Abstract
Background
Myofascial pain syndrome (MPS) is an important clinical condition that is characterized by chronic muscle pain and a myofascial trigger point (MTrP) located in a taut band (TB). Previous studies showed that EphrinB1 was involved in the regulation of pathological pain via EphB1 signalling, but whether EphrinB1-EphB1 plays a role in MTrP is not clear.
Methods
The present study analysed the levels of p-EphB1/p-EphB2/p-EphB3 in biopsies of MTrPs in the trapezius muscle of 11 MPS patients and seven healthy controls using a protein microarray kit. EphrinB1-Fc was injected intramuscularly to detect EphrinB1s/EphB1s signalling in peripheral sensitization. We applied a blunt strike to the left gastrocnemius muscles (GM) and eccentric exercise for 8 weeks with 4 weeks of recovery to analyse the function of EphrinB1/EphB1 in the muscle pain model.
Results
P-EphB1, p-EphB2, and p-EphB3 expression was highly increased in human muscles with MTrPs compared to healthy muscle. EphB1 (r = 0.723, n = 11, P < 0.05), EphB2 (r = 0.610, n = 11, P < 0.05), and EphB3 levels (r = 0.670, n = 11, P < 0.05) in the MPS group were significantly correlated with the numerical rating scale (NRS) in the MTrPs. Intramuscular injection of EphrinB1-Fc produces hyperalgesia, which can be partially prevented by pre-treatment with EphB1-Fc. The p-EphB1 contents in MTrPs of MPS animals were significantly higher than that among control animals (P < 0.01). Intramuscular administration of the EphB1 inhibitor EphB1-Fr significantly suppressed mechanical hyperalgesia.
Conclusions
The present study showed that the increased expression of p-EphB1/p-EphB2/p-EphB3 was related to MTrPs in patients with MPS. This report is the first study to examine the function of EphrinB1-EphB1 signalling in primary muscle afferent neurons in MPS patients and a rat animal model. This pathway may be one of the most important and promising targets for MPS.
Keywords: Myofascial pain syndrome, myofascial trigger point, EphrinB1-EphB1
Introduction
Myofascial pain syndrome (MPS) is one of the most common chronic pain symptoms, and it affects approximately 3% of patients in the United States.1,2
The following diagnostic criteria are used for MPS: 1) a regional hypersensitive spot (MTrP) in muscle; 2) a palpable taut band (TB) in painful muscles; 3) referred pain and restricted range of motion; and 4) recognition of pressure on MTrP evoking a current pain complaint.3 MTrP and TB are particularly critical for the diagnosis and characterization of MPS.4 MTrPs are defined as hypersensitive tender spots that are identified via palpation of taut bands (TBs) in skeletal muscle fibres, and these areas are a prerequisite for MPS diagnosis and therapy.5 The current treatments for MPS include various non-invasive and invasive methods. The non-invasive treatments of MTrPs include ultrasound, compression, analgesics, and nonsteroidal anti-inflammatory drugs, and the invasive treatments of MTrPs include dry needling (acupuncture), which involves the insertion of a needle directly into an MTrP and the subsequent injection of a material, such as botulinum toxin, local anaesthetics or corticosteroids.6,7 However, the efficacy and rationale remain controversial.8 Therefore, the identification of other mechanisms of MTrPs is crucial for the development of novel effective therapies.
The Eph receptor family constitutes the largest subfamily of receptor tyrosine kinases (RTKs), and it includes 14 members divided into two subgroups, EphA and EphB.9 The Ephrin/Eph receptor system participates in the pathophysiology of many types of pain, and Eph-selective agonists and antagonists modulate different types of pain, which may hold great therapeutic potential in various pain situations.9,10
EphBs also play an important role in the regulation of muscle contraction.11,12 Many studies showed that MTrPs are characterized by abnormal sarcomere contraction. Our previous study found that the phosphorylation levels of EphB1, EphB2, and EphB3 in muscle tissue biopsied at MTrP sites in MPS patients were higher than normal controls. However, the role of peripheral EphB1, EphB2, and EphB3 and their relationship with MTrPs in MPS patients is not known.
The present study tested the hypothesis that the EphrinB1/EphB1 system was involved in peripheral mechanisms of hyperalgesia in the MTrP region. To understand EphrinB1/EphB1 signalling in the pathogenic mechanism of MPS, we collected MPS patients and tried to develop a persistent muscle pain model with TB that mimicked the condition of MPS and examined the function of EphrinB1/EphB1 in muscle hyperalgesia.
Methods
Subjects
The procedure used to recruit MPS patients (M group) and healthy controls (C group) was described in previous publications.13 Patients with MTrPs were recruited for the study at the orthopaedics department of Qilu Hospital at Shandong University, Jinan, China. Eleven participants were recruited into the M group. Non-MPS controls were recruited via advertisements posted on notice boards in the hospital and around the community, and seven participants were included in the C group. The Ethics Committee of Qilu Hospital approved the study, and written informed consent was obtained from all subjects.
Briefly, the inclusion criteria were patients with MTrPs that was diagnosed according to the international consensus criteria14 and aged between 18 and 65 years. The following exclusion criteria were used: use of any type of opioid medication or medical history of systemic inflammatory diseases; previous neck trauma or surgery in the neck/shoulder area; neuropathic pain; chronic widespread muscle pain conditions; metabolic disease; high blood pressure; malignancy; and pregnancy. Physicians with more than 20 years of clinical experience in the MPS field clinically examined all subjects to minimize the inter-examiner variability.
Pain intensity
Approximately 1 hour prior to biopsy, each subject rated their pain intensity at the MTrPs using a numerical rating scale (NRS), which is a digital scale of 11 grades (0 to 10) with two endpoints: 0 indicates no pain, and 10 indicates the most severe pain.
Sample collection and proteomic analysis
A detailed description of the sample biopsy procedure is provided in our previous publication.13 Briefly, biopsies from the MTrPs were obtained using a disposable SuperCoreTM Biopsy instrument (ARGON) according to the international consensus on diagnostic criteria established in 2017.5 The tissues were rapidly frozen in liquid nitrogen and stored at –80°C. The expression of proteins was measured using the RayBio Human Phosphorylation Array Kit (catalogue no. AAH-PRTK-G1, RayBiotech, Inc., China) according to the manufacturer’s instructions.
Animals
The Animal Care and Use Committee of Shandong University approved the experiments. Six-week-old male Sprague-Dawley (SD) rats weighing 200–250 g were used in this study. The rats were maintained under a 12-hour light/12-hour dark cycle, room temperature of 24 °C and 20%-30% relative humidity. Three animals were included per cage, and the animals had free access to food and water.
Assessment of mechanical sensitivity
According to previous studies,15 the mechanical hyperalgesia of the rats was measured using a Randall-Selitto apparatus (Shandong Provincial Institute of Science and Technology, Jinan, Shandong, China) equipped with a round head probe (tip diameter: 8 mm) that sets the extraction threshold of the hindlimb subjected to mechanical stimulation to the muscle. Before the experiment, the animals were adapted to the laboratory environment for 7 days, and a mechanical pain behaviour test was performed during this period. During the behaviour test, the rats were restrained in a cylinder, and a Randall-Selitto probe was applied to the calves. The pressure was automatically increased until the animal withdrew its limbs. The pressure threshold was measured seven times at 2-minute intervals, and the average value after removing the minimum and maximum values was used as the nociceptive threshold.
Local injection
We performed intramuscular (i.m.) injections of EphrinB1-Fc to examine the direct pro-algesic effect of EphrinB1. Different concentrations of EphrinB1-Fr (0.02, 0.2, and 2 µg/µl, 30 µl, i.m., n = 4) were injected into the left gastrocnemius muscle of rats according to a previously published article.16 The same volume of PBS served as the control. Briefly, SD rats were briefly anaesthetized with isoflurane. The skin above the injection site was shaved and scrubbed with alcohol. A thin, indelible pen was used to mark the pierced area of the injected skin, and different concentrations of EphrinB1-Fr or vehicle were injected into the left gastrocnemius muscle.
We examined whether EphrinB1 played its role via its receptor EphB1. Under isoflurane anaesthesia, EphB1-Fc (2 µg/µl, 30 µl, i.m., n = 6) was injected into the left gastrocnemius muscle prior to the injection of EphrinB1 in the same spot. The same amount of PBS served as the control. The pressure threshold was measured before injection and 0.5 h, 1 h, 2 h, 4 h, 8 h and 24 h after injection. The doses of drug and the treatment durations are described in detail in the figure legends. EphrinB1-Fc and EphB1-Fc (Sigma, St. Louis, MO, USA) were dissolved in phosphate-buffered saline (PBS).
Model of MTrPs
We used an animal model that was previously reported by Huang el al.17,18
The schematic outline of the experimental workflow is showed in Figure 3(a). An active MTrP rat model was established by bluntly striking the left gastrocnemius muscle (GM) of SD rats followed by the performance of eccentric-based exercises for 8 weeks. The rats recovered for 4 weeks. Briefly, the marked left gastrocnemius muscles of the MTrP group were hit with a hand-made stick device that dropped from a height of 20 cm and had a kinetic energy of 2.352 J. The next day, the rats were placed on a treadmill (Sans, Nanjing, China) for 1.5 h with a downward angle of -16° and a speed of 16 m/min. The weight, limb movement, TB changes and behaviour of the rats were monitored. Active MTrPs were identified according to the 2017 International Consensus on Diagnostic Standards for Trigger Points of MPS: 1) mechanical sensitivity on TBs showing a rapid decrease; 2) a palpated TB; and 3) ability to elicit local twitch responses (LTRs) by needling.
Figure 3.
A rat model of taut band-associated persistent muscle pain. (a) Schematic illustration for the experimental design and procedures. (b) Time course of the mechanical withdrawal threshold of the stroked and control animals measured using the Randall-Selitto apparatus compared with the control group. The results are presented as the means ± SEMs. * * P < 0.01. (c) The time course of the incidence rate of palpable TBs in the MPS and control groups. (d) HE staining of the GM showing the histology of the stroked and control animals.
Before the experiment, basic withdrawal threshold to mechanical stimulation was measured for all rats as described previously. The withdrawal threshold was measured once weekly and checked after the 12th week. A TB palpation examination was performed once weekly for 12 weeks before the pharmacological experiment according to a previous study. The pharmacological experiment was started on the first day of week 13. The rats were randomly divided into three groups of 12 rats: MPS group + PBS, MPS group + EphB1-Fr, and Control group + PBS.
The effect of the EphB1 inhibitor EphB1-Fr (Sigma) on the hindlimb withdrawal threshold to mechanical stimulation was studied in animals with MTrPs in TB and control animals. EphB1-Fr was dissolved in 1 M PBS (2 µg/µl, i.m.) (30 µl × 3 points) and intramuscularly administered to animals with MTrPs (n = 6) or control animals (n = 6). After the intramuscular administration of EphB1-Fr, the mechanical withdrawal thresholds were measured at 0.5 h, 1 h, 2 h, 4 h, and 8 h using a Randal-Selitto apparatus. The effect of the administration of the same volume of the vehicle (1 M PBS), which was used as a control, was also measured.
Western blot analysis
Muscle tissues near the injection site in the left gastrocnemius were rapidly removed and stored in liquid nitrogen. The tissue samples were lysed in lysis buffer supplemented with protease and phosphatase inhibitors. The lysed homogenate was centrifuged at 12,000 rpm at 4 °C for 15 minutes. The supernatants were collected, dissolved in 4× sample buffer and denatured at 100 °C for 10 minutes. The proteins were separated using 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk powder for 1.5 h at room temperature and incubated overnight at 4 °C with the following primary antibodies: anti-Eph receptor B1 and Eph receptor B2 (phospho Y594 and Y604) (Abcam, ab61791, 1:1000). The membranes were washed with Tris-buffered saline Tween-20 (TBST) and incubated for 1.5 hours with the secondary antibody (1:5000) at room temperature. The blots were developed using a chemiluminescent reagent (Millipore).
Statistical analyses
Statistical analyses were performed using one-way analysis of variance (ANOVA) with repeated measures, two-way ANOVA with repeated measures followed by Holm-Sidak multiple comparison tests if warranted, Student’s t-test, or non-parametric test where appropriate. A p-value of <0.05 was considered significant. All data are expressed as the means ± standard errors (SEMs). The data were analysed using GraphPad Prism 7.0 statistical software.
Results
Baseline sample characteristics
Clinical and anthropometric data were obtained from 11 MPS patients and seven healthy controls. No significant differences in age, gender, height, weight, or body mass index (BMI) were found between the groups. The present pain intensity in the patients with MPS was significantly greater compared to the healthy subjects (P < 0.001; Table 1).
Figure 1.
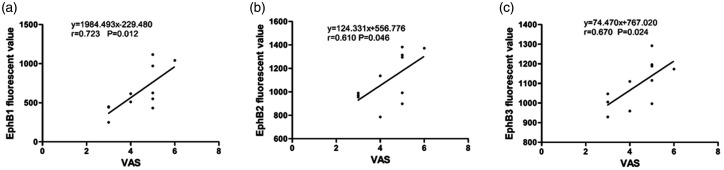
Correlation of p-EphB1, p- EphB2, p-EphB3 with numerical rating scale (NRS) in MPS patients derived from MTrPs site (n = 11). Higher levels of muscle (a) p-EphB1(r = 0.723, n = 11, P < 0.05); (b) p-EphB2(r = 0.610, n = 11, P < 0.05); (c) p-EphB3(r = 0.670, n = 11, P < 0.05) were correlated with higher values of NRS. NRS, numerical rating scale.
Correlations
EphB1 (r = 0.723, n = 11, P < 0.05), EphB2 (r = 0.610, n = 11, P < 0.05), and EphB3 levels (r = 0.670, n = 11, P < 0.05) in the M group significantly correlated with the numerical rating scale (NRS) in the MTrPs (Figure 1).
The intramuscular injection of EphrinB1-Fc causes muscle hyperalgesia
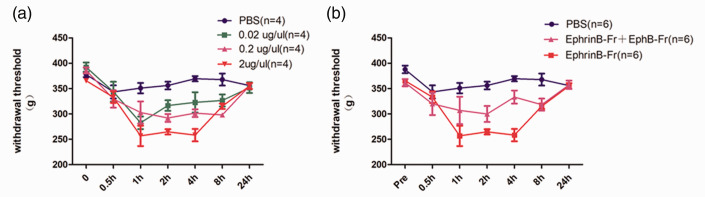
Muscle hyperalgesia was induced via the intramuscular injection of various doses of EphrinB1-Fc (0.02 µg/µl, 0.2 µg/µl, 2 µg/µl and PBS, 30 µ) into the left gastrocnemius muscle of SD rats. Thirty minutes after injection, the nociceptive threshold decreased in a dose-dependent manner, and the pain response lasted for at least 4 hours. Twenty-four hours after injection, the nociceptive threshold returned to baseline levels (Figure 2(a)). EphB1-Fc is a specific inhibitor of EphB1, and the intramuscular injection of EphB1-Fc (2 µg/µl, 30 µl) partially blocked the EphrinB1-Fc-induced nociceptive threshold decrease (Figure 2(b)). These results suggest that the EphrinB1-Fc-induced pain response was partially caused by the activation of EphB1 receptors in muscles.
Figure 2.
The intramuscular injection of EphrinB1-Fc caused dose- and time-dependent hyperalgesia. The paw withdrawal threshold was measured 0.5 h,1 h, 2 h, 4 h, 8 h and 24 h after the injection of EphrinB1-Fc. (a) Mechanical pain hypersensitivity was induced by the intramuscular injection of EphrinB1-Fc (0.02, 0.2 or 2 µg/µl), but the pain threshold did not change in the control group. The hyperalgesia induced by EphrinB1-Fc injection lasted for at least 4 hours, and 24 hours after injection, the withdrawal threshold returned to the baseline level of the control group (n=4 rats in each group). (b) EphB1-Fc (2 µg/µl) pretreatment partially prevented the mechanical hypersensitivity induced by EphrinB1-Fc (2 µg/µl) (n = 6 rats in each group).
Mechanical hyperalgesia, TB analysis and muscle histology in an animal model of MTrPs
Starting from the third week, the mechanical withdrawal thresholds measured using the Randall-Selitto apparatus in the TB sites of the MPS group (n = 10) were significantly lower than the control group (n = 5), and this difference lasted for 12 weeks (Figure 3(b)). No changes in the palpation or mechanical threshold were found in the control group. Two weeks after the first strike, the TBs were palpated subcutaneously in the middle gastrocnemius and were palpated weekly intervals from week 5 to 12 in the MPS group (n = 10) (Figure 3(c)).
Microscopic analysis of haematoxylin and eosin staining revealed that the muscle fibres in the control group were uniform in size, polygonal, and regularly arranged in the cross-sectional space (Figure 3(d)). Large, round muscle cells (contracture knots) were observed in the cross-sections of the MTrPs, and obvious inflammatory cell infiltration was also detected (black arrows in Figure 3(d)).
p-EphB1 increased from damaged muscle cells and the effects of an EphB1 inhibitor on muscular mechanical hyperalgesia
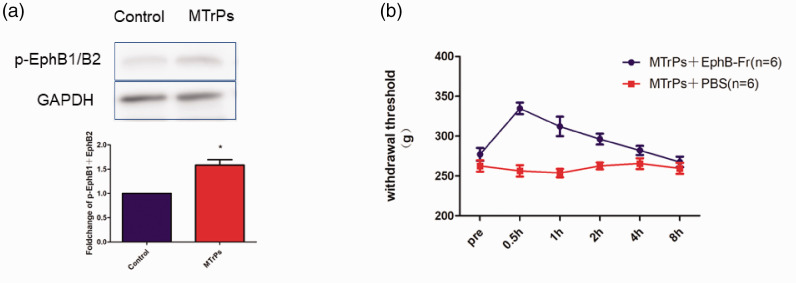
The expression of the p-EphB protein in the MTrPs was significantly upregulated compared to the control group on western blots (Figure 4(a)). The intramuscular administration of EphB1-Fr (n = 6) (2 µg/µl 30 µl × 3 points) resulted in significant recovery of mechanical hyperalgesia in the MPS group, as detected using a Randall-Selitto apparatus (Figure 4(b)), and this recovery continued for almost 4 hours. The intramuscular administration of PBS as a control did not induce any significant changes in the MTrP sites in the MPS (n = 6).
Figure 4.
The expression level of p-EphB1 was upregulated in M group and the peripheral effect of the EphB1 inhibitor EphB1-Fr on muscle hyperalgesia. The mechanical withdrawal threshold was measured before and 0.5, 1, 2, 4 h and 8 h after EphB-Fr (2 µg/µl 30 µl × 3 points) injection into the MTrP site in the GM of the M group and after the injection of vehicle (PBS).
Discussion
This study demonstrated the following results:
The p-EphB1/p-EphB2/p-EphB3 levels in biopsies from MTrP sites in the trapezius muscle strongly correlated with pain intensity in MPS patients;
The intramuscular injection of EphrinB1-Fc produced muscle hyperalgesia partially via activation of EphB receptors; and
p-EphB expression was increased in MTrP sites, and the injection of EphB1-Fr reduced pain behaviours in a rat MPS model.
Table 1.
Anthropometric data and data from the health questionnaire.
| Variables | CON (n = 11) | MPS (n = 7) | Statistics (p-value) |
|---|---|---|---|
| Age (years) | 53.57 ± 9.64 | 45.73 ± 8.43 | p < 0.05 |
| Height (cm) | 1.69 ± 0.06 | 1.66 ± 0.06 | p > 0.05 |
| Weight (kg) | 69.86 ± 9.79 | 65 ± 10.72 | p > 0.05 |
| BMI (kg/m2) | 24.33 ± 1.7 | 23.58 ± 2.74 | p > 0.05 |
| Pain intensity | 0 ± 0 | 4.36 ± 1.03 | p < 0.05 |
Abbreviations: BMI, body mass index; NA, not applicable.
The health questionnaire included questions concerning pain intensity. The results from the statistical analysis between the 2 groups are presented in the column furthest to the right.
Denotes statistical significance.
It is important to subject patients with clinical chronic pain to basic tests for classifying pain intensity and sensitivity, including the identification of pain areas and tenderness points. Proteins and other objective biomarkers in different tissues should be identified to promote and improve the diagnosis of chronic pain and elucidate the underlying mechanism. Notably, p-EphB1/p-EphB2/p-EphB3 levels in muscle biopsies from MTrP sites in the trapezius muscle were higher in MPS patients than healthy controls, and a significant correlation was found between pain intensity and p-EphB1/p-EphB2/p-EphB3 expression in patients with MPS. Several studies showed that acute and chronic pain change the expression and phosphorylation of EphB at the central level.9 However, the new findings from our study show that p-EphB expression in MTrP sites is significantly related to the present pain intensity in MPS patients, which indicates that Eph is involved in pain regulation at the peripheral level.
The secretion of sensitizers (e.g., cytokines, chemokines, and growth factors) participates in peripheral hypersensitivity and the maintenance of various types of pain.19 The Eph family is associated with the initiation, development and maintenance of pain sensitization at the peripheral level.10 Cao et al. confirmed that activation of the peripheral EphrinB/EphB signalling pathway caused hyperalgesia via MAPK-mediated mechanisms.10 Peripheral injection of EphB1-Fc in a formalin model of inflammatory pain inhibited EphB receptors to reduce hyperalgesia. Eph phosphorylation also regulates NMDA function in peripheral sensory neurons to mediate pain.20 As shown in our previous study, the phosphorylation level of EphB at MTrP sites was significantly higher than normal controls. To further verify whether EphB participated in the peripheral sensitization process of MTrPs, we established a model of MTrPs according to a previously published article. Long-lasting decreases in the mechanical withdrawal threshold with the development of TB and similar morphological changes in MTrPs in humans were found using this model, which indicates the success of the model. We did not perform electrophysiology studies because the accuracy of previous electrophysiological results remains controversial. The hypersensitive site in a palpable TB is more reflective of its nature. Our model used intramuscularly administered EphB-Fr, which is a specific inhibitor of EphB kinase, at the MTrP site to inhibit the activity of EphB. Many studies using21,22 pain models reported that EphB-Fr was effective for the treatment of inflammatory pain and the inhibition of hyperalgesia. EphB-Fr inhibited muscle hyperalgesia in the present model.
The possibility that EphB played a role in the central nervous system cannot be excluded in our research. However, the effect of EphB-Fr was more likely due to inhibition of the EphB signalling pathway in the surrounding nociceptors because an inhibitory effect was observed after the injection of EphB-Fr into the hyperirritable site. Several studies found Eph receptors and Ephrins in the nerve endings of humans and animals.10 Therefore, the current study was not able to determine whether EphB receptors were activated at nerve endings by exogenous EphrinB1-Fc. However, EphB and EphA receptors are expressed on DRG sensory neurons,21,23 which indicates that these sensory neurons in muscle have the potential ability to react to exogenous or endogenous EphrinB. All of these results suggest that this phenomenon may reasonably occur in peripheral sensory nerve endings. The Eph receptor and Ephrins are also expressed in peripheral T cells and muscle cells24,25 and may also function in inflammatory responses, and Ephrins/Eph receptors interact with a variety of membrane receptors to increase the release of various chemokines, neurotransmitters or growth factors, which simulates the conditions of peripheral nociceptor sensitization and pain.9 Eph enhanced the function of NMDA and upregulated its expression in the dorsal root ganglion, trigeminal ganglion or peripheral sensory neurons.20,26 The ionic NMDA receptor is an important part of the nociceptive signalling mechanism in the spinal dorsal horn and peripheral sensory neurons, and it is closely related to the main mechanism of pain development.27 Therefore, the Ephrin B/EphB signal may contribute to the sensitization of primary muscle afferents and the persistent muscle mechanical hyperalgesia observed in the present study.
Previous studies showed that the essence of MTrPs is the abnormal contraction of sarcomeres. Gerwin proposed that muscle pain may arise from ischaemia-induced inflammation in the muscle as a result of capillary compression by the taut bands. The widely accepted theory for the MTrP mechanism is based on the hypothesis28 that the excessive release of acetylcholine (ACh) from the motor nerve junction is the main cause of its contraction, which results in hypoxia, ATP energy crisis, the release of sensitizing substances, and pain. Once the cycle of abnormal contraction is broken, pain may be relieved. Our study showed that the expression of p-EphB on the muscle cell membrane of contracture sarcomeres was significantly increased (article under review). Several studies showed that Eph was located in the neuromuscular junction of the motor nerve in skeletal muscle, where it regulated the function of ACh,29,30 which controls muscle contraction. Although a few studies showed that Eph receptors regulated skeletal muscle contraction, many studies showed that Eph receptors regulated vascular and airway smooth muscles.11,12 Therefore, increased expression of EphB1 may enhance the activity of the motor endplate via upregulation of motor nerve fibre terminals and promote the formation of TBs where the MTrPs are located. Based on the role of EphrinB/EphB in peripheral sensitization, EphB1 may be involved in the mechanism of MTrP formation.
In summary, EphrinB1 induced muscle hyperalgesia via its high affinity receptor EphB1. Because EphB1 was also involved in the MTrP sites in MPS patients and an MTrP-like persistent muscle pain model, peripheral EphB1 administration may be a promising approach for the treatment of MPS. We will further investigate whether Eph affects skeletal muscle contraction and explore its possible molecular mechanisms in future research.
This study has some limitations. (1) We did not perform a correlation analysis between p-EphB1 levels and pressure pain thresholds. (2) We did not determine whether the activation of Eph led to the abnormal contraction of muscle fibres in our animal experiments, which requires further study. (3) The excessive number of noxious pinches in the animal experiments may be a harmful stimulation with unknown physiological effects.
Acknowledgment
We thank Dr. Yuchang Zhu for stimulating discussion on the subject. We also thank the different technical platforms of Qilu Hospital of Shandong University.
Footnotes
Author Contributions: Feihong Jin helped recruit all of the subjects and designed, performed, and analysed the antibody array experiments, performed behavioural tests and wrote the manuscript. Lianying Zhao helped analyse the data. Qiya Hu helped analyse the data. Feng Qi helped conceive the project and supervised all experiments.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by the National Natural Science Foundation of China (No. 81672250).
ORCID iDs: Feihong Jin https://orcid.org/0000-0002-9403-4169
Lianying Zhao https://orcid.org/0000-0002-6733-7092
References
- 1.Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician 2002; 65: 653–660. [PubMed] [Google Scholar]
- 2.Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep 2001; 5: 412–420. [DOI] [PubMed] [Google Scholar]
- 3.Travell JG, Simons DG. Myofascial pain and dysfunction: the trigger point manual. Vol. 1 Baltimore: Williams &Wilkins, 1983. [Google Scholar]
- 4.Gerwin RD, Shannon S, Hong CZ, Hubbard D, Gevirtz R. Interrater reliability in myofascial trigger point examination. Pain 1997; 69: 65–73. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-de-Las-Peñas C, Dommerholt J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: a Delphi study. Pain Med. 2018; 19: 142–150. [DOI] [PubMed] [Google Scholar]
- 6.Criscuolo CM. Interventional approaches to the management of myofascial pain syndrome. Curr Pain Headache Rep. 2001; 5: 407–411. [DOI] [PubMed] [Google Scholar]
- 7.Scott NA, Guo B, Barton PM, Gerwin RD. Trigger point injections for chronic non-malignant musculoskeletal pain: a systematic review. Pain Med. 2009; 10: 54–69. [DOI] [PubMed] [Google Scholar]
- 8.Levesque A, Ploteau S, Michel F, Siproudhis L, Bautrant E, Eggermont J, Rabischong B, Volteau C, Perrouin-Verbe MA, Labat JJ. Botulinum toxin infiltrations versus local anaesthetic infiltrations in pelvic floor myofascial pain: multicentre, randomized, double-blind study. Ann Phys Rehabil Med Epub ahead of print 22 January 2020. [DOI] [PubMed]
- 9.Vasileiou I, Adamakis I, Patsouris E, Theocharis S. Ephrins and pain. Expert Opin Ther Targets 2013; 17: 879–887. [DOI] [PubMed] [Google Scholar]
- 10.Cao J-L, Ruan J-P, Ling D-Y, Guan X-H, Bao Q, Yuan Y, Zhang L-C, Song X-J, Zeng Y-M. Activation of peripheral ephrinBs/EphBs signaling induces hyperalgesia through a MAPKs-mediated mechanism in mice. Pain 2008; 139: 617–631. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, Luo H, Thorin E, Tremblay J, Peng J, Lavoie JL, Wang Y, Qi S, Wu T, Wu J. Possible role of Efnb1 protein, a ligand of eph receptor tyrosine kinases, in modulating blood pressure. J Biol Chem 2012; 287: 15557–15569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target ras and rho proteins. Cell Signal 2004; 16: 655–666. [DOI] [PubMed] [Google Scholar]
- 13.Jin F, Guo Y, Wang Z, Badughaish A, Pan X, Zhang L, Qi F. The pathophysiological nature of sarcomeres in trigger points in patients with myofascial pain syndrome: A preliminary study. Eur J Pain 2020; 24(10): 1968–1978. [DOI] [PMC free article] [PubMed]
- 14.Gerwin R. Trigger point diagnosis: at last, the first word on consensus. Pain Med 2018; 19: 1–2. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi K, Ozaki N, Kawakita K, Itoh K, Mizumura K, Furukawa K, Yasui M, Hori K, Yi S-Q, Yamaguchi T, Sugiura Y. Involvement of NGF in the rat model of persistent muscle pain associated with taut band. J Pain 2011; 12: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez P, Green PG, Levine JD. Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain 2014; 155: 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L-H, Huang Q-M, Barbero M, Liu L, Nguyen T-T, Beretta-Piccoli M, Xu A-L, Ji L-J. Quantitative proteomics analysis to identify biomarkers of chronic myofascial pain and therapeutic targets of dry needling in a rat model of myofascial trigger points. J Pain Res 2019; 12: 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang QM, Ye G, Zhao ZY, Lv JJ, Tang L. Myoelectrical activity and muscle morphology in a rat model of myofascial trigger points induced by blunt trauma to the vastus medialis. Acupunct Med 2013; 31: 65–73. [DOI] [PubMed] [Google Scholar]
- 19.Rivat C, Sar C, Mechaly I, Leyris J-P, Diouloufet L, Sonrier C, Philipson Y, Lucas O, Mallié S, Jouvenel A, Tassou A, Haton H, Venteo S, Pin J-P, Trinquet E, Charrier-Savournin F, Mezghrani A, Joly W, Mion J, Schmitt M, Pattyn A, Marmigère F, Sokoloff P, Carroll P, Rognan D, Valmier J. Inhibition of neuronal FLT3 receptor tyrosine kinase alleviates peripheral neuropathic pain in mice. Nat Commun 2018; 9: 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanamura K, Washburn HR, Sheffler-Collins SI, Xia NL, Henderson N, Tillu DV, Hassler S, Spellman DS, Zhang G, Neubert TA, Price TJ, Dalva MB. Extracellular phosphorylation of a receptor tyrosine kinase controls synaptic localization of NMDA receptors and regulates pathological pain. PLoS Biol 2017; 15: e2002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song XJ, Zheng JH, Cao JL, Liu WT, Song XS, Huang ZJ. EphrinB-EphB receptor signaling contributes to neuropathic pain by regulating neural excitability and spinal synaptic plasticity in rats. Pain 2008; 139: 168–180. [DOI] [PubMed] [Google Scholar]
- 22.Deng XT, Wu MZ, Xu N, Ma PC, Song XJ. Activation of ephrinB-EphB receptor signalling in rat spinal cord contributes to maintenance of diabetic neuropathic pain. Eur J Pain 2017; 21: 278–288. [DOI] [PubMed] [Google Scholar]
- 23.Moss A, Alvares D, Meredith-Middleton J, Robinson M, Slater R, Hunt SP, Fitzgerald M. Ephrin-A4 inhibits sensory neurite outgrowth and is regulated by neonatal skin wounding. Eur J Neurosci 2005; 22: 2413–2421. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov AI, Romanovsky AA. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life 2006; 58: 389–394. [DOI] [PubMed] [Google Scholar]
- 25.Yu G, Luo H, Wu Y, Wu J. EphrinB1 is essential in T-cell-T-cell co-operation during T-cell activation. J Biol Chem 2004; 279: 55531–55539. [DOI] [PubMed] [Google Scholar]
- 26.Ma P, Chen P, Zhou Z-L, Mo R-F, Wu M, Song X-J. Activation of EphB receptors contributes to primary sensory neuron excitability by facilitating Ca2+ influx directly or through src kinase-mediated NMDA receptor phosphorylation. Pain 2020; 161: 1584–1596. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Saloman JL, Weiland G, Auh QS, Chung MK, Ro JY. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain 2012; 153: 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons’ integrated hypothesis of trigger point formation. Curr Pain Headache Rep 2004; 8: 468–475. [DOI] [PubMed] [Google Scholar]
- 29.Lai KO, Ip NY. Postsynaptic signaling of new players at the neuromuscular junction. J Neurocytol 2003; 32: 727–741. [DOI] [PubMed] [Google Scholar]
- 30.Lai KO, Chen Y, Po HM, Lok KC, Gong K, Ip NY. Identification of the jak/stat proteins as novel downstream targets of EphA4 signaling in muscle: implications in the regulation of acetylcholinesterase expression. J Biol Chem 2004; 279: 13383–13392. [DOI] [PubMed] [Google Scholar]