Abstract
Interoception refers to the representation of an organism’s internal states, and includes the processes by which it senses, interprets, integrates, and regulates signals from within itself. This review article presents a unified research framework and attempts to offer definitions for key terms to describe the processes involved in interoception. We elaborate on these definitions through illustrative research findings, and provide brief overviews of central aspects of interoception, including anatomy and function of neural and non-neural pathways, diseases and disorders, manipulations and interventions, and predictive modeling. We conclude with discussions on major research gaps and challenges.
Keywords: sympathetic, parasympathetic, vagal, spinal, insula, autonomic
The Emerging Science of Interoception at the NIH
Neuroscience has progressed tremendously in the last decades in clarifying how we sense and interact with the external world. On the sensory side, this line of research, sometimes referred to as “exteroception”, encompasses (according to most definitions) the primary sensory systems of vision, audition, olfaction, taste, and somatosensation. Less in known about the interoceptive system--the nervous system’s ability to represent our own internal world. On April 16 and 17 of 2019, the National Institutes of Health (NIH), Blueprint for Neuroscience Research convened a two-day workshop titled “The Science of Interoception and Its Roles in Nervous System Disorders.” At the workshop, a distinguished group of investigators highlighted recent findings and discussed a wide range of topics critical to the future of interoception research.
The workshop identified many critical knowledge gaps in areas related to interoceptive research, including: 1) ) characterization of functional circuits and interaction dynamics between central and peripheral nervous systems in physiological conditions; 2) delineation of the interaction between interoceptive networks involved in basic physiological processes (e.g., respiration, thirst, feeding, urination, metabolism) and other sensory, motor, reward, emotional, cognitive/memory, and social circuits; 3) determining the impact of central and peripheral disorders on interoceptive networks, and the effects of modulating interoceptive processes on associated diseases and disorders; and 4) the need for objective and quantitative assessments of interoception as well as effective technologies and approaches to measure and modulate interoceptive processes.
This article builds on these discussions and efforts to propose a unified framework of interoception science research by defining and describing several key terms involved. As context for these definitions and for providing some concrete examples of their implications, we also briefly review some key elements of interoceptive processing, from the angles of neuroanatomical analysis, function and dynamics, disease implications, potential interventions, computational modeling, and the integration of internal and external representations.
What is interoception?
The definition of interoception has evolved over the years. About 150 years ago, the concept was established through the identification of a set of physiological parameters that defined an organism’s normal internal state[1]. In the mid-20th century, the idea evolved to reflect the more dynamic concept of homeostasis[2]. More recently, interoception has been commonly referred to as the process by which the nervous system senses and integrates information about the inner state of the body[3].
Several issues regarding the definition of interoception require careful re-evaluation and clarification. First, whereas the terms “sensing” and “integrating” seem to imply a one-way communication to the brain from other organs, the links between brain and body are often bidirectional and also include communications from the brain to the other organs and in turn, modulation of the internal body signals sent back to the brain. Therefore, a more comprehensive definition of interoception should encompass the complex interplay between the brain and other organs necessary to monitor and regulate internal states. Secondly, the anatomical boundary that distinguishes interoceptive and exteroceptive signals requires a nuanced conversation. Conventional wisdom points to skin as the obvious border, with interoception referring to the processing of signals generated from within the body below the skin. For example, neural activities in subcutaneous tissues, including muscles and connective tissues, that contribute to proprioception, are a form of interoception[4]. As the anatomical boundaries between interoception and some forms of exteroception become blurred, such as in the case of proprioception and somatosensation, a complementary approach would be to assess whether the signals and the body’s responses represent, rather than originate from, the internal or external world. Gustation and taste also encounter the dilemma of sensing both the internal and external worlds, but the nutrient sensing in the gastrointestinal system is clearly more indicative of an internal status than an external world representation. Another example is the vestibular system, which is located in the same sensory organ as the auditory system but typically represents the internal world of an organism’s balance, thus belonging to interoception rather than exteroception[5].
Given these considerations, we propose a revised description of interoception that may more accurately reflect, we would argue, the bidirectional signal processing between the brain and the internal organs to represent the internal state of an organism. We recognize that this revised description significantly expands the traditional scope of interoception and may be different from the one used in much of the current literature. In this revised description, interoception includes the processes by which an organism senses, interprets, integrates, and regulates signals from within itself. Here, the action of “sensing” denotes communication from physiological systems outside of the central nervous system (CNS) to the CNS, through the commonly called ascending pathways, whereas the action of “regulating” refers to the communication from the brain to other physiological systems via descending pathways. The CNS, especially the brain, is primarily responsible for interpreting and integrating these signals into a representation of the internal world. One key difference between this revised definition and some of the more traditional definition of interoception is the inclusion of the descending body regulation component. The other key point is that the systems involved in processing signals about the internal environment include not only the peripheral nervous system and the CNS but also components of the vascular, endocrine, and immune systems. In the following sections, we describe key concepts related to interoceptive processes and illustrate aspects of the proposed expanded framework, which we hope will foster future venues for interoception research.
Interoceptive Signals, Interoceptors, and Sensing Processes
Interoception starts with interoceptive signals, which are signals originating from within an organism. Interoceptive signals can generally be categorized into three major types. The first type includes biochemical signals that range from inorganics, such as acidic ions, to organic molecules and small peptides. The second type includes mechanical forces that alter structures, such as cellular shape, through stretch or tissue extension. The third type are thermal and electromagnetic signals, which may be delivered in various wave frequencies across the electromagnetic spectrum.
Interoceptors are molecular sensors or receptors in neurons that directly detect these various interoceptive signals, and transduce them into electrical, hormonal, or other non-neural signals to be integrated and interpreted by the brain [6]. Interoceptors include chemoreceptors, humoral receptors, specialized mechanoreceptors, and free nerve endings or nociceptors[7]. It is important to note that the biochemical identity of most interoceptors remains largely unknown, with only a few specific examples described to date[8, 9]. One key challenge for interoception science is the development of systematic approaches to unravel the molecular identities of interoceptors.
Interoceptor location may determine whether the interoceptive signals are transmitted through the peripheral neural system or a non-neural system. For example, chemical interoceptors located on neurons inside the brain most likely receive interoceptive signals through non-neural pathways such as the circulatory or lymphatic systems. Classic neuroendocrine systems such as the hypothalamic–pituitary–adrenal (HPA) axis, hypothalamic–pituitary–gonadal axis (HPG), hypothalamic–pituitary–thyroid axis (HPT), and hypothalamic–neurohypophyseal system offer examples of interoception communication via non-neural systems. In contrast, some interoceptors, such as mechanical or thermal interoceptors, are expressed in peripheral nerve termini, to directly detect signals in local organs, induce the peripheral sensory ganglia to generate electrical signals, and thereby transmit the interoceptive information through the peripheral neural pathways to the brain (Figure 1).
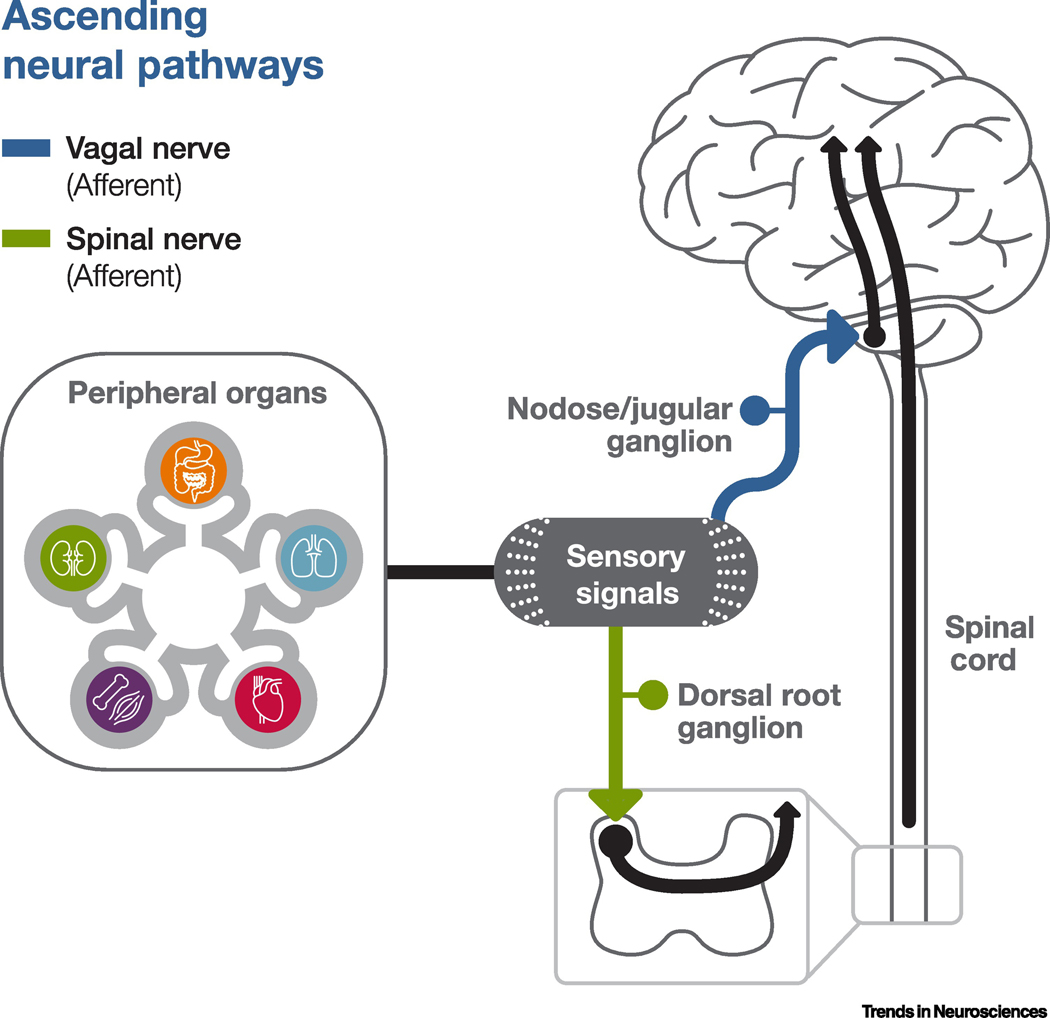
Figure 1. Illustrative diagram of sample ascending neural pathways of interoception.
This diagram only describes the ascending neural pathways connecting the peripheral internal organs with the brain. The non-neural ascending pathways are not included in this figure. Interoceptive sensory signals generated by the peripheral organs may be detected by molecular sensors or receptors, called interoceptors, present at the termini of the peripheral sensory ganglia. The sensory ganglia residing in the cranial/vagal pathways, such as nodose or jugular ganglia, often send projections to the nucleus tractus solitarii (NTS) of the brainstem through vagal afferents, sometimes referred to as ‘parasympathetic afferents.’ The sensory ganglia located along the spinal nerve pathway, often called dorsal root ganglia, send projections through the dorsal column of the spinal cord into the brain via spinal afferents, sometimes referred to as ‘sympathetic afferents.’
There are generally two major ascending peripheral neural or afferent pathways that transmit interoceptive signals to the CNS [10–13]. Signals in these two pathways commonly relay through two distinct types of peripheral sensory ganglia. Ganglia residing in the cranial/vagal pathways, such as nodose or jugular ganglia, often project to the nucleus tractus solitarii (NTS) of the brainstem; whereas dorsal root ganglia, located along the spinal nerve pathway, project information to the brain through the spinal cord[13]. Visceral afferents that travel along cranial nerves, including the vagus, can also be referred to as ‘parasympathetic afferents,’ whereas those that travel through the dorsal column of the spinal cord are often called ‘sympathetic afferents’ [10]. It is hypothesized that vagal afferents appear to carry primarily mechanoreceptor and chemosensory signals; spinal afferents carry signals related to temperature, pain, and tissue injury [12–15]. Some evidence has suggested that vagal and spinal afferents may represent opposing parasympathetic and sympathetic signals, and may thus interact within the brain’s interoceptive regions to inhibit each other [13]. However, much remains to be studied to assess the differences between these two types of ascending neural pathways and their impact.
Central interpreters and integrators of interoception include neurons in the CNS involved in processing, interpreting, and/or integrating interoceptive signals. Whether delivered through the humoral, lymphatic, or peripheral nervous systems, interoceptive information is often first processed in subcortical structures of the brain such as the medial NTS, the parabrachial nucleus (PB), and the ventromedial nucleus of the thalamus[4, 11, 12, 16, 17] (Figure 2). In turn, the neurons in these structures may project to higher brain regions including the hypothalamus, insula, anterior cingulate cortex, and somatosensory cortex for further integration and interpretation [5, 18–24].
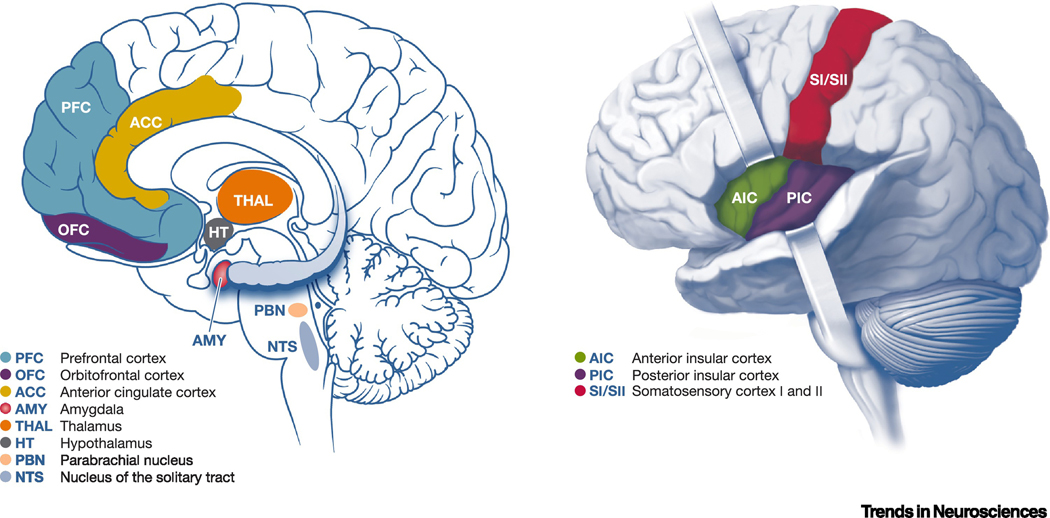
Figure 2. Brain regions involved in central processing of interoceptive signals.
The diagrams in this figure depict some of the various brain regions involved in central interpretation, integration, and regulation of interoceptive information. The figure on the left is a mid-sagittal view of the human brain, highlighting brain regions implicated in central processing of interceotion. The figure on the right is a lateral-saggital view of the brain highlighting the insula cortex, including anterior insular cortex (AIC) and posterior insular cortex (PIC), as well as somatonsensory cortex (SI/SII). Within the brain, interoceptive information is often first processed in subcortical structures such as the medial nucleus of the solitary tract (NTS), the parabrachial nucleus (PB), and then the ventromedial nucleus of the thalamus. Neurons in these structures project to other brain regions such as the hypothalamus and eventually to the insular cortex, a critical cortical node in the interoceptive system. Primary interoceptive information relayed from the ventromedial nucleus of the thalamus projects to PIC, where integration with exteroceptive sensorimotor and proprioceptive information from SI/II most likely occurs. AIC is most strongly connected to paralimbic cortical regions such as the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and prefrontal cortex (PFC), which may be involved in linking between interoceptive and emotional or cognitive states for further integration and interpretation, and possibly generation of regulatory signals sent back to lower brain regions involved in interoceptive descending efferent systems.
Early in the field’s history, the insula has emerged as a critical cortical node in the interoceptive system. Penfield’s neurosurgical stimulation experiments first connected insular cortex to visceral sensation, and neuroanatomical analyses confirmed a viscerotopic map in the insula [12, 13, 16, 25–27]. Although our understanding of anatomical and functional parcellation of the insula remains incomplete, studies in mammals ranging from rodents to humans consistently reveal a posterior-to-anterior topography in the insular interoceptive map [18, 27–29]. Primary interoceptive information is relayed from the ventromedial nucleus of the thalamus to the posterior insula, and integration with exteroceptive sensorimotor and proprioceptive information most likely occurs within the posterior and central regions [22, 30]. Anterior insular cortex (AIC) is most strongly connected to paralimbic cortical regions such as the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) and may be involved in connections between interoceptive and emotional or cognitive states [4, 16, 24, 26, 29, 31–33]. It is important to note some key anatomical differences in the insula across species [34]. The uniquely shaped and functionally mysterious layer 5 Von Economo and fork neurons are found primarily in the AIC and ACC of macaques, great apes and humans, as well as a number of other large and highly-social mammalian species such as elephants and whales [26, 29, 35]. The insula is considered to be part of the ventral salience network for arousal-based affective experience. The ventral salience network is largely homologous between monkeys and humans, but the dorsal network for attentional control is much more developed in humans[27, 30, 33, 36–38]. The insula also receives major inputs from the amygdala, but rodents and monkeys have markedly different organization of amygdalar nuclei[39, 40].
Processing of some interoceptive information may not require higher levels of cortical processing. In other cases, interoceptive signals may engage higher order processing at perceptual, cognitive, and/or affective levels, thus rising to the level of conscious awareness [6]. In humans, the insula is activated when individuals consciously attend to their own interoceptive states, suggesting that it may serve as a critical interoceptive hub for integrating and regulating signals from the internal and external environments [26]. Understanding how and when autonomic versus conscious processing of interoceptive information occurs remains an intriguing area for further in-depth investigations[7].
Regulation of Interoception
The concept of regulating interoception via descending pathways has not been incorporated into most definitions of interoception, despite the well-established ability of the CNS to generate signals that regulate the internal state [41, 42]. We will refer to these signals generated by the CNS, often in response to interoceptive input or cognitive and exteroceptive factors, to regulate the interoceptive processes, as “regulating signals of interoception”. Neurons in the CNS involved in generating these regulating signals can be called central regulators of interoception (Figure 3).
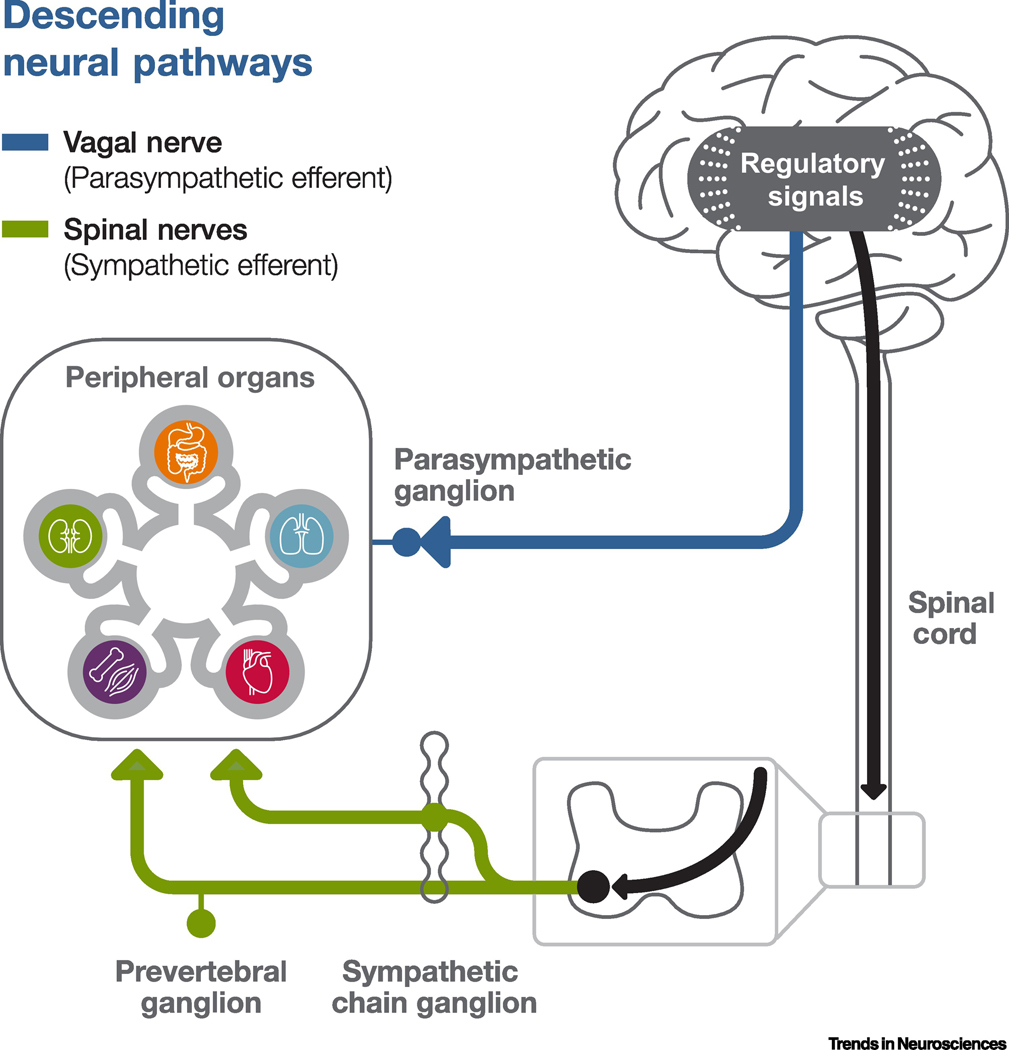
Figure 3. Illustrative diagram of sample descending neural pathways of interoception.
This diagram only describes the descending neural pathways connecting the brain with the peripheral internal organs. The non-neural descending pathways are not included in this figure. We refer to the signals generated by the CNS, often in response to interoceptive input, to regulate the interoceptive processes as “regulating signals of interoception”. Neurons in the CNS involved in generating these regulating signals can be called central regulators of interoception. The regulating signals may be communicated to the peripheral internal organs via descending spinal or vagal/cranial efferents. The spinal efferents are also referred to as the sympathetic efferents of the autonomic nervous system as they go through the spinal cord pathway and regulate sympathetic activities. After existing the spinal cord, some of the spinal efferents synapse onto the sympathetic chain ganglia, which then directly project onto the peripheral organ cells to regulate interoceptive signals or organ function. These sympathetic chain ganglia may thus be considered as the effector neurons in the interoceptive descending neural pathway. Other spinal efferents synapse onto prevertebral ganglia instead, which may act as effector neurons and project to the peripheral organ cells. The vagal/cranial efferents, also called the parasympathetic efferents of the autonomic nervous system, typically go through the nodose or jugular ganglia bundles and make synaptic connections onto parasympathetic ganglia, which are often located near the peripheral organs and act as effector neurons to regulate peripheral organ’s interoceptive signals or organ functions.
Regulating signals of interoception, similar to interoceptive signals, can be transmitted via both non-neural (e.g. humoral) and neural pathways (e.g. cranial/vagal or spinal efferents) that target the peripheral organs[23, 43]. In non-neural pathways, regulating signals are delivered to the peripheral organ via the vascular or lymphatic system and interact directly with the responding non-neural cells. In neural pathways, the final effectors, commonly called sympathetic or parasympathetic ganglion neurons, receive input from the brain through the spinal or vagal/cranial efferent nerves and directly synapse with the internal organ non-neural cells[43] (Figure 3). It is worth noting that the non-neural and neural pathways may interact, such that a regulating signal may be initially delivered through one pathway (e.g. a non-neural pathway) to impact the other pathway (e.g. neural pathway) before impacting its final effector target.
The primary function of the regulating signals of interoception is to regulate the generation and transmission of interoceptive signals of the targeted internal organs, which can be called effectors, and thus complete the circle of interoceptive processes. However, often the impact of the regulating signals may be best measured by the responses or changes of function in the target organs. It is therefore sometimes impossible to distinguish regulating interoceptive signals from body regulation. In our view, the inclusion of body regulation as a component of interoception science not only is necessary from a neuroanatomical perspective but also enables the development of more innovative methods to probe the functional impact of interoceptive processes, although we recognize that in the current literature, many have described body regulation and interoception as separate terms and concepts.
Gaps and Challenges of Research in Interoceptive Neural Circuits
The picture of the interoceptive nervous system sketched above (and see Figures 1, 2, and 3) is certainly incomplete. Many gaps and methodological challenges exist for studying neural circuits of interoception at neuroanatomical and functional levels.
The neuroanatomical techniques used to establish the knowledge base of interoception have primarily included histochemical and autoradiographic tract tracing, cytoarchitectonic analyses, and MRI-based diffusion tractography (DTI) [13, 31, 44, 45]. Notably, tract-tracing studies show highly collateralized and interconnected networks across all nodes between the periphery to the cortex [22, 46]. Additional experiments using tools such as trans-neuronal tracers and viral-based techniques are needed to establish a more detailed picture [17, 22]. Multiple gaps also exist between research focused on inputs from the periphery to brainstem and those focused on the insular cortex[47–49]. For instance, more attention needs to be paid to examining the thalamic relay(s) [45]. Additionally, much remains to be understood regarding the connections among the ascending and descending pathways, such as the hypothalamic endocrine connections and the connections from insula to other brain regions including somatosensory cortex and beyond. How do the neuronal and non-neural pathways connecting brain and periphery interact and influence each other[16]?
Converging and diverging projections are known to exist at all levels of interoception, but critical cellular and synaptic-level information is missing. Experiments focused on the molecular-level specificity and diversity of neuronal types within the interoceptive system have just begun to emerge [17, 50–52]. For instance, although attention has been paid to the different cytoarchitectonic regions of the insular cortex, is there a single representation of the body in the insula or might there be multiple, overlapping maps [26, 52]? Furthermore, it is important to note that properties and cell types within the insular cortex appear to differ substantially across species, limiting the applicability of some findings from rodents to human interoceptive health [34]. At the periphery level, transneuronal tracers have had limited application to date, consisting primarily of anterograde studies from scattered visceral organ systems [22, 46, 53–55]. Visceral afferents are low density, thin, unmyelinated fibers that can be difficult to visualize [56]. Focusing on cell type analysis and imaging of regions or nodes with high density of neurons, such as various types of peripheral sensory ganglia might offer a great opportunity to gain more comprehensive understanding [56–62]. Moreover, little is known about the diverse cell types involved in many of the key information processing nodes for interoception from peripheral ganglia to cortex, whereas the complexity of circuits within these nodes, such as the brainstem and hypothalamus, remain largely unmapped.
Functional techniques have been mostly limited to early studies of evoked potentials in vagotomized animals and very few human lesion case reports. A more recent collection of resting state and task-based fMRI studies in humans have revealed widespread visceral-brain coupling and placed insula function within the larger ‘salience network’ [27, 30, 33, 36–38, 63]. The insula and associated cortical and subcortical networks have been correlated with a wide range of possible functions related to interoception and allostasis, including visual perception, mental time-keeping, emotion, empathy, language and music perception, and self-awareness [23, 26, 27, 34, 38]. However, much remains to be understood about the many brain regions involved in interoception, especially whether specific neuronal populations in these regions are functioning as interpreters, integrators, or regulators of interoceptive information.
While interoception studies in humans currently rely primarily on fMRI BOLD and DTI structural imaging [16] to provide critical information about large scale function, these methodologies are fundamentally correlative. In-depth, mechanistic studies that link anatomical findings to function will be important to substantiate the roles of brain regions and domains in interoception. In human subject research, non-invasive methodologies such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), may offer opportunities to assess causal effects of brain regions in interoception [64]. However, the deep location of some of the brain regions involved in interoception, including the insular cortex make them difficult to image and stimulate [33]. Imaging studies in lesion patients, for instance AIC lesions, may also offer some causal evidence although requiring caution in their interpretation [24].
Functional Impact and Dynamics
Optimal sensing, interpretation, integration, and regulation of internal body signals, whether rising to the level of conscious awareness or not, are critical for many essential physiological functions, such as breathing, eating, drinking, micturition, and maintaining body temperature, as well as psychological experiences, ranging from a variety of feelings and emotions to motivations and adaptive behaviors [3]. In addition, interoceptive and exteroceptive processes may interact to orchestrate complex physiological and behavioral functions, such as in the stress response [65]. The functions of interoception may range from essential bodily functions, to high level cognitive and emotional behaviors[66], and can be roughly grouped into two categories, based on the direction of information flow.
The first category of interoception functions includes information flow from the body to the brain. For example, it is mostly unknown how affect --a brain state that can be described as a low-dimensional representation of high-dimensional behavioral state in the body [67]-- is influenced by interoception. However, recent work on gut and hippocampal function [17], which tested the hypothesis that specific interoceptive signals are transported via the vagus nerve to the hippocampus to influence memory function, provides an elegant example on how one could begin to address this challenge.
The second category of interoception functions includes information flow from the brain to the body to exert bodily effects. The voluntary urination model is a great example. The target output of voluntary urination is the urethral sphincter, which is somatically controlled by the pontine micturition center (PMC, or Barrington’s nucleus) of the brain [68], particularly corticotropin-releasing factor (CRF) labeled subpopulations of neurons in the PMC [69, 70]. Stimulation of CRF-labeled neurons in the PMC caused immediate urination in mice [69], suggesting that the PMC neurons are sufficient to drive behavior. When neural activity was blocked in CRF-labeled neurons in the PMC, voluntary urination was inhibited. Often, this category of interoceptive function utilizes autonomic nervous system pathways, sympathetic or parasympathetic.
In animal models, it is often possible to couple (1) neural activity manipulations, even at the single neuron level; (2) neural activity measurements, such as electrophysiological or physiological assays, and/or in vivo neural imaging; and (3) functional assessments to measure organ physiology and/or behavioral assays. Such experimental combinations have the potential to best support rigorous gain- and loss-of-function analyses of interoceptive neural circuits. In contrast, assessments of interoception in humans have been largely limited to a handful of approaches and remain mostly correlational. These approaches include heart beat measures [71–73], skin conductance responses [74, 75] and subjective self-report measures, such as interoceptive accuracy or awareness[76]. More recently, microneurography has emerged as an objective tool to probe interoceptive responses [77, 78]. If one includes body regulation as part of interoception research, additional physiological measures, such as blood pressure and baroreflex [79], may be explored to assess the functional impact of interoception. A key caveat in this context is that many organ functions are vital, making causal analyses challenging in human subject research of interoception. Furthermore, developing consistent experimental measures and metrics for comparable studies in humans and animal models will be critical for translating insights across animal model and human studies.
Dynamic changes in interoception pathways over the lifespan remain largely unexplored. A number of research groups have begun to study interoception in early life [80] and to examine how interoception changes with age[81–83], but relatively little is known about lifelong changes in interoception. One key challenge is the lack of a measure of interoceptive sensitivity suitable for non-verbal populations such as infants[80] or older adults with dementia [84]. In addition, conducting longitudinal studies and identifying milestones of interoception development will be necessary to uncover the changes in interoception across the lifespan.
Diseases and Disorders
Dysfunction of interoception has been increasingly recognized as an important component of many neurological, psychiatric, and behavioral disorders [3]. Altered structure, functional activity, or connectivity within the interoceptive neural network have been observed for instance in individuals with migraine and other types of chronic pain [85, 86], alcohol and substance use disorders[87], anxiety, depression and affective disorders [88, 89], posttraumatic stress disorder[90], obsessive compulsive disorder[91], autism spectrum disorder(ASD) [92], eating disorders[93], somatic symptom disorders[94], stroke and neurodegenerative diseases[95, 96], and may be a basis for comorbidity of neuropsychiatric and mental disorders[3, 97].
For example, irritable bowel syndrome, characterized by visceral hypersensitivity, is associated with disruptions in the endocrine and immune systems in the gut as well as changes in the cortical neuronal network of sensorimotor, salience, emotion, and arousal [54] and disrupted modulation of insular cortex [98] in the human brain. Similarly, obesity is associated with disrupted interoceptive states from the gut to the brain with altered functional connectivity of the insula [99] and distributed brain regions between the dorsal mid-insula, medial OFC, dorsal striatum, and ventral striatum[100].
Many psychiatric and neurodevelopmental disorders, such as schizophrenia[101], attention-deficit hyperactivity disorder[102], autism spectrum disorder, depression, and anxiety disorders, are not only linked to altered brain networks that are crucial for the integration of interoceptive signals to emotion processing and cognition but also exhibit a variety of physical symptoms[3]. For example, ASD is associated with dysregulated anterior insula connectivity and deficient emotional processing that may be due to aberrant prediction errors in interoceptive processing[72, 103]. At the same time, individuals with ASD often show interoceptive changes such as increased sensitivity to pain and gastrointestinal symptoms[104].
Similarly, addiction to alcohol or other substances of abuse may cause adaptation in the interoceptive network, which not only exacerbates stress and contribute to the alteration of emotion and reward processing, but also gives rise to comorbid conditions through central and peripheral interactions[105, 106]. A recent animal model study identified a genetic factor that links the neurocircuit adaptation of tobacco smoking to type 2 diabetes in humans[107], and provided evidence to show that the brain can control peripheral organ function while peripheral organs can regulate the addictive properties of nicotine. The gut-brain connection has also been shown to play a role in addictive behaviors and alcohol use disorders. For instance, ghrelin is a hunger hormone secreted in the stomach, and its concentration in individuals with alcohol use disorder correlates with alcohol drinking and predict alcohol relapse [108, 109]. In rodents, administration of ghrelin leads to an increase in alcohol reward and in alcohol intake, administration, and preference, while blockade of the ghrelin receptor resulted in reduction of these behaviors [108, 109]. Moreover, evidence suggests that ghrelin, delivered through the vascular system, may mediate the signaling from gut to brain via the vagus nerve [17, 110].
Although a wide range of diseases and disorders is related to interoception[3, 97], it is important to note that while pathophysiological and causal evidence has been generated for some of these conditions, in most of them, the links to interoceptive dysregulation is largely symptomatic and descriptive. Future pathophysiological studies in clinical populations as well as in appropriate animal models are necessary for deepening the understanding of the possible outcomes of dysregulated interoceptive processing.
Manipulations and Interventions
The complex interplay between the ascending and descending pathways of the interoceptive system provides many potential routes and methods for targeted interventions in the case of interoceptive dysfunction and related disorders. A multitude of approaches, ranging from non-invasive behavioral manipulations to specific pharmacological and neural stimulation interventions, have been considered for regulating interoceptive processes[111].
In general, these approaches can be categorized into three groups – behavioral, neural stimulation, and pharmacological - each with advantages and limitations. Behavioral interventions, such as meditation and cognitive behavioral therapy (CBT), are the least invasive and generally the safest among the various intervention[112]. These approaches utilize exteroceptive routes (e.g. sound, vision, somatosensation, and cognitive influence) to trigger activity in the brain and likely exert their effects on interoceptive body signals and function[113] through descending pathways. The limitations of these approaches include a lack of established therapeutic targets, relatively low potency, and delayed improvements[114]. Current neural stimulation approaches include TMS, tDCS, deep brain stimulation (DBS), vagal nerve stimulation (VNS), and transcutaneous electrical nerve stimulation (TENS) which target the spinal cord or peripheral nerves[115]. If the therapeutic targets are clear and specific, nerve stimulation can be a potent therapeutic with few side effects. However, therapeutic targets for most of these approaches have yet to be identified and validated. In addition, some approaches, such as DBS and VNS, may require invasive neurosurgery. Pharmacological interventions, such as blockade of the ghrelin receptor and/or of the peptide itself represent promising approaches, although additional translational work is needed in that regards [108, 109] The pharmacological approach could be particularly effective for targeting interoceptors, either within the brain, at the periphery, or along associated molecular signaling pathways. Like in most pharmacological approaches, off-target effects and related side effects pose major challenges.
Regardless of the type of interventional approaches, identifying, assessing, and validating the therapeutic targets through rigorous mechanistic studies in both humans and appropriate animal models will be critical for safety, efficacy, and effectiveness to treat interoception-related diseases and disorders. In addition, group and individual variables including sex, gender, age, and social factors are important elements to consider for interventional studies.
Predictive modeling
Computational modeling is an essential component of interoception research as it helps to formalize the empirical findings within a mathematical framework, as well as provide predictions for future experiments [116–118]. Several mathematical models are currently used to describe both the perceptual and body regulation aspects of interoception [117, 119], although the sensory or perceptual aspect of interoception and the body regulation aspect are often modeled as separate processes. It may be desirable to develop computational models that could capture and describe the proposed framework of interoception in this article. Additionally, modeling how interoceptive processes may be integrated with exteroceptive processes can provide a more comprehensive picture of how our nervous system interacts with the rest of the body to maintain function and support survival [119]. Modeling key parameters and outcomes that are accessible in experimental studies, such as “interoceptive accuracy” and “interoceptive awareness”[72, 76], can greatly facilitate the development and validation of computational models and should be strongly encouraged.
Concluding Remarks
While the foundations of the science of interoception were laid over 100 years ago, interoception research mostly regained a momentum in recent years, partly due to the availability of high-resolution, multi-modal tools for interrogating interoceptive processes. In this article, we outlined a proposed comprehensive framework of interoception science, which may help accelerate progress towards an integrative understanding of how we sense and regulate our internal states. Both conceptual and technical/methodological challenges remain (see Outstanding Questions), and our hope is that this review, along with other articles in this Special Issue, will offer stimulating ideas to enrich the emerging science of interoception.
Outstanding Questions:
Interoceptors are molecular sensors or receptors in neurons that directly detect various interoceptive signals, and transduce them into electrical, hormonal, or other non-neural signals to be integrated and interpreted by the brain. What are the molecular entities of these interoceptors? How can one systematically identify them?
What are the functional circuits of interoception? How do the central and peripheral nervous systems dynamically interact to support interoceptive processing, both in physiological and pathological conditions?
What is the impact of central or peripheral disorders on interoceptive networks? What are the effects of modulating interoceptive processes on diseases and disorders of the nervous system?
How can one develop more objective and quantitative assessments of interoception?
What are the most effective strategies for developing technologies and approaches to modulate interoceptive processes?
How do the interoceptive networks interact with other sensory, motor, reward, emotional, cognitive/memory circuits to regulate nervous system diseases and disorders?
Highlights:
Interoception refers to the representation of the internal world and includes the processes by which an organism senses, interprets, integrates, and regulates signals from within itself.
The brain communicates with internal organs via the peripheral nervous system and non-neuronal systems.
Key components of a unified research framework of interoception include: interoceptive signals; interoceptors; ascending and descending pathways; central interpreters; central integrators; central regulators; and interoceptive effectors.
In-depth mechanistic studies linking anatomical findings to function are important to define the roles of key elements of interoception.
Dysfunction of interoception may be an important component of many neurological, psychiatric, and behavioral disorders.
Better understanding of the neural basis of interoception may provide therapeutic targets for interoceptive dysfunction and related nervous system disorders.
Acknowledgement
The Workshop on “The Science of Interoception and Its Roles in Nervous System Disorders,” which served as an important foundation for this review article, was supported by the NIH Blueprint for Neuroscience Research and funding contributions from NCCIH, NIAAA, NIDCR, and OBSSR of the NIH. We would like to thank Dr. Paige Green from NCI, Dr. Michael Oshinsky from NINDS, and Dr. Marjorie Garvey from NIMH for their services in the workshop planning committee, the Directors and Coordination Committee members of the NIH Blueprint for Neuroscience Research Institute, Centers, and Offices for their support as well as thoughtful discussions on this effort, and Dr. Emmeline Edwards from NCCIH for her support and feedback for the workshop as well as the manuscript. In addition, we thank Catherine Law and Cindy Million for assistance in the revised manuscript. We would also like to thank all the invited speakers, panel discussants, and other workshop attendees for their intellectually stimulating presentations and discussions at the workshop. Lastly, we would like to thank Bryan Ewsichek from NCCIH for the graphic designs of figures 1 and 3 and the NIH Medical Arts for the graphic design of figure 2 in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cameron OG, Interoception: the inside story--a model for psychosomatic processes. Psychosom Med, 2001. 63(5): p. 697–710. [DOI] [PubMed] [Google Scholar]
- 2.Wiener N, The concept of homeostasis in medicine. Trans Stud Coll Physicians Phila, 1953. 20(3): p. 87–93. [PubMed] [Google Scholar]
- 3.Khalsa SS, et al. , Interoception and Mental Health: A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging, 2018. 3(6): p. 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuthill JC and Azim E, Proprioception. Curr Biol, 2018. 28(5): p. R194–R203. [DOI] [PubMed] [Google Scholar]
- 5.Shinder ME and Newlands SD, Sensory convergence in the parieto-insular vestibular cortex. J Neurophysiol, 2014. 111(12): p. 2445–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denton D, et al. , Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proc Natl Acad Sci U S A, 1999. 96(9): p. 5304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berntson GGB, et al. Neural Circuits of Interoception. this issue of Trends in Neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranade SS, et al. , Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature, 2014. 516(7529): p. 121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesler AT, et al. , The Role of PIEZO2 in Human Mechanosensation. N Engl J Med, 2016. 375(14): p. 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei N, Recent studies on intestinal vagal afferent innervation. Functional implications. J Auton Nerv Syst, 1983. 9(1): p. 199–206. [DOI] [PubMed] [Google Scholar]
- 11.Janig W, Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol, 1996. 42(1–2): p. 29–51. [DOI] [PubMed] [Google Scholar]
- 12.Saper CB, The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci, 2002. 25: p. 433–69. [DOI] [PubMed] [Google Scholar]
- 13.Craig AD, How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci, 2002. 3(8): p. 655–66. [DOI] [PubMed] [Google Scholar]
- 14.Barone FC, Zarco de Coronado I, and Wayner MJ, Gastric distension modulates hypothalamic neurons via a sympathetic afferent path through the mesencephalic periaqueductal gray. Brain Res Bull, 1995. 38(3): p. 239–51. [DOI] [PubMed] [Google Scholar]
- 15.Taher J, Farr S, and Adeli K, Central nervous system regulation of hepatic lipid and lipoprotein metabolism. Curr Opin Lipidol, 2017. 28(1): p. 32–38. [DOI] [PubMed] [Google Scholar]
- 16.Critchley HD and Harrison NA, Visceral influences on brain and behavior. Neuron, 2013. 77(4): p. 624–38. [DOI] [PubMed] [Google Scholar]
- 17.Suarez AN, et al. , Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat Commun, 2018. 9(1): p. 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cechetto DF and Saper CB, Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol, 1987. 262(1): p. 27–45. [DOI] [PubMed] [Google Scholar]
- 19.Cobos I and Seeley WW, Human von Economo neurons express transcription factors associated with Layer V subcerebral projection neurons. Cereb Cortex, 2015. 25(1): p. 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champagne D, Beaulieu J, and Drolet G, CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: a tract-tracing study. J Neuroendocrinol, 1998. 10(2): p. 119–31. [DOI] [PubMed] [Google Scholar]
- 21.Iwai H, et al. , Ascending parabrachio-thalamo-striatal pathways: potential circuits for integration of gustatory and oral motor functions. Neuroscience, 2015. 294: p. 1–13. [DOI] [PubMed] [Google Scholar]
- 22.Dum RP, Levinthal DJ, and Strick PL, The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci, 2009. 29(45): p. 14223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu X, et al. , Anterior insular cortex and emotional awareness. J Comp Neurol, 2013. 521(15): p. 3371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, et al. , Anterior insular cortex plays a critical role in interoceptive attention. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penfield W and Faulk ME Jr., The insula; further observations on its function. Brain, 1955. 78(4): p. 445–70. [DOI] [PubMed] [Google Scholar]
- 26.Craig AD, How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci, 2009. 10(1): p. 59–70. [DOI] [PubMed] [Google Scholar]
- 27.Azzalini D, Rebollo I, and Tallon-Baudry C, Visceral Signals Shape Brain Dynamics and Cognition. Trends Cogn Sci, 2019. 23(6): p. 488–509. [DOI] [PubMed] [Google Scholar]
- 28.Kelly C, et al. , A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage, 2012. 61(4): p. 1129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gogolla N, The insular cortex. Curr Biol, 2017. 27(12): p. R580–R586. [DOI] [PubMed] [Google Scholar]
- 30.King AB, et al. , Human forebrain activation by visceral stimuli. J Comp Neurol, 1999. 413(4): p. 572–82. [PubMed] [Google Scholar]
- 31.Mufson EJ and Mesulam MM, Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol, 1982. 212(1): p. 23–37. [DOI] [PubMed] [Google Scholar]
- 32.Khalsa SS, et al. , The pathways of interoceptive awareness. Nat Neurosci, 2009. 12(12): p. 1494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uddin LQ, et al. , Structure and Function of the Human Insula. J Clin Neurophysiol, 2017. 34(4): p. 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butti C and Hof PR, The insular cortex: a comparative perspective. Brain Struct Funct, 2010. 214(5–6): p. 477–93. [DOI] [PubMed] [Google Scholar]
- 35.Evrard HC, Forro T, and Logothetis NK, Von Economo neurons in the anterior insula of the macaque monkey. Neuron, 2012. 74(3): p. 482–9. [DOI] [PubMed] [Google Scholar]
- 36.Seeley WW, et al. , Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 2007. 27(9): p. 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurth F, et al. , A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct, 2010. 214(5–6): p. 519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleckner IR, et al. , Evidence for a Large-Scale Brain System Supporting Allostasis and Interoception in Humans. Nat Hum Behav, 2017. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesulam MM and Mufson EJ, Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol, 1982. 212(1): p. 38–52. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LW and Petrovich GD, What is the amygdala? Trends Neurosci, 1998. 21(8): p. 323–31. [DOI] [PubMed] [Google Scholar]
- 41.Li MM, et al. , The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits. Neuron, 2019. 102(3): p. 653–667 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taggart P, et al. , Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Auton Neurosci, 2016. 199: p. 54–65. [DOI] [PubMed] [Google Scholar]
- 43.Janig W and Habler HJ, Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic and protective body functions. Prog Brain Res, 2000. 122: p. 351–67. [DOI] [PubMed] [Google Scholar]
- 44.Mesulam MM and Mufson EJ, Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol, 1982. 212(1): p. 1–22. [DOI] [PubMed] [Google Scholar]
- 45.Mufson EJ and Mesulam MM, Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol, 1984. 227(1): p. 109–20. [DOI] [PubMed] [Google Scholar]
- 46.Dum RP, Levinthal DJ, and Strick PL, The mind-body problem: Circuits that link the cerebral cortex to the adrenal medulla. Proc Natl Acad Sci U S A, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruggiero DA, et al. , A role of insular cortex in cardiovascular function. J Comp Neurol, 1987. 257(2): p. 189–207. [DOI] [PubMed] [Google Scholar]
- 48.Gehrlach DA, et al. , Aversive state processing in the posterior insular cortex. Nat Neurosci, 2019. 22(9): p. 1424–1437. [DOI] [PubMed] [Google Scholar]
- 49.Livneh Y, et al. , Estimation of Current and Future Physiological States in Insular Cortex. Neuron, 2020. 105(6): p. 1094–1111 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaelberer MM, et al. , A gut-brain neural circuit for nutrient sensory transduction. Science, 2018. 361(6408). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, et al. , Transcriptomic Landscape of von Economo Neurons in Human Anterior Cingulate Cortex Revealed by Microdissected-Cell RNA Sequencing. Cereb Cortex, 2019. 29(2): p. 838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evrard HC, The Organization of the Primate Insular Cortex. Front Neuroanat, 2019. 13: p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rinaman L and Schwartz G, Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci, 2004. 24(11): p. 2782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rinaman L, Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res, 2010. 1350: p. 18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGovern AE, et al. , Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience, 2012. 207: p. 148–66. [DOI] [PubMed] [Google Scholar]
- 56.Wang W, Optogenetic manipulation of ENS - The brain in the gut. Life Sci, 2018. 192: p. 18–25. [DOI] [PubMed] [Google Scholar]
- 57.Udit S and Gautron L, Molecular anatomy of the gut-brain axis revealed with transgenic technologies: implications in metabolic research. Front Neurosci, 2013. 7: p. 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kupari J, et al. , An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep, 2019. 27(8): p. 2508–2523 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang RB, et al. , Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell, 2015. 161(3): p. 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams EK, et al. , Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell, 2016. 166(1): p. 209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han W, et al. , A Neural Circuit for Gut-Induced Reward. Cell, 2018. 175(3): p. 665–678 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajendran PS, et al. , Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat Commun, 2019. 10(1): p. 1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Critchley HD, et al. , Neural systems supporting interoceptive awareness. Nat Neurosci, 2004. 7(2): p. 189–95. [DOI] [PubMed] [Google Scholar]
- 64.Malik S, et al. , Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [(11)C] PHNO-PET pilot trial in healthy humans. Brain Imaging Behav, 2018. 12(5): p. 1306–1317. [DOI] [PubMed] [Google Scholar]
- 65.Mayer EA and Collins SM, Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology, 2002. 122(7): p. 2032–48. [DOI] [PubMed] [Google Scholar]
- 66.Quigley KSK, et al. Functions of Interoception: From Energy Regulation to Experience of the Self. this issue of Trends in Neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrett LF and Bliss-Moreau E, Affect as a Psychological Primitive. Adv Exp Soc Psychol, 2009. 41: p. 167–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pavcovich LA and Valentino RJ, Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington’s nucleus. Neurosci Lett, 1995. 196(3): p. 185–8. [DOI] [PubMed] [Google Scholar]
- 69.Hou XH, et al. , Central Control Circuit for Context-Dependent Micturition. Cell, 2016. 167(1): p. 73–86 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valentino RJ, et al. , Evidence for widespread afferents to Barrington’s nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience, 1994. 62(1): p. 125–43. [DOI] [PubMed] [Google Scholar]
- 71.Brener J and Ring C, Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philos Trans R Soc Lond B Biol Sci, 2016. 371(1708). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garfinkel SN, et al. , Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biol Psychol, 2016. 114: p. 117–26. [DOI] [PubMed] [Google Scholar]
- 73.Garfinkel SN and Critchley HD, Threat and the Body: How the Heart Supports Fear Processing. Trends Cogn Sci, 2016. 20(1): p. 34–46. [DOI] [PubMed] [Google Scholar]
- 74.D’Alonzo M, et al. , Modulation of Body Representation Impacts on Efferent Autonomic Activity. J Cogn Neurosci, 2020. 32(6): p. 1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaman J, et al. , The use of stimulus perception to account for variability in skin conductance responses to interoceptive stimuli. Psychophysiology, 2020. 57(3): p. e13494. [DOI] [PubMed] [Google Scholar]
- 76.Mehling W, Differentiating attention styles and regulatory aspects of self-reported interoceptive sensibility. Philos Trans R Soc Lond B Biol Sci, 2016. 371(1708). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park J, Middlekauff HR, and Campese VM, Abnormal sympathetic reactivity to the cold pressor test in overweight humans. Am J Hypertens, 2012. 25(12): p. 1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ackerley R and Watkins RH, Microneurography as a tool to study the function of individual C-fiber afferents in humans: responses from nociceptors, thermoreceptors, and mechanoreceptors. J Neurophysiol, 2018. 120(6): p. 2834–2846. [DOI] [PubMed] [Google Scholar]
- 79.Freeman R and Chapleau MW, Testing the autonomic nervous system. Handb Clin Neurol, 2013. 115: p. 115–36. [DOI] [PubMed] [Google Scholar]
- 80.Maister L, Tang T, and Tsakiris M, Neurobehavioral evidence of interoceptive sensitivity in early infancy. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khalsa SS, Rudrauf D, and Tranel D, Interoceptive awareness declines with age. Psychophysiology, 2009. 46(6): p. 1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mikkelsen MB, et al. , Emotional reactivity and interoceptive sensitivity: Exploring the role of age. Psychon Bull Rev, 2019. 26(4): p. 1440–1448. [DOI] [PubMed] [Google Scholar]
- 83.Li D, et al. , Adolescent development of insula-dependent interoceptive regulation. Dev Sci, 2017. 20(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toller G, et al. , Individual differences in socioemotional sensitivity are an index of salience network function. Cortex, 2018. 103: p. 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maleki N, et al. , Female migraineurs show lack of insular thinning with age. Pain, 2015. 156(7): p. 1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suarez-Roca H, et al. , Contribution of Baroreceptor Function to Pain Perception and Perioperative Outcomes. Anesthesiology, 2019. 130(4): p. 634–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Migliorini R, et al. , What do you feel? Adolescent drug and alcohol users show altered brain response to pleasant interoceptive stimuli. Drug Alcohol Depend, 2013. 133(2): p. 661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Avery JA, et al. , Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry, 2014. 76(3): p. 258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grossi D, et al. , Altered functional connectivity of interoception in illness anxiety disorder. Cortex, 2017. 86: p. 22–32. [DOI] [PubMed] [Google Scholar]
- 90.Simmons A, et al. , Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med, 2009. 71(4): p. 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stern ER, et al. , The buildup of an urge in obsessive-compulsive disorder: Behavioral and neuroimaging correlates. Hum Brain Mapp, 2020. 41(6): p. 1611–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hatfield TR, et al. , Autism spectrum disorder and interoception: Abnormalities in global integration? Autism, 2019. 23(1): p. 212–222. [DOI] [PubMed] [Google Scholar]
- 93.Donofry SD, et al. , Alterations in emotion generation and regulation neurocircuitry in depression and eating disorders: A comparative review of structural and functional neuroimaging studies. Neurosci Biobehav Rev, 2016. 68: p. 911–927. [DOI] [PubMed] [Google Scholar]
- 94.Harshaw C, Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol Bull, 2015. 141(2): p. 311–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garcia-Cordero I, et al. , Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos Trans R Soc Lond B Biol Sci, 2016. 371(1708). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcia-Cordero I, et al. , Stroke and Neurodegeneration Induce Different Connectivity Aberrations in the Insula. Stroke, 2015. 46(9): p. 2673–7. [DOI] [PubMed] [Google Scholar]
- 97.Bonaz BL, et al. Diseases, Disorders, and Comorbidities of Interoception. this issue of Trends in Neurosciences. [DOI] [PubMed] [Google Scholar]
- 98.Elsenbruch S, et al. , Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology, 2010. 139(4): p. 1310–9. [DOI] [PubMed] [Google Scholar]
- 99.Kanoski SE and Grill HJ, Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol Psychiatry, 2017. 81(9): p. 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avery JA, et al. , Obesity is associated with altered mid-insula functional connectivity to limbic regions underlying appetitive responses to foods. J Psychopharmacol, 2017. 31(11): p. 1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ardizzi M, et al. , Interoception and Positive Symptoms in Schizophrenia. Front Hum Neurosci, 2016. 10: p. 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kutscheidt K, et al. , Interoceptive awareness in patients with attentiondeficit/hyperactivity disorder (ADHD). Atten Defic Hyperact Disord, 2019. 11(4): p. 395–401. [DOI] [PubMed] [Google Scholar]
- 103.Uddin LQ and Menon V, The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev, 2009. 33(8): p. 1198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu X, et al. , Heightened brain response to pain anticipation in high-functioning adults with autism spectrum disorder. Eur J Neurosci, 2018. 47(6): p. 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herman AM and Duka T, Facets of impulsivity and alcohol use: What role do emotions play? Neurosci Biobehav Rev, 2019. 106: p. 202–216. [DOI] [PubMed] [Google Scholar]
- 106.Goldstein RZ, et al. , The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci, 2009. 13(9): p. 372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duncan A, et al. , Habenular TCF7L2 links nicotine addiction to diabetes. Nature, 2019. 574(7778): p. 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farokhnia M, et al. , Ghrelin: From a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav, 2019. 204: p. 49–57. [DOI] [PubMed] [Google Scholar]
- 109.Morris LS, Voon V, and Leggio L, Stress, Motivation, and the Gut-Brain Axis: A Focus on the Ghrelin System and Alcohol Use Disorder. Alcohol Clin Exp Res, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suarez AN, Noble EE, and Kanoski SE, Regulation of Memory Function by Feeding-Relevant Biological Systems: Following the Breadcrumbs to the Hippocampus. Front Mol Neurosci, 2019. 12: p. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weng HL, et al. , Interventions and Manipulations of Interoception. this issue of Trends in Neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barrera TL, et al. , A review of cognitive behavioral therapy for panic disorder in patients with chronic obstructive pulmonary disease: the rationale for interoceptive exposure. J Clin Psychol Med Settings, 2014. 21(2): p. 144–54. [DOI] [PubMed] [Google Scholar]
- 113.Freeman SC, et al. , Component network meta-analysis identifies the most effective components of psychological preparation for adults undergoing surgery under general anesthesia. J Clin Epidemiol, 2018. 98: p. 105–116. [DOI] [PubMed] [Google Scholar]
- 114.Ehde DM, Dillworth TM, and Turner JA, Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol, 2014. 69(2): p. 153–66. [DOI] [PubMed] [Google Scholar]
- 115.Moisset X, Lanteri-Minet M, and Fontaine D, Neurostimulation methods in the treatment of chronic pain. J Neural Transm (Vienna), 2020. 127(4): p. 673–686. [DOI] [PubMed] [Google Scholar]
- 116.Seth AK, Suzuki K, and Critchley HD, An interoceptive predictive coding model of conscious presence. Front Psychol, 2011. 2: p. 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Owens AP, et al. , Interoceptive inference: From computational neuroscience to clinic. Neurosci Biobehav Rev, 2018. 90: p. 174–183. [DOI] [PubMed] [Google Scholar]
- 118.Di Lernia D, et al. , Feel the Time. Time Perception as a Function of Interoceptive Processing. Front Hum Neurosci, 2018. 12: p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petzschner FHG, et al. Computational Models of Interoception and Body Regulation. this issue of Trends in Neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]