Abstract
We present a case of milker’s nodules in a 41-year-old rancher from Saskatchewan, Canada, with secondary complications consisting of papular erythema and lymphadenopathy.
Keywords: Milker’s nodule, pseudocowpox virus, parapoxvirus, farmyard pox, Saskatchewan
Introduction
Pseudocowpox virus is a parapoxvirus that typically infects the teats and udders of cattle, but is also capable of causing cutaneous infection in humans. Transmission generally occurs through direct inoculation through a break in the skin. Development of lesions in humans is known as milker’s nodules, or pseudocowpox. Cutaneous infection is normally self-limiting but in some cases can result in secondary complications that include cutaneous eruptions, secondary bacterial infection, lymphadenopathy, and lymphangitis.1,2 It is clinically indistinguishable from other farmyard pox viruses and can appear clinically similar to other more severe zoonotic infections, necessitating the use of molecular methods for correct diagnosis.3 We report a case of milker’s nodules in a rancher from Saskatchewan with secondary complications consisting of papular erythema and lymphadenopathy.
Case report
A 41-year-old otherwise healthy male rancher was referred to the dermatology clinic for evaluation of two non-healing lesions on his right middle finger. Approximately 1 month prior to being seen in our clinic, the patient reported scraping his finger on a cow’s tooth when manually administering medication. One week later, he developed an itchy, pruritic, raised purple lesion several millimeters in size. Over the following 2 weeks, the lesion progressed to a fluid-filled lesion 1 cm in diameter, and an accompanying smaller lesion developed (Figure 1).
Figure 1.
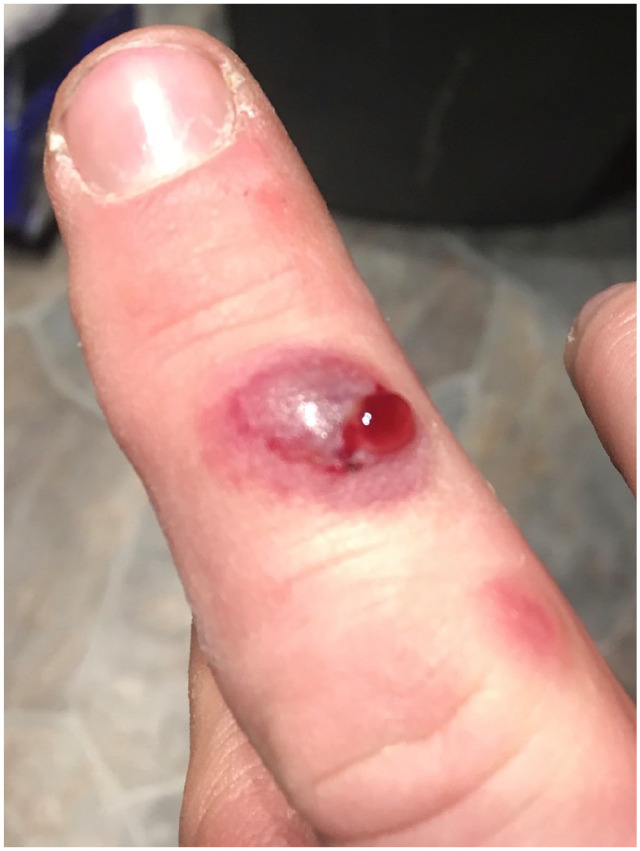
Milker’s nodules on right hand. Acute weeping nodule and papulonodule.
The patient visited a primary care physician, who lanced the lesion and drained sanguineous fluid. Swabs were sent for Gram stain, culture, and polymerase chain reaction (PCR). The following week, the patient was seen by the dermatology service. At the time of examination there was a 1.0 cm crusted necrotic vesiculonodule with surrounding erythema on the middle phalanx, and an accompanying proximal smaller lesion on the medial edge of the proximal interphalangeal joint of the same finger (Figure 2). A 3-mm punch biopsy was taken from the lesion and sent for deep fungal and mycobacterial culture.
Figure 2.
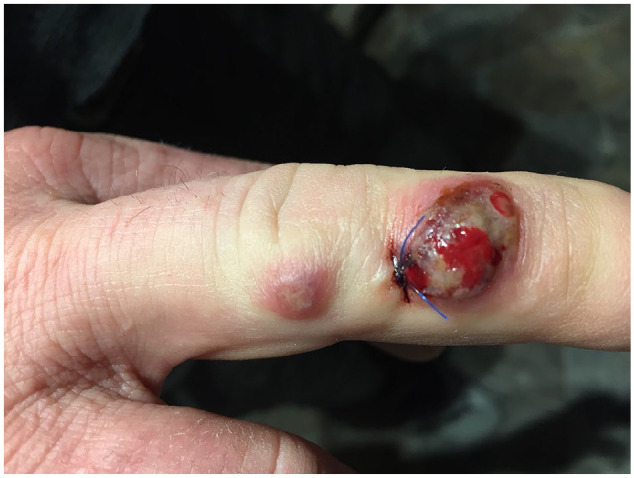
Milker’s nodules, post-punch biopsy.
Considering the patient’s profession and specific animal exposures, in addition to milker’s nodule, the differential diagnosis included bovine papular stomatitis, cutaneous anthrax, sporotrichosis, and other zoonotic infections including cowpox and vaccinia. The patient was started on doxycycline 100 mg BID due to concern about possible anthrax.
Approximately 1 month after initial development of the first lesion, and 4 days after beginning the doxycycline, the patient developed an intensely pruritic symmetrical papular erythemic eruption on the face, ears, and dorsum of both hands (Figure 3), as well as right axillary lymphadenopathy. His doxycycline was discontinued, and both the eruption and lymphadenopathy resolved 1 week later.
Figure 3.
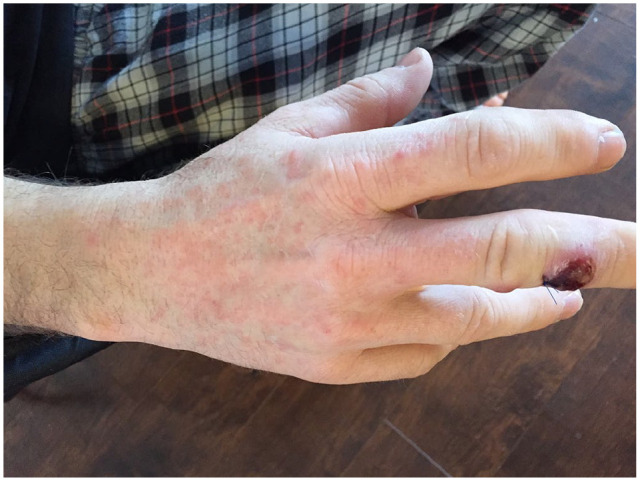
Papular erythema.
Laboratory PCR confirmed virus from the parapoxvirus genus, and DNA sequencing yielded pseudocowpox virus species. Table 1 displays the PCR primers that were used for determination of genus and species.3–5 The patient was counseled regarding the self-limiting nature of the condition.
Table 1.
PCR primers utilized for determination of parapoxvirus genus and pseudocowpox species.
| Parapoxvirus primer set3 • PPVF: 5’ TCG ATG CGG TGC AGC AC 3’ • PPVR: 5’ GCG GCG TAT TCT TCT CGG AC 3’ • PPVP: 5’ FAM-TGC GGT AGA AGC C-MGB 3’ |
| qORF primer set4 • qorfF: 5’ CAG CAG AGC CGC GTG AA 3’ • qorfR: 5’ CAT GAA CCG CTA CAA CAC CTT CT 3’ • qorfP: 5’ FAM-CAC CTT CGG CTC CAC-MGB 3’ |
| Pan-parapoxvirus primer set5 • PPP1: 5′ GTC GTC CAC GAT GAG CAG CT 3′ • PPP4: 5′ GCG AGT CCG AGA AGA ATA CG 3′ |
PCR: polymerase chain reaction; qPCR: qualitative polymerase chain reaction.
In follow-up, the patient reported that the site of the initial lesion had remained tender to palpation for approximately 4 months, with subsequent resolution. The lesion healed with mild scarring (Figure 4).
Figure 4.
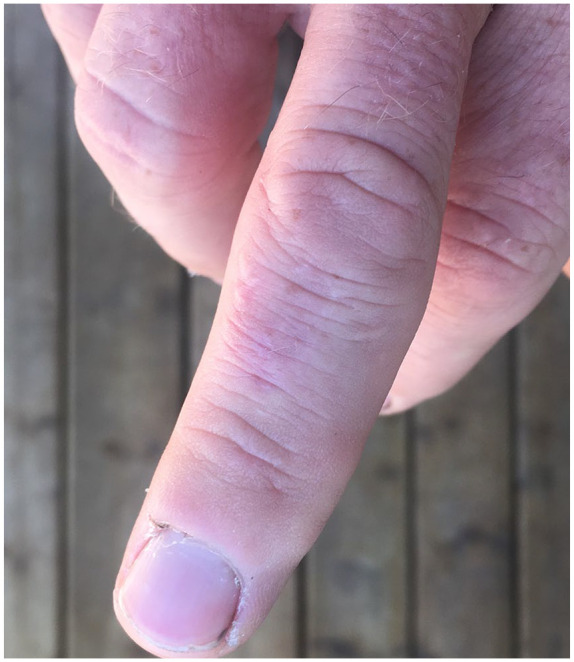
Mild scarring, 7 months following nodule development.
Discussion
Parapoxviruses are DNA viruses that belong to the poxvirus family. They are commonly associated with ruminant animals but are also capable of causing infection in humans. Lesions are characterized by mild papules and erosions on the muzzle, oral mucosa, and udder. Of the domestic ruminants, orf virus infects goats and sheep, whereas bovine papular stomatitis virus and pseudocowpox virus infect the mouth and udders of cattle.
In humans, lesions caused by these viruses are clinically indistinguishable, which has led to the use of the general term “farmyard pox” to describe infection. Farmyard pox generally follows a benign albeit protracted course, although more severe infection can occur in individuals with immune compromise. The differential diagnosis is extremely broad, and infection can appear clinically similar to other more severe zoonotic infections such as cutaneous anthrax. Correct diagnosis is therefore important. Clinical context and specific environmental and animal exposures can help to narrow the differential, and definitive diagnosis can be determined through PCR assay and viral-specific DNA sequencing.3
After an incubation period of 5–15 days, pseudocowpox infection normally results in 1 to 4 lesions on the area of contact, which evolve through six stages, each lasting approximately 6 days:2,6 maculopapular, target lesion, acute weeping nodule, regenerative dry stage, papillomatous, and regression with dry crust to complete resolution. It is a self-limited disease and typically heals without scarring.1
Secondary complications can include cutaneous eruptions, bacterial infection, lymphadenopathy, and lymphangitis.1,2 Secondary eruptions in milker’s nodules include papulo-urticaria, morbilliform exanthema, erythema nodosum, and erythema multiforme, including the bullous form.7 Time from initial lesion to secondary eruption occurs usually between 6 and 17 days, and typically resolves within 1–2 weeks.7,8 Prior treatment with antibiotics has been noted in some but not all cases of farmyard pox, suggesting that many secondary eruptions are likely attributable to an immune stimulus from the virus.8,9
Our patient’s presentation and clinical course was generally consistent with that of cases reviewed in the literature, with the exception of the timing of secondary cutaneous eruption occurring a month rather than several weeks later. This secondary eruption had been attributed to a reaction to the doxycycline due to the time correlation and its photo distribution; however, it also may have been a hypersensitivity reaction to the virus. Our patient experienced unilateral axillary lymphadenopathy secondary to the milker’s nodules; timing of lymphadenopathy, though noted as a rare occurence,1 has been less well described in the literature.
This case report highlights the importance of considering parapoxvirus infections as potential causes of hand lesions in Canadian farmers. Clinical context and molecular analysis are important for the correct diagnosis of milker’s nodules and further avoidance of unnecessary treatment. This case also highlights potential areas of investigation regarding the relative contribution of antibiotics, host factors, and viral factors to secondary eruptions in cases of pseudocowpox virus infection.
Acknowledgments
The authors would like to thank our patient and his family for their interest and permission to describe this case report.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval and informed consent: Signed informed consent was obtained from the patient for information and images to be published.
ORCID iD: Zoë C Phillips  https://orcid.org/0000-0003-2444-504X
https://orcid.org/0000-0003-2444-504X
References
- 1. Werchniak AE, Herfort OP, Farrell TJ, et al. Milker’s nodule in a healthy young woman. J Am Acad Dermatol 2003; 49(5): 910–911. [DOI] [PubMed] [Google Scholar]
- 2. Diven DG. An overview of poxviruses. J Am Acad Dermatol 2001; 44(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 3. Nitsche A, Buttner M, Wilhelm S, et al. Real-time PCR detection of parapoxvirus DNA. Clin Chem 2006; 52(2): 316–319. [DOI] [PubMed] [Google Scholar]
- 4. Gallina L, Dal Pozzo F, Mc Innes CJ, et al. A real time PCR assay for the detection and quantification of orf virus. J Virol Methods 2006; 134(1-2): 140–145. [DOI] [PubMed] [Google Scholar]
- 5. Inoshima Y, Morooka A, Sentsui H. Detection and diagnosis of parapoxvirus by the polymerase chain reaction. J Virol Methods 2000; 84(2): 201–208. [DOI] [PubMed] [Google Scholar]
- 6. Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol 2018; 32(4): 537–541. [DOI] [PubMed] [Google Scholar]
- 7. Kuokkanen K, Launis J, Morttinen A. Erythema nodosum and erythema multiforme associated with milker’s nodules. Acta Derm Venereol 1976; 56(1): 69–72. [PubMed] [Google Scholar]
- 8. Sonck CE. Milker’s nodules with allergic secondary eruptions. Acta Allergol 1951; 4(3): 241–252. [DOI] [PubMed] [Google Scholar]
- 9. Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect 2015; 143(2): 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]


