Abstract
Objectives:
Streptococcus pyogenes or group A streptococcus (GAS) is a human specific pathogen that annually infects over 700 million individuals. GAS strains of type emm28 are an abundant cause of invasive infections in Europe and North America.
Methods:
We conducted a population-based study on bacteraemic emm28 GAS cases in Finland, from 1995 to 2015. Whole-genome sequencing (WGS) was used to genetically characterize the bacterial isolates. Bayesian analysis of the population structure was used to define genetic clades. Register-linkage analysis was performed to test for association of emm28 GAS with delivery- or postpartum-related infections. A genome-wide association study was used to search for DNA sequences associated with delivery or puerperal infections.
Results:
Among 3060 bacteraemic cases reported during the study period, 714 were caused by emm28. Women comprised a majority of cases (59 %, 422/714), and were significantly over-represented (84.4 %, 162/192, p < 0.0001) among cases in the childbearing age group (20–40 years). Register-linkage analysis revealed strong association (p < 0.0001) of emm28 bacteraemias with delivery and puerperium. In this register-linkage analysis, 120 women with GAS bacteraemia were identified and linked to delivery, infections during delivery or puerperium time. Among these the proportion of cases caused by emm28 was significantly higher than any other emm type (55.8%, 67/120, p < 0.0001). Among the four genetic subclades identified, SC1B has dominated among the bacteraemic cases since 2000. Altogether 620 of 653 (94.9%) isolates belonged to SC1B. No specific sequence or genetic clade was found nonrandomly associated with delivery or puerperal infections.
Conclusions:
Women of childbearing age were significantly overrepresented among bacteraemic emm28 GAS cases, and in particular were strongly associated with delivery and puerperium cases over the 21 years studied. The molecular mechanisms behind these associations are unclear and warrant further investigation.
Keywords: Bacteraemic, Group A streptococcus, Puerperal sepsis, Streptococcus pyogenes, Whole-genome sequencing
Introduction
Streptococcus pyogenes or group A streptococcus (GAS), is a strict human pathogen capable of causing a wide spectrum of infections from mild pharyngitis to severe life-threating conditions such as bacteraemia. Global morbidity and mortality caused by GAS infections is substantial, causing over 700 million infections and over 500 thousand deaths annually [1]. Although far less frequent than mild superficial infections, mortality for invasive GAS infections such as bacteraemia can be very high [2].
The major surface antigen produced by GAS is the M protein, encoded by the emm gene. GAS strains are classified into emm types and subtypes, based on DNA sequence variation in the variable region of the emm gene. At present more than 240 emm types have been recognized. The distribution of GAS emm types causing infections fluctuates geographically and temporally [3]. Population-based whole-genome sequencing (WGS) studies on GAS isolates have revealed important molecular genetic evolution in the bacterial genome that have increased the pathogenicity and enhanced the spread of newly emergent GAS clones [4,5].
In Finland, the overall incidence of invasive GAS cases has steadily increased since 1995. Importantly the incidence of invasive GAS infections has increased from 2.22 cases per 100,000 inhabitants in 2000 to 3.26/100,000 inhabitants in 2015 [6].
Emm28 strains have consistently remained among the three most prevalent emm types causing invasive GAS infections in Finland since 2003 [7–9]. Other numerically dominant emm types in invasive GAS infections since 2008 have been emm1 and emm89 [8]. A predominance of emm28 invasive GAS infections has also been observed in several other countries, including other Nordic countries, namely Sweden, Norway and Iceland [7,10–14]. For mainly unknown molecular reasons, emm28 GAS strains are frequently associated with infections in young women of childbearing age, especially in maternal postpartum infections encountered after delivery [7,15–17]. Previously, it was reported that in the age group of 30–39 years invasive GAS emm28 infections were strongly associated with women (80%, p < 0.001) [7]. In Finland the fertility rate for women in the 20- to 40-year age group is substantially higher than for younger and older age groups [18].
Recently, we performed a comprehensive WGS-based population genomic analysis of a large cohort of 2101 invasive emm28 isolates collected from six countries, including Finland [19]. The primary goal of the current study was to further analyse the Finnish isolates by investigating the population genomic data in conjunction with demographic, temporal and spatial information of the cases.
Additionally, we searched for pregnancy- or delivery-linked clinical information among bacteraemic emm28 cases and analysed it in relation to the population genomic structure. Here we report a comprehensive, population-based study of 714 GAS emm28 cases covering 21 years.
Methods
Bacterial isolates
Since 1995, all clinical microbiological laboratories from Finland are required to report each GAS isolate cultured from blood and/or cerebrospinal fluid to the National Infectious Disease Register (NIDR) maintained by the Finnish institute for health and welfare (THL) [9].
Clinical microbiological laboratories submit the corresponding GAS isolate to the National Reference Laboratory (NRL). All isolates sent to the NRL are emm typed according to the guidelines of the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA; http://www.cdc.gov/streplab/protocol-emm-type.html). Emm type has been assigned for all bacteraemic GAS cases since 2003. Before that, T-typing was used to serotype GAS isolates. Isolates assigned as T28 were emm sequenced to determine the emm type [20]. In this study, all 714 bacteremic GAS emm28 strains isolated between 1995 and 2015 from the NRL strain collection were investigated.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing (AST) was performed for 684 emm28 GAS isolates. AST was performed using the disc diffusion method according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (version 5.0). Antimicrobial susceptibility was tested against erythromycin, clindamycin, tetracycline and levofloxacin.
Data analysis
Annual incidence rates for bacteraemic emm28 cases were calculated using population data obtained from Statistic Finland, a public authority, which informs the national statistical service (https://www.stat.fi/index_en.html). To analyse for association of bacteraemic GAS isolates with infections related to delivery or puerperium, we performed a register-linkage study between NIDR and the Hospital Discharge Register maintained by THL. Included were all 267 bacteraemic GAS cases in women aged 20–40 years during years 2004–2015. For these cases, International Classification of Diseases v10 (ICD-10) codes related to delivery, or infections related to delivery or puerperium or GAS (Supplementary Table S1) within a period of 30 days before to 30 days after the isolation of a bacteremic GAS were searched for from the Hospital Discharge Register.
Chi-squared test (Prism 4, GraphPad Software) was used to compare the proportions of women with delivery or infections related to puerperium to those without. The difference was considered significant if the p-value was <0.05.
WGS and genome wide association study
DNA extraction, library preparation and WGS using an Illumina NextSeq550 instrument were performed as described previously [4]. Genome analysis was performed as described previously [19]. In brief (see Supplementary Material for detailed methods for WGS and genome-wide association study (GWAS)), after removal of artifacts and low-quality bases, base call corrected reads were aligned to emm28 reference genome MGAS6180. Single nucleotide polymorphisms (SNPs) residing in the core chromosome after correction for recombination were used to infer phylogeny and to cluster genetically related strains, as described previously [19].
Ethics
The study was conducted in accordance with the Declaration of Helsinki and national and institutional standards. Ethical committee clearance was not needed for this retrospective register-based study. Permission for this study was obtained from the Finnish Institute for Health and Welfare (THL/1121/5.05.00/2017).
Results
Invasive GAS cases
Altogether 3060 bacteraemic GAS cases were reported to NIDR from January 1995 to December 2015. Of these, 714 (714/3060, 23.3%) cases with available corresponding strains were caused by type emm28. The yearly proportions and the corresponding incidences of emm28 cases among all bacteraemic GAS cases are shown in Fig. 1. The yearly proportion of emm28 GAS cases varied between 8.8% (seven out of 80 GAS bacteraemias) in 1997 and 46.2% (55/119) in 2003.
Fig. 1.
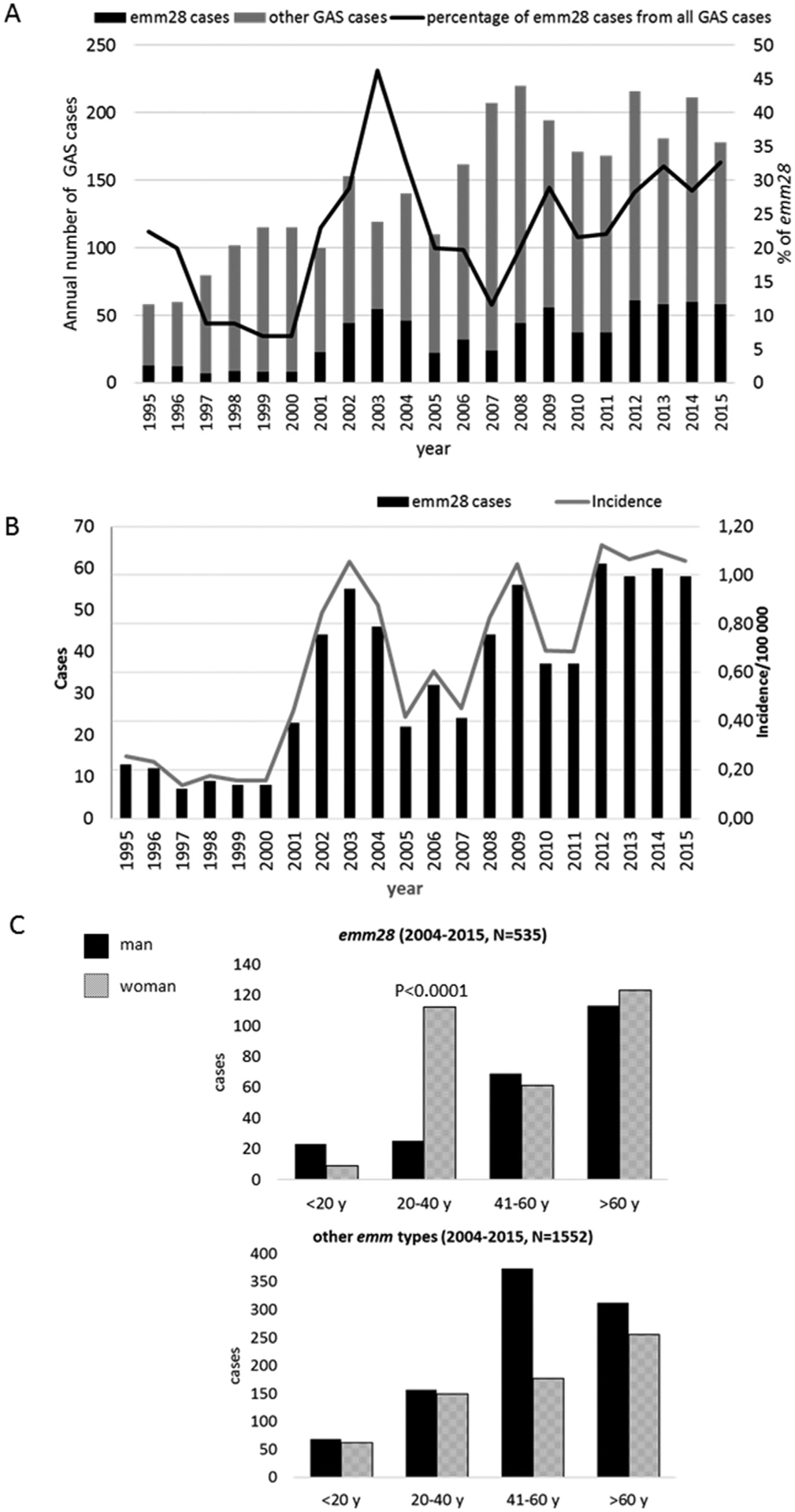
(a) Bacteraemic group A streptococcus (GAS) cases reported to the National Infectious Disease Register between 1995 and 2015, and the proportion of emm28 GAS cases in relation to all cases. Emm typing has been used as reference typing method for all GAS isolates since 2003 (n total = 3060). (b) Annual number of cases and incidence of bacteraemic emm28 GAS cases, Finland, 1995–2015 (n total = 714). (c) Total number of bacteraemic GAS cases in Finland between 2004 and 2015 by gender and age groups in type emm28 (upper part) and the rest of the emm types (lower part).
The median age of emm28 bacteraemic GAS cases was 54 years (range, 13 days to 94 years). Women accounted for 59% of cases (422 women from altogether 714 GAS cases), men 40% (283/714), and for 1% (9/714) gender data were not available. To investigate for an association of emm28 infections with women of childbearing age, we divided the cohort into four age groups (<20, 20–40, 41–60 and > 60 years). Women were significantly overrepresented (p < 0.0001) in the 20- to 40-year childbearing age group. From altogether 192 cases in this age group, 162 were women, which comprises 84.4% of the cases. In all other age groups combined (n = 512), the distribution of cases among men and women was relatively equal, altogether 252 men (252/512, 49 %) and 260 women (260/512, 51 %) (Fig. 1(c)). The same comparison was done with all non-emm28 bacteraemic GAS infections in Finland for years 2004–2015. No similar overabundance was observed (Fig. 1(c)).
Antimicrobial susceptibility testing was performed for altogether 684 emm28 GAS isolates. Resistance against all tested antibiotics remained low; erythromycin 1.9 % (n = 13), clindamycin 0.9 % (n = 6), tetracycline 0.7 % (n = 5) and levofloxacin 1.3 % (n = 9) of resistance, respectively.
Register-based linkage analysis
To explore the nature of the overabundance of bacteraemic emm28 infections among women of childbearing age, a register-based linkage analysis was performed. Specific ICD-10 codes linked to deliveries, infection related to delivery or puerperium, temporally linked to the bacteraemic GAS infection were searched (Supplementary Table S1). Altogether 267 cases were included in the analysis. These cases were caused by 30 different emm types (Table 1). Emm28 was the most frequently observed emm type, representing 42.3% (n = 113) of the cases. Of the 267 cases, 112 had an ICD-10 code indicative of a delivery and from these, in 63 cases (58%) the infection was caused by emm28 (p < 0.0001, Table 1). Among cases with ICD-10 codes for puerperal sepsis or infections related to delivery or puerperium time, emm28 was the most frequently observed emm type (55.3%, 52/94, p = 0.0015, Table 1). Searching for any of the ICD-10 codes related to delivery, infections during delivery or puerperium time, identified 120 cases, and among these the proportion caused by emm28 was highly significant (55.8%, 67/120, p < 0.0001, Table 1).
Table 1.
Register-linkage analysis was performed for all invasive GAS cases (n = 267) recorded in women aged 20–40 years between 2004 and 2015
| emm type | All n (%) | Delivery n (%) | Infections related to delivery or puerperium, n (%) | Delivery or infection related to delivery or puerperium, n (%) |
|---|---|---|---|---|
| emm28 | 113 (42.3) | 63 (56.3)* | 52 (55.3)* | 67 (55.8)* |
| emm89 | 39 (14.6) | 13 (11.6) | 13 (13.8) | 15 (12.5) |
| emm12 | 16 (6.0) | 5 (4.4) | 5 (5.3) | 7 (5.8) |
| emm1 | 22 (8.2) | 9 (8.0) | 6 (6.4) | 9 (7.5) |
| emm4 | 14 (5.2) | 9 (8.0) | 8 (8.5) | 9 (7.5) |
| emm75 | 8 (3.0) | 2 (1.8) | 2 (2.1) | 2 (1.7) |
| other emm types** | 55 (20.6) | 11 (9.8) | 8 (8.5) | 11 (9.2) |
| Total | 267 | 112 | 94 | 120 |
The Hospital Discharge Register was used to search for specific ICD-10 codes related to delivery, or infections related to delivery or puerperium (Supplementary Table S1). Table 1 summarizes the distribution of emm types in cases for which specific ICD-10 codes were recorded.
p < 0.05.
other emm types (number of cases): emm119.1 (7), emm84 (6), emm27G.6 (4), emm50 (4), emm77 (4), emm118 (3), emm22 (3), emm66 (3), emm25 (2), emm110.1 (2), emm73(2), emm81 (2), emm78.3 (2), emm104 (1), emm102.3 (1), emm11 (1), emm112.2 (1), emm33 (1), emm2 (1), emm60.1 (1), emm177 (1), emm87 (1), emm79.2 (1), emm8 (1).
WGS analysis
Genomic analysis was performed for all available Finnish bacteraemic emm28 GAS isolates (n = 714; Supplementary Table S2) from 1995 to 2015. Of these, 704 were also part of the large study of bacteraemic emm28 GAS isolates from six countries [19]. The Finnish strains were sequenced to a mean depth of 207-fold coverage (range 34- to 815-fold). To analyse the genome-wide distribution of single nucleotide polymorphisms (SNPs) and genetic relationships among the strains, core chromosomal SNPs were used. Two isolates were very distant genetic outliers and were excluded from further analysis. Among the 712 remaining isolates, in the aggregate, there were 5110 SNPs identified in the recombination-filtered core genome (that is, with SNPs acquired by horizontal gene transfer removed from the core genome). Bayesian analysis of population structure (BAPS) hierarchically clustered the strains in two major primary clades (1 and 2), and into four secondary subclades (designated SC1A, SC1B, SC2A and SC2B) [19]. A majority of the 712 Finnish strains are part of Clade 1 (n 699) of which the majority are part of SC1B (n = 628) (Fig. 2(a)). Only a minor proportion of the Finnish strains belongs to Clade 2 (n = 13). From the temporal data of isolates, it was discovered that SC1B started to predominate after year 2000 by replacing SC1A entirely (Fig. 2(a)).
Fig. 2.
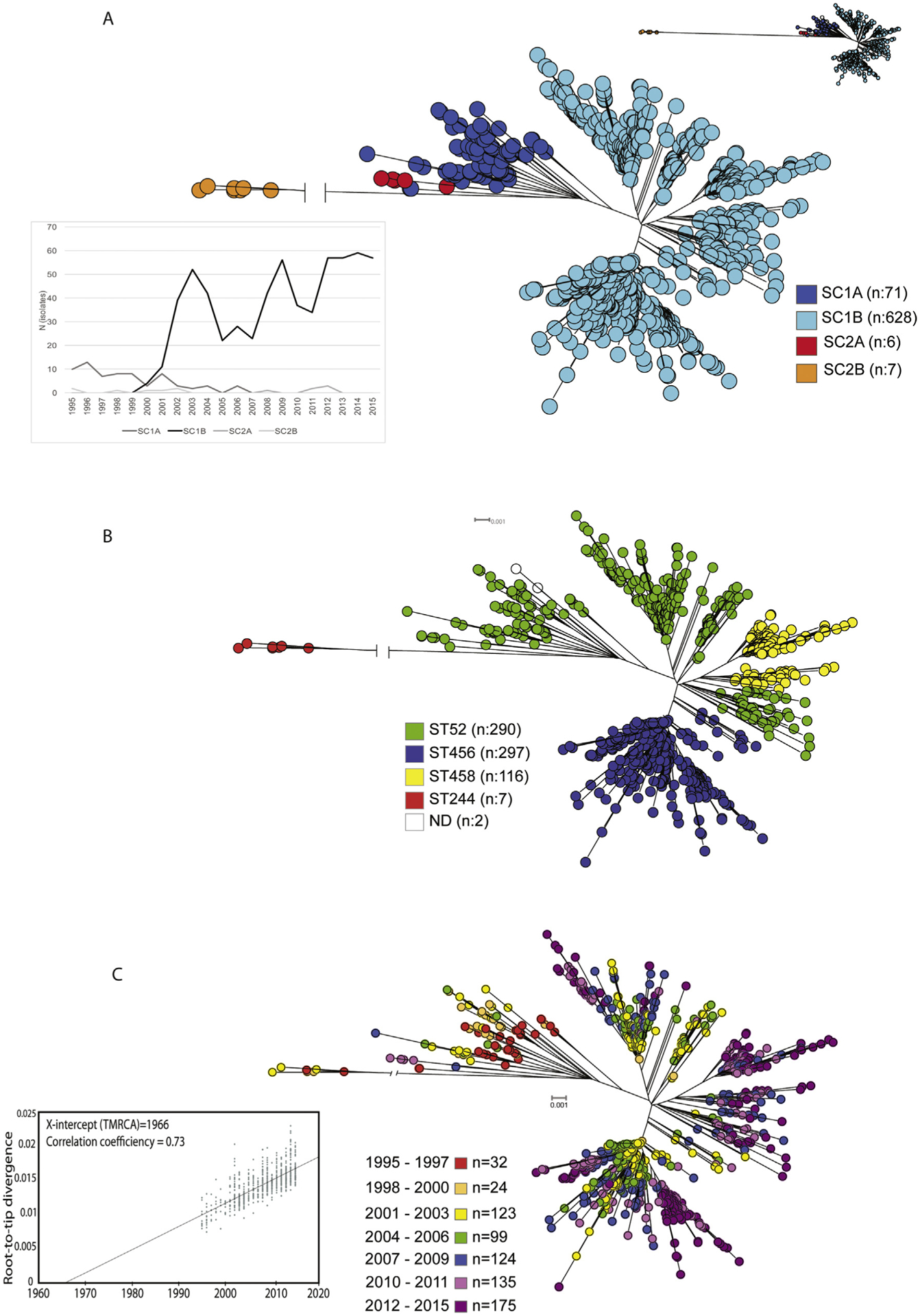
(a) Neighbour-joining tree of 712 emm28 isolates with 5110 core single nucleotide polymorphisms (SNPs). Two primary clades 1 and 2 were further divided to subclades (SC) A and B. Lower left part of the figure: expansion of SC 1B in the early 2000s. (b) Multilocus sequence typing (MLST) distribution in neighbour-joining tree of 712 emm28 group A streptococcus (GAS) isolates. (c) Temporal association of the 712 emm28 GAS isolates with isolation year in neighbour-joining tree. Left corner: a root-to-tip genetic distance for 705 Finnish isolates after excluding isolates (n = 7) with the region of recombination to constrain the inference to primarily vertically inherited SNPs. The X-intercept is the time of origin of the most recent common ancestor (TMRCA = 1966).
Multilocus sequence typing (MLST) types of the strains were determined from the WGS data. Four sequence types (STs) were discovered, ST52, ST456, ST45 and ST244, respectively (Fig. 2(b)). ST52 and ST456 predominated covering 82.4 % (n = 587) of the strains.
With our comprehensive population-based collection covering 21 years, we were able to study temporal dynamics of the population in relation to the phylogenetic tree. There was a clear temporal correlation observed with inferred phylogeny (Fig. 2(c)). A clear shift in the phylogeny is seen with the change from SC1A to SC1B in the early 2000s. Although there is a significant non-random association between GAS infections in women of childbearing age and emm28, strains isolated from women in the 20- to 40-year age group were found randomly distributed relative to the emm28 isolate phylogenetic tree (Fig. 3).
Fig. 3.
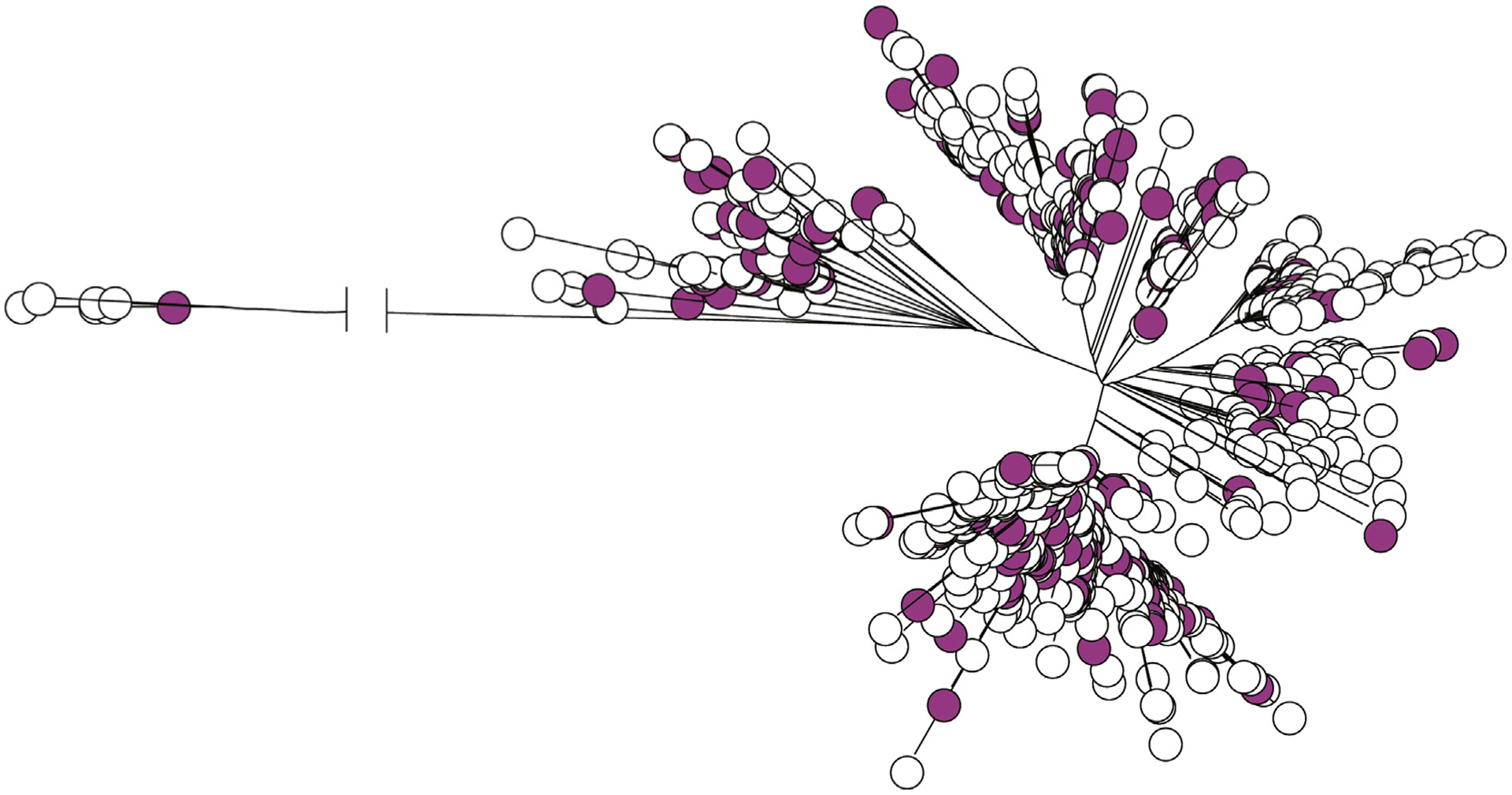
Distribution of isolates from women aged 20–40 years (magenta circles, n = 162) in the neighbour-joining tree of all 712 group A streptococcus (GAS) isolates included in the study.
We performed a further GWAS analysis on 136 de novo assembled emm28 genomes. Using this approach, no genetic content of the isolates was found significant associated with infections related to delivery or puerperium (data not shown).
Discussion
In this study we showed that in Finland, young women of childbearing age are significantly over-represented in bacteraemic S. pyogenes cases caused by emm28. With further register-linkage analysis, we observed a clear association of emm28 GAS bacteraemias with deliveries and puerperal infections in young women. In addition, with WGS data, we show that the genome of emm28 GAS has evolved during the 21 years studied.
S. pyogenes is an important human pathogen with a wide disease spectrum and global dissemination. The resurgence of invasive GAS cases has been reported in several countries [2,21]. In the early 2000s, increased incidence rates were reported especially due emm1 and emm89 [5,8,10,22,23]. In Finland, despite epidemic waves caused by emm1 and emm89, the predominant emm type causing invasive GAS infections since 2004 has been emm28 [8,24]. Our study consists of a genome-wide analysis of bacteraemic emm28 GAS isolates collected during 21 years and an in-depth analysis of invasive infections in young women of childbearing age.
Emm28 accounted almost one-fourth (23.3 %) of all invasive GAS cases reported during the study period. Previous epidemiological studies on invasive GAS cases in Finland for years 2004–2013 [8,24] found emm28 GAS as the most prevalent emm type. Similar to Finland, several other countries report emm28 GAS as one of the most common types in invasive infections [10,11].
Several predisposing factors have been described for invasive GAS infections. These include age extremes, ethnicity, underlying conditions such as heart disease [25]. In general, male gender is considered as a risk factor for invasive GAS infection [2,25]. On the contrary, in women, puerperal sepsis is a rather rare but severe disease condition, and GAS is one of the well-recognized causatives for it [16,17,26]. In a large study conducted in the USA, the incidence of pregnancy- and postpartum-related severe GAS infections were 0.2 and 0.55/1000 woman-years, respectively [26]. In the Strep-EURO study which included 11 European countries, in 4% of invasive GAS cases the disease manifestation was puerperal sepsis [7]. Similarly, in a Finnish study covering 10 years of bacteraemic GAS cases in one Health district, in 8% of the cases the presenting clinical manifestation was puerperal sepsis [27]. For postpartum women, the risk for infection caused by GAS is 20-fold higher compared with non-pregnant women [26]. In our cohort, emm28 GAS was clearly associated with delivery and infections related to delivery or puerperium (Table 1). Our result is supported by previous studies on the association of emm28 GAS with invasive postpartum infections [15,16,26].
In the WGS analysis of the isolates, SC1B was discovered as the predominant emm28 genetic lineage in Finland. The proportion of SC1B has increased also in other countries including the USA, Iceland and Norway [19]. It remains uncertain whether the displacement of SC1A with SC1B was a random effect or happened due to the effect a specific SNPs.
Previous studies on emm89 and emm1 have shown that acquisition of genetic material with horizontal gene transfer (HGT) has resulted in the increased production of secreted toxins and led to a more virulent phenotype of the bacteria [4,28]. A similar type of HGT event was discovered in emm28 GAS isolates in Clade 2, which resulted in the acquisition of 28.0-kilobase genetic block from Streptococcus dysgalactiae subspecies equisimilis that encodes secreted toxins NAD + -glycohydrolase (SPN) and streptolysin O [19]. The frequency of Clade 2 increased in the USA from early 2000 but not in other countries included in that study. Only a scarce proportion of the Finnish isolates were part of Clade 2 and all were isolated before 2000 (Fig. 2(a)).
We did not find any subset of the emm28 isolates studied or differing locations in the genome that were non-randomly associated with cases related to puerperal sepsis or delivery. This finding suggests that there is no distinct clone of the emm28 isolates studied, which would cause these specific infections.
The strength of our study is clearly the nationwide nature of the collection which includes all bacteraemic emm28 GAS cases from Finland. Our study emphasizes the observation that delivery- and postpartum-linked infections caused by GAS are a noteworthy clinical manifestation in Finland, and emm28 has a significant role in these. With our comprehensive population-based collection covering 21 years, we were able to study temporal dynamics of the population in relation to the phylogenetic tree. There are certain limitations in this study as well. Our search for clinical risk factor data in young women with GAS bacteraemia was limited only to certain disease manifestations recorded by a limited number of ICD-codes. A different type of study design might identify more cases increasing the power of the analysis. The number of strains included in the GWAS analysis was limited and it might affect the power of the analysis.
There are several factors that might contribute to the postpartum GAS infections such as altered immune status of the mother, disruption of the tissues, spread of the pathogen from an another infection site such as pharyngitis and through nosocomial exposure [29]. None the less, none of these factors as such explains the high prevalence of emm28 in the infections. The emm28 GAS genome contains an integrative conjugative element called region of difference (RD)2, which is thought to be horizontally acquired from group B streptococci (GBS) [30,31]. RD2 encodes several surface proteins, which may play a role in the tissue tropism by attaching to endometrial epithelial cells [32,33]. However, vaginal GAS colonization during pregnancy is known be to very low, less than 1% [34,35], and most likely it does not explain the prevalence of postpartum emm28 GAS infections. Asymptomatic carriage of GAS on skin or throat are more common (5–30% of the population), and community carriage might predispose to postpartum infections [29].
In conclusion, a significant association between bacteraemic emm28 GAS, young women and infections related to delivery and puerperium was identified. This is a first descriptive study of emm28 GAS bacteraemias from Finland and the results warrant further studies to identify the possible underlying mechanism behind the association of emm28 GAS with maternal infections.
Supplementary Material
Acknowledgements
The authors thank Jari Jalava and Carita Savoilainen-Kopra from the Finnish Institute for Health and Welfare for fruitful discussions and scientific advice with this study. Johanna Vilhonen and Anna Aromaa are thanked for the help to select ICD-10 codes used in this study. Minna Lamppu, Heli Laaksonen and Kai Puhakainen are acknowledged for technical support with the strains. We acknowledge all clinical microbiology laboratories for submitting isolates to National Reference Laboratory. Parts of this study were presented as a poster at the Annual meeting of the Nordic Society of Clinical Microbiology and Infectious Diseases (NSCMID) in Torshavn, Faroe Islands, 2017.
Footnotes
Transparency declaration
The authors declare that they have no conflicts of interest. This study was supported by the Nordic Society of Clinical Microbiology and Infectious Diseases (SSAC Foundation, SLS-688641, to J.V.), Academy of Finland (grant no 308482 to J.V.) and the Maud Kuistila Memorial Foundation (to K.G.Y.H), and Fondren Foundation, Houston Methodist Hospital and Research Institute, and National Institutes of Health (grants AI139369–01 and AI146771–01 to J.M.M.).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.04.004.
References
- [1].Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685–94. November. [DOI] [PubMed] [Google Scholar]
- [2].Steer AC, Lamagni T, Curtis N, Carapetis JR. Invasive group a streptococcal disease: epidemiology, pathogenesis and management. Drugs 2012;72:1213–27. June 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis 2009;9:611–6. October. [DOI] [PubMed] [Google Scholar]
- [4].Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci U S A 2014;111:E1768–76. April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Turner CE, Abbott J, Lamagni T, Holden MT, David S, Jones MD, et al. Emergence of a new highly successful acapsular Group A Streptococcus clade of genotype emm89 in the United Kingdom. MBio 2015;6. July 14 e00622–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Finnish institute for health and welfare. National infectious disease register. January 2020. Available at: https://www.thl.fi/ttr/gen/rpt/tilastot.html.
- [7].Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 2009;47:1155–65. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Smit PW, Lindholm L, Lyytikainen O, Jalava J, Patari-Sampo A, Vuopio J. Epidemiology and emm types of invasive group A streptococcal infections in Finland, 2008–2013. Eur J Clin Microbiol Infect Dis 2015;34:2131–6. October. [DOI] [PubMed] [Google Scholar]
- [9].Latronico F, Nasser W, Puhakainen K, Ollgren J, Hyyrylainen HL, Beres SB, et al. Genomic tracks behind spread of bacteremic Group A Streptococcus type emm89 in Finland, 2004–2014. J Infect Dis 2016;214:1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gherardi G, Vitali LA, Creti R. Prevalent emm types among invasive GAS in Europe and North America since year 2000. Front Public Health 2018;6:59. March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nelson GE, Pondo T, Toews KA, Farley MM, Lindegren ML, Lynfield R, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis 2016;63:478–86. August 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Naseer U, Steinbakk M, Blystad H, Caugant DA. Epidemiology of invasive group A streptococcal infections in Norway 2010–2014: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2016;35:1639–48. October. [DOI] [PubMed] [Google Scholar]
- [13].Darenberg J, Henriques-Normark B, Lepp T, Tegmark-Wisell K, Tegnell A, Widgren K. Increased incidence of invasive group A streptococcal infections in Sweden, January 2012-February 2013. Euro Surveill 2013;18:20443. April 4. [DOI] [PubMed] [Google Scholar]
- [14].Olafsdottir LB, Erlendsdóttir H, Melo-Cristino J, Weinberger DM, Ramirez M, Kristinsson KG, et al. Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Eurosurveillance 2014;19:20784. [PubMed] [Google Scholar]
- [15].Colman G, Tanna A, Efstratiou A, Gaworzewska ET. The serotypes of Streptococcus pyogenes present in Britain during 1980–1990 and their association with disease. J Med Microbiol 1993;39:165–78. September. [DOI] [PubMed] [Google Scholar]
- [16].Chuang I, Van Beneden C, Beall B, Schuchat A. Population-based surveillance for postpartum invasive group a streptococcus infections, 1995–2000. Clin Infect Dis 2002;35:665–70. September 15. [DOI] [PubMed] [Google Scholar]
- [17].Hamilton SM, Stevens DL, Bryant AE. Pregnancy-related group a streptococcal infections: temporal relationships between bacterial acquisition, infection onset, clinical findings, and outcome. Clin Infect Dis 2013;57:870–6. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roustaei Z, Raisanen S, Gissler M, Heinonen S. Fertility rates and the post-ponement of first births: a descriptive study with Finnish population data. BMJ Open 2019;9. January 15 e026336-2018–026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kachroo P, Eraso JM, Beres SB, Olsen RJ, Zhu L, Nasser W, et al. Integrated analysis of population genomics, transcriptomics and virulence provides novel insights into Streptococcus pyogenes pathogenesis. Nat Genet 2019;51:548–59. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Siljander T, Toropainen M, Muotiala A, Hoe NP, Musser JM, Vuopio-Varkila J. Emm typing of invasive T28 group A streptococci, 1995–2004, Finland. J Med Microbiol 2006;55:1701–6. December. [DOI] [PubMed] [Google Scholar]
- [21].Barnett TC, Bowen AC, Carapetis JR. The fall and rise of Group A Streptococcus diseases. Epidemiol Infect 2018:1–6. August 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beres SB, Kachroo P, Nasser W, Olsen RJ, Zhu L, Flores AR, et al. Transcriptome remodeling contributes to epidemic disease caused by the human pathogen Streptococcus pyogenes. MBio 2016;7. 10.1128/mBio.00403-16. May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beres SB, Olsen RJ, Ojeda Saavedra M, Ure R, Reynolds A, Lindsay DSJ, et al. Genome sequence analysis of emm89 Streptococcus pyogenes strains causing infections in Scotland, 2010–2016. J Med Microbiol 2017;66:1765–73. December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Siljander T, Lyytikainen O, Vahakuopus S, Snellman M, Jalava J, Vuopio J. Epidemiology, outcome and emm types of invasive group A streptococcal infections in Finland. Eur J Clin Microbiol Infect Dis 2010;29:1229–35. October. [DOI] [PubMed] [Google Scholar]
- [25].Efstratiou A, Lamagni T. Epidemiology of Streptococcus pyogenes. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations Oklahoma city (OK); 2016. [Google Scholar]
- [26].Deutscher M, Lewis M, Zell ER, Taylor TH Jr, Van Beneden C, Schrag S, et al. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis 2011;53:114–23. July 15. [DOI] [PubMed] [Google Scholar]
- [27].Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjanen J. Clinical presentations and epidemiology of beta-haemolytic streptococcal bacteraemia: a population-based study. Clin Microbiol Infect 2009;15:286–8. March. [DOI] [PubMed] [Google Scholar]
- [28].Zhu L, Olsen RJ, Nasser W, de la Riva Morales I, Musser JM. Trading capsule for increased cytotoxin production: contribution to virulence of a newly emerged clade of emm89 Streptococcus pyogenes. MBio 2015;6. October 6 e01378–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mason KL, Aronoff DM. Postpartum group A Streptococcus sepsis and maternal immunology. Am J Reprod Immunol 2012;67:91–100. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Green NM, Zhang S, Porcella SF, Nagiec MJ, Barbian KD, Beres SB, et al. Genome sequence of a serotype M28 strain of group A streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J Infect Dis 2005;192:760–70. September 1. [DOI] [PubMed] [Google Scholar]
- [31].Sitkiewicz I, Green NM, Guo N, Mereghetti L, Musser JM. Lateral gene transfer of streptococcal ICE element RD2 (region of difference 2) encoding secreted proteins. BMC Microbiol 2011;11. April 1 65-2180-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weckel A, Ahamada D, Bellais S, Mehats C, Plainvert C, Longo M, et al. The N-terminal domain of the R28 protein promotes emm28 group A Streptococcus adhesion to host cells via direct binding to three integrins. J Biol Chem 2018;293:16006–18. October 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stalhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol Microbiol 1999;33:208–19. July. [DOI] [PubMed] [Google Scholar]
- [34].Mead PB, Winn WC. Vaginal-rectal colonization with group A streptococci in late pregnancy. Infect Dis Obstet Gynecol 2000;8:217–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saab J, Bell SM, Lahra MM. Vaginal carriage rate of streptococcal pyogenes in 1600 pregnant women. Pathology 2012;44:567–8. October. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


