Abstract
Immunohistochemical localization of indoleamine 2,3-dioxygenase-1 and indoleamine 2,3-dioxygenase-2, the first and rate-limiting enzyme in tryptophan metabolism along the kynurenine pathway, has been studied in order to better understand the physiological significance of these enzymes at the maternal-fetal interface of human pregnancy with a gestational age of 7 weeks (n = 1) and term placentas (37-40 weeks of gestation, n = 5). Indoleamine 2,3-dioxygenase-1 protein immunoreactivity was found in glandular epithelium of the decidua and the endothelium of the fetal blood vessels in the villous stroma with some additional positive cells in the villous core and in the decidua. The syncytiotrophoblast stained strongly for indoleamine 2,3-dioxygenase-2. Immunoreactivity of kynurenine, the immediate downstream product of indoleamine 2,3-dioxygenase-mediated tryptophan metabolism, showed the same localization as that of indoleamine 2,3-dioxygenase-1 and indoleamine 2,3-dioxygenase-2, suggesting these are functional enzymes. Interferon-γ added to placental villous explant culture markedly stimulated expression level of both mRNA and immunoreactivity of indoleamine 2,3-dioxygenase-1. The different cellular expression and interferon-γ sensitivity of these enzymes at the maternal-fetal interface suggests distinct physiological roles for each enzyme in normal human viviparity.
Keywords: Indoleamine 2,3-dioxygenase-1; indoleamine 2,3-dioxygenase-2; interferon-γ; maternal-fetal interface; human placenta
Introduction
The enzyme indoleamine 2,3-dioxygenase (IDO) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of various indoleamines derivatives (e.g. tryptophan, 5-hydroxytryptophan, tryptamine, and serotonin) upon the insertion of 2 atoms of molecular oxygen.1 IDO is widely expressed in a variety of tissues of mammals. One tissue with particularly high activity is the human placenta.2 It is primarily induced by interferon-γ (IFN-γ) and other pro-inflammatory stimulants are also effective.3 By reducing the availability of tryptophan, IDO is thought to inhibit intracellular and other infections,4 the growth of malignant cells,5 and immune cell function.6,7 An isoform of IDO was identified and assigned the name indoleamine 2,3-dioxygenase-2 (IDO2).8,9 The original IDO has now been referred to as indoleamine 2,3-dioxygenase-1 (IDO1). Although the 2 proteins have similar enzymatic activities, their different expression patterns within tissues suggests a distinct role for each protein.8,10
With regard to the role of placental IDO Munn et al11 established the proposal in mouse that expression of this enzyme at the maternal-fetal interface regulates the maternal immune response to the fetal allograft and prevents its immunological rejection. It has been demonstrated that indoleamine 2,3-dioxygenase production by macrophages7 and dendritic cells6 results in the inhibition of lymphocyte proliferation due to tryptophan depletion by this enzyme. We have shown that the same mechanisms are present in the human placenta which are able to regulate CD4 positive T lymphocyte proliferation.12 Thus, by catabolizing tryptophan, the mouse conceptus suppresses T cell activity and defends itself against rejection.11 However, several issues arise from their hypothesis. How is the developing fetus well supplied with tryptophan when cells at the maternal-fetal interface degrade it?13 It is possible that IDO mediated tryptophan catabolism may produce an immunosuppressive metabolite. Quinolinic acid for example is a potent neuroexcitatory toxin that putatively could serve as a mediator of cell destruction in a variety of neurodegenerative disorders.14 Additionally IDO mediated tryptophan catabolism consumes oxygen radicals1 and this might influence T cell responsiveness. More recently it has become possible that tryptophan depletion is not advantageous in pregnancy and Badawy has proposed the utilization concept.15-17
In the first trimester placenta immunohistochemical staining for IDO was found in syncytiotrophoblast, extravillous cytotrophoblast and macrophages in the villous stroma. Staining was also seen in the glandular epithelium and stromal cells of the first trimester decidua.18 Sedlmayr et al,19 Sedlmayr and Blaschitz,20 and Ligam et al21 also found IDO expressed in the glandular epithelium and in endothelial cells with some positive cells in the decidual stroma. They did however find staining of the syncytiotrophoblast was comparatively rare. Interestingly both these studies used the same monoclonal antibody to indoleamine 2,3-dioxygenase.22 The question of trophoblastic expression of IDO is still a continuous controversial issue.
As mentioned above, a second IDO isozyme (IDO2) was identified and expression of IDO2 mRNA was confirmed in the human placental tissue.9,23 In other tissues IDO2 protein localization shows a different pattern from that of IDO1.8,10 Hitherto no report has yet been appeared concerning IDO2 protein localization at the maternal-fetal interface of human pregnancy. In this study we have mapped the immunohistochemical distribution of the IDO1 and IDO2 protein at the human maternal-fetal interface using both a rare early pregnancy sample from a women at 7 weeks of gestation who underwent hysterectomy for cervical cancer with gestational sac in utero as well as term placentas.
Materials and Methods
Tissue collection and processing
The specimen was collected from an early human pregnancy that included the entire uterus removed for medical reasons at 7 weeks of gestation in situ. The patient was diagnosed with cervical cancer and chose surgical treatment with hysterectomy. She consented to have her surgical specimen used for research, and the uterus and the pregnancy tissues were removed intact. Samples of term placenta (37-40 weeks, n = 5) were obtained directly after delivery from women without any complications other than cephalopelvic disproportion requiring repeat Cesarean section. These term samples were obtained with informed consent. Collection and study were approved by the Ethics Review Committee of Graduate School of Biomedical Sciences, Hiroshima University (reference number; Hi-222, date of approval; 14 October 2018).
Immunohistochemistry
Freshly dissected tissues were fixed in neutral buffered formalin, embedded in paraffin and 4 µm sections were cut onto adhesive slides and dried at 37°C in an oven overnight. Sections were heated at 60°C for 15 minutes and then standard deparaffinization with xylene was performed. Antigen retrieval was carried out using an electric kettle at 98°C for 40 minutes in 0.2 M citrate buffer at pH6.0. Inactivation of endogenous peroxidase activity was performed in 0.3% H2O2/methanol at room temperature for 20 minutes. Sections were blocked with horse serum (for mouse antibodies) or donkey serum (for rabbit antibodies) at room temperature for 30 minutes. Primary antibodies were diluted as IDO1 (#86630; Cell Signaling Technology, Danvers, MA, USA); 1/4000, IDO2 (NBP2-46021; Novus Biologicals, Centennial, CO, USA); 1/2000,
L-kynurenine (ab36922; Abcam, Cambridge, UK); 1/1000, CD68 (#76437; Cell Signaling Technology, Danvers, MA, USA); 1/5000, mouse-isotype control (02-6502; Thermo Fisher Scientific, Waltham, MA USA) and rabbit-isotype control (#3900; Cell Signaling Technology, Danvers, MA, USA); depending on Abs concentration. Sections were incubated with primary antibodies overnight at 4°C. Slides were washed 3 times in phosphate-buffered saline (PBS) and exposed to biotinylated antibodies at room temperature for 30 minutes, then to the streptavidin-HRP antibody at room temperature for an additional 30 minutes (Streptavidin-Biotin Complex Peroxidase kit: 30462-30; Nakalai Tesque, Kyoto, Japan). Color development utilized DAB (Peroxidase Stain DAB kit: 25985-50; Nakalai Tesque, Kyoto, Japan) and routine hematoxylin staining was performed at room temperature for 5 minutes.
Culture of villous tissue
Term human placental chorionic villi were cultured in vitro using a standard technique24 as described previously.25 The tissue was washed 3 times with ice-cold PBS containing 100 unit/ml penicillin and 100 unit/ml streptomycin and dissected into small pieces (a single piece was approximately 5 mg). All these procedures were carried out at 4°C. Three pieces of dissected tissue were placed on a polyester mesh (Netwell 500 micron mesh, Costar, NY, USA) and cultured in 35 mm plastic culture dish at 37°C in an atmosphere of 5% CO2 and 95% air for 36 hours. The culture medium used was DMEM medium with addition of 5% fetal bovine serum, 100 unit/ml penicillin, 100 unit/ml streptomycin, and 1000 unit/ml IFN-γ or vehicle. Cultures were conducted in triplicate. The number of placentae used in each study is mentioned in the figure legends. For immunohistochemistry cultured villous tissue samples were fixed, sectioned, and stained as described above for non-cultured tissue.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR) analysis
IDO1 and IDO2 gene expression were analyzed by RT-PCR using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal standard. Total RNA was extracted from chorionic villi with QIAshredder (79654; Qiagen, Tokyo, Japan) and RNeasy Mini Kit (74134; Qiagen, Tokyo, Japan), and single-strand cDNA was synthesized with ReverTraAce kit (FSK-101; Takara, Shiga, Japan) according to the manufacture’s protocol. The primers used in the subsequent RT-PCR were as follows:
IDO1, forward, 5′-ACCAGAGGAGCAGACTACAA-3′ and backward, 5′-GTGATGCATCCCAGAACTAGAC-3′;
IDO2, forward, 5′-GGGCTGATGTATGAAGGAGTTT-3′ and backward, 5’-GTGCTGAGTGGATGTCTTCTATG-3′;
GAPDH, forward, 5′-GTCTCCTCTGACTTCAACAGCG-3′ and backward, 5′-ACCACCCTGTTGCTGTAGCCAA-3′.
The expected size of the PCR products were 282 bp for IDO1, 196 bp for IDO2, and 131 bp for GAPDH. To control for DNA contamination, reactions were run without RNA or with RNA in the absence of the reverse transcriptase and revealed no amplified product (data not shown). The synthesized cDNA (0.05 μg equivalent to RNA) was used for PCR amplification (KAPATaq Extra HS Ready Mix: KK3606; Nippon Genetics, Tokyo, Japan). The PCR conditions were: 94°C for 3 minutes, 60°C for 1 minutes and 72°C for 2 minutes; then 30 cycles (for IDO1 and GAPDH) and 32 cycles (for IDO2) of 94°C for 30 seconds, 60°C for 30 seconds and 72°C for 30 seconds; followed by a 10 minutes final extension at 72°C. PCR products were separated on a 1.5% agarose gel which was stained with ethidium bromide. For quantitative PCR, 6 µl of a diluted (1/10) reverse transcription product was used for quantitative PCR with SYBR Green I in 25 ml total volume (Applied Biosystems Power SYBR Green Master Mix: 4368577; Thermo Fisher Scientific, Waltham, MA, USA). The primers used for quantitative PCR are the same as those for conventional PCR. The relative gene expression was analyzed by the 2−∆∆CT methods using GAPDH as an internal control. Statistical analysis was performed using the Mann-Whitney U-test with Bonferroni correction.
Chemicals
Human recombinant IFN-γ was obtained from Pepro Tech (AF-300-02; NJ, USA), tissue culture supplements were from Fujifilm (Tokyo, Japan). All chemicals were of the highest purity commercially available.
Results
Immunohistochemistry of IDO1, IDO2, and kynurenine
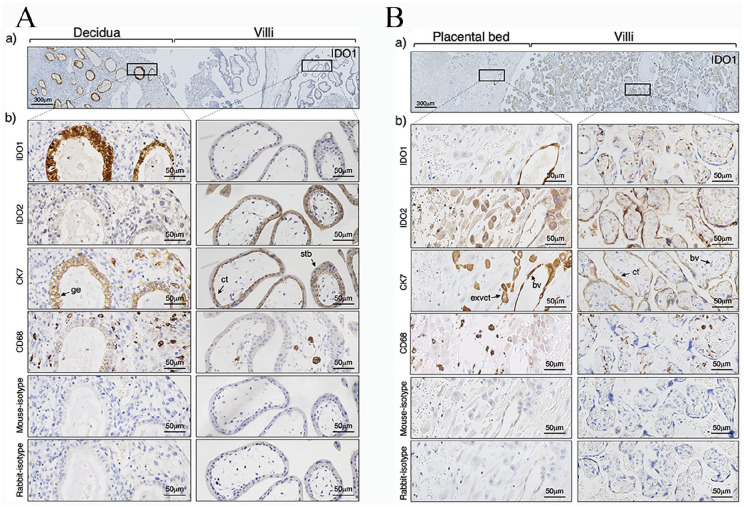
Serial sections were stained with antibodies to indoleamine IDO1, IDO2, and kynurenine with mouse or rabbit immunoglobulin as the primary antibody control. Figure 1A shows the section of an implantation site of 7 weeks of gestation including the placental-decidual interface. There was strong staining for IDO1 in the decidual specifically of the glandular epithelium with weaker staining on some cells. IDO2 protein immunoreactivity was seen to be obviously expressed on the syncytiotrophoblast in the villi of 7 weeks of gestation. In the term placenta (Figure 1B), the maternal blood vessel endothelium of the decidua was positive for IDO1. The extravillous cytotrophoblast in the term placental bed also expressed IDO2. In the term villi, there is obvious staining for IDO1 in the villous core, specifically of the endothelium of the fetal blood vessels and of macrophages (CD68 positive). There is continued expression of IDO2 in the syncytiotrophoblast of the term villi.
Figure 1.
Immunohistochemical localization of indoleamine 2,3-dioxygenase at the maternal-fetal interface of human placenta. Immunostaining for indoleamine 2,3-dioxygenase-1 (IDO1), indoleamine 2,3-dioxygenase-2 (IDO2), cytokeratin 7 (CK7), CD68, rabbit-isotype control, and mouse-isotype control were performed. (A) Implantation site of 7 weeks of gestation. (B) Term chorionic villi and placental bed. (a) Lower magnification of the maternal-fetal interface. Scale bar: 300 µm. (b) Higher magnification of the same area of the specimens depicted in (a). Scale bar: 50 µm. The results presented are from a single representative experiment performed with one 7 weeks sample and 5 term placentas.
Abbreviations: bv, blood vessel; cct, chorionic cytotrophoblast; exvct, extravillous cytotrophoblast; ge, glandular epithelium; ma, placental macrophage; stb, syncytiotrophoblast.
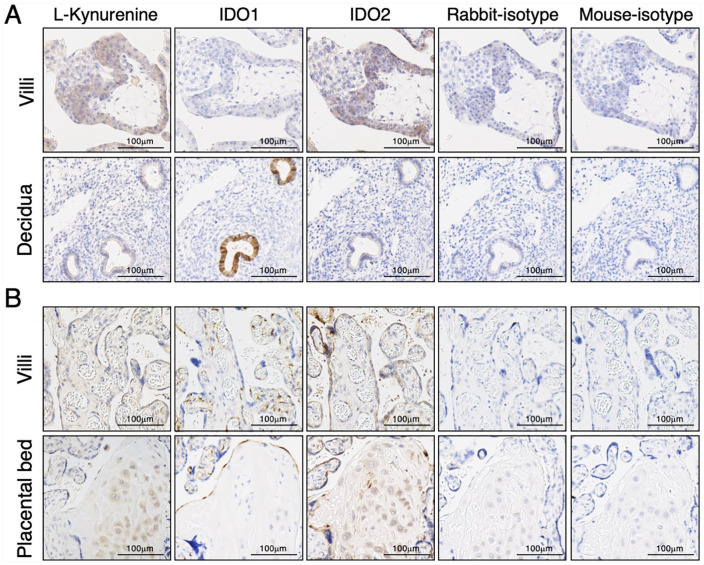
Immunostaining for kynurenine (Figure 2), the immediate downstream product of IDO mediated tryptophan catabolism, was found in the syncytiotrophoblast and in the glandular epithelium and some cells (extravillous cytotrophoblasts) in the decidua. These kynurenine immunoreactivity had essentially the same localization as that of IDO1 and IDO2.
Figure 2.
Immunohistochemistry of kynurenine at the maternal-fetal interface of human placenta. Immunostaining for kynurenine, indoleamine 2,3-dioxygenase-1 (IDO1), indoleamine 2,3-dioxygenase-2 (IDO2), rabbit-isotype control, and mouse-isotype control were performed as described in the section “Materials and Methods.” (A) Implantation site of 7 weeks of gestation. (B) Term chorionic villi and placental bed. The results presented are from a single representative experiment performed with one 7 weeks sample and 5 term placentas. Scale bars represent 100 µm.
Effect of IFN-γ on IDO1 and IDO2 expression in cultured placental villous tissue
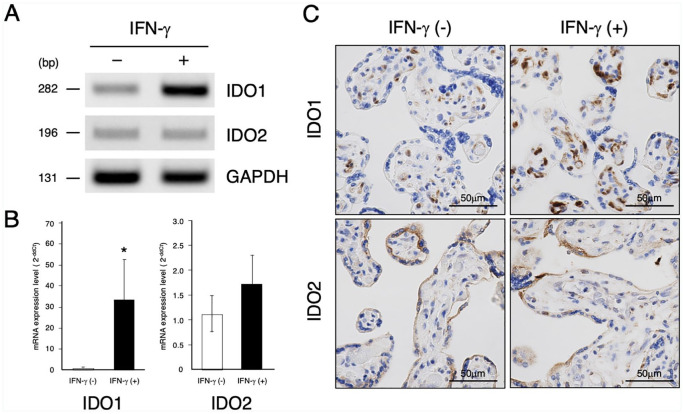
In order to examine the role of IFN-γ as a regulator of IDO expression in placental villous tissue, IDO mRNA level, and immunoreactivity after treatment of IFN-γ were determined by RT-PCR and immunohistochemistry, respectively. A single band of the expected size for either IDO1 (282 bp) or IDO2 (196 bp) was detected by RT-PCR (Figure 3A). Treatment of IFN-γ induced marked increase of IDO1 mRNA expression (Figure 3B). In contrast, IFN-γ showed no significant stimulatory effect on IDO2 mRNA expression level. Figure 3C shows immunohistochemistry of the section of villous explants cultured with or without IFN-γ. Immunoreactivity of IDO1 was observed in the villous core, specifically of the fetal vessel endothelial cells and of some cells which are probably macrophages. In the IFN-γ treated culture, the large numbers of stromal cells and endothelial cells were more strongly positive for IDO1. The syncytiotrophoblast was positive for IDO2, but in contrast to IDO1, immunoreactivity of IDO2 was not enhanced following IFN-γ treatment.
Figure 3.
Effect of IFN-γ on indoleamine 2,3-dioxygenase expression in cultured chorionic villi. Chorionic villous explants from term placenta were cultured with 1000 unit/ml IFN-γ or vehicle for 36 hours. (A) RT-PCR. The relative abundance of indoleamine 2,3-dioxygenase-1 (IDO1), indoleamine 2,3-dioxygenase-2 (IDO2), and glyceraldehyde phosphate 3-dehydrogenase (GAPDH) mRNA were analyzed by RT-PCR as described in the section “Materials and Methods.” The results presented are representative of 3 separate experiments performed with 3 placentas yielding similar results. (B) Relative quantitation. IDO1, IDO2, and GAPDH mRNA expression were analyzed by quantitative RT-PCR as described in the section “Materials and Methods.” Data represent the mean ± SD of 3 separate experiments performed with 3 placentas. *Significantly different from values for villous explants cultured with vehicle alone (P < .05). (C) Immunocytochemistry. Cultured villous explant stained for IDO1 and IDO2 as described in the section “Materials and Methods.” The results presented are representative of 3 separate experiments performed with 3 placentas. Scale bars represent 100 µm.
Discussion
In this paper we have studied the cellular localization of IDO1 and IDO2, a more recently identified isoform of IDO, at the human maternal-fetal interface both in the 7 weeks and in the term placenta. A rare pregnancy sample at 7 weeks of gestation was obtained from hysterectomy for cervical cancer with her pregnancy in situ. The section of this sample includes the placental-decidual interface. Hitherto there is no information available on the cellular distribution of IDO2 at the human maternal-fetal interface.
Obvious expression of IDO1 and IDO2 is seen at the maternal-fetal interface both in the 7 weeks and in the term placenta (Figure 1). There is strong expression of IDO1 on the glandular epithelium in the decidua of 7 weeks of gestation. The cellular expression of IDO2 in the 7 week placenta shows obvious syncytiotrophoblastic expression. At term there is continued expression of IDO2 in the syncytiotrophoblast. There is more obvious staining for IDO1 in the term villous core compared to the first trimester villous tissue, specifically of fetal vessel endothelial cells and of CD68 positive macrophages. There is also staining for IDO1 in the decidua specifically of endothelium of maternal blood vessels. The extravillous cytotrophoblast in the term placental bed also expressed IDO2.
In our previous study we used monoclonal antibody to human IDO prepared by Takikawa et al22 and demonstrated that obvious trophoblastic expression of IDO with some immunoreactivity in the villous core and striking expression in the glandular epithelium with some positive cells in the decidual stroma of the first trimester and the term placenta.18 However our results of immunohistochemistry were in distinct contrast to those of Sedlmayr et al,19 Sedlmayr and Blaschitz,20 and Santoso et al26 Sedlmayer et al found no IDO staining of placental villi in the first trimester and Santoso et al did not demonstrate consistent IDO expression on syncytiotrophoblast at term. They however showed IDO strongly expressed in the glandular epithelium with some positive cells in the decidual stroma. We attributed these discrepant results to the methodological differences (e.g. concentration of antibody, antigen retrieval method) used in each study.18
In the present study we use the antibody to either human IDO1 or IDO2 raised against a recombinant protein for each enzyme respectively for the immunohistochemical analysis and demonstrated obvious syncytiotrophoblastic expression of IDO2 at the maternal-fetal interface both in the first trimester and in the term. The previous studies including ours were conducted before discovery of IDO2 and the authors used the monoclonal antibody to IDO prepared by Takikawa et al22 for immunohistochemical analysis.18,19,21,26 Since Takikawa’s antibody to human IDO was raised by using human IDO protein purified from human placenta, it is possible that this antibody can react with both IDO1 and IDO2 protein.
Expression of IDO2 in syncytiotrophoblasts is also confirmed functionally by immunohistochemistry of kynurenine (Figure 2). Kynurenine is the major degradation product of tryptophan by IDO. Immunoreactivity of kynurenine showed essentially the same localization as that of IDO1 and IDO2, and kynurenine immunoreactivity was more prominent in syncytiotrophoblasts where IDO2 is expressed as compared with cells expressing IDO1. However, IDO2 enzyme activity has been reported to be less compared with IDO1 activity in recombinant protein assay and cellular model in vitro.27,28 This is probably because amount of kynurenine is not only influenced by IDO activity, but also by downstream enzymes of the kynurenine pathway. Even more, kynurenine might even be produced somewhere else and taken up by certain cells. Another tryptophan catabolizing enzyme through the kynurenine pathway is tryptophan 2,3-dioxygenase (TDO).29 Although information of the localization and role of TDO in the human placenta is still limited,30 it is possible that the observed kynurenine immunoreactivity can also be attributed to TDO activity.
It has well been demonstrated that IFN-γ induces the expression of IDO1 mRNA and protein, however its effect on IDO2 expression is still controversial either at the mRNA or protein level.8,9,31-33 In the present study we show stimulation by IFN-γ of IDO1 mRNA expression yet no effect on IDO2 mRNA (Figure 3B) as reported previously.8,32 We also find striking changes in IDO1 immunoreactivity of the villous stroma following culture of villous explants with IFN-γ (Figure 3C). The endothelium of the fetal blood vessels and the presumptive macrophages become even more markedly immuno-positive for IDO1, whereas the immunoreactivity of IDO2 on the syncytiotrophoblast shows no detectable changes compared with control material incubated in the absence of this cytokine. These results are consistent with those of our earlier study using monoclonal antibody to human IDO prepared by Takikawa in which we showed striking enhancement of immunoreactivity of the cells within the stroma and no changes in syncytiotrophoblast following culture of villous explants with IFN-γ.18
It has been shown that in early pregnancy there is a particularly dense infiltrate of activated T cells and natural killer cells in the decidua having a different phenotype from peripheral blood natural killer cells34; and that decidual natural killer cells also produce a wide variety of cytokines.35 Secretion of interferon-γ by these immune cells has been confirmed.36 It is possible that cytokines produced by these cells regulate IDO expression and form a cytokine network at the site of placentation. The factors regulating IDO2 expression at the human maternal-fetal interface are clearly in need of further investigation.
Since the expression of IDO2 has not been studied in detail at the human maternal-fetal interface, its biological role is still yet to be defined. The findings of our present study, that the distribution and induction of IDO2 are dissimilar to those of IDO1 at the human maternal-fetal interface, suggest involvement of IDO2 in normal human pregnancy. Because the identification of IDO2 is so recent, the physiological role of IDO has not been classified into that of IDO1 and of IDO2 and the wording IDO may imply collective IDO functional activity in most published studies.
With regard to the physiological role of IDO in normal pregnancy, Munn et al11 have proposed the hypothesis that the expression of this enzyme in the placenta is crucial to prevention of immunological rejection of the fetal allograft. Thus IDO dependent localized depletion of tryptophan at the site of placentation has been proposed to be the mechanism of the suppression of maternal T cells which attack the conceptus.7 Badawy has recently hypothesized the tryptophan utilization concept in pregnancy, namely tryptophan metabolites through kynurenine pathway are key regulators of immune system responsiveness.15-17 However it has been demonstrated that allogeneic mating in which both parents were genetically IDO deficient can produced normal litters.37 The authors suggested that compensatory or redundant immunosuppressive mechanisms protected allogeneic fetuses in IDO deficient mice. This study was conducted before discovery of IDO2. It is possible to assume that animals they used were deficient in IDO1 and IDO2 expressed in syncytiotrophoblasts which we show in the present study may compensate for IDO1 enzyme activity and promote tryptophan depletion at the maternal-fetal interface. We suggest that IDO2 expressed in syncytiotrophoblasts is the isoform responsible for preventing allogeneic fetal rejection. It is possible to test this assumption by investigating whether fetal rejection can be induced with IDO2 inhibitor, 1-methyl-D-tryptophan,9 in IDO1 gene knockout mice. In tumor biology it has also been suggested that the IDO2 enzyme may be involved in immune evasion by tumor.9
Other physiological roles of IDO in the human placenta have proposed as follows; IDO expressed in the endothelium of the fetal blood vessels in the villous stroma and of spiral arteries in the decidua reduces the biosynthesis of vasoconstrictor serotonin and may contribute to maintaining feto-placental blood flow necessary for physiological placentation, placental function and fetal growth.38,39 It has also been demonstrated that a kynurenine pathway metabolite has a role of inducing vasodilatation.38,40,41 That expression and activity of IDO were significantly reduced in the placenta of pre-eclampsia has been demonstrated.38,41-43 This may be a connection between IDO mediated vasodilation and pre-eclampsia and/or intrauterine growth retardation. Recently, Broekhuizen et al41 demonstrated that increased tryptophan uptake might compensate for reduced IDO1 expression and induces IDO1 mediated relaxation in preeclamptic placental arteries. This has led to the hypothesis that increasing kynurenine pathway activity mediated by IDO may offer a new treatment strategy for preeclampsia.44 It has been shown in vitro model that IDO mediated tryptophan depletion induces apoptosis of extravillous trophoblast cells, thereby controlling trophoblast invasion and leading to normal placentation.45 We have also shown using cesarean scar pregnancy specimen that IDO expressed in the decidua may control extravillos cytotrophoblast invasion at the site of implantation and absence of its expression may be involved in the pathogenesis of over-invaded placenta.46 In addition, IDO1 in the utero-placental unit may provide a mechanism of local antimicrobial activity.19,47
From these we would like to suggest IDO1 expressed in the endothelial cells of the fetal blood vessels and of spiral arteries may contribute to maintaining feto-placental blood flow and IDO1 in the decidua may control extravillous cytotrophoblast invasion at the site of implantation and lead to the normal placentation; IDO2 in the syncytiotrophoblasts may be responsible for regulating maternal immune response to the fetal allograft at the maternal-fetal interface.
Although there are still fundamental questions about the role of IDO in human pregnancy, we think that the data provided here by studying IDO1 and IDO2 expression and their regulation at the maternal fetal interface may help address some of these. The most straightforward experimental strategy to delineate the significance of these enzymes in mammalian reproductive physiology might be use of knock out animals for IDO1 and/or IDO2.
Acknowledgments
We thank Dr C.A.R. Boyd, Brasenose College, Oxford for valuable comments.
Footnotes
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We are grateful to the Grant-in-Aid for Scientific Research (17K11238), Japan Society for the Promotion of Science for financial support.
Declaration of conflicting interests:The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: YK and JS designed experiments. IK collected a clinical sample. JS and IK performed the experiments. YK and JS analyzed the data and wrote the manuscript.
ORCID iDs: Yoshiki Kudo  https://orcid.org/0000-0001-6542-3408
https://orcid.org/0000-0001-6542-3408
Iemasa Koh  https://orcid.org/0000-0003-4617-1377
https://orcid.org/0000-0003-4617-1377
References
- 1. Yoshida R, Hayaishi O. Indoleamine 2,3-dioxygenase. Methods Enzymol. 1987;142:188-195. [DOI] [PubMed] [Google Scholar]
- 2. Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem J. 1985;230:635-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516-2522. [PubMed] [Google Scholar]
- 4. Daubener W, MacKenzie CR. IFN-gamma activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv Exp Med Biol. 1999;467:517-524. [DOI] [PubMed] [Google Scholar]
- 5. Burke F, Knowles RG, East N, Balkwill FR. The role of indoleamine 2,3-dioxygenase in the anti-tumour activity of human interferon-gamma in vivo. Int J Cancer. 1995;60:115-122. [DOI] [PubMed] [Google Scholar]
- 6. Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596-3599. [DOI] [PubMed] [Google Scholar]
- 7. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203-213. [DOI] [PubMed] [Google Scholar]
- 9. Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082-7087. [DOI] [PubMed] [Google Scholar]
- 10. Fukunaga M, Yamamoto Y, Kawasoe M, et al. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: the absence of IDO1 upregulates IDO2 expression in the epididymis. J Histochem Cytochem. 2012;60:854-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191-1193. [DOI] [PubMed] [Google Scholar]
- 12. Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Tryptophan degradation by human placental indoleamine 2,3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001;535:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kudo Y, Boyd CAR. The physiology of immune evasion during pregnancy; the critical role of placental tryptophan metabolism and transport. Pflugers Arch. 2001;442:639-641. [DOI] [PubMed] [Google Scholar]
- 14. Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309-379. [PubMed] [Google Scholar]
- 15. Badawy AA. The tryptophan utilization concept in pregnancy. Obstet Gynecol Sci. 2014;57:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Badawy AA. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015;35:e00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badawy AA, Namboodiri AM, Moffett JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci (Lond). 2016;130:1327-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kudo Y, Boyd CAR, Spyropoulou I, et al. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. 2004;61:87-98. [DOI] [PubMed] [Google Scholar]
- 19. Sedlmayr P, Blaschitz A, Wintersteiger R, et al. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol Hum Reprod. 2002;8:385-391. [DOI] [PubMed] [Google Scholar]
- 20. Sedlmayr P, Blaschitz A. Placental expression of indoleamine 2,3-dioxygenase. Wien Med Wochenschr. 2012;162:214-219. [DOI] [PubMed] [Google Scholar]
- 21. Ligam P, Manuelpillai U, Wallace EM, Walker D. Localisation of indoleamine 2,3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: implications for role of the kynurenine pathway in pregnancy. Placenta. 2005;26:498-504. [DOI] [PubMed] [Google Scholar]
- 22. Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041-2048. [PubMed] [Google Scholar]
- 23. Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152-2154. [DOI] [PubMed] [Google Scholar]
- 24. Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol. 2001;280:R1116-R1122. [DOI] [PubMed] [Google Scholar]
- 25. Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Modulation of indoleamine 2,3-dioxygenase by interferon-γ in human placental chorionic villi. Mol Hum Reprod. 2000;6:369-374. [DOI] [PubMed] [Google Scholar]
- 26. Santoso DIS, Rogers P, Wallace EM, Manuelpillai U, Walker D, Subakir SB. Localization of indoleamine 2,3-dioxygenase and 4-hydroxynonenal in normal and pre-eclamptic placentae. Placenta. 2002;23:373-379. [DOI] [PubMed] [Google Scholar]
- 27. Austin CJ, Mailu BM, Maghzal GJ, et al. Biochemical characteristics and inhibitor selectivity of mouse indoleamine 2,3-dioxygenase-2. Amino Acids. 2010;39:565-578. [DOI] [PubMed] [Google Scholar]
- 28. Yuasa HJ, Ball HJ, Ho YF, et al. Characterization and evolution of vertebrate indoleamine 2, 3-dioxygenases IDOs from monotremes and marsupials. Comp Biochem Physiol B Biochem Mol Biol. 2009;153:137-144. [PubMed] [Google Scholar]
- 29. Badawy AA. Possible involvement of the enhanced tryptophan pyrrolase activity in the corticosterone- and starvation-induced increases in concentrations of nicotinamide-adenine dinucleotides (phosphates) in rat liver. Biochem J. 1981;196:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dharane Nee Ligam P, Manuelpillai U, Wallace E, Walker DW. NFκB-dependent increase of kynurenine pathway activity in human placenta: inhibition by sulfasalazine. Placenta. 2010;31:997-1002. [DOI] [PubMed] [Google Scholar]
- 31. Croitoru-Lamoury J, Lamoury FM, Caristo M, et al. Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One. 2011;6:e14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520-3530. [DOI] [PubMed] [Google Scholar]
- 33. Lob S, Konigsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update. 1998;4:480-485. [DOI] [PubMed] [Google Scholar]
- 35. Jokhi PP, King A, Sharkey AM, Smith SK, Loke YW. Screening for cytokine messenger ribonucleic acids in purified human decidual lymphocyte populations by the reverse-transcriptase polymerase chain reaction. J Immunol. 1994;153:4427-4435. [PubMed] [Google Scholar]
- 36. Ho HN, Chao KH, Chen CK, Yang YS, Huang SC. Activation status of T and NK cells in the endometrium throughout menstrual cycle and normal and abnormal early pregnancy. Hum Immunol. 1996;49:130-136. [DOI] [PubMed] [Google Scholar]
- 37. Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67-77. [DOI] [PubMed] [Google Scholar]
- 38. Zardoya-Laguardia P, Blaschitz A, Hirschmugl B, et al. Endothelial indoleamine 2,3-dioxygenase-1 regulates the placental vascular tone and is deficient in intrauterine growth restriction and pre-eclampsia. Sci Rep. 2018;8:5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blaschitz A, Gauster M, Fuchs D, et al. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS One. 2011;6:e21774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stanley CP, Maghzal GJ, Ayer A, et al. Singlet molecular oxygen regulates vascular tone and blood pressure in inflammation. Nature. 2019;566:548-552. [DOI] [PubMed] [Google Scholar]
- 41. Broekhuizen M, Klein T, Hitzerd E, et al. l-Tryptophan-Induced vasodilation is enhanced in preeclampsia: studies on its uptake and metabolism in the human placenta. Hypertension. 2020;76:184-194. [DOI] [PubMed] [Google Scholar]
- 42. Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Decreased tryptophan catabolism by placental indoleamine 2,3- dioxygenase in preeclampsia. Am J Obstet Gynecol. 2003;188:719-726. [DOI] [PubMed] [Google Scholar]
- 43. Dunn WB, Brown M, Worton SA, et al. Changes in the metabolic footprint of placental explant-conditioned culture medium identifies metabolic disturbances related to hypoxia and pre-eclampsia. Placenta. 2009;30:974-980. [DOI] [PubMed] [Google Scholar]
- 44. Worton SA, Greenwood SL, Wareing M, Heazell AE, Myers J. The kynurenine pathway; a new target for treating maternal features of preeclampsia? Placenta. 2019;84:44-49. [DOI] [PubMed] [Google Scholar]
- 45. Reister F, Frank HG, Kingdom JC, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143-1152. [DOI] [PubMed] [Google Scholar]
- 46. Kudo Y, Koh I, Yamazaki T, Oomori Y, Mukai Y, Sugimoto J. Indoleamine 2,3-dioxygenase and trophoblast invasion in caesarean scar pregnancy: implications for the aetiopathogenesis of placenta accreta spectrum. J Reprod Immunol. 2020;138:103099. [DOI] [PubMed] [Google Scholar]
- 47. Sedlmayr P, Blaschitz A, Stocker R. The role of placental tryptophan catabolism. Front Immunol. 2014;5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]