Abstract
Background and aims:
Recently, several clinical trials have shown that increased glycated hemoglobin (HbA1c) level is correlated with poor clinical outcomes in ischemic stroke patients after thrombolysis and possibly after mechanical thrombectomy. However, the effect of HbA1c on posterior circulation large vessel occlusion (PCLVO) patients treated with endovascular thrombectomy (EVT) remains unclear. This multicenter study assessed the association between the HbA1c levels and clinical outcomes in patients with PCLVO after EVT.
Methods:
We studied 385 PCLVO ischemic stroke patients included in the EVT for acute basilar artery occlusion study (BASILAR). Patients were divided into a high HbA1c level group (HbA1c >6.5%) and a low HbA1c level group (HbA1c ⩽6.5%). The efficacy outcome was a 90-day favorable functional outcome (modified Rankin Scale 0–3). The safety outcomes included symptomatic intracerebral hemorrhage and mortality at 90 days after EVT.
Results:
The frequency of a favorable outcome in patients with an HbA1c ⩽6.5% was significantly higher than that in the HbA1c >6.5% group (41.2% versus 26.2%, p = 0.001). In multivariate analysis with adjusted confounders, high HbA1c levels and favorable outcomes were significantly negatively correlated. There was also a significant association between high HbA1c levels and mortality after 3 months. The negative effects of high HbA1c levels on functional status after 3 months were exacerbated in patients aged ⩾65 years.
Conclusion:
Our multicenter study suggests that a higher serum HbA1c level (HbA1c >6.5%) is an independent predictor of a 90-day poor outcome and mortality in patients with PCLVO after EVT, particularly in those aged ⩾65 years.
Clinical Trial Registry identifier: ChiCTR1800014759.
Keywords: endovascular treatment, glycated hemoglobin, posterior circulation
Introduction
Hyperglycemia in patients with acute ischemic stroke (AIS) is a well-established predictor of poor clinical outcomes. It occurs in approximately one-third of AIS patients.1 A significant association was reported between hyperglycemia at admission and adverse prognosis in patients with emergent large vessel occlusion after mechanical thrombectomy.2 Hyperglycemia not only can aggravate ischemic brain injury by enhancing intracellular acidosis in the penumbra and increasing free radical formation but also can damage cerebral autoregulation and the blood–brain barrier, leading to ischemia–reperfusion injury.3,4
However, serum glucose fluctuates during the day, for reasons that include exercise, the level of blood insulin, diet, and medical treatment.5 Inflammatory responses and an acute stress reaction may elevate the serum glucose levels, which could be negative prognostic factors.1,6,7 By contrast, glycated hemoglobin (HbA1c), a widely used glycemic control parameter, reflects the average blood glucose level over several months before the examination and is less susceptible to recent changes.5 Recent researches have shown that higher HbA1c levels may increase the risk of ischemic stroke and new ischemic lesions in patients with AIS.8–10 Other studies have reported an independent association between a higher HbA1c level and poor prognosis after intravenous thrombolysis.11 However, only a few studies with a small sample size and single-center studies have investigated the relationship between HbA1c and clinical outcomes in patients who had undergone endovascular treatment.12,13 To the best of our knowledge, this association has not been examined in patients with acute posterior circulatory endovascular treatment (EVT).
In our multicenter prospective cohort study, we aimed to analyze the relationship between the HbA1c levels and clinical outcomes in patients with posterior circulation occlusion of large vessels (PCLVO) after EVT.
Methods
Study population
Our patients were selected from the EVT for acute basilar artery occlusion study (BASILAR), a nationwide prospective cohort study including 47 comprehensive stroke centers in China that tested the efficacy and safety of EVT in patients with AIS due to basilar artery occlusion. All patients or their legally authorized representatives provided signed informed consent and their HbA1c levels were systematically measured before admission. BASILAR was approved by the China Ethics Committee of Registering Clinical Trials (approval number CHiECRCT-20180023) and is registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn;ChiCTR1800014759). The details of this registration study have been published previously.14
The main inclusion criteria were as follows: (1) age ⩾18 years; (2) presentation of estimated time of PCLVO ⩽24 h; (3) presence of PCLVO on magnetic resonance angiography (MRA), computed tomographic angiography (CTA), or digital subtraction angiography; (4) EVT performed within 24 h of estimated time of PCLVO; (5) performance of tests to determine the HbA1c levels; and (6) informed consent was signed.
Patients were excluded when they (1) had a premorbid Modified Rankin Scale (mRS) >2; (2) evidence of cerebral hemorrhage by neuroimaging; (3) incomplete clinical data, including laboratory data; and (4) pregnant or lactating women and those patients with a serious, terminal illness.
Treatments
Patients received standard medical treatment after excluding intracranial hemorrhage by non-contrast computed tomography (CT). The use of recombinant tissue plasminogen activator (rt-PA) or urokinase, anticoagulation, and antiplatelet drugs were in accordance with the guidelines for the management of AIS.15,16 Patients were treated with EVT using stent retrievers, balloon angioplasty thromboaspiration, stenting, intra-arterial thrombolysis, or multiple combinations of these approaches within 24 h of the estimated time of PCLVO.
Data collection
We recorded the following data for analysis: baseline information, laboratory data, risk factors for stroke, imaging performance, and EVT characteristics. Details of the data are available in Tables 1 and 2.
Table 1.
Baseline clinical and biochemical characteristics, according to HbA1c levels.
| HbA1c ⩽6.5% n = 255 |
HbA1c >6.5% n = 130 |
p valuea | |
|---|---|---|---|
| Age, years, mean ± SD | 64.7 ± 11.5 | 62.4 ± 11.1 | 0.033 |
| Men, n (%) | 190 (74.5) | 99 (76.2) | 0.724 |
| History of risk factors, n (%) | |||
| Hypertension | 165 (64.7) | 108 (83.1) | <0.001 |
| DM | 20 (7.8) | 103 (79.2) | <0.001 |
| Dyslipidemia | 85 (33.3) | 56 (43.1) | 0.061 |
| Atrial fibrillation | 52 (20.4) | 23 (17.7) | 0.527 |
| Cerebral infarction | 48 (18.8) | 34 (26.2) | 0.097 |
| Smoking | 102 (40.0) | 50 (38.5) | 0.77 |
| TIA | 4 (1.6) | 2 (1.5) | 0.982 |
| SBP, mmHg, mean ± SD | 148.7 ± 27.0 | 154.1 ± 23.1 | 0.028 |
| Biochemical variables, mean ± SD | |||
| Total cholesterol, mmol/L | 4.7 ± 1.4 | 4.8 ± 1.5 | 0.964 |
| LDL-C, mmol/L | 3.0 ± 1.2 | 2.9 ± 1.0 | 0.626 |
| Triglyceride, mmol/L | 1.4 ± 1.0 | 2.1 ± 2.7 | 0.477 |
| HDL-C, mmol/L | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.994 |
| Admission serum glucose, mmol/L | 7.3 ± 2.2 | 11.0 ± 3.4 | <0.001 |
| TOAST classification, n (%) | 0.465 | ||
| LAA | 170 (66.7) | 96 (73.8) | |
| CE | 67 (26.3) | 27 (20.8) | |
| SOE | 5 (2.0) | 3 (2.3) | |
| SUE | 13 (5.1) | 4 (3.1) | |
| Initial NIHSS | 22.9 ± 10.2 | 24.4 ± 9.7 | <0.001 |
| Initial pc-ASPECTS | 8.0 ± 1.6 | 7.6 ± 1.8 | <0.001 |
Continuous variables were compared between groups using Student t tests. The χ2 test was used for non-continuous variables.
CE, cardioembolism; DM, diabetes mellitus; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LAA, large artery atherosclerosis; LDL-C, low-density lipoprotein cholesterol; NIHSS, National Institutes of Health Stroke Scale; pc-ASPECTS, posterior circulation Alberta Stroke Program Early CT Score; SBP, systolic blood pressure; SOE, stroke of other determined cause; SUE, stroke of undetermined cause; TIA, transient ischemic attack; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Table 2.
Procedural-related characteristics, according to HbA1c levels.
| HbA1c ⩽6.5% n = 255 |
HbA1c >6.5% n = 130 |
p value | |
|---|---|---|---|
| Occlusion site, n (%) | 0.108 | ||
| BA distal | 77 (30.2) | 34 (26.2) | |
| BA middle | 79 (31.0) | 36 (27.7) | |
| BA proximal | 50 (19.6) | 26 (20.0) | |
| V4 | 43 (16.9) | 34 (26.2) | |
| PCA | 6 (2.4) | 0 (0) | |
| mTICI, n (%) | 0.109 | ||
| mTICI 0–2a | 40 (15.7) | 29 (22.3) | |
| mTICI 2b–3a | 215 (84.3) | 101 (77.7) | |
| Anesthesia, n (%)b | 0.214 | ||
| General | 80 (31.6) | 49 (38.0) | |
| Local | 173 (68.4) | 80 (62.0) | |
| ASITN/SIR, n (%) | 0.991 | ||
| ASITN/SIR 0–1 | 145 (56.9) | 74 (56.9) | |
| ASITN/SIR 2–4c | 110 (43.1) | 56 (43.1) | |
| Time variables, min, mean ± SD | |||
| Onset to puncture time | 419.3 ± 306.3 | 422.1 ± 459.9 | 0.023 |
| Onset to recanalization time | 535.0 ± 316.0 | 541.3 ± 462.9 | 0.001 |
| Puncture to recanalization time | 115.7 ± 64.9 | 119.3 ± 69.3 | <0.001 |
| Intravenous thrombolysis, n (%) | 40 (15.7) | 23 (17.7) | 0.615 |
| Intracerebral hemorrhage, n (%) | 6 (2.4) | 1 (0.8%) | 0.271 |
A TICI score of 2b or 3 indicates complete recanalization.
Data were not available in three patients (two patients in HbA1c ⩽6.5% group and one patient in HbA1c >6.5%).
An ASITN/SIR score of 2–4 indicates favorable collateral compensation.
ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; BA, basilar artery; HbA1c, glycated hemoglobin; mTICI, modified thrombolysis in cerebral infarction; PCA, posterior cerebral artery; V4, V4 segment of vertebral artery.
Patients were divided into two groups according to their plasma HbA1c levels, measured at admission. The high HbA1c level group was defined as a HbA1c >6.5%, which meets the current criteria for diagnosis of diabetes.5 The low HbA1c level group consisted of patients with HbA1c ⩽6.5%. The degree of recanalization was evaluated at the end of the procedure. Successful reperfusion after EVT was defined as modified Thrombolysis in Cerebral Infarction (mTICI) scores of 2b or 3.17 The patients also underwent non-contrast CT to identify intracerebral hemorrhage. CTA or MRA was performed within 48 h after EVT to determine vessel patency. Relevant imaging data were evaluated by the imaging core laboratory.
Outcome measurements
The efficacy clinical outcome was assessed by the mRS scores at 90 days. A favorable functional outcome was defined as a mRS score between 0 and 3 (indicating the ability to walk without assistance, 0–3 is preferred over 0–2 for posterior stroke, which carries a worse prognosis than those of anterior circulation).
The safety clinical outcomes included symptomatic intracerebral hemorrhage (sICH) and mortality at 90 days after EVT. sICH was defined as a newly observed intracranial hemorrhage leading to an increase of ⩾4 points on the National Institutes of Health Stroke Scale (NIHSS) score before worsening or increase of ⩾2 points in one category.
Statistical analysis
Data analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Differences between the two groups were tested using the χ2 test for categorical values and the t-test or Fisher’s exact test for continuous values. We defined a p value <0.05 as statistically significant.
The assessment of the odds ratios (ORs) and 95% confidence intervals (CIs) for clinical outcomes used a multivariable logistic regression analysis. We adjusted the variables based on clinical significance, including age, systolic blood pressure (SBP), NIHSS score, onset to puncture time, occlusion site, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology score, and posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS). Subgroups included mTICI (0–2a or 2b–3), pc-ASPECTS ⩽7 or 7 < pc- ASPECTS ⩽10, NIHSS score (⩽25 or >25), sex, and age (⩽65 or >65 years). We also analyzed the heterogeneity of the HbA1c effect within subgroups.
Results
Patient characteristics
Our multicenter prospective study examined the data of 385 patients with PCLVO treated with EVT after AIS. The data were obtained from the BASILAR database in China. There were 255 patients with a HbA1c level ⩽6.5% and 130 patients with a HbA1c level >6.5%. All patients had follow-ups at 3 months. Twenty-seven patients (21.8%) had no history of diabetes mellitus (DM) in the high HbA1c level group, but should be diagnosed as occult DM.5 We found that hypertension, SBP, serum glucose level at admission, and initial NIHSS scores were higher in patients with HbA1c levels >6.5% and initial pc-ASPECTS were lower in this group. Table 1 presents the associations between the HbA1c levels and biochemical and clinical characteristics.
Characteristics of endovascular procedures
Intravenous thrombolysis was performed in 40 patients (15.7%) with a HbA1c level ⩽6.5% and in 23 patients (17.7%) with a HbA1c level >6.5%. Basilar artery occlusion was found in 206 patients (80.7%) in the HbA1c ⩽6.5% group and in 96 patients (73.8%) in the HbA1c >6.5% group. Poor collateral flow (American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology 0–1) was found in 145 patients (56.9%) with a HbA1c ⩽6.5% and 74 patients (56.9%) with a HbA1c >6.5%. Successful reperfusion (mTICI 2b or 3) was achieved in 316 patients. Onset to puncture time, onset to recanalization time, puncture to recanalization time, and initial NIHSS scores were higher in the high HbA1c level group. Table 2 describes the procedural characteristics.
Efficacy outcomes
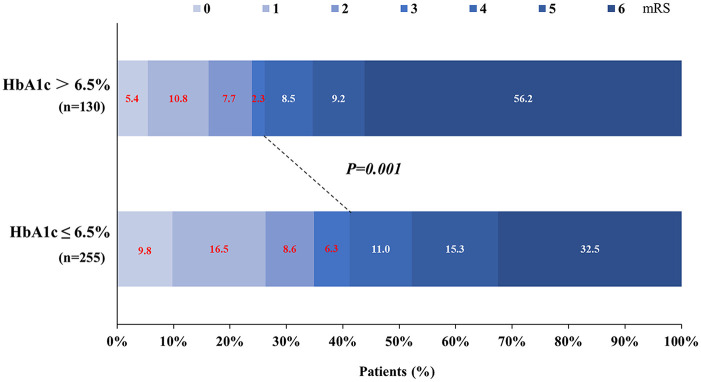
There were 139 patients who had a favorable outcome (mRS of 0 to 3). The frequency of favorable outcomes in patients with a HbA1c ⩽6.5% was significantly higher than that in the HbA1c >6.5% group (41.2% versus 26.2%, p = 0.001; Figure 1). In a multivariate analysis with adjusted confounders, high HbA1c levels and favorable outcomes were found to be significantly negatively correlated (OR, 0.52; 95% CI, 0.28 − 0.94; p = 0.032; Table 3).
Figure 1.
Primary outcomes according to glycated hemoglobin (HbA1c) levels. Distribution of modified Rankin Scale (mRS) scores at 3 months in patients treated with endovascular treatment.
Table 3.
Association between high HbA1c levels (HbA1c >6.5%) and outcomes.
| Clinical outcomes | HbA1c ⩽6.5% n = 255 n (%) |
HbA1c >6.5% n = 130 n (%) |
Adjusted ORa (95% CI) | p valueb |
|---|---|---|---|---|
| Efficacy outcomes at 3 months | ||||
| Good outcome, mRS, 0–3 | 105 (41.2) | 34 (26.2) | 0.52 (0.28–0.94) | 0.032 |
| Safety outcomes | ||||
| 3-month mortality | 83 (32.5) | 73 (56.2) | 2.89 (1.68–4.99) | <0.001 |
| Symptomatic intracranial hemorrhagec | 12 (4.8) | 10 (7.9) | 1.59 (0.62–4.05) | 0.330 |
The multiple logistic regression test was used to analyze ORs. Adjusted variables: age, systolic blood pressure, National Institutes of Health Stroke Scale, onset to puncture time, occlusion site, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology, Alberta Stroke Program Early CT Score.
The Bonferroni correction method was applied to multiple comparisons using a p value <0.05/number of comparisons as a threshold for statistical significance.
Data were not available in seven patients (four patients in HbA1c ⩽6.5% group and three patients in HbA1c >6.5%).
CI, confidence interval; HbA1c, glycated hemoglobin; mRS, modified Rankin Scale; OR, odds ratio.
Safety outcomes
In the multivariable analysis, there was also a significant association between the high HbA1c levels and mortality after 3 months (OR, 2.89; 95% CI, 1.68–4.99; p < 0.001). We found no correlation between the high HbA1c levels and sICH.
Subgroup analyses
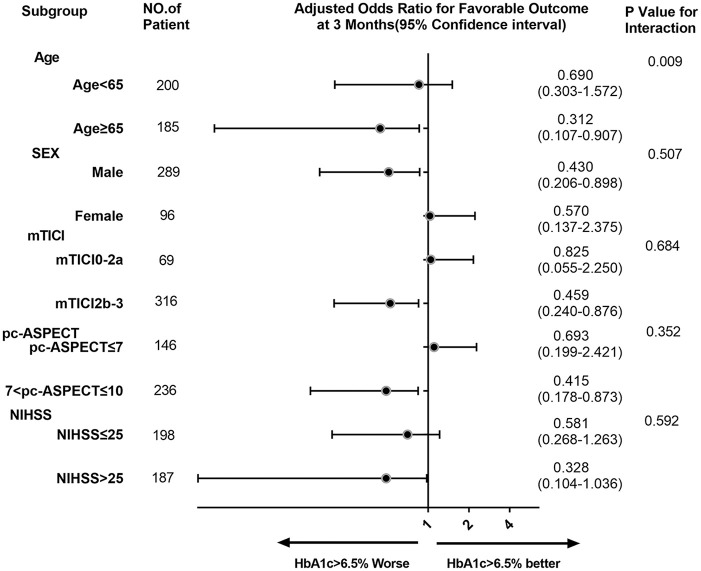
There was significant heterogeneity between the patients aged <65 years and patients aged ⩾65 years in OR for favorable outcome in subgroup analyses. In the age subgroup, a high HbA1c level in those aged ⩾65 years was associated with lower odds for favorable outcome at 3 months than in the <65 years group. Heterogeneity was not affected by the high HbA1c levels in the other prespecified subgroups. Subgroup analysis is shown in Figures 2 and 3.
Figure 2.
Subgroup analyses of primary outcomes. The forest plot shows the differences in odds ratios for favorable outcomes (defined as modified Rankin Scale score of 0–3) at 3 months in the prespecified subgroups. Adjusted variables: age, systolic blood pressure, National Institutes of Health Stroke Scale (NIHSS), puncture to recanalization time, occlusion site, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology, posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS).
CT, computed tomography; HbA1c, glycated hemoglobin; mTICI, modified Thrombolysis in Cerebral Infarction.
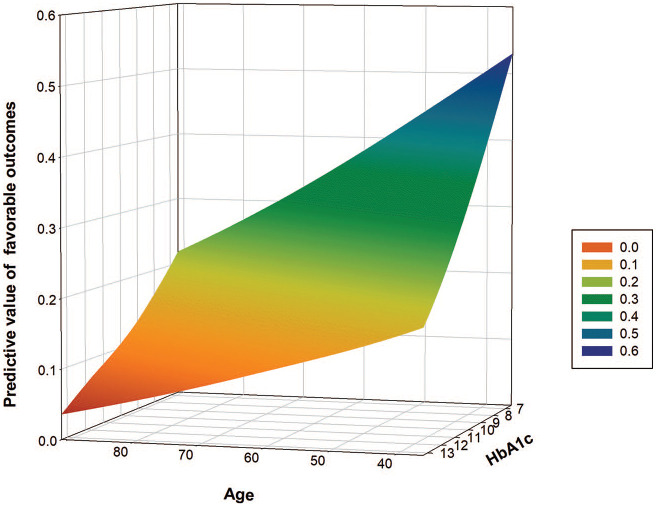
Figure 3.
Association between age and predictive value of favorable outcomes in the high glycated hemoglobin (HbA1c) level group.
Discussion
This multicenter cohort study, to the best of our knowledge, is the first to examine the association between the HbA1c levels and functional outcomes among PCLVO patients treated with EVT. Our data indicated that the higher HbA1c levels (>6.5%) were associated with a poor functional outcome and higher mortality at 3 months. The correlation remained statistically significant after adjusting for confounding factors.
High HbA1c levels reflect a chronic hyperglycemia status and abnormal glucose metabolism.18 Compared with acute hyperglycemia, chronic hyperglycemia might be less related to inflammatory and stress responses.1 Given the link between chronic hyperglycemia and the mechanism of injury to the nervous system in AIS patients,12,19,20 previous studies have emphasized the importance of intensive pre-stroke glycemic control, which may lead to better outcomes after performing reperfusion therapies.13,21 Our results regarding the HbA1c levels and clinical outcome also supported this view. In our study, there were some patients with HbA1c levels >6.5% who were not diagnosed with diabetes according to the present standards before stroke.22 A previous study by O’Sullivan et al.23 found a higher mortality rate in undiagnosed than in already diagnosed patients with DM after emergency vascular surgical procedures. This may suggest that the regular serum glucose control and testing are necessary for a better prognosis in diagnosed and undiagnosed patients with DM.12
Previous researchers have reported that higher HbA1c levels were correlated with worse clinical outcomes in patients with AIS after intravenous rt-PA.11,24 Compared with intravenous rt-PA, EVT treatment for AIS had a higher reperfusion rate and did not increase the mortality rates or the risk for sICH.25,26 Therefore, recent studies have investigated the association between HbA1c levels and clinical outcomes, and found that high HbA1c levels had a negative effect on clinical outcomes.12,13 Our findings were consistent with those obtained from previous studies on functional outcome. However, our study further highlighted the effect of HbA1c levels on posterior circulation large vessel occlusion treated with EVT.
A previous study on cardiovascular disease showed that high HbA1c was associated with increased all-cause mortality.27 However, recent findings regarding the relationship between the HbA1c levels and mortality after ischemic stroke were controversial. Specifically, some reports have suggested that there was no association, while others have revealed a detrimental effect in patients with high HbA1c levels.28,29 In the present study, we found that high HbA1c level was a significant predictive factor of 3 months mortality in patients with PCLVO after EVT (OR, 2.89; 95% CI, 1.68–4.99; p < 0.001). This discrepancy may be attributed to the different study populations. Further researches are needed in the future to define this association.
In our study, successful reperfusion (mTICI, 2b or 3) was achieved in 215 (84.3%) and 101 (77.7%) patients in the HbA1c ⩽6.5% and HbA1c >6.5% groups, respectively. Although the recanalization rates appeared to be higher in the normal HbA1c level group, the difference between the two groups was not statistically significant (p = 0.109). Thomas et al.30 found that the futile recanalization was significantly higher in the posterior than in the anterior circulation. These findings may suggest that the protective effect of normoglycemia does not seem to be attributed to high recanalization rates.
A previous post hoc analysis of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution Study 2 trial demonstrated that age was an independent predictor of adverse functional outcomes.31 Similarly, our observations also suggested that the negative effects of higher HbA1c levels on the functional status at the 3 month follow-up were exacerbated in patients aged ⩾65 years. These results may suggest that more caution should be taken in elderly patients treated with EVT, especially in those with PCLVO.
Limitations
Our study had some limitations. First, as it was an observational study, there may be inevitable biases. However, our prospective data collection from multiple centers minimized the risk of bias. Although we have adjusted for confounding factors in a multivariate analysis, unmeasured residual confounding factors may still exist. Moreover, because of the lack of data on diabetes typing and fasting glucose, we were unable to analyze their effects on the patients’ prognosis.
Conclusion
In summary, a high HbA1c level may be an independent predictor of poor prognosis at 3 months in patients with PCLVO after EVT. These findings may help physicians to identify the PCLVO patients that may benefit more or less from EVT. Further confirmation of this finding or potential methods to reduce this risk should be examined in a well-designed prospective, randomized trial.
Acknowledgments
We would like to thank Prof. WenJje Zi for helping us to perform statistical analyses. We would also like to thank Editage (www.editage.cn) for English language editing. We are grateful to all the co-investigators for their dedication to the study.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Army Medical University Clinical Medical Research Talent Training Program (Nos. 2019XLC2008, 2019XLC3016, 2018XLC2013, and 2018XLC3039), National Science Fund for Distinguished Young Scholars (No. 81525008), Chongqing Major Disease Prevention and Control Technology Research Project (No. 2019ZX001), and Major Clinical Innovation Technology Project of the Second Affiliated Hospital of the Army Military Medical University (No. 2018JSLC0017), Chongqing Natural Science Foundation (No.cstc2020jcyj-msxmX0926) and Jilin Province Science and Technology Innovation Center Construction Project (No.20190902002TC).
ORCID iD: Shouchun Wang  https://orcid.org/0000-0002-6570-4530
https://orcid.org/0000-0002-6570-4530
Contributor Information
Feixue Yue, Department of Neurology, The First Hospital of Jilin University, Changchun, Jilin, China.
Zhongxiu Wang, Department of Neurology, The First Hospital of Jilin University, Changchun, Jilin, China.
Jie Pu, Department of Neurology, Hubei Province People’s Hospital, Wuhan, China.
Min Zhang, Department of Neurology, Chinese Medical Hospital of Maoming, Maoming, China.
Yong Liu, Department of Neurology, Liu’an Affiliated Hospital of Anhui Medical University, Liu’an, China.
Hongxing Han, Department of Neurology, Linyi People’s Hospital, Linyi, China.
Wenhua Liu, Department of Neurology, Wuhan No. 1 Hospital, Wuhan, China.
Xianjun Wang, Department of Neurology, Linyi People’s Hospital, Linyi, China.
Rongzong Li, Department of Neurology, The 924th Hospital of The People’s Liberation Army, Guilin, China.
Dongzhang Xue, Department of Neurology, The 902th Hospital of The People’s Liberation Army, Bangfu, China.
Jiaming Cao, Department of Neurology, The 904th Hospital of The People’s Liberation Army, Wuxi, China.
Zhizhong Yan, Department of Neurology, The 904th Hospital of The People’s Liberation Army, Wuxi, China.
Guozhong Niu, Department of Neurology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Hao Zhang, Department of Neurology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Haitao Guan, Department of Neurology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Hongliang Zeng, Department of Neurology, Ganzhou People’s Hospital, Ganzhou, China.
Feng You, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Qian Yang, Department of Neurology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China.
Wenjie Zi, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Yi Zhang, Department of Neurology, The First People’s Hospital of Chenzhou, Chenzhou, China.
Zetao Shao, Department of Neurology, Changle People’s Hospital, Weifang, China.
Jincheng Liu, Department of Neurology, The First People’s Hospital of Xiangyang, Hubei Medical University, Xiangyang, China.
Jun Sun, Department of Neurology, Nanyang Central Hospital, Nanyang, China.
Shouchun Wang, Department of Neurology, The First Hospital of Jilin University, No. 1 Xinmin Street, Changchun, Jilin 130021, China.
References
- 1. Luitse MJ, Biessels GJ, Rutten GE, et al. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 2012; 11: 261–271. [DOI] [PubMed] [Google Scholar]
- 2. Goyal N, Tsivgoulis G, Pandhi A, et al. Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J Neurointerv Surg 2018; 10: 112–117. [DOI] [PubMed] [Google Scholar]
- 3. Suh SW, Shin BS, Ma H, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol 2008; 64: 654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desilles JP, Syvannarath V, Ollivier V, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke 2017; 48: 1932–1940. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(Suppl. 1): S13–S27. [DOI] [PubMed] [Google Scholar]
- 6. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009; 373: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kruyt ND, Biessels GJ, Devries JH, et al. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 2010; 6: 145–155. [DOI] [PubMed] [Google Scholar]
- 8. Oh HG, Rhee EJ, Kim TW, et al. Higher glycated hemoglobin level is associated with increased risk for ischemic stroke in non-diabetic Korean male adults. Diabetes Metab J 2011; 35: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitsios JP, Ekinci EI, Mitsios GP, et al. Relationship between glycated hemoglobin and stroke risk: a systematic review and meta-analysis. J Am Heart Assoc 2018; 7: e007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braemswig TB, Nolte CH, Fiebach JB, et al. Early new ischemic lesions located outside the initially affected vascular territory appear more often in stroke patients with elevated glycated hemoglobin (HbA1c). Front Neurol 2017; 8: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu SY, Cao WF, Wu LF, et al. Effect of glycated hemoglobin index and mean arterial pressure on acute ischemic stroke prognosis after intravenous thrombolysis with recombinant tissue plasminogen activator. Medicine 2018; 97: e13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi KH, Kim JH, Kang KW, et al. HbA1c (glycated hemoglobin) levels and clinical outcome post-mechanical thrombectomy in patients with large vessel occlusion. Stroke 2019; 50: e225. [DOI] [PubMed] [Google Scholar]
- 13. Diprose WK, Wang M, Mcfetridge A, et al. Glycated hemoglobin (HbA1c) and outcome following endovascular thrombectomy for ischemic stroke. J Neurointerv Surg 2019; 12: 30–32. [DOI] [PubMed] [Google Scholar]
- 14. Zi W, Qiu Z, Wu D, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol 2020; 77: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 16. Ming L, Mao-lin H, Chuan-qiang P, et al. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2014. Chin J Neurol 2015; 48: 246–257. [Google Scholar]
- 17. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007; 50: 2239–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou Q, Zuo Z, Michel P, et al. Influence of chronic hyperglycemia on cerebral microvascular remodeling: an in vivo study using perfusion computed tomography in acute ischemic stroke patients. Stroke 2013; 44: 3557–3560. [DOI] [PubMed] [Google Scholar]
- 20. Umemura T, Kawamura T, Hotta N. Pathogenesis and neuroimaging of cerebral large and small vessel disease in type 2 diabetes: a possible link between cerebral and retinal microvascular abnormalities. J Diabetes Invest 2017; 8: 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamouchi M, Matsuki T, Hata J, et al. Prestroke glycemic control is associated with the functional outcome in acute ischemic stroke: the Fukuoka Stroke Registry. Stroke 2011; 42: 2788–2794. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 2019; 42(Suppl. 1): S13–S28. [DOI] [PubMed] [Google Scholar]
- 23. O’sullivan CJ, Hynes N, Mahendran B, et al. Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg 2006; 32: 188–197. [DOI] [PubMed] [Google Scholar]
- 24. Masrur S, Cox M, Bhatt DL, et al. Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post-thrombolysis: findings from get with the guidelines-stroke. J Am Heart Assoc 2015; 4: e002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 26. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 27. Sakurai M, Saitoh S, Miura K, et al. HbA1c and the risks for all-cause and cardiovascular mortality in the general Japanese population: NIPPON DATA90. Diabetes Care 2013; 36: 3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roquer J, Giralt-Steinhauer E, Cerdà G, et al. Glycated hemoglobin value combined with initial glucose levels for evaluating mortality risk in patients with ischemic stroke. Cerebrovasc Dis 2015; 40: 244–250. [DOI] [PubMed] [Google Scholar]
- 29. Lew J, Thijs V, Churilov L, et al. Using routine HbA1c measurements in stroke and the associations of dysglycaemia with stroke outcomes. J Diabetes Complications 2018; 32: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 30. Thomas R, Johannes K, Panagiotis CL, et al. Mechanical thrombectomy for basilar artery occlusion: efficacy, outcomes, and futile recanalization in comparison with the anterior circulation. J Neurointerv Surg 2019; 11: 1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liggins JT, Yoo AJ, Mishra NK, et al. A score based on age and DWI volume predicts poor outcome following endovascular treatment for acute ischemic stroke. Int J Stroke 2015; 10: 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]