Through flow cytometry and qPCR technology, the promoter vector for high expression of eGFP protein in HEK293F cells was optimized and verified with hepatitis B surface antigen. The CAG promoter vector can not only effectively increase and maintain the expression level of recombinant proteins, but also increase the expression of transgenes under low temperature conditions.

Keywords: CAG, expression vector, HEK293F cells, promoter, transgene expression
Abstract
The vast majority of therapeutic recombinant proteins are produced in mammalian cell lines. However, proteins generated in nonhuman cell lines, such as Chinese hamster ovary (CHO) cells, are decorated with human‐like glycan structures that differ from those of human cells, and these may induce immunogenic responses in human cells. Human embryonic kidney cells (HEK293F) are also extensively used as hosts for the expression of recombinant therapeutic proteins, but their utility is limited by the low expression of transgenes in these cells. Here, we investigated recombinant protein expression from eight frequently used promoters in transfected HEK293F cells. The expression levels and stability of the transgenes were evaluated by flow cytometry and qRT‐PCR. The most efficient expression (in terms of both mRNA and protein yields) was achieved using a cytomegalovirus (CMV) major immediate‐early enhancer combined with the chicken beta‐actin promoter (CAG) promoter, as compared to all other tested promoters under both transient and stable transfection conditions. In addition, application of mild hypothermia (i.e., 33 °C) after transfection improved the positive effect of the CMV enhancer fused to the chicken beta‐actin promoter (CAG promoter) on enhanced green fluorescent protein (eGFP) expression. Although the temperature sensitivity of the CMV promoter is greater than that of CAG promoter, recombinant protein levels were still highest when expression was driven by the CAG promoter. When eGFP was replaced with hepatitis B surface antigen, the CAG promoter still showed the highest transgene expression. In conclusion, our data show that the CAG promoter is a strong promoter for recombinant protein expression in HEK293F cells.
Abbreviations
- CAG promoter
cytomegalovirus (CMV) enhancer fused to the chicken beta‐actin promoter
- CHEF‐1α
Chinese hamster elongation factor‐1α
- CHO
Chinese hamster ovary
- CMV
cytomegalovirus
- eGFP
enhanced green fluorescent protein
- HBsAg
hepatitis B surface antigen
- HEF‐1α
human elongation factor‐1α
- HEK
human embryonic kidney
- MAR
matrix attachment region
- MFI
median fluorescence intensity
- PGK
phosphoglycerate kinase
- POI
protein of interest
- SV40
Simian virus 40
Approximately 80% of recombinant therapeutic proteins and antibodies are produced in mammalian cells due to the requirement for post‐translational modifications that are similar to those in human cells [1, 2, 3, 4]. Several cell lines are commonly used to produce recombinant therapeutic proteins, including Chinese hamster ovary (CHO) cells, human embryonic kidney (HEK)293 cells, and SP2/0 and NS0 mouse myeloma cells [5]. However, recombinant proteins produced in nonhuman mammalian cells are decorated with two human‐like glycan structures that differ from those of human cells, namely N‐glycolylneuraminic acid and galactose‐alpha‐1,3‐galactose group. In human cells, these proteins can be immunogenic, as antibodies are sometimes produced against these two glycan structures [6, 7]. This immunogenicity can be avoided by producing recombinant proteins in HEK293F cells [8, 9]. In addition, it is easy to rapidly produce recombinant proteins through transient expression using HEK293F cells [10, 11, 12, 13].
Although HEK293 cells have some merits in terms of the production of recombinant therapeutic proteins, low protein yield is an issue that needs to be resolved. One effective strategy to improve transgene expression levels is optimization of the expression vector components [14, 15, 16, 17]. The human elongation factor‐1α (HEF‐1α) [18, 19], mouse phosphoglycerate kinase (PGK), and CMV promoter [20] as well as other natural promoters have been used to drive recombinant protein expression in mammalian cell lines [21]. However, the yield of transgenes driven by the CMV promoter decreased with culture time due to complex transcriptional silencing and DNA methylation issues [22, 23]. In addition to natural promoters, artificial promoters have also been used to promote stable transgene expression. For example, the cytomegalovirus (CMV) enhancer fused to the chicken beta‐actin promoter (CAG) promoter is a robust artificial construct composed of a CMV enhancer combined with the chicken‐actin promoter [24].
Studies have shown that the use of low culture temperatures is an effective strategy for improving the expression of foreign proteins [25]. Reducing the cell culture temperature after transfection can further enhance the yields of recombinant proteins in CHO cells [26, 27, 28]. Although the mechanism underlying this phenomenon is poorly understood, at low temperatures, the S100 calcium‐binding protein A6 promoter showed higher expression levels than the Simian virus 40 (SV40) promoter [29]. In HEK293F cells, lower culture temperatures have a similar effect on transgene expression [30].
Here, we investigated the impact of eight promoters, CAG, HEF‐1α, CMV mutant, Chinese hamster elongation factor‐1α (CHEF‐1α), CAG enhancer, mouse CMV, PGK, and CMV, on transgene expression in HEK293F cells. We further explored the effect of mild low‐temperature culture on the expression of recombinant protein in transfected HEK293 cells.
Materials and methods
Vector construction
The pEGFP‐eGFP‐matrix attachment region (MAR) plasmid, which contains the CMV promoter and MAR element, was previously constructed in our laboratory [31, 32, 33, 34, 35, 36]. The CAG (GenBank accession no: GU299216.1, position 3–1664), HEF‐1α (accession no: AY188393.1, position 10964–12623), CMV mutant, CHEF‐1α (accession no: KY447299.1, position 12–1346), CAG enhancer (accession no: AJ575208.1, position 100–386), mouse CMV (accession no: KT343252.1, position 1103–1625), and PGK (accession no: KJ175229.1, position 641–1195) [37] promoters were synthesized and used to replace the CMV promoter in the pEGFP‐eGFP vector (Fig. 1).
Fig. 1.
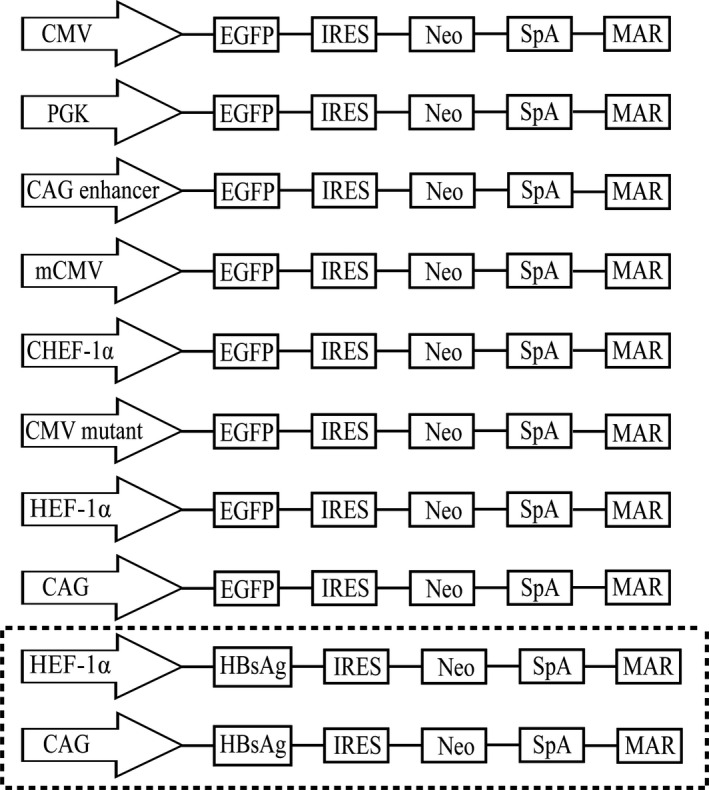
Schematic representation of vectors containing different promoters, CMV mutant, and CAG enhancer element. The constructed vectors containing different elements were used in this study.
To evaluate the effect of each promoter on the expression of a protein of interest (POI), the enhanced green fluorescent protein (eGFP) gene was replaced with hepatitis B surface antigen (HBsAg) (accession no: ABY65392.1) in vectors containing the CMV enhancer and CHEF1‐α (Fig. 1). A codon‐optimized HBsAg gene was synthesized and then inserted into the pIRES‐neo vector.
Cell culture and transfection
HEK293F cells were cultured in Dulbecco's Modified Eagle's medium/12 (ProteinEasy, Xinxiang, China) supplemented with 10% FBS (ProteinEasy) and 1% penicillin and streptomycin (ProteinEasy) in a humidified incubator at 37 °C with 5% CO2. Cells were seeded at a density of 2 × 105 cells/well into 24‐well plates and incubated overnight. The next day, when the cells reached 80% confluence, they were transfected with the vectors mentioned above with Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Approximately 48 h after transfection, the cells were screened and cultured in medium supplemented with blasticidin (Sangon Biotech, Shanghai, China) for ~ 2 weeks and then collected for analysis.
To further increase the expression of eGFP, cells were moved from 37 to 33 °C after transient transfection. Seven days later, eGFP was detected by flow cytometry to analyze the protein expression yield under mild, low‐temperature conditions. High‐expression vectors containing HBsAg were transfected in the same way as the vectors containing eGFP. At 48 h post‐transfection, the cultures were centrifuged to pellet cells, which were transferred to serum‐free medium (ProteinEasy) in a CO2 isothermal shaking incubator. After 5 days, the supernatant was collected by centrifugation, and the following analysis was performed.
Transfection efficiency and transient expression analysis
Transfection efficiency was assessed by eGFP antibody labeling. At 48 h after transfection, HEK293F cells were resuspended with a prechilled GFP antibody suspension to determine the percentage of cells that were successfully transfected, that is, the transfection efficiency, which was expressed as the ratio of eGFP‐positive cells to the total cells. To accurately analyze transient expression levels, HEK293F cells were collected, and the green fluorescence of the cells was observed at 200× magnification using a 530/15 bandpass filter at an emission wavelength of 530 nm and an excitation length of 480 nm, and the median fluorescence intensity (MFI) of eGFP was evaluated using a Guava EasyCyte 8HT flow cytometer (Millipore Sigma, Darmstadt, Germany). Fluorescence intensity was positively correlated with the promoter's driving effect on the expression of eGFP protein.
Stable expression analysis
At 48 h post‐transfection, cells were passaged and the transfectants were stabilized by incubation with 20 μg·mL−1 blasticidin. After 14 and 30 days of incubation, the fluorescence intensity of the cells was observed under a fluorescence microscope, and eGFP expression was assessed by flow cytometry using a Guava EasyCyte™ 8HT flow cytometer and flow jo software (version 7.6; Tree Star, Ashland, OR, USA).
At 48 h after transfection, when the HEK293F cells transfected with the HBsAg‐containing vectors reached 90% confluence, the cells were harvested with 0.25% trypsin/EDTA and transferred to protein‐free, serum‐free HEK293F suspension medium (ProteinEasy). The appropriate concentration of blasticidin was added, and the cells were placed in a 125 mL Corning shake flask (Sigma # 431255) containing 20 mL of culture medium and incubated for 14 days. A population of stably transfected cells was cultured, and when the cell density reached 8 × 106/mL, the supernatant was collected to measure the expression levels of HBsAg. All experiments were performed in triplicate.
RNA extraction and quantitative real‐time PCR
Total RNA was extracted from each vector‐transfected cell line and was reverse transcribed using a cDNA Synthesis Kit (ProteinEasy) according to the manufacturer’s instructions. The PCR primers used for GAPDH [38] were as follows: (forward, 5′‐GAGAGACCCTCACTGCTG‐3′ and reverse, 5′‐GATGGTACATGACAAGGTGC‐3′; HBsAg forward, 5′‐TTGGCCAAAATTCG‐CAGTCC‐3′ and reverse, 5′‐TGAGGCATAGCAGCAGGATG‐3′. PCR was carried out according to standard procedures, and qRT‐PCR was performed in a PikoReal™ 2.2 Real‐Time PCR system (Thermo Scientific, Waltham, MA, USA).
Western blot analysis
When the cell density in the shake flask reached 6 × 106/mL, the supernatant was collected, and HBsAg expression was analyzed by immunoblotting, and a small amount of each sample was saved for ELISA. The supernatant was mixed with 5× SDS sample buffer and boiled for 10 min. Then, a 10 µL aliquot of each sample was separated by SDS/PAGE on a 15% gel and transferred to a nitrocellulose membrane by electroblotting. The membrane was incubated with a 1 : 1000 dilution of anti‐Hepatitis B virus surface antigen antibody (ab9193; Abcam Inc., Cambridge, MA, USA) followed by a 1 : 5000 dilution of a secondary goat anti‐horse antibody conjugated to alkaline phosphatase (Jackson Immuno Research Laboratory, West Grove, PA, USA). Densitometric analysis was performed using imagej v2.1.4.7 software (National Institutes of Health, Bethesda, MD, USA).
ELISA
The cell supernatant was collected, and HBsAg production was determined by using an ELISA kit according to the manufacturer’s instructions (Meimian Biotechnology, Yancheng, Jiangsu, China). Then, the absorbance values of the treated Microlon ELISA plates were detected with a microplate reader (Synergy HT; BIOTEK, Broadview, IL, USA).
Statistical analysis
All experimental data were analyzed using spss 18.0 software (SPSS Inc., Chicago, IL, USA). Data are reported as mean ± SD. All experiments were performed three times, and t‐tests were used for comparisons. Differences with P values < 0.05 were considered statistically significant.
Results
Transfection efficiency and transient transgene expression
The transfection efficiency of the eight promoter constructs in HEK293 cells was evaluated. Vectors containing the CAG promoter had the highest transfection efficiency (81.3%), followed by HEF1‐α (75.1%), CMV mutant (67.7%), CHEF1‐α (63.4%), and CMV (59.1%; Fig. 2B). The lengths of the CAG, HEF1‐α, CMV mutant, CHEF‐1α, CAG enhancer, mouse CMV, PGK, and CMV promoter inserts were 1662, 1659, 589, 1335, 287, 523, 555, and 589 bp, respectively. Given that each vector contains different cis‐acting elements, their lengths are different. Therefore, we evaluated the correlation between vector length and transfection efficiency. The results showed that the transfection efficiency did not vary with the size of the vectors, indicating that the length of the promoter is not the most crucial factor affecting transfection efficiency.
Fig. 2.
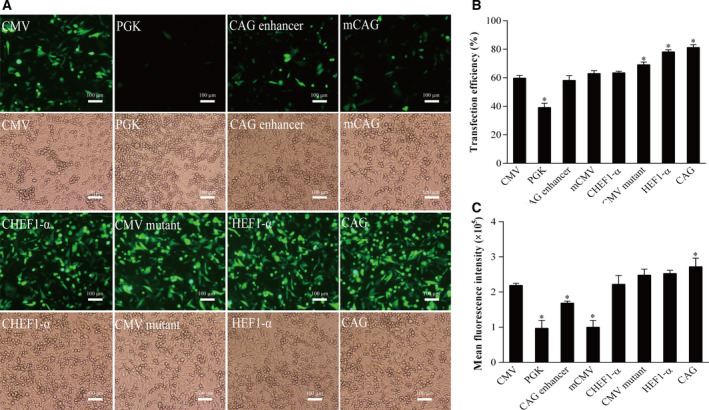
The transient expression of eGFP of the vectors containing different promoter elements in HEK293F cells. (A) The fluorescence spectrum of eGFP was observed after transfection for 48 h under the fluorescence microscope, scale bars: 100 μm. (B) Transfection efficiency. Transfection efficiency was detected using eGFP antibody analysis. (C) Transient eGFP expression was measured by flow cytometry 48 h post‐transfection. The eGFP MFI of transient transfected cells containing different promoters were detected by flow cytometry. All the experiments were repeated three times. SEM is indicated (Student's t‐test, *P < 0.05).
Next, the transient transgene expression levels of eGFP from the eight promoters constructs was evaluated. At 48 h after transfection, the eGFP fluorescence intensity of HEK293F cells transfected with vectors containing eGFP under the control of various promoters was observed under a fluorescence microscope (Fig. 2A). The results indicated that the CAG, HEF‐1α, CHEF‐1α, and CMV mutant promoters enhanced GFP transgene fluorescence intensity compared to that from the CMV promoter. Of the eight tested promoters, the CAG promoter showed the strongest fluorescence. The MFI was detected by flow cytometry, and the results indicated that compared with the CMV promoter, the fluorescence enhancement was highest for the CAG promoter (Fig. 2C), followed by HEF1‐α, CMV mutant, CHEF‐1α, and CAG enhancer. In addition, the expression levels from the mouse CMV and PGK vectors were lower than that from the CMV promoter. The driving effect of the CAG promoter on transgene expression was significantly higher than that of the other seven promoters.
Recombinant protein expression levels in stably transfected cells
We tested different concentrations of blasticidin to select the appropriate concentration for screening stably transfected HEK293F cells and found that 20 μg·mL−1 was an effective concentration. After 14 days of blasticidin selection, stably transfected cell lines were obtained. On the 14th and 30th days (Fig. 3A), stable eGFP expression from each promoter vector was detected by fluorescence microscopy and flow cytometry. The results of the fluorescence analysis indicated that CAG and HEF1‐α showed the highest fluorescence after 14 and 30 days of blasticidin selection, followed by CMV mutant and CMV, while fluorescence from CHEF1‐α, CAG enhancer, mouse CMV, and PGK was weaker. The flow cytometry results showed that the CAG, HEF1‐α, CMV mutant, and CHEF1‐α promoters had a strong effect on protein expression, which was superior to that of the CAG enhancer, mouse CMV, PGK, and CMV promoters. Among them, the CAG and HEF1‐α promoters had the highest eGFP levels (Fig. 3B). Although blasticidin selection takes longer, transgene expression levels are higher. However, this was not the case for the mouse CMV and PGK promoters, which may be due to the weak driving effect of the promoter on transgene expression. Selection with blasticidin caused the cells to lose fluorescence, which is not conducive to stable recombinant protein expression.
Fig. 3.
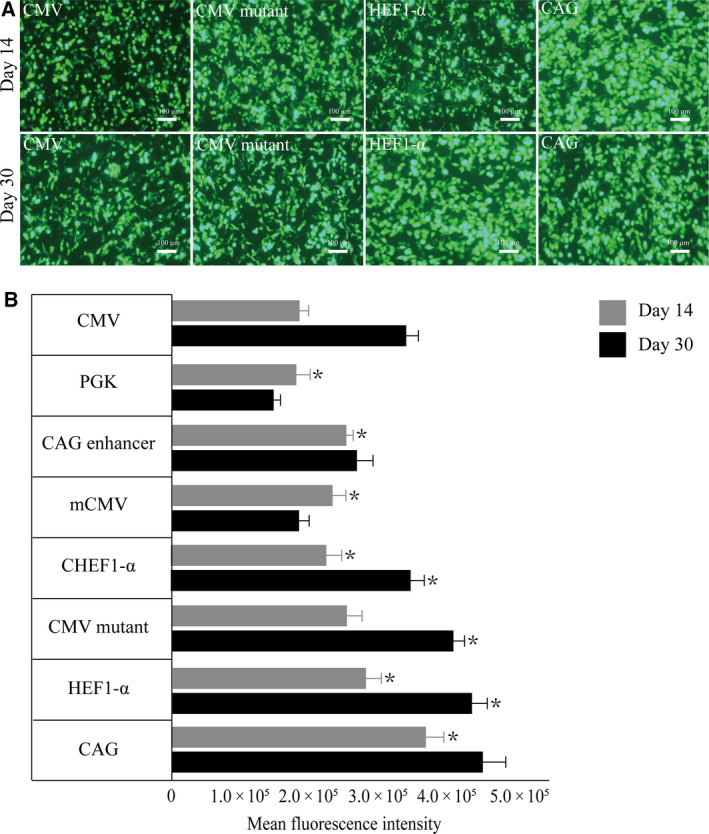
Cells were collected 14 and 30 days after transfection, respectively, and eGFP expression levels were detected by fluorescence microscopy and flow cytometry. (A) The fluorescent expression level was detected at 14 and 30 days after transfected with four high‐expression promoter: CAG, HEF1‐α, CMV mutant, and CMV, scale bars: 100 μm. (B) Flow cytometry analysis of eGFP stable expression at 14 and 30 days after transfection, and the eGFP MFI was normalized to CMV promoter. All the experiments were repeated three times. SEM is indicated (Student’s t‐test, *P < 0.05).
eGFP mRNA expression
The mRNA expression level can reflect the recombinant protein expression level. After transfecting the cells with vectors containing the CAG, HEF‐1α, CMV mutations, and CHEF‐1α promoter constructs, quantitative real‐time PCR was performed. The Ct values were determined (Fig. 4A), and mRNA expression levels were measured. The CAG promoter showed the highest eGFP mRNA expression levels, followed by the HEF‐1α, CMV mutation, and CHEF‐1α promoters (Fig. 4B). The eGFP mRNA expression levels were consistent with the protein expression levels, but the fold increase differed.
Fig. 4.
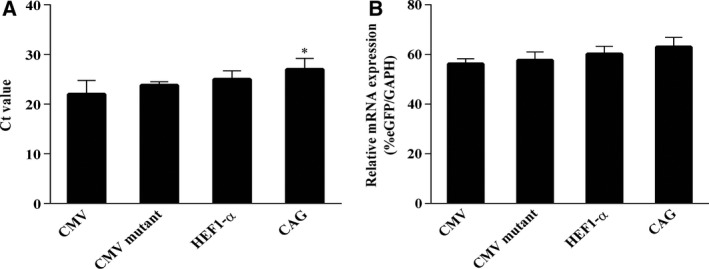
eGFP expression at mRNA level in cells transfected with CAG, HEF‐1α, CMV mutant, and CMV promoters at 14 days after transfection. (A) The mRNA expressions of report gene (eGFP) and internal reference gene (GAPDH) were measured by qRT‐PCR. (B) The mRNA expression levels of cells were calculated using the percentage of eGFP/GAPDH. All the experiments were repeated three times. SEM is indicated (Student’s t‐test, *P < 0.05).
The effects of mild low temperature on protein expression
Next, we tested whether mild low‐temperature culture conditions could enhance eGFP expression. A previous study showed that reduced expression during the initial cell growth phase at low temperature (i.e., 33 °C) was followed by a boost in expression in production phase [29]. After transient transfection of the eGFP vectors at 37 °C, the cells were immediately transferred to 33 °C and incubated for 12, 24, and 48 h, while the control was maintained at 37 °C. Since the low‐temperature culture at 33 °C grew slower than the control culture at 37 °C, the low‐temperature culture was incubated for 24 h after transfection (Fig. 5). Fluorescence microscopy showed that changing the temperature from 37 to 33 °C led to an increase in the intensity of the green fluorescence in the cells. Flow cytometry showed that the protein expression levels were higher than those in control cells after 7 days. At 48 h after transfection, the culture temperature was changed from 37 to 33 °C, and eGFP expression decreased. Therefore, we believe that changing the incubation temperature from 37 to 33 °C at 24 h after transfection can promote the expression of eGFP.
Fig. 5.
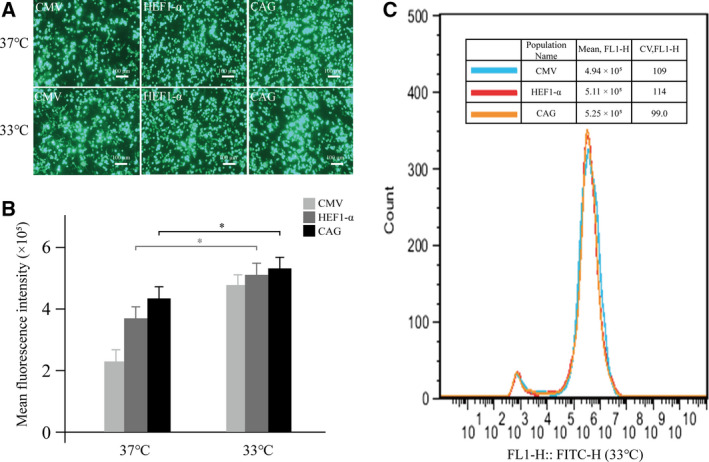
Effects of mild hypothermia on the expression of eGFP under different conditions. (A) Cells were transferred from 37 to 33 °C for 24 h and cultured for 7 days post‐transfection. The fluorescence intensity was observed under a fluorescence microscope, scale bars: 100 μm. (B) Cells were transferred from 37 to 33 °C for 7 days at 12, 24, and 48 h post‐transfection, and the expression levels of eGFP were detected by flow cytometry. (C) The eGFP MFI was determined by flow cytometry in a stably transfected cell pool with three highly expressed promoters, CAG, HEF‐α, and CMV mutant. The culture temperature was transferred from 37 to 33 °C 24 h after transfection. Cells were collected after 30 days, and eGFP fluorescence was measured by FACSCalibur. All the experiments were repeated three times. SEM is indicated (Student’s t‐test, *P < 0.05).
Expression of HBsAg
To assess the influence of the promoter on the expression of a POI, we selected HBsAg and detected its expression in HEK293F cells in serum‐free medium. Western blotting confirmed that HBsAg expression driven by the CAG promoter was higher than that driven by the HEF‐1α promoter (Fig. 6A). ELISA showed that cells transfected with the CAG‐driven vector produced the highest HBsAg levels (14.5 mg·L−1; Fig. 6B), while cells transfected with the HEF‐1α‐driven vector produced 11.4 mg·L−1 HBsAg (Fig. 6B). Cells transfected with the control vector, containing the CMV promoter, produced 9.3 mg·L−1 HBsAg, indicating the protein yield can be increased significantly by changing the promoter component of the expression vector.
Fig. 6.
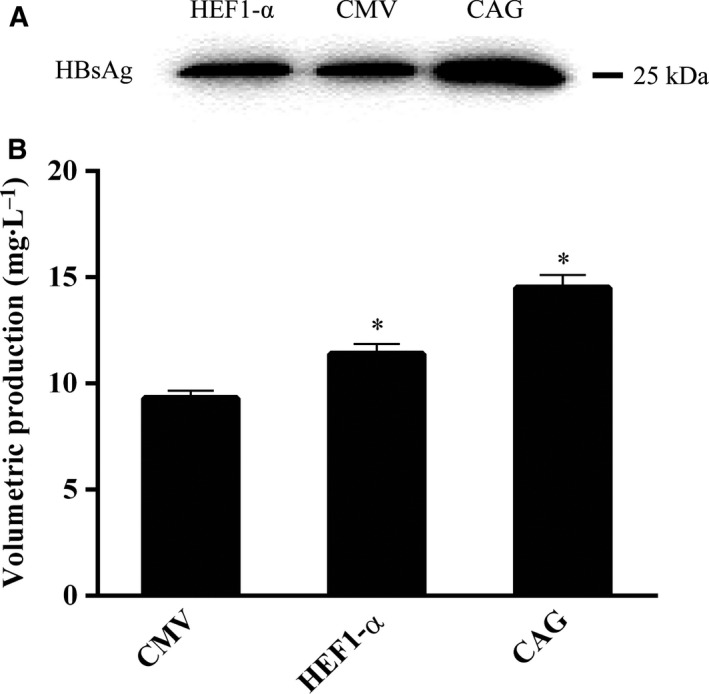
The expression of HBsAg protein. (A) The western blot of the HBsAg. (B) ELISA determined the yield of HBsAg. All the experiments were repeated three times. SEM is indicated (Student's t‐test, *P < 0.05).
Discussion
The promoter is a critical element in an expression vector, and selection of an optimal promoter can increase the expression and improve the stability of a gene of interest [39]. The CMV promoter is the most commonly used promoter for the production of recombinant proteins [40]. However, the CMV promoter has many potential methylation sites, which may lead to reduced recombinant protein production [41, 42]. In this study, the CMV promoter was used in the control vector to evaluate the transgene expression levels from seven other promoter‐driven vectors.
Of the eight promoters investigated here, the CAG enhancer was the shortest, followed by the mouse CMV and PGK promoters. Flow cytometry after transient transfection showed the highest eGFP gene expression levels from the CAG promoter but not the CAG enhancer. This indicated that the length of the promoter fragment is not the only factor affecting the efficiency of transfection. Since the CAG promoter vector had the highest transfection efficiency, in our opinion, the structure of the promoter itself is a key factor affecting transfection efficiency.
Cytomegalovirus is the most commonly used promoter for recombinant proteins, but due to presence of methylation site mutations, it lacks long‐term stability [43]. Therefore, it is crucial to find a more stable and efficient promoter. The HEF1‐α promoter can drive high levels of long‐term transgene expression in a lentiviral vector‐mediated system [44]. However, in the present study, it was not the most effective promoter. The results showed that among the eight tested commonly used promoters, the activity of the artificial CAG promoter construct was higher than the activity of the CMV and HEF‐α promoters in HEK93 cells. Expression levels of both the transient and stable eGFP transgenes driven by the CAG promoter were the highest, and the stability of the CAG vector was higher than that of the HEF1‐α and other promoter vectors.
Promoters are DNA elements that initiate the transcription of specific genes and are the key factors in determining the strength and time dynamics of transcription [28]. The results of the qRT‐PCR analysis showed that CAG drives the highest mRNA transcription levels in HEK93 cells, which were higher than the levels from other promoters such as HEF1‐α and CMV, and there was a linear relationship between eGFP mRNA and protein expression levels, indicating that the transgene expression levels are related to the transcription levels from the promoter vectors.
It has been reported that lowering the culture temperature to increase protein expression levels does not affect the function and activity of the recombinant protein [45]. We found that reducing the temperature of the culture from 37 to 33 °C at 24 h after transient transfection can effectively increase the expression level of recombinant proteins from promoter vectors in HEK293F cells. Flow cytometry showed that among the eight tested promoter vectors, low‐temperature treatment had the most obvious effect on transgene expression from the CMV promoter when compared with that of the control vector without low‐temperature treatment. However, the MFI of the CMV vector was still lower than that of the CAG vector. Based on this, we speculate that the CMV promoter was more temperature sensitive than the other promoters, but the CAG vector had the highest transgene expression level. Although low temperature can increase the expression levels of various recombinant proteins, it reduces the cell growth rate and inhibits cell division [46], so the culture temperature cannot be lower than 33 °C. In addition, the cells cannot be transferred to a low‐temperature environment too soon after transfection. This approach may be particularly useful when it is challenging to use chemical‐based expression methods for recombinant proteins.
To further examine the function of a strong promoter on a POI, we investigated the expression of HBsAg driven by the CAG and HEF1‐α promoters in serum‐free medium. The results indicated that the level of HBsAg produced by the CAG promoter was markedly higher than that produced by the CHEF1‐α promoter in HEK293 cells, further confirming the effects of the CAG promoter.
In conclusion, we first identified the impact of the CAG promoter on the expression of recombinant proteins as both transient and stable transgenes in HEK293F cells. Then, we found that a mild low temperature (i.e., 33 °C) increased the activity of the CMV and CAG promoters in HEK293F cells and increased recombinant protein productivity.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
T‐YW and XW conceived and designed the project, Y‐YD acquired the data, Y‐LJ and C‐PZ analyzed and interpreted the data, Y‐YD and YL wrote the paper.
Acknowledgements
This work was supported by the Grants from Basic Research Foundation of Key Scientific Research of Universities in Henan Province (No. 20zx013), Major Science and Technology projects of Henan Province (No. 191110311500), the Key Scientific Research Projects in Universities of Henan Province (No. 19A350008), the Key Science and Technology Project of Henan (192102310149).
Yuanyuan Dou and Yan Lin are Co‐first authors
Data Accessibility
Data and details of the analyses are available from the corresponding author upon reasonable request.
References
- 1. Zhu J (2012) Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv 30, 1158–1170. [DOI] [PubMed] [Google Scholar]
- 2. Lalonde ME and Durocher Y (2017) Therapeutic glycoprotein production in mammalian cells. J Biotechnol 251, 128–140. [DOI] [PubMed] [Google Scholar]
- 3. Xu DH, Wang XY, Jia YL, Wang TY, Tian ZW, Feng X and Zhang YN (2018) SV40 intron, a potent strong intron element that effectively increases transgene expression in transfected Chinese hamster ovary cells. J Cell Mol Med 22, 2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Datta P, Linhardt RJ and Sharfstein ST (2013) An 'omics approach towards CHO cell engineering. Biotechnol Bioeng 110, 1255–1271. [DOI] [PubMed] [Google Scholar]
- 5. Chin CL, Goh JB, Srinivasan H, Liu KI, Gowher A, Shanmugam R, Lim HL, Choo M, Tang WQ, Tan AH et al (2019) A human expression system based on HEK293 for the stable production of recombinant erythropoietin. Sci Rep 9, 16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghaderi D, Zhang M, Hurtado‐Ziola N and Varki A (2012) Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non‐human sialylation. Biotechnol Genet Eng Rev 28, 147–175. [DOI] [PubMed] [Google Scholar]
- 7. Diaz SL, Padler‐Karavani V, Ghaderi D, Hurtado‐Ziola N, Yu H, Chen X, Brinkman‐Van der Linden EC, Varki A and Varki NM (2009) Sensitive and specific detection of the non‐human sialic Acid N‐glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS One 4, e4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Go EP, Liao HX, Alam SM, Hua D, Haynes BF and Desaire H (2013) Characterization of host‐cell line specific glycosylation profiles of early transmitted/founder HIV‐1 gp120 envelope proteins. J Proteome Res 12, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fliedl L, Grillari J and Grillari‐Voglauer R (2015) Human cell lines for the production of recombinant proteins: on the horizon. N Biotechnol 32, 673–679. [DOI] [PubMed] [Google Scholar]
- 10. Yu DY, Lee SY and Lee GM (2018) Glutamine synthetase gene knockout‐human embryonic kidney 293E cells for stable production of monoclonal antibodies. Biotechnol Bioeng 115, 1367–1372. [DOI] [PubMed] [Google Scholar]
- 11. Mensah EO, Guo XY, Gao XD and Fujita M (2019) Establishment of DHFR‐deficient HEK293 cells for high yield of therapeutic glycoproteins. J Biosci Bioeng 128, 487–494. [DOI] [PubMed] [Google Scholar]
- 12. L'Abbe D, Bisson L, Gervais C, Grazzini E and Durocher Y (2018) Transient gene expression in suspension HEK293‐EBNA1 cells. Methods Mol Biol 1850, 1–16. [DOI] [PubMed] [Google Scholar]
- 13. Lee CP, Ko AM, Chiang SL, Lu CY, Tsai EM and Ko YC (2018) Regulatory elements in vectors containing the ctEF‐1alpha first intron and double enhancers for an efficient recombinant protein expression system. Sci Rep 8, 15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bayat H, Hossienzadeh S, Pourmaleki E, Ahani R and Rahimpour A (2018) Evaluation of different vector design strategies for the expression of recombinant monoclonal antibody in CHO cells. Prep Biochem Biotechnol 48, 160–164. [DOI] [PubMed] [Google Scholar]
- 15. Hagedorn C, Antoniou MN and Lipps HJ (2013) Genomic cis‐acting sequences improve expression and establishment of a nonviral vector. Mol Ther Nucleic Acids 2, e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saunders F, Sweeney B, Antoniou MN, Stephens P and Cain K (2015) Chromatin function modifying elements in an industrial antibody production platform–comparison of UCOE, MAR, STAR and cHS4 elements. PLoS One 10, e0120096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho SC, Mariati , Yeo JHM, Fang SG and Yang Y (2015) Impact of using different promoters and matrix attachment regions on recombinant protein expression level and stability in stably transfected CHO cells. Mol Biotechnol 57, 138–144. [DOI] [PubMed] [Google Scholar]
- 18. Running Deer J and Allison DS (2004) High‐level expression of proteins in mammalian cells using transcription regulatory sequences from the Chinese hamster EF‐1alpha gene. Biotechnol Prog 20, 880–889. [DOI] [PubMed] [Google Scholar]
- 19. Chan KK, Wu SM, Nissom PM, Oh SK and Choo AB (2008) Generation of high‐level stable transgene expressing human embryonic stem cell lines using Chinese hamster elongation factor‐1 alpha promoter system. Stem Cells Dev 17, 825–836. [DOI] [PubMed] [Google Scholar]
- 20. Rita Costa A, Elisa Rodrigues M, Henriques M, Azeredo J and Oliveira R (2010) Guidelines to cell engineering for monoclonal antibody production. Eur J Pharm Biopharm 74, 127–138. [DOI] [PubMed] [Google Scholar]
- 21. Dighe N, Khoury M, Mattar C, Chong M, Choolani M, Chen J, Antoniou MN and Chan JK (2014) Long‐term reproducible expression in human fetal liver hematopoietic stem cells with a UCOE‐based lentiviral vector. PLoS One 9, e104805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly LE, Martinez‐De Luna RI and El‐Hodiri HM (2016) Autoregulation of retinal homeobox (rax) gene promoter activity through a highly conserved genomic element. Genesis 54, 562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ford KL, Baumgartner K, Henricot B, Bailey AM and Foster GD (2016) A native promoter and inclusion of an intron is necessary for efficient expression of GFP or mRFP in Armillaria mellea . Sci Rep 6, 29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farokhimanesh S, Rahbarizadeh F, Rasaee MJ, Kamali A and Mashkani B (2010) Hybrid promoters directed tBid gene expression to breast cancer cells by transcriptional targeting. Biotechnol Prog 26, 505–511. [DOI] [PubMed] [Google Scholar]
- 25. Yoon SK, Kim SH and Lee GM (2003) Effect of low culture temperature on specific productivity and transcription level of anti‐4‐1BB antibody in recombinant Chinese hamster ovary cells. Biotechnol Prog 19, 1383–1386. [DOI] [PubMed] [Google Scholar]
- 26. Wang K, Zhang T, Chen J, Liu C, Tang J and Xie Q (2018) The effect of culture temperature on the aggregation of recombinant TNFR‐Fc is regulated by the PERK‐eIF2a pathway in CHO cells. Protein Pept Lett 25, 570–579. [DOI] [PubMed] [Google Scholar]
- 27. Sunley K, Tharmalingam T and Butler M (2008) CHO cells adapted to hypothermic growth produce high yields of recombinant beta‐interferon. Biotechnol Prog 24, 898–906. [DOI] [PubMed] [Google Scholar]
- 28. Estes B, Hsu YR, Tam LT, Sheng J, Stevens J and Haldankar R (2015) Uncovering methods for the prevention of protein aggregation and improvement of product quality in a transient expression system. Biotechnol Prog 31, 258–267. [DOI] [PubMed] [Google Scholar]
- 29. Thaisuchat H, Baumann M, Pontiller J, Hesse F and Ernst W (2011) Identification of a novel temperature sensitive promoter in CHO cells. BMC Biotechnol 11, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin CY, Huang Z, Wen W, Wu A, Wang C and Niu L (2015) Enhancing protein expression in HEK‐293 cells by lowering culture temperature. PLoS One 10, e0123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang TY, Yang R, Qin C, Wang L and Yang XJ (2008) Enhanced expression of transgene in CHO cells using matrix attachment region. Cell Biol Int 32, 1279–1283. [DOI] [PubMed] [Google Scholar]
- 32. Wang XY, Zhang JH, Zhang X, Sun QL, Zhao CP and Wang TY (2016) Impact of different promoters on episomal vectors harbouring characteristic motifs of matrix attachment regions. Sci Rep 6, 26446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian ZW, Xu DH, Wang TY, Wang XY, Xu HY, Zhao CP and Xu GH (2018) Identification of a potent MAR element from the human genome and assessment of its activity in stably transfected CHO cells. J Cell Mol Med 22, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang XY, Zhang JH, Sun QL, Yao ZY, Deng BG, Guo WY, Wang L, Dong WH, Wang F, Zhao CP et al (2015) Characteristic element of matrix attachment region mediates vector attachment and enhances nerve growth factor expression in Chinese hamster ovary cells. Genet Mol Res 14, 9191–9199. [DOI] [PubMed] [Google Scholar]
- 35. Wang TY, Wang L, Yang YX, Zhao CP, Jia YL, Li Q, Zhang JH, Peng YY, Wang M, Xu HY et al (2016) Cell compatibility of an eposimal vector mediated by the characteristic motifs of matrix attachment regions. Curr Gene Ther 16, 271–277. [DOI] [PubMed] [Google Scholar]
- 36. Sun QL, Zhao CP, Chen SN, Wang L and Wang TY (2016) Molecular characterization of a human matrix attachment region that improves transgene expression in CHO cells. Gene 582, 168–172. [DOI] [PubMed] [Google Scholar]
- 37. Wang W, Guo X, Li YM, Wang XY, Yang XJ, Wang YF and Wang TY (2018) Enhanced transgene expression using cis‐acting elements combined with the EF1 promoter in a mammalian expression system. Eur J Pharm Sci 123, 539–545. [DOI] [PubMed] [Google Scholar]
- 38. Song J, Zhang X, Ge Q, Yuan C, Chu L, Liang HF, Liao Z, Liu Q, Zhang Z and Zhang B (2018) CRISPR/Cas9‐mediated knockout of HBsAg inhibits proliferation and tumorigenicity of HBV‐positive hepatocellular carcinoma cells. J Cell Biochem 119, 8419–8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pham PL, Kamen A and Durocher Y (2006) Large‐scale transfection of mammalian cells for the fast production of recombinant protein. Mol Biotechnol 34, 225–237. [DOI] [PubMed] [Google Scholar]
- 40. Humby MS and O'Connor CM (2015) Human cytomegalovirus US28 is important for latent infection of hematopoietic progenitor cells. J Virol 90, 2959–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moritz B, Becker PB and Gopfert U (2015) CMV promoter mutants with a reduced propensity to productivity loss in CHO cells. Sci Rep 5, 16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin Y, Li Z, Wang T, Wang X, Wang L, Dong W, Jing C and Yang X (2015) MAR characteristic motifs mediate episomal vector in CHO cells. Gene 559, 137–143. [DOI] [PubMed] [Google Scholar]
- 43. Yang C, Shang X, Cheng L, Yang L, Liu X, Bai C, Wei Z, Hua J and Li G (2017) DNMT 1 maintains hypermethylation of CAG promoter specific region and prevents expression of exogenous gene in fat‐1 transgenic sheep. PLoS One 12, e0171442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montiel‐Equihua CA, Zhang L, Knight S, Saadeh H, Scholz S, Carmo M, Alonso‐Ferrero ME, Blundell MP, Monkeviciute A, Schulz R et al (2012) The beta‐globin locus control region in combination with the EF1alpha short promoter allows enhanced lentiviral vector‐mediated erythroid gene expression with conserved multilineage activity. Mol Ther 20, 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fujii KK, Taga Y, Sakai T, Ito S, Hattori S, Nagata K and Koide T (2019) Lowering the culture temperature corrects collagen abnormalities caused by HSP47 gene knockout. Sci Rep 9, 17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hendrick V, Winnepenninckx P, Abdelkafi C, Vandeputte O, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G and Werenne J (2001) Increased productivity of recombinant tissular plasminogen activator (t‐PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology 36, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and details of the analyses are available from the corresponding author upon reasonable request.


