA‐DQA1, HLA‐DQB1, HLA‐DPA1 and HLA‐DPB1 alleles to present D2R 15‐mer peptides encompassing the full‐length protein was predicted using the Immune Epitope Database (IEDB). The top 10 percentile ranks are shown on the y‐axis and represent the binding affinity of D2R peptides to HLA‐D. A lower percentile rank is indicative of a higher binding affinity to a HLA‐D molecule. Shaded areas denote the peptide pool which elicited CD4+T‐cell activation in that patient using the CD25/CD134 assay (green = peptide pool 4A; blue = peptide pool 5B; purple = peptide pool 10B; red arrow indicates peptides within blue shaded area).
In addition to recognising the peptide of an HLA‐peptide complex, T cells must also recognise the specific HLA variant in the complex for full activation. Hence, certain HLA genotypes may predispose a patient to D2R‐specific T‐cell activation. HLA‐DPA1*01:03:01 and HLA‐DPB1*04:01:01 were notably over‐represented in D2R‐specific T‐cell‐positive patients (Supplementary table 4). These two genotypes were prevalent in 6/8 (75%) patients and, moreover, co‐existed in a patient, suggesting that both genotypes may play a contributing role (Supplementary tables 3 and 4). There was also a higher occurrence of HLA‐DQA1*01:02:01 (3/8; 37.5%) and HLA‐DQB1*03:01:01 (3/8; 37.5%; Supplementary table 4).
D2R‐specific T‐cell activation in patients with movement and psychiatric disorders was associated with a pro‐inflammatory cytokine secretion profile
A hallmark of activated and functional T cells is the secretion of cytokines which modulate the inflammatory response. As elevated pro‐inflammatory cytokines were reported in other neuroinflammatory diseases, 33 , 34 , 40 , 41 , 42 we determined whether D2R‐specific T‐cell activation corresponded with an increase in pro‐inflammatory cytokines. We characterised the cytokine milieu of the supernatant of PBMCs stimulated with the D2R sub‐pools of immunodominant master pools 4, 5 and 10. When the combined cytokine response to all sub‐pools in patients was compared to the normalised median of controls, pro‐inflammatory cytokine characteristics of T helper 1 (Th1) and T helper 17 (Th17) cells were notably elevated in 6/6 D2R‐specific T‐cell‐positive patients (Figure 3a). In particular, IL‐6 was 4‐ to 41‐fold higher in 5/6 patients and IL‐17A was 7‐ to 122‐fold higher in 4/6 patients than controls (n = 11). Sub‐pools of D2R also induced increased secretion of IL‐2 (4/6), IFN‐γ (5/6), TNF (4/6) and, to a lesser extent, IL‐17F (3/6; Figure 3a). Conversely, the concentration of anti‐inflammatory T helper 2 (Th2) cytokines IL‐4, IL‐5, IL‐10 and IL‐13 was consistently low in all D2R‐specific T‐cell‐positive patients and similar to controls (Figure 3b). Further analysis showed that elevated secretion of these six pro‐inflammatory cytokines was predominantly induced by sub‐pools 4A, 5B and 10B (Figure 4). Moreover, in each patient, increased cytokine secretion was associated with the sub‐pool, which induced D2R‐specific T‐cell activation (Figure 1c and Figure 4).
Figure 3.
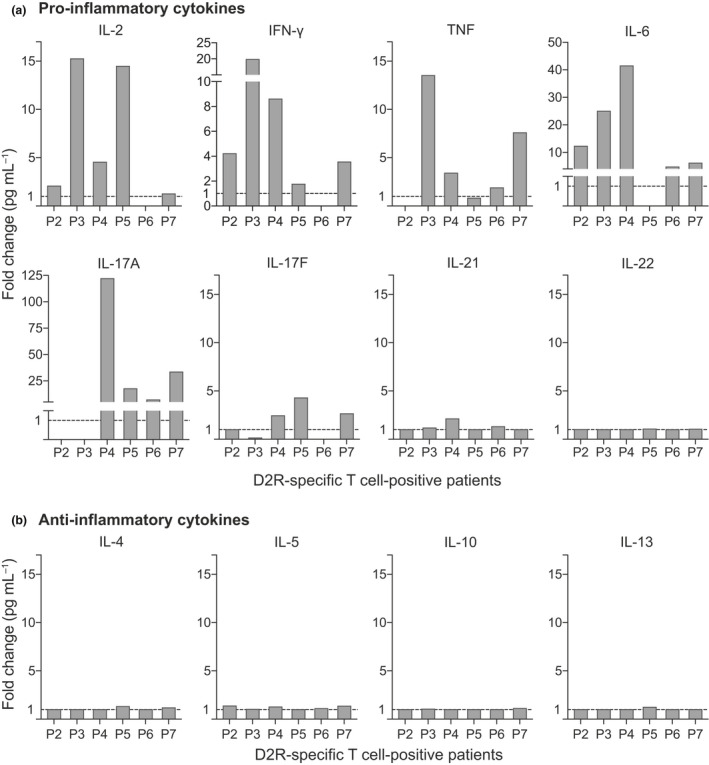
Dopamine‐2 receptor (D2R)‐specific T‐cell‐positive patients exhibited a pro‐inflammatory cytokine profile. Peripheral blood mononuclear cells of D2R‐specific T‐cell‐positive patients (n = 6) were stimulated with immunodominant sub‐pools 4, 5 and 10. The supernatant of each sub‐pool stimulation was tested once in duplicates on a multiplex cytometric bead array and IFN‐γ ELISA to characterise the cytokine secretions. Compared to the controls (dashed line = normalised median of controls), D2R‐specific T‐cell‐positive patients had (a) elevated levels of pro‐inflammatory cytokines IL‐2 (4/6), IFN‐γ (1/3), TNF (4/6), IL‐6 (5/6), IL‐17A (4/6) and IL‐17F (3/6), and slightly higher IL‐21 (1/6). (b) Contrastingly, D2R‐specific T‐cell‐positive patients had similar concentration of Th2 cytokines (6/6) as the controls.
Figure 4.
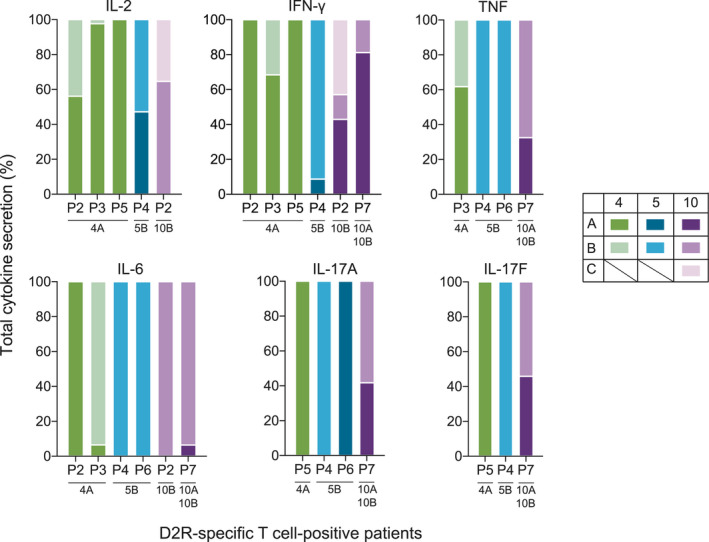
An elevated pro‐inflammatory cytokine secretion was associated with the dopamine‐2 receptor (D2R) peptide sub‐pools which also activated D2R‐specific T cells. Peripheral blood mononuclear cells (PBMCs) from D2R‐specific T‐cell‐positive patients (n = 6) were stimulated with the sub‐pools of immunodominant master pools 4, 5 and 10. The supernatant of each sub‐pool stimulation was tested in duplicates on a multiplex cytometric bead array and IFN‐γ ELISA to characterise the cytokine secretions. Elevated levels of pro‐inflammatory cytokines in each patient were found to be predominantly secreted when the PBMCs were stimulated by D2R sub‐pools 4A, 5B and 10B as compared to the other sub‐pools 4B, 5A, 10A and 10C. These sub‐pools that induced these increased cytokine secretions largely corresponded to the sub‐pools that also elicited activation of D2R‐specific T cells, as specified beneath each patient.
Discussion
It is widely accepted that there is a pathogenic inflammatory response in movement and psychiatric disorders, such as NMDAR antibody‐associated encephalitis and Sydenham's chorea. 1 , 2 , 3 , 4 , 5 , 6 , 7 Additionally, there is an emerging role of the immune system in neurodevelopmental disorders such as Tourette's syndrome and associated neuropsychiatric disorders. 43 Given these compelling findings, we aimed to study the immune dysfunction in paediatric patients with a range of acute and chronic movement and psychiatric disorders of autoimmune and neurodevelopmental aetiology, and a promising target of immune attack in these disorders is D2R. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Herein, we identified and characterised autoreactive D2R‐specific T cells in a subset of paediatric patients with movement and psychiatric disorders. Three discrete immunodominant regions of D2R induced CD4+ T‐cell activation, corresponding to aa121–131, aa171–181 and aa396–416 that are localised on the second intracellular loop, second and third extracellular loops and the seventh transmembrane domain. These regions were predicted to bind with high affinity to the HLA‐D genotype of D2R‐specific T‐cell‐positive patients, and T‐cell activation positively correlated with elevated levels of pro‐inflammatory cytokines. Together, these findings support a role for autoreactive T cells in the pathogenesis of a subgroup of movement and psychiatric disorders.
In this single‐centre study, D2R‐specific T cells were detected in patients with a variety of acute and chronic movement and psychiatric disorders. Acute chorea was the most common presentation in D2R‐specific T‐cell‐positive patients with probable autoimmunity. On the other hand, D2R‐specific T‐cell‐positive patients with neurodevelopmental disorders had a chronic disease course and Tourette's syndrome concomitant with OCD was the prominent feature. This result supports the existing paradigm of dopamine dysregulation in Tourette's syndrome 17 , 44 , 45 and is consistent with the recent report of altered frequencies in functional subsets of circulating CD4+ T cells in paediatric patients with OCD. 46 While PANS was a common feature in our cohort and recent findings have suggested neuroinflammatory involvement, 25 D2R‐specific T cells were not observed in these patients. D2R‐specific T cells were identified in both an acute course and chronic course of movement and psychiatric disorders, suggesting different modes of T‐cell activity in different disease courses. Activated D2R‐specific T cells in acute autoimmune movement and psychiatric disorders suggest a recent encounter with its cognate antigen. In chronic neurodevelopmental disorders, however, activated D2R‐specific T cells may reflect a persistently active immune system. Interestingly, D2R‐specific T cells in patients with neurodevelopmental disorders recognised more immunodominant regions of D2R than in patients with autoimmune movement and psychiatric disorders. While there were more patients with neurodevelopmental disorders, this difference may represent epitope spreading in the chronic course of neurodevelopmental disorders. Although we did not analyse serial samples, Vaknin‐Debminsky and colleagues have demonstrated in a longitudinal study that T cells in patients with NMOSD gradually recognised a broader range of aquaporin‐4 (AQP4) peptides. 33 A similar expansion in autoreactive T‐cell antigen specificity over time has been observed in MS and type 1 diabetes. 47 , 48
Dopamine‐2 receptor has been previously described as an immune target of antibodies. However, patients positive for D2R‐specific T cells were all seronegative for D2R antibodies. This finding adds to the growing reports of neuroimmune encephalitis cases that are negative for known neuronal antibodies, but are responsive to immunotherapy. 49 , 50 D2R antibodies can be detected with methodologies such as an ELISA 13 , 15 , 16 , 22 , 25 ; however, the live cell‐based assay used in this study is a widely accepted method for neuronal antibody detection. Thus, the detection of D2R‐specific T cells may define a subset of movement and psychiatric disorders. Conversely, D2R antibody seropositivity in our cohort was not associated with activated D2R‐specific T cells. Despite D2R antibodies being IgG, 17 an antibody isotype dependent on interactions with CD4+ T follicular helper (Tfh) cells, the absence of D2R‐specific CD4+ T cells in these patients can be attributed to certain factors. Unlike antibodies, which are readily accessible in the peripheral blood, Tfh cells predominantly reside in secondary lymph nodes. 51 , 52 Hence, the time of peripheral blood sampling can affect the detection of antigen‐specific CD4+ T cells. This factor is compounded by the rarity of antigen‐specific T cells in the peripheral blood 53 and challenges the sensitivity of a detection test. Nevertheless, the CD25/CD134 assay that we utilised has been shown to be more sensitive and specific than other T‐cell detection methods in identifying rare antigen‐specific memory T cells. 39 , 54 , 55
T‐cell autoreactivity against D2R demonstrated by the CD25/CD134 assay was corroborated by computational predictions of HLA binding to D2R peptides. The three D2R immunodominant regions identified in the in vitro assay were predicted to bind with high affinity to the HLA‐D genotype of patients who harboured D2R‐specific T cells. This finding underscores the immunogenicity of these immunodominant regions, as a complete activation of antigen‐specific T cells requires recognition of the entire HLA–peptide complex, and further supports the immune dysregulation hypothesis. It was notable that HLA‐DPA1*01:03:01 and HLA‐DPB1*04:01:01 were over‐represented in patients with D2R‐specific T cells and demonstrated haplotypic association. These two alleles were prevalent in Polynesian and Amerindian populations, and HLA‐DPB1*04:01:01 was also observed in some oriental and Caucasoid groups. 56 , 57 , 58 , 59 , 60 , 61 In comparison, our cohort consisted of a mixed population who were not of Polynesian or Amerindian background. Specific HLA alleles and linked haplotypes have been strongly associated with other neurological neuroimmune diseases such as leucine‐rich glioma‐inactivated 1 (LGI1), contactin‐associated protein‐like 2 (CASPR2) and N‐methyl‐d‐aspartic acid receptor (NMDAR) antibody‐associated encephalitis. 62 , 63 , 64 The prevalent HLA alleles in these disorders were HLA‐DRB1*07 linked to HLA‐DRB4, HLA‐DRB1*11:01 and HLA‐DRB1*16:02, respectively. Variations in HLA associations across these encephalitides and in patients with D2R‐specific T cells highlight the contribution of different genetic factors to the genetic susceptibility of these conditions. Strong genetic associations cannot be established in our small cohort of D2R‐specific T‐cell‐positive patients, but the frequent occurrence of these particular alleles in our patients warrants future large‐scale studies.
The pro‐inflammatory cytokine profile in patients with activated D2R‐specific T cells suggested that these cells exhibited a Th1 and Th17 bias. There was a pronounced increase in IL‐2, IFN‐ γ, TNF, IL‐6, IL‐17A and IL‐17F in D2R‐specific T‐cell‐positive patients, while an anti‐inflammatory cytokine secretion was comparable to controls. While these pro‐inflammatory cytokines can be secreted by different immune cells, they are characteristic of Th1 and Th17 cells and their elevated levels were associated with D2R‐specific T‐cell activation. Although we studied the peripheral response of D2R‐specific T cells, Th1 and Th17 signature cytokines have been reported to be the markers of intrathecal inflammation. 41 Th1 and Th17 cells and their associated cytokines have been strongly implicated in a range of neuroinflammatory diseases, including NMDAR antibody‐associated encephalitis and demyelinating disorders such as MS, NMOSD and acute disseminated encephalomyelitis. 33 , 34 , 40 , 41 , 65 Despite the varied phenomenology, neuroimmune encephalitis and demyelinating disorders both positively correlated with Th1 and Th17 cytokines, and the cytokine profile of these immune‐mediated aetiologies differed from an infectious aetiology. 41 , 66 These pro‐inflammatory cytokines can contribute to neuroinflammation by various mechanisms including breakdown of the blood–brain barrier (BBB) and attracting other immune cells, such as neutrophils and macrophages. 27 , 67
In addition to a role in neuroinflammation, it is plausible that D2R‐specific T cells can interfere with immune system functions as dopamine receptors are widely expressed by immune cells. 10 , 11 , 68 , 69 , 70 , 71 Dopaminergic signalling can modulate immunological processes, such as T‐ and B‐cell interactions in antibody production, activation of naïve T cells, inhibition of stimulated T cells, suppression of regulatory T cells, cytokine secretion, and cellular trafficking and chemotactic migration of T cells. 10 , 11 , 72 , 73 , 74 Some studies have reported abnormalities in the expression of dopamine receptors on immune cells or impaired immune function in neurological and psychiatric disorders, including schizophrenia, Parkinson's disease and Tourette's syndrome. 75 , 76 , 77 , 78 , 79 It can then be hypothesised that patients in this study may have abnormal D2R expression on immune cells or that autoreactive D2R‐specific T cells may target immune cells, which express, process and present dopamine receptors. These factors can contribute to a defective immune system and subsequent deleterious effects on the CNS.
The mechanisms that give rise to autoreactive T cells remain an important, yet unanswered question. Molecular mimicry has often been proposed and theorises that cross‐reactivity between microbial structures and host proteins results in an immune response misdirected to the host. This theory has been supported by the evidence that there was 90% homology between the T‐cell epitope of AQP4 and the bacterium Clostridium perfringens, 34 and the induction of a Th17‐biased response in mice when infected with Clostridium. 80 A similar phenomenon has been described wherein a D2R antibody epitope shared a sequence with an unknown protein of Penicillium, 19 and a streptococcal infection led to the production of cross‐reactive autoantibodies in Sydenham's chorea. 13 , 81 , 82 Sequence homology between T‐cell immunodominant regions of D2R and microbial structures was not observed in this study (data not shown). Some D2R‐specific T‐cell‐positive patients, however, exhibited disease exacerbation upon infection, suggesting that an activated immune response could erroneously target self‐proteins as a bystander effect of fighting an infection. 25 , 83 This notion has been demonstrated in a murine model wherein repeated infections led to the expansion and migration of Th17 cells from the peripheral lymph nodes to the brain. 26 Once in the brain, these reactive T cells induced secretion of IL‐17A, activation of microglia and breakdown of the BBB, which could augment neuroinflammation. Additionally, an impaired innate immune response may contribute to autoimmunity. For instance, monocytes in NMOSD patients had elevated IL‐6 secretion and expression of co‐stimulatory molecules that could perpetuate a Th17 response. 34 An activated complement system and microglia have also been increasingly recognised for their role in neuroinflammation in diseases such as MS, Tourette's syndrome, Parkinson's disease and schizophrenia. 84 , 85 , 86 , 87 , 88 Our study explored CD4+ helper T cells; however, CD8+ cytotoxic T cells have been implicated in the pathophysiology of MS, Rasmussen's encephalitis and paraneoplastic syndromes. 29 , 30 , 31 , 35 , 36 , 37 , 38 Likewise, CD8+ T cells may also have a contributing role in D2R‐specific T‐cell autoimmunity through activation and subsequent secretion of pro‐inflammatory cytokines. Given these observations, future studies are needed to evaluate the role of innate and cytotoxic T‐cell immune dysfunction in neuroimmune movement and psychiatric disorders.
Identifying and characterising the neuroinflammation associated with a reactive T‐cell response offer novel alternatives for the treatment of movement and psychiatric disorders. Strategies may include inhibiting T‐cell proliferation, restricting T‐cell trafficking or targeting cytokines produced by T cells. 89 , 90 An example of the latter is tocilizumab, a monoclonal antibody that targets the receptor of IL‐6, a cytokine notably elevated in patients positive for D2R‐specific T cells. Interestingly, tocilizumab has been effective in the treatment of neuroimmune encephalitis and NMOSD patients unresponsive to standard immunotherapies. 91 , 92 , 93 , 94 The co‐stimulatory molecule CD134 on activated T cells is another viable treatment target. Blockade of CD134 with a monoclonal antibody decreased mononuclear cell infiltration into the spinal cord of experimental autoimmune encephalomyelitis mouse models and reduced pro‐inflammatory response in a murine model of rheumatoid arthritis. 95 , 96 Further studies are needed to assess the implications of these results on patient treatments.
In summary, we have identified and characterised pro‐inflammatory D2R‐specific T cells in patients with movement and psychiatric disorders who are negative for D2R antibodies. Notably, these T cells are associated with a Th1 and Th17 phenotype, suggesting a T‐cell‐driven immune response. This suggests that autoreactive D2R‐specific T cells may be a hallmark of a subgroup of movement and psychiatric disorders from both an autoimmune origin and neurodevelopmental origin and prompts further investigation into their pathogenic role. Further knowledge can aid in discriminating patients who are phenotypically similar and consequently encourage treatment regimes towards T‐cell‐directed therapies for improved patient outcomes.
Methods
Subjects
This study investigated paediatric patients (n = 24) seen from 2016 to 2019 at The Children's Hospital at Westmead, Sydney, Australia. Patients presented with a range of movement and psychiatric disorders that were of suspected autoimmune aetiology (n = 7) 43 or neurodevelopmental origin (n = 17) 97 (Table 2). All patients were symptomatic at the time of blood sampling and were not on immunotherapy. Patients with Sydenham's chorea (n = 5) fulfilled the revised Jones criteria of acute rheumatic fever and the criteria defined by Cardoso et al. 98 , 99 All patients with Tourette's syndrome (n = 17) fulfilled the DSM‐IV criteria for this disorder. All patients with PANS (n = 7) fulfilled the criteria developed by Chang et al. 100 The control cohort (n = 16; seven males; median age = 11.5 years; range = 6–16 years) consisted of children with neurological disorders (n = 7; 4/7 confirmed genetic, 3/7 suspected genetic brain disease), children investigated for growth failure (n = 8) and a child with coeliac disease (n = 1). The ethics approval for this study (NEAF 12/SCHN/395) was granted by the human research ethics committees of the Sydney Children's Hospital Network. Written informed consent was obtained from all participants or their carers.
Table 2.
Summary of demographics and clinical characteristics of patients with movement and psychiatric disorders classified by aetiology
| Autoimmune | Neurodevelopmental | |
|---|---|---|
| Number of participants | 7 a | 17 |
| Male:female | 3:4 | 9:8 |
| Median age at sampling (range; years) | 12 (10–17) | 11 (3–16) |
| Median disease duration at sampling (range; years) | 0.17 (0.04–12) | 3 (0.04–12) |
| Clinical characteristics | ||
| Tourette's syndrome only, n | 0 | 2 |
| Tourette's syndrome and OCD, n | 0 | 15 b |
| Sydenham's chorea | 5 | 0 |
| Other neuropsychiatric features and movement disorders, n | Basal ganglia encephalitis 2 | Anorexia 1; anxiety 4; autism 4; ADHD 2; depression 4; fatigue 1; mood 1; ODD 1; sensory 1 |
| Course of disease, n | ||
| Acute | 6 | 1 |
| Chronic | 1 | 16 |
ADHD, attention‐deficit/hyperactivity disorder; OCD, obsessive–compulsive disorder; ODD, oppositional defiant disorder
The seven autoimmune patients fulfilled the criteria for Sydenham's chorea (n = 5) according to the revised Jones criteria of acute rheumatic fever and the criteria by Cardoso et al. 98 , 99 or basal ganglia encephalitis (n = 2) with specific basal ganglia inflammation, as described. 17
Seven patients with Tourette's syndrome had acute infection‐provoked onset consistent with paediatric acute‐onset neuropsychiatric syndrome.
Peptides and antigens
A library of 87 peptides were synthesised based on the complete sequence of the human D2R protein (Mimotopes, Australia; Supplementary table 1). Each 15‐mer peptide overlapped by 10 amino acids and had > 85% purity. Excluding 20 peptides that were insoluble mainly because of their hydrophobicity, the peptides were sequentially grouped into 10 master pools for whole blood cultures (Supplementary table 1). Select master pools were further split into sub‐pools of two or three peptides for PBMC cultures (Supplementary table 2). Each peptide was used at a final concentration of 9 μg mL−1. Cells were stimulated with two positive control antigens: 1 μg mL−1 of staphylococcus enterotoxin B (SEB; Sigma‐Aldrich, St Louis, MO, USA), a super‐antigen which binds to a subset of T cells in a non‐antigen‐specific manner and induces substantial activation and proliferation, and 2 μg mL−1 of tetanus toxoid (TT; Enzo Life Sciences, Farmingdale, NY, USA), a recall antigen which requires processing and presentation by antigen‐presenting cells in association with HLA class II molecules to induce activation of TT‐specific T cells via the T‐cell receptor.
CD25/CD134 assay for the detection of D2R‐specific T cells
To identify activated D2R‐specific CD4+ T cells, blood was collected concurrently for CD25/CD134 assay with whole blood 39 , 101 and PBMCs. PBMCs were isolated by density gradient centrifugation using the Ficoll‐Paque PLUS (GE Healthcare Lifesciences, Chicago, IL, USA).
Fresh sodium heparinised whole blood was diluted with Iscove's Modified Dulbecco's Media (IMDM; 1:1; Gibco, Gaithersburg, MD, USA) and cultured in sterile 24‐well tissue culture plate with media alone (unstimulated), positive control antigens (SEB and TT) or D2R peptide master pools. In four patients with limited sample, D2R peptide master pool 1 and pool 7 were not tested as they were previously shown not to elicit a T‐cell activation. Similarly, cryopreserved PBMCs were cultured in 96‐well U‐bottom tissue culture plates at 5 × 105 cells well−1 (200 μL well−1) in RPMI (Gibco) supplemented with 10% heat‐inactivated human AB serum (Sigma‐Aldrich), penicillin and streptomycin. Each PBMC sample was cultured with complete media alone, TT and D2R sub‐pools of the master pools previously shown to elicit T‐cell activation in the whole blood CD25/CD134 assay. Whole blood and PBMC cultures were incubated for 42–44 h at 37°C in a humidified atmosphere of 5% CO2.
After 42–44 h of culture, 200 μL of whole blood or PBMC cultures was stained with mouse anti‐human CD3‐V450, CD4‐FITC, CD25‐APC and CD134‐PE (BD Biosciences, San Jose, CA, USA) for 15 min at room temperature (Supplementary table 5). Red blood cells in whole blood samples were lysed with BD Pharm Lyse (BD Biosciences), as per the manufacturer's protocol. Cells were acquired on a five‐laser BD LSR II Flow Cytometer (BD Biosciences). Data were analysed with FlowJo 10.4.1 (TreeStar, FLowJoTM Software, Ashland, OR, USA), Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism (GraphPad Software, San Diego, CA, USA). CD3+CD4+ helper T cells were analysed for co‐expression of CD134 and high CD25 based on the negative (unstimulated) and positive (SEB and TT) controls included in each experiment (Supplementary figure 3). A minimum of 40 000 and 30 000 lymphocytes were analysed for the whole blood and PBMC cultures, respectively. The signal of the unstimulated condition was subtracted from the antigen‐stimulated conditions to remove background. In whole blood cultures, a positive D2R‐specific T‐cell activation was established if the frequency of CD25+CD134+CD4+ T cells exceeded a threshold set by the mean + 3SD of the control cohort. One control was excluded from the control cohort of pools 1, 4 and 10 as they were an outlier as per the Grubbs test.
HLA genotyping and HLA‐D2R peptide‐binding prediction
HLA class II molecules are crucial in presenting an antigen to CD4+ T cells. The genes encoding HLA class II proteins are highly polymorphic and, unsurprisingly, have been commonly associated with autoreactive CD4+ T cells. Given this, HLA class II genotyping was performed in patients shown to harbour D2R‐specific T cells (n = 8) using the AlloSeq Tx assay (CareDx, Brisbane, CA, USA). HLA alleles were fully resolved to the second or third field as defined by the IPD‐IMGT/HLA database (http://hla.alleles.org/nomenclature/naming.html).
The online server tool MHC‐II prediction from the Immune Epitope Database (IEDB; http://tools.iedb.org/mhcii/) was used to assess the binding prediction of D2R peptides to class II HLA alleles of each patient. With the exception of new alleles or one allele not documented in IEDB, all possible allele combinations for each class and both chromosomes were examined, resulting in two combinations for HLA‐DR and four for HLA‐DQ and HLA‐DP. The sequence of the full protein was inputted and analysed as 15‐mer peptides overlapping by 10 amino acids. Peptides were considered predicted binders if the consensus percentile rank was < 10%. 102
Cytokine secretion profiling
Following 42–44 h of stimulation, the supernatant of the PBMC cultures was collected and frozen at −80°C for later use. The supernatant of six D2R‐specific T‐cell‐positive patients was analysed for secreted cytokines with the LEGENDPlex Human Th Cytokine Panel 13‐plex Kit (BioLegend, San Diego, CA, USA) and an IFN‐γ enzyme‐linked immunosorbent assay (ELISA; Mabtech Stockholm, Sweden) used as per the manufacturer's protocol. The following cytokines were quantified: IL‐2, IL‐4, IL‐5, IL‐6, IL‐9, IL‐10, IL‐13, IL‐17A, IL‐17F, IL‐21, IL‐22, IFN‐γ and TNF. All samples were measured undiluted and acquired on BD LSR II and BD FACSCanto II. Data were analysed with LEGENDPlex Data Analysis Software v8 (BioLegend), Excel and GraphPad Prism.
Flow cytometry live cell‐based assay for the detection of D2R antibodies
A flow cytometry live cell‐based assay was used to detect the binding of patient serum antibodies against surface human D2R, an accepted method of neuronal antibody detection, 4 and was performed as previously described. 17 , 18 , 19 , 21 Briefly, transfected live HEK293 cells expressing D2R were incubated with serum (1:50), 103 followed by staining with Alexa Fluor 647‐conjugated anti‐human IgG (H + L; Thermo Fisher Scientific, Waltham, MA, USA). Cells were acquired live using the high‐throughput system on a five‐laser BD LSR II Flow Cytometer and analysed with FlowJo 10.4.1, Excel and GraphPad Prism. The threshold was calculated as the mean + 3SD of the control cohort, and a patient was reported positive for D2R antibodies if they were above this threshold in at least two of three independent experiments. Only 23 of 24 patients were tested as the serum of one patient was not available. Antibodies against other dopamine receptor subtypes were not detected in our previous study and therefore were not tested. 17
Statistics
Subject groups were compared with non‐parametric two‐tailed Mann–Whitney U‐tests or Kruskal–Wallis tests, where appropriate, using GraphPad Prism 7. Statistical significance was determined to be P < 0.05.
Author contributions
Deepti Pilli: Conceptualization; Investigation; Methodology; Writing‐original draft; Writing‐review & editing. Alicia Zou: Formal analysis; Investigation; Methodology; Writing‐review & editing. Ruebena Dawes: Formal analysis; Investigation; Writing‐original draft. Joseph A Lopez: Formal analysis; Investigation; Methodology; Writing‐review & editing. Fiona Tea: Formal analysis; Investigation; Methodology; Writing‐review & editing. Ganesha Liyanage: Formal analysis; Investigation; Methodology; Writing‐review & editing. Fiona X.Z. Lee: Investigation; Methodology; Writing‐review & editing. Vera Merheb: Investigation; Methodology; Writing‐review & editing. Samuel Houston: Data curation; Methodology; Software; Writing‐review & editing. Aleha Pillay: Data curation; Formal analysis; Methodology; Writing‐review & editing. Hannah Jones: Formal analysis; Investigation; Writing‐review & editing. Sudarshini Ramanathan: Investigation; Methodology; Writing‐review & editing. Shekeeb Mohammad: Data curation; Formal analysis; Investigation; Writing‐review & editing. Anthony D Kelleher: Formal analysis; Investigation; Methodology; Writing‐review & editing. Stephen I. Alexander: Formal analysis; Investigation; Methodology; Writing‐review & editing. Russell C Dale: Formal analysis; Investigation; Writing‐review & editing. Fabienne Brilot: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Writing‐review & editing.
Conflict of interest
DP reports funding from the Neville Brown Scholarship (Australia). SR reports fellowship research funding from the National Health and Medical Research Council (Australia). RCD and FB have received research funding from the Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, the Petre Foundation and the National Health Medical Research Council (Australia). They have received honoraria from Biogen Idec and Merck Serono as invited speakers. AZ, RD, JAL, FT, GL, FXZL, VM, SDH, AP, HFJ, SM, ADK and SIA declare no competing interests.
Supporting information
Acknowledgments
We thank all the patients and families who participated in this study. We thank Dr Suat Dervish, Dr Edwin Lau and Dr Maggie Wang for providing access and services at the Flow Cytometry Core Facility, which are supported by the Westmead Research Hub, the Cancer Institute New South Wales, the National Health and Medical Research Council and the Ian Potter Foundation. This work was supported by the Australian National Health and Medical Research Council [APP1078643] (NHRMC, Australia), Petre Foundation (Australia) and the Sydney Research Excellence Initiative Grant (University of Sydney, Australia).
References
- 1. Balint B, Vincent A, Meinck HM, Irani SR, Bhatia KP. Movement disorders with neuronal antibodies: syndromic approach, genetic parallels and pathophysiology. Brain 2018; 141: 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cellucci T, Van Mater H, Graus F et al Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm 2020; 7: e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dalmau J, Graus F. Antibody‐mediated encephalitis. N Engl J Med 2018; 378: 840–851. [DOI] [PubMed] [Google Scholar]
- 4. Graus F, Titulaer MJ, Balu R et al A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry 2019; 10: e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pollak TA, Lennox BR, Muller S et al Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry 2020; 7: 93–108. [DOI] [PubMed] [Google Scholar]
- 7. Varley J, Taylor J, Irani SR. Autoantibody‐mediated diseases of the CNS: structure, dysfunction and therapy. Neuropharmacology 2018; 132: 71–82. [DOI] [PubMed] [Google Scholar]
- 8. Mishra A, Singh S, Shukla S. Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson's disease. J Exp Neurosci 2018; 12: 10.1177/1179069518779829. eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol 2019; 39: 31–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf) 2016; 216: 42–89. [DOI] [PubMed] [Google Scholar]
- 11. Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun 2010; 24: 525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Addabbo F, Baglioni V, Schrag A et al Anti‐dopamine D2 receptor antibodies in chronic tic disorders. Dev Med Child Neurol 2020; 62: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 13. Ben‐Pazi H, Stoner JA, Cunningham MW. Dopamine receptor autoantibodies correlate with symptoms in Sydenham's chorea. PLoS One 2013; 8: e73516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brilot F, Merheb V, Ding A, Murphy T, Dale RC. Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology 2011; 76: 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brimberg L, Benhar I, Mascaro‐Blanco A et al Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology 2012; 37: 2076–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox CJ, Sharma M, Leckman JF et al Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J Immunol 2013; 191: 5524–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dale RC, Merheb V, Pillai S et al Antibodies to surface dopamine‐2 receptor in autoimmune movement and psychiatric disorders. Brain 2012; 135: 3453–3468. [DOI] [PubMed] [Google Scholar]
- 18. Pathmanandavel K, Starling J, Merheb V et al Antibodies to surface dopamine‐2 receptor and N‐methyl‐D‐aspartate receptor in the first episode of acute psychosis in children. Biol Psychiatry 2015; 77: 537–547. [DOI] [PubMed] [Google Scholar]
- 19. Sinmaz N, Tea F, Pilli D et al Dopamine‐2 receptor extracellular N‐terminus regulates receptor surface availability and is the target of human pathogenic antibodies from children with movement and psychiatric disorders. Acta Neuropathol Commun 2016; 4: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody‐mediated cell signaling in the pathogenesis of Sydenham's chorea. Autoimmunity 2006; 39: 21–29. [DOI] [PubMed] [Google Scholar]
- 21. Amatoury M, Merheb V, Langer J, Wang XM, Dale RC, Brilot F. High‐throughput flow cytometry cell‐based assay to detect antibodies to N‐methyl‐D‐aspartate receptor or dopamine‐2 receptor in human serum. J Vis Exp 2013; 81: e50935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Connery K, Tippett M, Delhey LM et al Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl Psychiatry 2018; 8: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohammad SS, Paget SP, Dale RC. Current therapies and therapeutic decision making for childhood‐onset movement disorders. Mov Disord 2019; 34: 637–656. [DOI] [PubMed] [Google Scholar]
- 24. Perlmutter SJ, Leitman SF, Garvey MA et al Therapeutic plasma exchange and intravenous immunoglobulin for obsessive‐compulsive disorder and tic disorders in childhood. Lancet 1999; 354: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 25. Shimasaki C, Frye RE, Trifiletti R et al Evaluation of the Cunningham Panel in pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) and pediatric acute‐onset neuropsychiatric syndrome (PANS): changes in antineuronal antibody titers parallel changes in patient symptoms. J Neuroimmunol 2020; 339: 577138. [DOI] [PubMed] [Google Scholar]
- 26. Dileepan T, Smith ED, Knowland D et al Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal‐specific Th17 cells. J Clin Invest 2016; 126: 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 2009; 9: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Louveau A, Smirnov I, Keyes TJ et al Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 30. Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 2015; 14: 406–419. [DOI] [PubMed] [Google Scholar]
- 31. Kaskow BJ, Baecher‐Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8: a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicolas P, Ruiz A, Cobo‐Calvo A et al The balance in T follicular helper cell subsets is altered in neuromyelitis optica spectrum disorder patients and restored by rituximab. Front Immunol 2019; 10: 2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaknin‐Dembinsky A, Brill L, Kassis I et al T‐cell responses to distinct AQP4 peptides in patients with neuromyelitis optica (NMO). Mult Scler Relat Disord 2016; 6: 28–36. [DOI] [PubMed] [Google Scholar]
- 34. Varrin‐Doyer M, Spencer CM, Schulze‐Topphoff U et al Aquaporin 4‐specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol 2012; 72: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bien CG, Urbach H, Deckert M et al Diagnosis and staging of Rasmussen's encephalitis by serial MRI and histopathology. Neurology 2002; 58: 250. [DOI] [PubMed] [Google Scholar]
- 36. Tekgul H, Polat M, Kitis O et al T‐cell subsets and interleukin‐6 response in Rasmussen's encephalitis. Pediatr Neurol 2005; 33: 39–45. [DOI] [PubMed] [Google Scholar]
- 37. Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7: 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melzer N, Meuth SG, Wiendl H. Paraneoplastic and non‐paraneoplastic autoimmunity to neurons in the central nervous system. J Neurol 2013; 260: 1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zaunders JJ, Munier ML, Seddiki N et al High levels of human antigen‐specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J Immunol 2009; 183: 2827–2836. [DOI] [PubMed] [Google Scholar]
- 40. Byun JI, Lee ST, Moon J et al Distinct intrathecal interleukin‐17/interleukin‐6 activation in anti‐N‐methyl‐d‐aspartate receptor encephalitis. J Neuroimmunol 2016; 297: 141–147. [DOI] [PubMed] [Google Scholar]
- 41. Kothur K, Wienholt L, Mohammad SS et al Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating. Anti‐NMDAR and enteroviral encephalitis. PLoS One 2016; 11: e0161656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang HH, Dai YQ, Qiu W et al Interleukin‐17‐secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci 2011; 18: 1313–1317. [DOI] [PubMed] [Google Scholar]
- 43. Dale RC, Gorman MP, Lim M. Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr Opin Neurol 2017; 30: 334–344. [DOI] [PubMed] [Google Scholar]
- 44. Buse J, Schoenefeld K, Munchau A, Roessner V. Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci Biobehav Rev 2013; 37: 1069–1084. [DOI] [PubMed] [Google Scholar]
- 45. Felling RJ, Singer HS. Neurobiology of tourette syndrome: current status and need for further investigation. J Neurosci 2011; 31: 12387–12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodriguez N, Morer A, Gonzalez‐Navarro EA et al Altered frequencies of Th17 and Treg cells in children and adolescents with obsessive‐compulsive disorder. Brain Behav Immun 2019; 81: 608–616. [DOI] [PubMed] [Google Scholar]
- 47. Coppieters KT, Dotta F, Amirian N et al Demonstration of islet‐autoreactive CD8 T cells in insulitic lesions from recent onset and long‐term type 1 diabetes patients. J Exp Med 2012; 209: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Davies S, Nicholson T, Laura M, Giovannoni G, Altmann DM. Spread of T lymphocyte immune responses to myelin epitopes with duration of multiple sclerosis. J Neuropathol Exp Neurol 2005; 64: 371–377. [DOI] [PubMed] [Google Scholar]
- 49. Kalman B. Autoimmune encephalitides: a broadening field of treatable conditions. Neurologist 2017; 22: 1–13. [DOI] [PubMed] [Google Scholar]
- 50. Ramanathan S, Al‐Diwani A, Waters P, Irani SR. The autoantibody‐mediated encephalitides: from clinical observations to molecular pathogenesis. J Neurol 2019. 10.1007/s00415-019-09590-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chevalier N, Jarrossay D, Ho E et al CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol 2011; 186: 5556–5568. [DOI] [PubMed] [Google Scholar]
- 52. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bacher P, Scheffold A. Flow‐cytometric analysis of rare antigen‐specific T cells. Cytometry Part A 2013; 83A: 692–701. [DOI] [PubMed] [Google Scholar]
- 54. Dan JM, Arlehamn CSL, Weiskopf D et al A cytokine‐independent approach to identify antigen‐specific human germinal center T follicular helper cells and rare antigen‐specific CD4+ T cells in blood. J Immunol 2016; 197: 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reiss S, Baxter AE, Cirelli KM et al Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen‐specific CD4 T cells. PLoS One 2017; 12: e0186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Velickovic ZM, Carter JM. HLA‐DPA1 and DPB1 polymorphism in four Pacific Islands populations determined by sequencing based typing. Tissue Antigens 2001; 57: 493–501. [DOI] [PubMed] [Google Scholar]
- 57. Hu W, Tang L, Wang J et al Polymorphism of HLA‐DRB1, ‐DQB1 and ‐DPB1 genes in Bai ethnic group in southwestern China. Tissue Antigens 2008; 72: 474–477. [DOI] [PubMed] [Google Scholar]
- 58. Tracey MC, Carter JM. Class II HLA allele polymorphism: DRB1, DQB1 and DPB1 alleles and haplotypes in the New Zealand Maori population. Tissue Antigens 2006; 68: 297–302. [DOI] [PubMed] [Google Scholar]
- 59. Wang B, Hu W, Wang J et al HLA‐DPB1 polymorphism in Blang and Puyi ethnic groups of Southwest China inferred from sequence‐based typing. Tissue Antigens 2008; 71: 81–84. [DOI] [PubMed] [Google Scholar]
- 60. Williams RC, Knowler WC, Shuldiner AR et al Next generation sequencing and the classical HLA loci in full heritage Pima Indians of Arizona: defining the core HLA variation for North American Paleo‐Indians. Hum Immunol 2019; 80: 955–965. [DOI] [PubMed] [Google Scholar]
- 61. Skibola CF, Akers NK, Conde L et al Multi‐locus HLA class I and II allele and haplotype associations with follicular lymphoma. Tissue Antigens 2012; 79: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Binks S, Varley J, Lee W et al Distinct HLA associations of LGI1 and CASPR2‐antibody diseases. Brain 2018; 141: 2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shu Y, Qiu W, Zheng J et al HLA class II allele DRB1*16:02 is associated with anti‐NMDAR encephalitis. J Neurol Neurosurg Psychiatry 2019; 90: 652–658. [DOI] [PubMed] [Google Scholar]
- 64. van Sonderen A, Roelen DL, Stoop JA et al Anti‐LGI1 encephalitis is strongly associated with HLA‐DR7 and HLA‐DRB4. Ann Neurol 2017; 81: 193–198. [DOI] [PubMed] [Google Scholar]
- 65. Brucklacher‐Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009; 132: 3329–3341. [DOI] [PubMed] [Google Scholar]
- 66. Michael BD, Griffiths MJ, Granerod J et al Characteristic cytokine and chemokine profiles in encephalitis of infectious, immune‐mediated, and unknown aetiology. PLoS One 2016; 11: e0146288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huppert J, Closhen D, Croxford A et al Cellular mechanisms of IL‐17‐induced blood‐brain barrier disruption. FASEB J 2010; 24: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 68. Amenta F, Bronzetti E, Felici L, Ricci A, Tayebati SK. Dopamine D2‐like receptors on human peripheral blood lymphocytes: a radioligand binding assay and immunocytochemical study. J Auton Pharmacol 1999; 19: 151–159. [DOI] [PubMed] [Google Scholar]
- 69. Keren A, Gilhar A, Ullmann Y et al Instantaneous depolarization of T cells via dopamine receptors, and inhibition of activated T cells of Psoriasis patients and inflamed human skin, by D1‐like receptor agonist: fenoldopam. Immunology 2019; 158: 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kustrimovic N, Rasini E, Legnaro M, Marino F, Cosentino M. Expression of dopaminergic receptors on human CD4+ T lymphocytes: flow cytometric analysis of naive and memory subsets and relevance for the neuroimmunology of neurodegenerative disease. J Neuroimmune Pharmacol 2014; 9: 302–312. [DOI] [PubMed] [Google Scholar]
- 71. McKenna F, McLaughlin PJ, Lewis BJ et al Dopamine receptor expression on human T‐ and B‐lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol 2002; 132: 34–40. [DOI] [PubMed] [Google Scholar]
- 72. Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T‐cells and triggers the selective secretion of either IL‐10, TNFα or both. J Neuroimmunol 2005; 169: 161–171. [DOI] [PubMed] [Google Scholar]
- 73. Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates β1 integrin function. Eur J Immunol 2001; 31: 3504–3512. [DOI] [PubMed] [Google Scholar]
- 74. Saussez S, Laumbacher B, Chantrain G et al Towards Neuroimmunotherapy for Cancer: the neurotransmitters glutamate, dopamine and GnRH‐II augment substantially the ability of T cells of few Head and Neck cancer patients to perform spontaneous migration, chemotactic migration and migration towards the autologous tumor, and also elevate markedly the expression of CD3zeta and CD3epsilon TCR‐associated chains. J Neural Transm 2014; 121: 1007–1027. [DOI] [PubMed] [Google Scholar]
- 75. Boneberg EM, von Seydlitz E, Propster K, Watzl H, Rockstroh B, Illges H. D3 dopamine receptor mRNA is elevated in T cells of schizophrenic patients whereas D4 dopamine receptor mRNA is reduced in CD4+ ‐T cells. J Neuroimmunol 2006; 173: 180–187. [DOI] [PubMed] [Google Scholar]
- 76. Ferrari M, Termine C, Franciotta D et al Dopaminergic receptor D5 mRNA expression is increased in circulating lymphocytes of Tourette syndrome patients. J Psychiatr Res 2008; 43: 24–29. [DOI] [PubMed] [Google Scholar]
- 77. Ilani T, Ben‐Shachar D, Strous RD et al A peripheral marker for schizophrenia: increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci USA 2001; 98: 625–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson's disease. Neurology 1996; 46: 791–795. [DOI] [PubMed] [Google Scholar]
- 79. Wandinger KP, Hagenah JM, Kluter H, Rothermundt M, Peters M, Vieregge P. Effects of amantadine treatment on in vitro production of interleukin‐2 in de‐novo patients with idiopathic Parkinson's disease. J Neuroimmunol 1999; 98: 214–220. [DOI] [PubMed] [Google Scholar]
- 80. Farkas AM, Panea C, Goto Y et al Induction of Th17 cells by segmented filamentous bacteria in the murine intestine. J Immunol Methods 2015; 421: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cunningham MW. Rheumatic fever, autoimmunity, and molecular mimicry: the streptococcal connection. Int Rev Immunol 2014; 33: 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody‐mediated neuronal cell signaling in Sydenham chorea. Nat Med 2003; 9: 914–920. [DOI] [PubMed] [Google Scholar]
- 83. Labrie V, Brundin L. Harbingers of mental disease‐infections associated with an increased risk for neuropsychiatric illness in children. JAMA Psychiatry 2019; 76: 237–238. [DOI] [PubMed] [Google Scholar]
- 84. Carpanini SM, Torvell M, Morgan BP. Therapeutic inhibition of the complement system in diseases of the central nervous system. Front Immunol 2019; 10: e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hakobyan S, Boyajyan A, Sim RB. Classical pathway complement activity in schizophrenia. Neurosci Lett 2005; 374: 35–37. [DOI] [PubMed] [Google Scholar]
- 86. Lennington JB, Coppola G, Kataoka‐Sasaki Y et al Transcriptome analysis of the human striatum in tourette syndrome. Biol Psychiatry 2016; 79: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shastri A, Bonifati DM, Kishore U. Innate immunity and neuroinflammation. Mediators Inflamm 2013; 2013: 342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 2004; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shin Y‐W, Lee S‐T, Park K‐I et al Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord 2017; 11: e1756285617722347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Steward‐Tharp SM, Song Y‐j, Siegel RM, O'Shea JJ. New insights into T cell biology and T cell‐directed therapy for autoimmunity, inflammation, and immunosuppression. Ann N Y Acad Sci 2010; 1183: 123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ayzenberg I, Kleiter I, Schroder A et al Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti‐CD20 therapy. JAMA Neurol 2013; 70: 394–397. [DOI] [PubMed] [Google Scholar]
- 92. Kieseier BC, Stuve O, Dehmel T et al Disease amelioration with tocilizumab in a treatment‐resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol 2013; 70: 390–393. [DOI] [PubMed] [Google Scholar]
- 93. Lee WJ, Lee ST, Moon J et al Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort Study. Neurotherapeutics 2016; 13: 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Randell RL, Adams AV, Van Mater H. Tocilizumab in refractory autoimmune encephalitis: a series of pediatric cases. Pediatr Neurol 2018; 86: 66–68. [DOI] [PubMed] [Google Scholar]
- 95. Jiang J, Liu C, Liu M et al OX40 signaling is involved in the autoactivation of CD4+CD28‐ T cells and contributes to the pathogenesis of autoimmune arthritis. Arthritis Res Ther 2017; 19: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nohara C, Akiba H, Nakajima A et al Amelioration of experimental autoimmune encephalomyelitis with anti‐OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol 2001; 166: 2108–2115. [DOI] [PubMed] [Google Scholar]
- 97. Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. Lancet Psychiatry 2017; 4: 339–346. [DOI] [PubMed] [Google Scholar]
- 98. Gewitz MH, Baltimore RS, Tani LY et al Revision of the jones criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography. Circulation 2015; 131: 1806–1818. [DOI] [PubMed] [Google Scholar]
- 99. Cardoso F, Eduardo C, Silva AP, Mota CC. Chorea in fifty consecutive patients with rheumatic fever. Mov Disord 1997; 12: 701–703. [DOI] [PubMed] [Google Scholar]
- 100. Chang K, Frankovich J, Cooperstock M et al Clinical evaluation of youth with pediatric acute‐onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol 2015; 25: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tong WWY, Shepherd K, Garland S et al Human papillomavirus 16‐specific T‐cell responses and spontaneous regression of anal high‐grade squamous intraepithelial lesions. J Infect Dis 2015; 211: 405–415. [DOI] [PubMed] [Google Scholar]
- 102. Fleri W, Paul S, Dhanda SK et al The immune epitope database and analysis resource in epitope discovery and synthetic vaccine design. Front Immunol 2017; 8: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tea F, Pilli D, Ramanathan S et al Effects of the positive threshold and data analysis on human MOG antibody detection by live flow cytometry. Front Immunol 2020; 11: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


