Abstract
Reactive Fe(III) minerals can influence methane (CH4) emissions by inhibiting microbial methanogenesis or by stimulating anaerobic CH4 oxidation. The balance between Fe(III) reduction, methanogenesis, and CH4 oxidation in ferruginous Archean and Paleoproterozoic oceans would have controlled CH4 fluxes to the atmosphere, thereby regulating the capacity for CH4 to warm the early Earth under the Faint Young Sun. We studied CH4 and Fe cycling in anoxic incubations of ferruginous sediment from the ancient ocean analogue Lake Matano, Indonesia, over three successive transfers (500 days in total). Iron reduction, methanogenesis, CH4 oxidation, and microbial taxonomy were monitored in treatments amended with ferrihydrite or goethite. After three dilutions, Fe(III) reduction persisted only in bottles with ferrihydrite. Enhanced CH4 production was observed in the presence of goethite, highlighting the potential for reactive Fe(III) oxides to inhibit methanogenesis. Supplementing the media with hydrogen, nickel and selenium did not stimulate methanogenesis. There was limited evidence for Fe(III)-dependent CH4 oxidation, although some incubations displayed CH4-stimulated Fe(III) reduction. 16S rRNA profiles continuously changed over the course of enrichment, with ultimate dominance of unclassified members of the order Desulfuromonadales in all treatments. Microbial diversity decreased markedly over the course of incubation, with subtle differences between ferrihydrite and goethite amendments. These results suggest that Fe(III) oxide mineralogy and availability of electron donors could have led to spatial separation of Fe(III)-reducing and methanogenic microbial communities in ferruginous marine sediments, potentially explaining the persistence of CH4 as a greenhouse gas throughout the first half of Earth history.
1 |. INTRODUCTION
Elevated atmospheric methane (CH4; 100–1,000 ppmv vs. ~2 ppmv in the modern atmosphere) likely played an important role in the first half of Earth history by helping warm Earth’s surface temperature enough to sustain liquid water under considerably lower solar radiation (Haqq-Misra, Domagal-Goldman, Kasting, & Kasting, 2008; Kasting, 2005; Pavlov, Kasting, Brown, Rages, & Freedman, 2000; Roberson, Roadt, Halevy, & Kasting, 2011). During this time, the main source of CH4 was likely hydrogenotrophic methanogenesis (CO2 + 4 H2 → CH4 + 2H2O (Ueno, Yamada, Yoshida, Maruyama, & Isozaki, 2006; Battistuzzi, Feijao, & Hedges, 2004)) from anoxic oceans, which were ferruginous for most of the Archean and Paleoproterozoic eons (Poulton & Canfield, 2011). In these seas, a “ferrous wheel” would have cycled iron from dissolved Fe2+ to Fe(III) oxides via microbial photoferrotrophy (and/or abiotic photo-oxidation; Kappler, Pasquero, Konhauser, & Newman, 2005; Crowe, Jones, et al., 2008), and then back to Fe2+ via microbial Fe(III) respiration (Craddock & Dauphas, 2011; Johnson, Beard, & Roden, 2008; Konhauser, Newman, & Kappler, 2005; Vargas, Kashefi, Blunt-Harris, & Lovley, 1998).
Ferruginous oceans could have influenced CH4 cycling by several mechanisms. It is well established that Fe(III)-reducing bacteria have higher affinity for H2 than hydrogenotrophic methanogens and will therefore outcompete them in the presence of poorly crystalline Fe(III) oxides (e.g., ferrihydrite; Lovley & Phillips, 1987; Lovley & Goodwin, 1988; Zhou, Xu, Yang, & Zhuang, 2014) (note that Fe(III)-reducing bacteria also outcompete acetoclastic methanogens (Lovley & Phillips, 1986), but acetoclastic methanogenesis likely evolved much later in Earth history (Fournier & Gogarten, 2008)). In addition, evidence is accumulating that Fe(III) oxides can mediate or stimulate microbial CH4 oxidation, either as the direct oxidant (Ettwig et al., 2016; Gal’chenko, 2004), or indirectly by regenerating sulfate by oxidization of reduced sulfur compounds (Sivan, Antler, Turchyn, Marlow, & Orphan, 2014).
The putative microbial metabolism of CH4 oxidation coupled to Fe(III) reduction is thermodynamically favorable with ferrihydrite () and goethite () as terminal electron acceptors (Caldwell et al., 2008; Zehnder & Brock, 1980). Based on the chemical equations and free energy yields above, we would expect to observe a stoichiometric ratio of 1 CH4 oxidized per 8 Fe(III) reduced and preferential use of ferrihydrite over goethite as the electron acceptor. Accumulating geochemical evidence for microbial CH4 oxidation coupled to, or stimulated by, Fe(III) reduction is widespread across modern anoxic ecosystems and anaerobic digester communities (Amos et al., 2012; Beal, House, & Orphan, 2009; Crowe et al., 2011; Egger et al., 2015; Fu et al., 2016; Noroi, Thamdrup, & Schubert, 2013; Riedinger et al., 2014; Rooze, Egger, Tsandev, & Slomp, 2016; Segarra, Comerford, Slaughter, & Joye, 2013; Sivan et al., 2011, 2014; Sturm et al., 2015; Zehnder & Brock, 1980), and a recent study reported simultaneous CH4 oxidation and ferrihydrite reduction in a 1:8 ratio in an archaea-dominated enrichment culture (Ettwig et al., 2016).
Despite the possible importance of coupled Fe(III) and CH4 cycling in the Archean and Paleoproterozoic eons, long-term studies of Fe(III) reduction under low organic carbon and high CH4 conditions remain sparse. Lake Matano, Indonesia, is one of the only modern analogues for the ferruginous Archean ocean (Crowe, Jones, et al., 2008). Despite the abundance of Fe(III) oxides that might be expected to suppress methanogenesis, CH4 accumulates to 1.4 mm in anoxic deep waters (Crowe, Roberts, Weisener, & Fowle, 2007; Crowe, Jones, et al., 2008; Crowe, O’Neill, et al., 2008; Crowe, et al., 2011; Kuntz, Laakso, Schrag, & Crowe, 2015). Methanotrophy is a key carbon fixation process in Lake Matano’s oxic-anoxic transition zone, and the dearth of other oxidants (<100 nm nitrate and sulfate) suggests that Fe(III) might be the terminal electron acceptor in methanotrophy (Crowe et al., 2011; Sturm et al., 2015). In this study, we examined the influence of CH4 and Fe(III) mineral speciation on rates of Fe(III) reduction, methanogenesis, and CH4 oxidation, and microbial community composition, over three successive dilutions (500 total days of incubation) of anoxic Lake Matano sediments.
2 |. MATERIALS AND METHODS
2.1 |. Sample collection and storage
A 15-cm sediment core from 200 m water depth in Lake Matano, Sulawesi Island, Indonesia (2°26’S, 121°15’E; in situ sediment temperature ~27°C), was sampled in November 2014 and subsampled at 5-cm increments. Sediments from 0–5 to 5–10 cm depth were fluffy and black, and 10–15 cm was dark gray. Sediments were sealed in gastight bags with no headspace (Hansen, Thamdrup, & Jørgensen, 2000) and stored at 4°C until incubations began in March 2015.
2.2 |. Enrichment medium and substrate synthesis
A modified artificial freshwater medium lacking nitrate and sulfate was developed based on the pore water composition of Lake Matano sediments (S.A. Crowe and D.A. Fowle, unpublished work). The medium contained 825 μm MgCl2, 550 μm CaCO3, 3 mm NaHCO3, 3.5 μm K2HPO4, 5 μm Na2HPO4, 225 μm NH4Cl, 1 μm CuCl2, 1.5 μm Na2 MoO4, 2.5 μm CoCl2, 23 μm MnCl2, 4 μm ZnCl2, 9.4 μm FeCl3 and mm Na2 NTA, 0.07 μm vitamin B12, 0.4 μm biotin, and 68.5 μm thiamine. Filter-sterilized vitamin solutions were added after autoclaving. Ferrihydrite (Fe(OH)3) and goethite (FeOOH) were synthesized as described in Schwertmann & Cornell (1991) and added to enrichments to 10 mm as described below.
2.3 |. Inoculation of enrichment and amendments
The sediment was pre-treated for 36 days at 30°C in 100% N2 headspace to deplete endogenous organic carbon, electron donors, and reactive electron acceptors. After pre-treatment, sediment from the 0- to 5-cm-depth layer was inoculated in a ratio of sediment to medium of 1:5 (v/v) in an anoxic chamber (97% N2 and 3% H2; Coy Laboratory Products, Grass Lake, MI, USA). Sediment slurry (35 ml) was aliquoted into 70-ml sterile serum bottles, stoppered with sterile butyl stoppers (Geo-Microbial Technologies, Ochelata, OK, USA; pre-boiled in 0.1 N NaOH) and crimped with aluminum seals. Ferric iron was added either as ferrihydrite or goethite to 10 mm. Bottles were purged with 99.99% N2 for 1 hr, and CH4 amendments were injected with 10 ml 99.99% CH4 and 5 ml 99% 13CH4 (Cambridge Isotope Laboratories, Tewksbury, MA, USA). Controls were autoclaved at 121°C for 1 hr on day 0 and again on day 6 of the 1° enrichment. All treatments were duplicated, and bottles were incubated in the dark at 30°C with shaking at 200 rpm.
After 50 days, the volume of all cultures was reduced to 5 ml, and 30 ml of fresh media was added to each bottle, constituting a sixfold dilution. These 2° enrichments were amended with approximately 10 mm of either ferrihydrite or goethite. All bottles were purged with 99.99% N2 for 1 hr, and all bottles except N2 controls were injected with 8 ml 99.99% CH4 and 2 ml 99% 13CH4. Controls were autoclaved again at 121°C for 1 hr. dl-Methionine (10 μm) was added as a sulfur source. After 303 days, cultures were diluted 10-fold with fresh media into new serum bottles (3° enrichment) with the same substrate and headspace composition as the 2° enrichment. A schematic of the incubation and dilutions is shown at the top of Figures 1–3.
FIGURE 1.
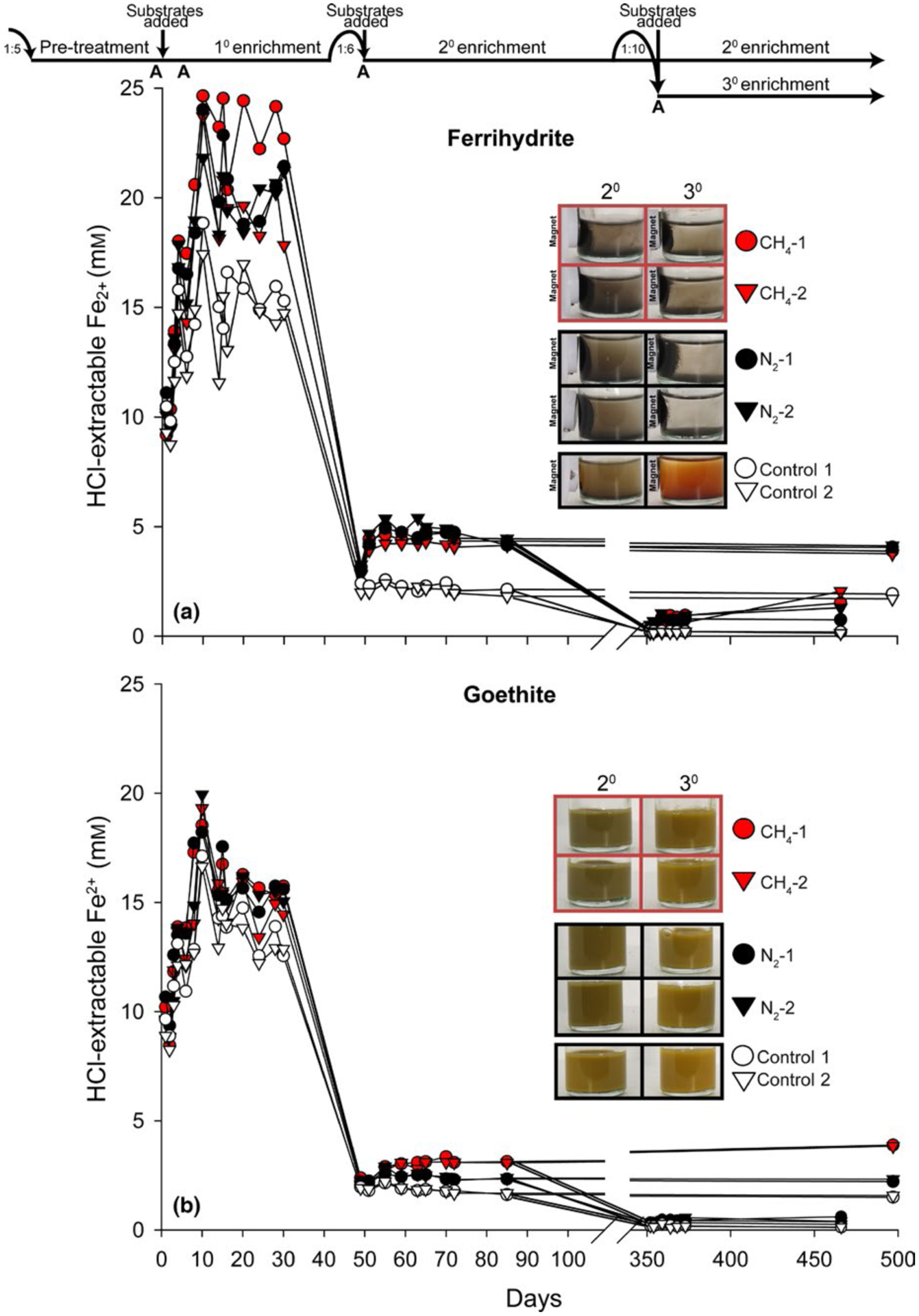
HCl-extractable Fe2+ for sediment enrichments with (a) ferrihydrite and (b) goethite over 497 days. Timeline at top shows transfer dates and dilution ratios. “A” represents days that controls were autoclaved. Red and black symbols represent treatments with and without CH4, respectively. White symbols represent autoclaved controls. All treatments were run in duplicate (circle and triangle symbols). Photographs depict 2° and 3° enrichment bottles on day 497 with evidence for magnetic mineral formation in live treatments amended with ferrihydrite
FIGURE 3.
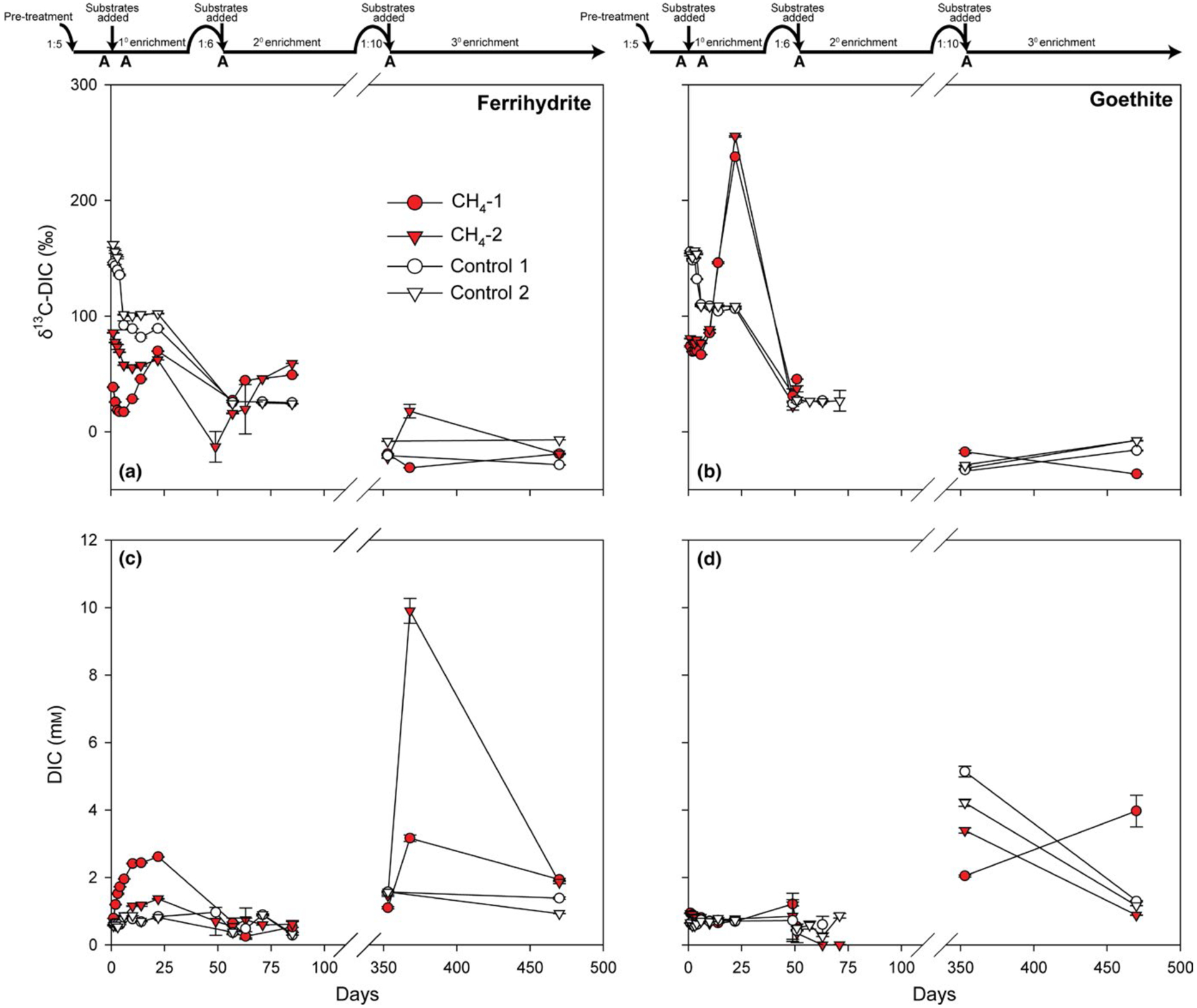
Dissolved inorganic carbon (DIC) isotopic composition and concentration for sediment enrichments amended with 13CH4 and either (a,c) ferrihydrite or (b,d) goethite. Timeline at top shows transfer dates and dilution ratios. “A” represents days that controls were autoclaved. Red and white symbols represent live treatments and autoclaved controls, respectively. Errors bars represent standard deviation of triplicate measurements. Calculated methane oxidation rates for the 1° enrichment were 1.7 and 1.1 μm CH4/day ferrihydrite bottles 1 and 2, respectively, and 0.2 and 0.8 μm CH4/day for goethite bottles 1 and 2, respectively. Isotopic data are not plotted for DIC concentrations 0.5 mm. Rate calculations were not possible for the 2° enrichment due to low/variable DIC
After an additional 220 days, goethite-amended N2 cultures were diluted 25-fold with fresh anoxic media into new serum bottles. Cultures received either 10 mm goethite or no Fe(III). A subset of cultures received 5 ml of 99.99% H2 (20% headspace) while all others had 100% N2 headspace. Controls were autoclaved at 121°C. After 48 days, an anoxic solution of nickel (Ni) and selenium (Se) was added to all bottles, yielding final concentrations of 1 μm Ni and 1 μm Se.
2.4 |. HCl-extractable Fe2+ and Fe3+ and soluble Fe2+
Samples were taken from each bottle in the anoxic chamber using a 21-gauge needle (BD PrecisionGlide™) and plastic syringe. Plasticware was stored in the anoxic chamber for at least 24 hr to minimize O2 sample contamination. For HCl-extractable Fe2+ analyses, 100 μl of sediment slurry was extracted with 400 μl 0.6 N HCl in the dark for 1 hr, followed by centrifugation at 10 000 g for 1 min, injection of 10 μl of supernatant into 990 μl of 10 mm FerroZine reagent in 46 mm HEPES (pH 8.3), and measurement of absorbance at 562 nm (Stookey, 1970). For HCl-extractable Fe3+, 100 μl of sediment slurry was incubated overnight in 0.5 N HCl and 0.25 m NH2OH-HCl in the dark, followed by centrifugation and measurement as above, and subtraction of HCl-extractable Fe2+ as in Kostka and Luther (1994).
2.5 |. Methane oxidation
Samples were collected for δ13C-DIC analysis by 0.2-μm membrane filtration of medium into crimp top autosampler vials (Thermo Scientific National Target LoVial) and analysis as described in Brandes (2009). Rates of 13CH4 oxidation to 13C-DIC were calculated over the linear period of δ13C-DIC increase based on the method in Scheller, Yi, Chadwick, McGlynn, and Orphan (2016). First, the δ13C-DIC values were converted into fractional abundances (13F = (13C/12C+13C)), and then DIC production from CH4 oxidation was calculated using the following formula:
where [DIC]n and 13Fn are equal to the total DIC concentration (mm) and fractional abundance of 13C in the DIC at time n, respectively. [DIC]0 and 13F0 are the total DIC concentration (mm) and fractional abundance of DIC at time 0, respectively, and 13FCH4 is the fractional abundance of 13C in the CH4.
2.6 |. Headspace methane
Headspace (50 μl) was sampled using a gastight syringe and injected into a gas chromatograph (SRI Instruments 8610C, Torrance, CA, USA) with a HayeSep N column and flame ionization detector to measure headspace CH4 concentrations. A CH4 standard (1,000 PPM; Airgas, Radnor Township, PA, USA) was used for calibration.
2.7 |. Inductively coupled plasma mass spectrometry
Total dissolved Ni and Se concentrations were measured using inductively coupled plasma mass spectrometry (ICP-MS). To determine the amounts of Ni and Se supplied by the media and Fe(III) oxides, aliquots of media were dispensed in serum bottles, purged with 99.99% N2, and amended with 10 mm goethite or ferrihydrite in the same manner as enrichments. Stoppers were penetrated multiple times with 21-gauge stainless steel needles (BD PrecisionGlide™) to mimic the effect of sampling on enrichment cultures. All samples for ICP-MS were filtered through 0.2-μm-pore polypropylene syringe filters and diluted in 2% trace metal grade HNO3 (Fisher Scientific, Inc., Pittsburgh, PA, USA) containing scandium and yttrium as internal standards to account for instrument drift. Calibration standards were prepared from certified stock solutions of Ni (CertiPREP) and Se (BDH), and a blank and calibration standard were measured periodically as quality controls. The measurement detection limits, calculated as three times the standard deviation of the blank (n = 8), were 7 and 128 nm for Ni and Se, respectively.
2.8 |. 16S rRNA gene amplicon sequencing
Samples (2 ml) of sediment used for inoculating incubations (hereafter, “sediment inoculum”) were taken in February 2015 (prior to pre-treatment) and after incubation for 15 days (1° enrichment), 72 days (2° enrichment) and 469 days (3° enrichment). Nucleic acid was extracted and purified using a MO BIO PowerSoil Isolation Kit following the manufacturer’s protocol and MO BIO UltraClean® 15 Purification Kit (MO BIO Laboratories, Carlsbad, CA, USA). 16S rRNA gene amplicons were synthesized from extracted DNA with V4 region-specific barcoded primers F515 and R806 (Caporaso et al., 2011) appended with Illumina-specific adapters according to Kozich, Westcott, Baxter, Highlander, and Schloss (2013) using a Bio-Rad C1000 Touch Thermocycler and QIAGEN Taq PCR Master Mix. Thermal cycling conditions were as follows: initial denaturing at 94°C (5 min), 35 cycles of denaturing at 94°C (40 s), primer annealing at 55°C (40 s), and primer extension at 68°C (30 s). Amplicons were checked for correct size (~400 bp) on a 1% agarose gel and purified using Diffinity RapidTips. Amplicon concentrations were determined on a Qubit™ (ThermoFisher, Waltham, MA, USA) fluorometer. Amplicons were pooled at equimolar concentrations (4 nmol) and sequenced on an Illumina MiSeq running miseq control software v.2.4.0.4 (Illumina, San Diego, CA, USA) using a 500-cycle MiSeq reagent kit v2 with a 5% PhiX genomic library control, as described by Kozich et al. (2013). Sequences were deposited as NCBI accession numbers SAMN04532568–04532641 and SAMN05915184–05915222.
2.9 |. 16S rRNA gene amplicon sequence analysis
Demultiplexed amplicon read pairs were quality-trimmed with Trim Galore (Babraham Bioinformatics, http://www.bioinformatics.babraham.ac.uk/projects/) using a base Phred33 score threshold of Q25 and a minimum length cutoff of 100 bp. Reads were then analyzed using mothur (Schloss et al., 2009) following its MiSeq standard operating procedure. High-quality paired reads were merged and screened to remove sequences of incorrect length and those with high numbers of ambiguous base calls. Merged reads were dereplicated and aligned to the ARB SILVA database (release 123; available at http://www.mothur.org/wiki/Silva_reference_alignment). Sequences with incorrect alignment and those with homopolymers longer than 8 bp were filtered out. Unique sequences and their frequency in each sample were identified, and then a pre-clustering algorithm was used to further denoise sequences within each sample. Sequences were then chimera-checked using UCHIME (Edgar, Haas, Clemente, Quince, & Knight, 2011). Reads were clustered into operational taxonomic units (OTUs) at 97% similarity based on uncorrected pairwise distance matrices. OTUs were classified using SILVA reference taxonomy database (release 123, available at http://www.mothur.org/wiki/Silva_reference_files). Chao 1 (species richness), phylogenetic diversity, and Shannon index (species evenness) estimates were generated using mothur after normalization to 4,000 sequences per sample.
3 |. RESULTS
3.1 |. Iron reduction
3.1.1 |. 1° Enrichment
Over the first 10 days of incubation, HCl-extractable Fe2+ increased from 10 to 25 mm in ferrihydrite treatments (Figure 1a) and from 10 to 20 mm in goethite treatments (1–2 mm/day; Figure 1b). From day 6 to 10, HCl-extractable Fe3+ (7 and 12 mm in ferrihydrite and goethite treatments, respectively) was completely consumed in all bottles except autoclaved controls with ferrihydrite (data not shown). Iron reduction rates were identical with and without CH4 (Figure 1a,b). Initial autoclaving did not suppress Fe(III) reduction. A second round of autoclaving on day 6 slightly suppressed further activity. From day 10 to day 28, HCl-extractable Fe2+ fluctuated in ferrihydrite treatments (Figure 1a) and declined slightly in goethite treatments (Figure 1b). Soluble Fe2+ was consistently <1% of HCl-extractable Fe2+, and sediment-free controls did not reduce Fe(III) (data not shown).
3.1.2 |. 2° Enrichment
After 1:6 dilution and 10 mm ferrihydrite addition on day 50, HCl-extractable Fe2+ increased from 3 to 4 mm over two days and then remained constant through the final time point (day 497) in bottles with and without added CH4 (Figure 1a). After 10 mm goethite addition on day 50, HCl-extractable Fe2+ increased from 2 to 3 mm after a two-day lag period. Thereafter, HCl-extractable Fe2+ rose to 4 mm by day 497 in goethite treatments with added CH4; without CH4, HCl-extractable Fe2+ dropped back to 2 mm (Figure 1b). Autoclaved controls had no activity. Black magnetic minerals formed in all ferrihydrite treatments except autoclaved controls (Figure 1a). No magnetic minerals formed in goethite treatments (Figure 1b).
3.1.3 |. 3° Enrichment
After 1:10 dilution and addition of 10 mm ferrihydrite on day 352, HCl-extractable Fe2+ doubled in the first week in N2 treatments and bottle CH4-1 (Figure 1a). Bottle CH4-2 displayed similar activity after a two-week lag period. Over an additional 100 days (day 466), HCl-extractable Fe2+ increased to 2 mm. Goethite treatments and autoclaved controls had minimal activity (Figure 1b). As in the 2° enrichment, magnetic minerals formed in the presence of ferrihydrite (Figure 1a), but not goethite (Figure 1b).
3.2 |. Trace metal concentrations
Total dissolved Ni averaged 41 ± 20 nm in fresh basal growth media and was neither affected by Fe(III) oxide additions nor by puncturing of stainless steel needles through stoppers into culture liquid (Table S1). Dissolution of ferrihydrite in HNO3 liberated significant Ni (2.5 μm; Table S1). Nickel was higher in enrichment cultures than in basal media: 96–286 and 54–134 nm with ferrihydrite and goethite, respectively; Table S2). Selenium was consistently below the detection limit (<128 nm) in growth media and enrichment culture.
3.3 |. Methane production
Goethite treatments consistently displayed higher CH4 production than those with ferrihydrite (Figure 2). In the 1° goethite enrichment, methanogenesis (13–19 μm CH4/day) coincided with the period of Fe(III) reduction and stopped after HCl-extractable Fe3+ was completely consumed on day 10. Methanogenesis persisted throughout the 3° goethite enrichment (3 μm CH4/day). Negligible CH4 was produced in the presence of ferrihydrite, except for the final time point for bottle N2-2 in the 3° enrichment.
FIGURE 2.
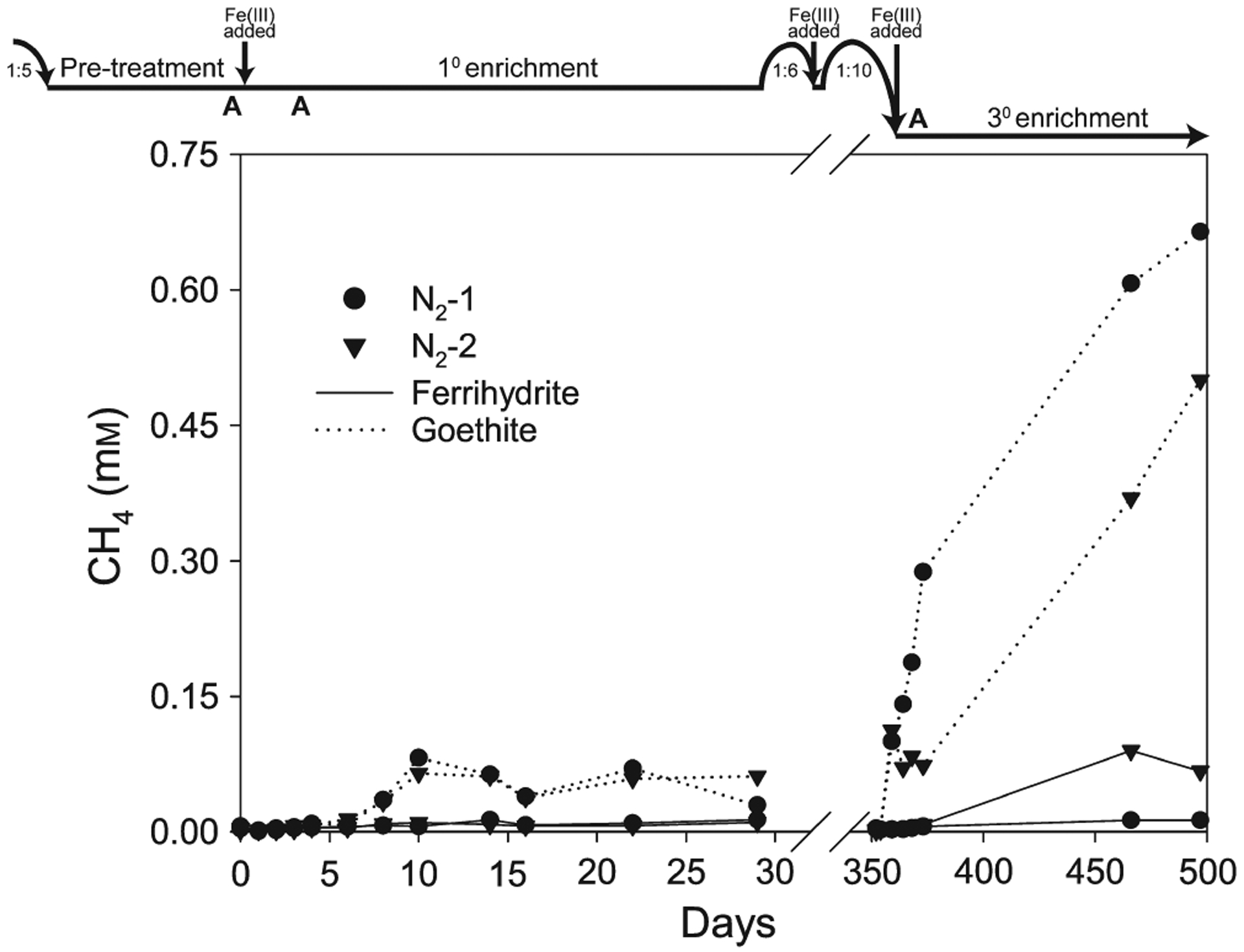
Accumulation of CH4 in the headspace of sediment enrichments. Timeline at top shows transfer dates and dilution ratios. Solid and dotted lines represent ferrihydrite and goethite treatments, respectively. All treatments were run in duplicate (circle and triangle symbols). Original headspace was 100% N2
In an additional 4° enrichment (days 571–663), we tested the effect of H2, Ni, and Se amendments, as well as no Fe(III) controls, on CH4 production in goethite treatments (Figure S1). As in previous enrichments, Bottle 1 consistently produced more CH4 (17 μm CH4/day) than Bottle 2. There were no significant differences in CH4 production with and without 20% H2 headspace and 10 mm goethite. Like in previous enrichments with goethite, minimal Fe(III) reduction was observed (data not shown). No CH4 was produced in any of the treatments between day 619, when 1 μm Ni and Se were added, and day 663.
3.4 |. Methane oxidation
3.4.1 |. 1° Enrichment
13C incorporation into DIC began on day 6 in both ferrihydrite and goethite treatments and continued for the remainder of the sampling period (Figure 3a,b). Ferrihydrite treatments showed lower 13C-DIC enrichment but higher total DIC production (totaling to 1–2 μm CH4 oxidized/day) than goethite treatments, which had greatest δ13C enrichment but decreasing DIC concentrations, making calculation of CH4 oxidation rates impossible. Autoclaved controls showed neither 13C incorporation nor DIC production.
3.4.2 |. 2° Enrichment
Both ferrihydrite treatments displayed 13C enrichment (Figure 3a), but declining DIC, precluding calculation of CH4 oxidation rates. Initial pH of 8 declined to 7.6, 6.7 and 6 in the autoclaved, N2 and CH4 treatments, respectively. DIC in goethite treatments with CH4 dropped to undetectable values within three weeks, suggesting sampling or analytical error, which precluded accurate isotopic measurement at these time points. These data are thus not considered further. Autoclaved and N2 controls did not show pH changes.
3.4.3 |. 3° Enrichment
Bottle CH4-2 with ferrihydrite was the only treatment with significant 13C incorporation into DIC over the first 15 days (Figure 3a). Over the same interval, DIC increased in both ferrihydrite-amended bottles (yielding CH4 oxidation rates of 32 and 7 μm/day in bottles 1 and 2, respectively) and pH dropped from 8.2 to 7.1 and 7.9 in bottles 1 and 2, respectively. By day 470, 13C enrichment and DIC concentrations in both ferrihydrite-amended bottles had returned to a level similar to that at the start of the 3° enrichment. Autoclaved controls did not exhibit any change in DIC and pH. Goethite treatments had initial DIC concentrations (3–5 mm) higher than those in previous enrichments. In the goethite-amended autoclaved controls and bottle CH4-1, DIC concentrations dropped over the 3° enrichment. Only goethite-amended bottle CH4-2 increased in DIC, without concurrent 13C enrichment. Large DIC variability implies that reported rates may be underestimates if declining pH led to outgassing of 13C-DIC into the headspace CO2 pool.
3.5 |. Microbial taxonomy
3.5.1 |. Inoculum
16S rRNA gene amplicons from the sediment inoculum were dominated by Bathyarchaeota (25%), formerly Miscellaneous Crenarchaeotal Group, and unclassified Archaea (11%; Figure 4).
FIGURE 4.
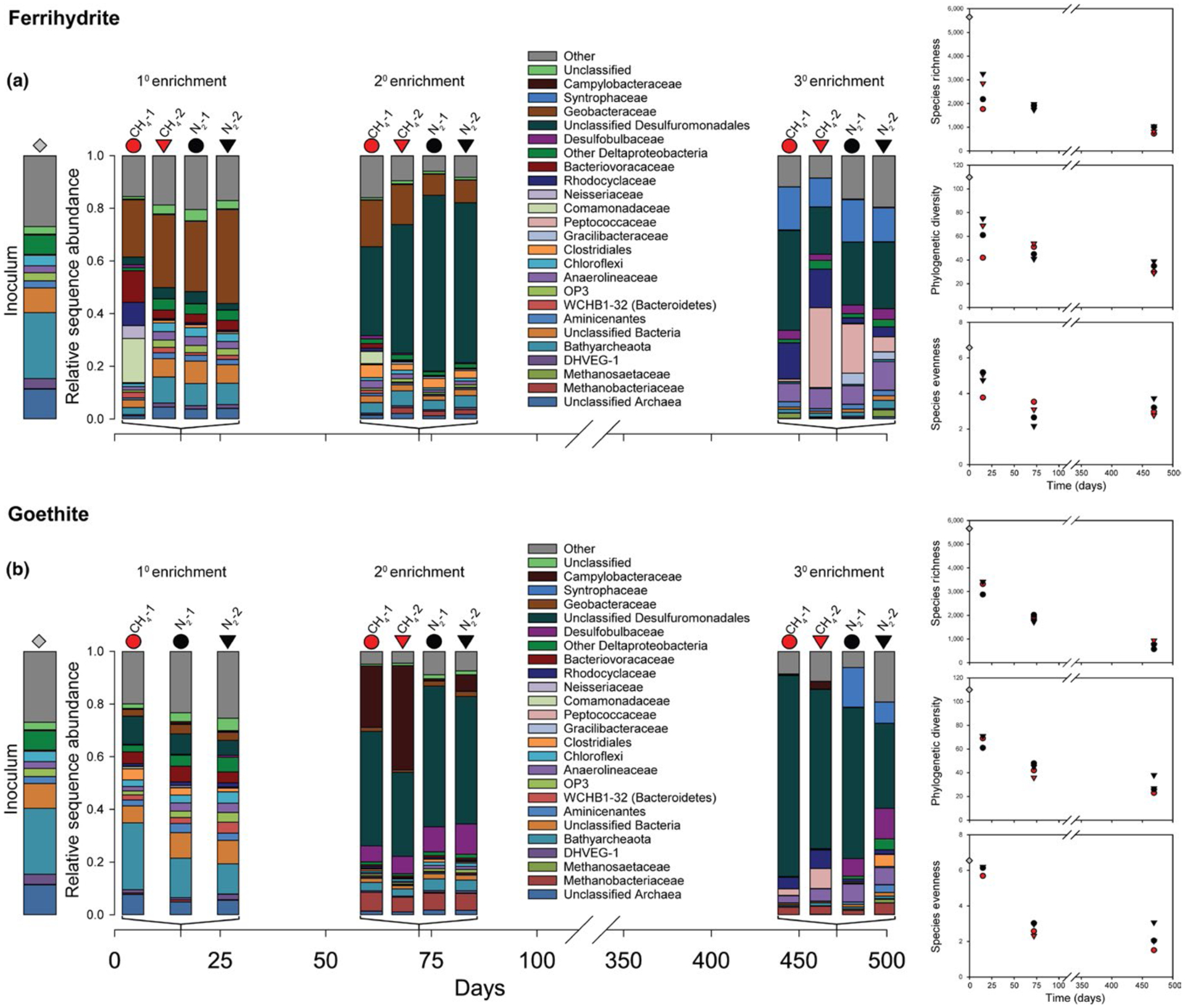
16S rRNA gene diversity and phylogenetic diversity for inoculum and sediment enrichments amended with (a) ferrihydrite and (b) goethite. Samples were taken on day 15 (1° enrichment), 72 (2° enrichment), and 469 (3° enrichment). Red and black symbols represent treatments with and without CH4, respectively. Gray diamonds represent inoculum samples. All treatments were run in duplicate (circle and triangle symbols). Species richness, phylogenetic diversity, and species evenness for the sediment inoculum and enrichments normalized to 4,000 sequences per sample are shown to the right of bar charts
3.5.2 |. 1° Enrichment
Species richness, evenness, and phylogenetic diversity decreased relative to the inoculum in all treatments (Figure 4). Geobacteraceae (Deltaproteobacteria) became dominant (22–36%) in all ferrihydrite treatments (Figure 4a); the dominant OTU had 97% similarity to Geothermobacter sp. Ferrihydrite-amended bottle CH4-1 was enriched in Betaproteobacteria, specifically Comamonadaceae (17%) and Rhodocyclaceae (9%; Figure 4a). Bathyarchaeota persisted in goethite treatments (11–25%; Figure 4b).
3.5.3 |. 2° Enrichment
All treatments declined further in species richness, evenness, and phylogenetic diversity. Unclassified Desulfuromondales dominated both ferrihydrite and goethite enrichments (34–68%). The dominant OTU had 98% similarity to Geobacter hephaestius/Geobacter lovleyi. Geobacteraceae declined in ferrihydrite enrichments (2%–18%; Figure 4a). Campylobacteraceae (Epsilonproteobacteria), a trace constituent of the inoculum and 1° enrichment, were enriched in goethite treatments with CH4 (23%–40%); the dominant OTU had 98% similarity to Sulfurospirillum barnesii (Figure 4b). The most abundant methanogenic Euryarchaeota family, Methanobacteriaceae, comprised 1%–2% and 6%–7% of sequences in ferrihydrite and goethite treatments, respectively; the dominant OTU had 100% similarity to Methanobacterium flexile. Bathyarchaeota were depleted compared to the 1° enrichment (Figure 4).
3.5.4 |. 3° Enrichment
Species richness, evenness, and phylogenetic diversity continued to decline (Figure 4a,b). Unclassified Desulfuromondales dominated goethite treatments (32%–76%; Figure 4b) and were less abundant in ferrihydrite treatments (18%–38%); as in the 2° enrichment, the dominant OTU had 98% identity to G. hephaestius/G. lovleyi. Rhodocyclaceae were more abundant in ferrihydrite treatments with CH4 (14%–15%) than with N2 (2%–4%; Figure 4a); the dominant OTU had 100% similarity to Azospira oryzae/Dechlorosoma suillum. Peptococcaceae (Firmicutes) were most abundant in bottle CH4-2 with ferrihydrite (30%); the dominant OTU had 96% similarity to uncultured members of the genus Thermincola. Syntrophaceae (Deltaproteobacteria) were enriched in all ferrihydrite treatments (11%–16%) and goethite treatments with N2 (8%–15%); the dominant OTU had 97% similarity to Smithella propionica. Methanobacteriaceae comprised 1%–4% of sequences in goethite treatments and were absent from ferrihydrite treatments; as in the 2° enrichment, the dominant OTU had 100% identity to M. flexile.
4 |. DISCUSSION
4.1 |. Fe(III) reduction rates in long-term ferruginous sediment incubations
Initial rates of HCl-extractable Fe2+ production (1–2 mm/day) in the 1° enrichment were similar to those from freshwater wetlands with organic carbon as the electron donor (Jensen, Thamdrup, Rysgaard, Holmer, & Fossing, 2003; Kostka, Roychoudhury, & Van Cappellen, 2002; Roden & Wetzel, 2002). Despite replenishment of Fe(III) substrates, activity declined with each successive transfer, likely reflecting organic carbon limitation. The next most thermodynamically favorable electron donor, H2, could have been supplied by fermenters (such as Syntrophaceae in the 3° enrichment), but would ultimately still require a source of organic carbon. Some of our incubations display evidence for CH4, the next most thermodynamically favorable electron donor, as a source of electrons for Fe(III) reduction (e.g., higher Fe2+ yields with CH4 addition with ferrihydrite in 1° and 3° enrichments, and with goethite in the 2° enrichment; see further discussion below).
Higher Fe(III) reduction rates were maintained on ferrihydrite than goethite, consistent with its higher energetic yield and (typically) greater surface area. Magnetic mineral formation was likely due to adsorption of Fe2+ onto ferrihydrite followed by solid-state conversion of ferrihydrite to magnetite (Hansel et al., 2003). As the HCl-extraction method does not dissolve magnetite and magnetite-adsorbed Fe2+ (Poulton & Canfield, 2005), it is possible that our Fe(III) reduction rates based on HCl-extractable Fe(II) production were underestimates of the total Fe(III) reduction.
4.2 |. Fe(III) oxide mineralogy controls methane production and methanogen taxonomy
Our observation of higher rates of methanogenesis in goethite vs. ferrihydrite amendments is consistent with prior results showing that bacteria that reduce ferrihydrite better outcompete methanogenic archaea for H2 and acetate than those that reduce more crystalline Fe(III) oxides, including goethite (Hori, Müller, Igarashi, Conrad, & Friedrich, 2010; Lovley & Goodwin, 1988; Lovley & Phillips, 1987; Roden & Wetzel, 1996; Zhou et al., 2014). This outcompetition is also broadly supported by taxonomic shifts in our enrichment cultures. In particular, anaerobic heterotrophs such as Geothermobacter sp. (Kashefi, Holmes, Baross, & Lovley, 2003) were enriched in ferrihydrite treatments by day 15 and may have outcompeted other microbes for organic carbon sources.
Higher abundances of Methanobacteriaceae (0.1%–1% and 1%–4% on days 15 and 469, respectively) in goethite than ferrihydrite treatments (≤0.1%) suggest that CH4 in goethite treatments came from the substrates used by Methanobacteriaceae (H2/CO2, formate, or CO). Addition of H2 did not stimulate additional methanogenesis in the 4° amendment, implying another limiting substrate or growth condition. The ferrihydrite treatment (bottle 2) that produced CH4 by day 469 contained 3% Methanosaetaceae; the most dominant OTU had 98% similarity to Methanosaeta concilii, in agreement with observations from the Lake Matano water column (Crowe et al., 2011). Methanosaeta spp. produce CH4 from acetate, or from H2/CO2 via direct interspecies electron transfer with Geobacter (Rotaru et al., 2014).
4.3 |. Fe(III)-dependent CH4 oxidation
Enrichments were established under conditions thought to be favorable for Fe(III)-dependent CH4 oxidation, with Fe(III) oxides and CH4 as the most abundant electron acceptors and donors, respectively. In the 1° enrichment, incorporation of 13CH4 into DIC overlapped with the second phase of Fe(III) reduction (days 6–10), but calculating the stoichiometry of CH4 oxidized to Fe(III) reduced posed a challenge due to similar rates of Fe(III) reduction with and without added CH4 and in autoclaved controls. 13C-DIC enrichment from the back reaction of hydrogenotrophic methanogenesis (Zehnder & Brock, 1979) was ruled out because CH4 oxidation continued after Fe(III) reduction and methanogenesis stopped at day 10. Therefore, CH4 was likely oxidized by an electron acceptor other than Fe(III) (e.g., O2, Mn(IV), , ) in the 1° enrichment, likely supplied by residual sediment or inadvertent introduction of air.
During the first 15 days of the 3° enrichment, rates of CH4 oxidation and HCl-extractable Fe2+ production were similar (~10–20 μm/day) and roughly consistent with the low rates presented in Ettwig et al. (2016) that yielded a 1:8 ratio. However, the lack of multiple time points for the interval of simultaneous Fe(III) reduction and CH4 oxidation, as well as similar initial rates of Fe(III) reduction with and without CH4 throughout this interval, prevents us from attributing this activity to Fe(III)-dependent CH4 oxidation with high confidence.
It is notable that the two incubations with the highest rates of CH4 oxidation (ferrihydrite bottle 1 in 1° and 3° enrichments) were also the only treatments with very different microbial community compositions relative to other bottles in the same enrichment. In the 1° enrichment, ferrihydrite bottle 1 was enriched in Betaproteobacteria (Comamonadaceae and Rhodocyclaceae). In the 3° enrichment, the CH4-1 sample had less Peptococcaceae than other ferrihydrite incubations. By day 469, the betaproteobacterium A. oryzae/D. suillum, a member of the Rhodocyclaceae family, was more abundant in both of the CH4 vs. N2 treatments. The potential role of this microbe in CH4 cycling remains unclear, as laboratory cultures of this species are not known to oxidize CH4. Notably, related members of the Betaproteobacteria, including the genera Azospira and Comamonas found here, are typically facultative anaerobes that can use alternative electron acceptors like , , or perchlorate (Reinhold-Hurek & Hurek, 2015; Willems, 2014). As such, these Betaproteobacteria are poised to respond to enhanced electron acceptor supply that accompanies pulse of O2. Anecdotally, Betaproteobacteria are frequently associated with environments that are characterized by fluctuating redox conditions and periodic exposure to O2 (Converse, McKinley, Resch, & Roden, 2015). Thus, their growth in our incubations may be a response to trace O2 introduction. It is also possible that the growth of novel organisms capable of high rates of Fe(III)-dependent CH4 oxidation was inhibited by other unidentified factors, potentially related to the batch-style incubations, the use of butyl rubber stoppers (Niemann et al., 2015), or the lack of a critical substrate in the enrichment medium.
4.4 |. Effect of Fe(III) oxide and carbon substrates on microbial community diversity
The microbial community underwent multiple shifts over the 500-day incubation, with an overall decrease in species members, evenness, and phylogenetic diversity, likely in response to declining organic carbon. By the 3° enrichment, species evenness was consistently lower in each goethite-amended treatment than in the respective ferrihydrite-amended treatment. This could mean that the greater energetic yield of ferrihydrite reduction fosters higher diversity, or that the higher reactivity of ferrihydrite allowed it to be utilized by more organisms than goethite.
All of the most enriched taxa in our enrichments comprised ≤0.1% of the inoculum community and have members that reduce Fe(III) in laboratory cultures. Within those taxa, the most abundant OTUs were closely related to organisms capable of Fe(III) reduction (Geothermobacter sp., G. hephaestius/G. lovleyi, Thermincola sp., and S. barnesii) (Kashefi et al., 2003; Stolz et al., 1999; Zavarzina et al., 2007). Desulfuromonadales was the only metal-reducing taxon that was continuously present in all enrichments (3%–11%, 34%–53%, and 18%–76% in the, 1°, 2°, and 3° enrichment, respectively). Other taxa differed significantly in their abundance over the course of incubation. Geobacteraceae was enriched at day 15 with ferrihydrite (22%–36%) but had declined in abundance by day 72 (8%–18%). Still other taxa, including Rhodocyclaceae and Peptococcaceae, were enriched in the presence of ferrihydrite at day 469. In goethite treatments, Campylobacteraceae (23%–40%) were enriched at day 72, but were minimal at day 469. The succession of different metal-reducing taxa may be due to the changing availability of electron donors (e.g., H2 and organic C). Enrichment of Syntrophaceae, known for their syntrophic fermentative interactions, suggests the establishment of syntrophy in the 3° enrichment in response to depletion of electron donors.
4.5 |. Nickel sources
Enrichment cultures contained ~2–10× more total dissolved Ni than the basal growth medium. The inoculum (~60 nm in Lake Matano deep water; Crowe, O’Neill, et al., 2008) would not have significantly contributed to the Ni pool past the 1° enrichment, and repeated needle exposure had no effect on Ni concentrations. The Ni source to enrichment cultures was likely partial ferrihydrite dissolution, as ferrihydrite readily scavenges Ni from solution (Zegeye et al., 2012), while its dissolution liberates Ni (Table S1; Crowe, O’Neill, et al., 2007). Slow Ni leaching from silicate glass during extended contact between microbes and the serum bottles could have contributed another source of Ni in microbial enrichments vs. abiotic controls (Hausrath, Liermann, House, Ferry, & Brantley, 2007).
4.6 |. Geobiological implications
Our results point to a mineralogical control on Fe(III) reduction, methanogenesis, and microbial community composition and diversity, under conditions of severe organic carbon limitation. These conditions likely existed in Archean and Paleoproterozoic oceans with relatively low amounts of primary production (Farquhar, Zerkle, & Bekker, 2011; Knoll, Bergmann, & Strauss, 2016). We posit that the relative abundance and distribution of Fe(III) phases in marine sediments would have impacted methanogenesis rates in the Archean and Paleoproterozoic. Sediments below shallow water columns were likely fed by abundant amorphous Fe(III) from photoferrotrophic activity, resulting in rapid sedimentation of amorphous Fe(III) phases (e.g., ferrihydrite). These Fe(III) oxides could have supported diverse Fe(III)-reducing communities that outcompeted other taxa such as methanogens for limited carbon and nutrients.
Conversely, slow deposition and aging of ferrihydrite to goethite could have limited both the abundance and diversity of Fe(III)-reducing microbes in sediments, allowing for more organic carbon remineralization via methanogenesis than Fe(III) reduction, as recently calculated for Lake Matano (Crowe et al., 2011; Kuntz et al., 2015). In the open ocean, organic carbon and Fe(III) would likely have been consumed before reaching sediments, leaving behind more crystalline Fe(III) phases. Importantly, the role of Fe(III)-driven CH4 oxidation appears limited given our experimental results, although we cannot rule out this pathway given that some of our data suggest it may operate at low rates.
Availability of trace metal nutrients is another important consideration in potential controls on ancient CH4 and Fe cycling. Measurements of Ni/Fe ratios in ancient marine sediments indicate that total dissolved Ni decreased from ~400 nm before 2.7 Ga to 200 nm between 2.7 and 2.5 Ga, to modern levels of 2–11 nm at ~0.5 Ga, assuming that the Fe(III) minerals in Archean sediments were of biological origin (Eickhoff et al., 2014; Konhauser et al., 2009, 2015). It is likely that abundant Ni would have been bound and sequestered in Fe(III) oxide-rich sediments. Rapid and widespread Archean redox cycling of Fe(III) could have served as constant source of Ni for methanogenic communities. The influence of changing availability of Se (Stüeken, Buick, & Anbar, 2015) and other trace nutrients on methanogenesis rates through time remains open for further exploration.
Overall, our results support a model for a sustained CH4 greenhouse in the Archean and Paleoproterozoic due to emissions from ferruginous oceans with spatially segregated habitats of bacterial reduction of reactive Fe(III) oxides and methanogenesis in the presence of less reactive Fe(III) phases. Rates of Fe(III) deposition, aging, and recrystallization may thus have played an important role in regulating the preservation of sedimentary Fe(III), the production of CH4, and the ecology and diversity of the biosphere during the first half of Earth history. By the mid-Proterozoic, rising seawater sulfate likely stimulated anaerobic CH4 oxidation, thereby minimizing marine CH4 emissions and the CH4 greenhouse (Olson, Reinhard, & Lyons, 2016).
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by NASA Exobiology grant NNX14AJ87G. Support was also provided by a Center for Dark Energy Biosphere Investigations (NSF-CDEBI OCE-0939564) small research grant and supported by the NASA Astrobiology Institute (NNA15BB03A). This is C-DEBI contribution 365. SAC was supported through NSERC CRC, CFI, and Discovery grants. We thank Miles Mobley and Johnny Striepen for assistance with laboratory incubations, and Nadia Szeinbaum, Liz Percak-Dennett, Martial Taillefert, and Joel Kostka for helpful discussions.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- Amos R, Bekins B, Cozzarelli I, Voytek M, Kirshtein J, Jones E, & Blowes D (2012). Evidence for iron-mediated anaerobic methane oxidation in a crude oil-contaminated aquifer. Geobiology, 10, 506–517. [DOI] [PubMed] [Google Scholar]
- Battistuzzi FU, Feijao A, & Hedges SB (2004). A genomic timescale of prokaryote evolution: Insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evolutionary Biology, 4, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal EJ, House CH, & Orphan VJ (2009). Manganese- and iron-dependent marine methane oxidation. Science, 325, 184–187. [DOI] [PubMed] [Google Scholar]
- Brandes JA (2009). Rapid and precise δ13C measurement of dissolved inorganic carbon in natural waters using liquid chromatography coupled to an isotope-ratio mass spectrometer. Limnology and Oceanography: Methods, 7, 730–739. [Google Scholar]
- Caldwell SL, Laidler JR, Brewer EA, Eberly JO, Sandborgh SC, & Colwell FS (2008). Anaerobic oxidation of methane: Mechanisms, bioenergetics, and the ecology of associated microorganisms. Environmental Science & Technology, 42, 6791–6799. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, … Knight R (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the USA, 108, 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse BJ, McKinley JP, Resch CT, & Roden EE (2015). Microbial mineral colonization across a subsurface redox transition zone. Frontiers in Microbiology, 6, 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock PR, & Dauphas N (2011). Iron and carbon isotope evidence for microbial iron respiration throughout the Archean. Earth and Planetary Science Letters, 303, 121–132. [Google Scholar]
- Crowe SA, Jones C, Katsev S, Magen CD, O’Neill AH, Sturm A, … Sundby BR (2008). Photoferrotrophs thrive in an Archean Ocean analogue. Proceedings of the National Academy of Sciences of the USA, 105, 15938–15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S, Katsev S, Leslie K, Sturm A, Magen C, Nomosatryo S, … Roberts J (2011). The methane cycle in ferruginous Lake Matano. Geobiology, 9, 61–78. [DOI] [PubMed] [Google Scholar]
- Crowe SA, O’Neill AH, Katsev S, Hehanussa P, Haffner GD, Sundby B, … Fowle DA (2008). The biogeochemistry of tropical lakes: A case study from Lake Matano, Indonesia. Limnology Oceanography, 53, 319–331. [Google Scholar]
- Crowe SA, O’Neill AH, Kulczycki E, Weisener CG, Roberts JA, & Fowle DA (2007). Reductive dissolution of trace metals from sediments. Geomicrobiology Journal, 24, 157–165. [Google Scholar]
- Crowe S, Roberts J, Weisener C, & Fowle D (2007). Alteration of iron-rich lacustrine sediments by dissimilatory iron-reducing bacteria. Geobiology, 5, 63–73. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, & Knight R (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27, 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Rasigraf O, Sapart CLJ, Jilbert T, Jetten MS, Röckmann T, … Ettwig KF (2015). Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environmental Science & Technology, 49, 277–283. [DOI] [PubMed] [Google Scholar]
- Eickhoff M, Obst M, Schröder C, Hitchcock AP, Tyliszczak T, Martinez RE, … Kappler A (2014). Nickel partitioning in biogenic and abiogenic ferrihydrite: The influence of silica and implications for ancient environments. Geochimica et Cosmochimica Acta, 140, 65–79. [Google Scholar]
- Ettwig KF, Zhu B, Speth D, Keltjens JT, Jetten MSM, & Kartal B (2016). Archaea catalyze iron-dependent anaerobic oxidation of methane. Proceedings of the National Academy of Sciences of the USA, 113, 12792–12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar J, Zerkle AL, & Bekker A (2011). Geological constraints on the origin of oxygenic photosynthesis. Photosynthesis Research, 107, 11–36. [DOI] [PubMed] [Google Scholar]
- Fournier GP, & Gogarten JP (2008). Evolution of acetoclastic methanogenesis in Methanosarcina via horizontal gene transfer from cellulolytic Clostridia. Journal of Bacteriology, 190, 1124–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Li S-W, Ding Z-W, Ding J, Lu Y-Z, & Zeng RJ (2016). Iron reduction in the DAMO/Shewanella oneidensis MR-1 coculture system and the fate of Fe(II). Water Research, 88, 808–815. [DOI] [PubMed] [Google Scholar]
- Gal’chenko V (2004). On the problem of anaerobic methane oxidation. Microbiology, 73, 599–608. [Google Scholar]
- Hansel CM, Benner SG, Neiss J, Dohnalkova A, Kukkadapu RK, & Fendorf S (2003). Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochimica et Cosmochimica Acta, 67, 2977–2992. [Google Scholar]
- Hansen JW, Thamdrup B, & Jørgensen BB (2000). Anoxic incubation of sediment in gas-tight plastic bags: A method for biogeochemical process studies. Marine Ecology Progress Series, 208, 273–282. [Google Scholar]
- Haqq-Misra JD, Domagal-Goldman SD, Kasting PJ, & Kasting JF (2008). A revised, hazy methane greenhouse for the Archean Earth. Astrobiology, 8, 1127–1137. [DOI] [PubMed] [Google Scholar]
- Hausrath EM, Liermann LJ, House CH, Ferry JG, & Brantley SL (2007). The effect of methanogen growth on mineral substrates: Will Ni markers of methanogen based communities be detectable in the rock record? Geobiology, 5, 49–61. [DOI] [PubMed] [Google Scholar]
- Hori T, Müller A, Igarashi Y, Conrad R, & Friedrich MW (2010). Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. The ISME Journal, 4, 267–278. [DOI] [PubMed] [Google Scholar]
- Jensen MM, Thamdrup B, Rysgaard S, Holmer M, & Fossing H (2003). Rates and regulation of microbial iron reduction in sediments of the Baltic-North Sea transition. Biogeochemistry, 65, 295–317. [Google Scholar]
- Johnson CM, Beard BL, & Roden EE (2008). The iron isotope fingerprints of redox and biogeochemical cycling in modern and ancient Earth. Annual Reviews of Earth and Planetary Sciences, 36, 457–493. [Google Scholar]
- Kappler A, Pasquero C, Konhauser KO, & Newman DK (2005). Deposition of banded iron formations by anoxygenic phototrophic Fe(II)-oxidizing bacteria. Geology, 33, 865–868. [Google Scholar]
- Kashefi K, Holmes DE, Baross JA, & Lovley DR (2003). Thermophily in the Geobacteraceae: Geothermobacter ehrlichii gen. nov., sp. nov., a novel thermophilic member of the Geobacteraceae from the “Bag City” hydrothermal Vent. Applied and Environmental Microbiology, 69, 2985–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasting JF (2005). Methane and climate during the Precambrian era. Precambrian Research, 137, 119–129. [Google Scholar]
- Knoll AH, Bergmann KD, & Strauss JV (2016). Life: The first two billion years. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371, 20150493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhauser K, Newman D, & Kappler A (2005). The potential significance of microbial Fe(III) reduction during deposition of Precambrian banded iron formations. Geobiology, 3, 167–177. [Google Scholar]
- Konhauser KO, Pecoits E, Lalonde SV, Papineau D, Nisbet EG, Barley ME, … Kamber BS (2009). Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature, 458, 750–753. [DOI] [PubMed] [Google Scholar]
- Konhauser KO, Robbins LJ, Pecoits E, Peacock C, Kappler A, & Lalonde SV (2015). The Archean nickel famine revisited. Astrobiology, 15, 804–815. [DOI] [PubMed] [Google Scholar]
- Kostka JE, & Luther GW (1994). Partitioning and speciation of solid phase iron in saltmarsh sediments. Geochemica Cosmochimica Acta, 58, 1701–1710. [Google Scholar]
- Kostka JE, Roychoudhury A, & Van Cappellen P (2002). Rates and controls of anaerobic microbial respiration across spatial and temporal gradients in saltmarsh sediments. Biogeochemistry, 60, 49–76. [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, & Schloss PD (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and Environmental Microbiology, 79, 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz LB, Laakso TA, Schrag DP, & Crowe SA (2015). Modeling the carbon cycle in Lake Matano. Geobiology, 13, 454–461. [DOI] [PubMed] [Google Scholar]
- Lovley DR, & Goodwin S (1988). Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochimica et Cosmochimica Acta, 52, 2993–3003. [Google Scholar]
- Lovley D, & Phillips E (1986). Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Applied and Environmental Microbiology, 51, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, & Phillips EJ (1987). Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Applied and Environmental Microbiology, 53, 2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H, Steinle L, Blees J, Bussmann I, Treude T, Krause S, … Lehmann MF (2015). Toxic effects of lab-grade butyl rubber stoppers on aerobic methane oxidation. Limnology and Oceanography: Methods, 13, 40–52. [Google Scholar]
- Noroi KA, Thamdrup B, & Schubert CJ (2013). Anaerobic oxidation of methane in an iron-rich Danish freshwater lake sediment. Limnology and Oceanography, 58, 546–554. [Google Scholar]
- Olson SL, Reinhard CT, & Lyons TW (2016). Limited role for methane in the mid-Proterozoic greenhouse. Proceedings of the National Academy of Sciences of the USA, 113, 11447–11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov AA, Kasting JF, Brown LL, Rages KA, & Freedman R (2000). Greenhouse warming by CH4 in the atmosphere of early Earth. Journal of Geophysical Research: Planets, 105, 11981–11990. [DOI] [PubMed] [Google Scholar]
- Poulton SW, & Canfield DE (2005). Development of a sequential extraction procedure for iron: Implications for iron partitioning in continentally derived particulates. Chemical Geology, 214, 209–221. [Google Scholar]
- Poulton SW, & Canfield DE (2011). Ferruginous conditions: A dominant feature of the ocean through Earth’s history. Elements, 7, 107–112. [Google Scholar]
- Reinhold-Hurek B, & Hurek T (2015) Azospira Bergey’s Manual of Systematics of Archaea and Bacteria, Vol. 1–3. Hoboken, NJ: John Wiley & Sons, Inc. in association with Bergey’s Manual Trust. [Google Scholar]
- Riedinger N, Formolo M, Lyons T, Henkel S, Beck A, & Kasten S (2014). An inorganic geochemical argument for coupled anaerobic oxidation of methane and iron reduction in marine sediments. Geobiology, 12, 172–181. [DOI] [PubMed] [Google Scholar]
- Roberson AL, Roadt J, Halevy I, & Kasting J (2011). Greenhouse warming by nitrous oxide and methane in the Proterozoic Eon. Geobiology, 9, 313–320. [DOI] [PubMed] [Google Scholar]
- Roden EE, & Wetzel RG (1996). Organic carbon oxidation and suppression of methane production by microbial Fe (III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnology and Oceanography, 41, 1733–1748. [Google Scholar]
- Roden EE, & Wetzel RG (2002). Kinetics of microbial Fe (III) oxide reduction in freshwater wetland sediments. Limnology and Oceanography, 47, 198–211. [Google Scholar]
- Rooze J, Egger M, Tsandev I, & Slomp CP (2016). Iron-dependent anaerobic oxidation of methane in coastal surface sediments: Potential controls and impact. Limnology and Oceanography, 6, S267–S282. [Google Scholar]
- Rotaru A-E, Shrestha PM, Liu F, Shrestha M, Shrestha D, Embree M, … Lovley DR (2014). A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy & Environmental Science, 7, 408–415. [Google Scholar]
- Scheller S, Yi H, Chadwick GL, McGlynn SE, & Orphan VJ (2016). Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science, 351, 703–707. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, … Robinson CJ (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertmann U, & Cornell RM. (1991). Iron oxides in the laboratory (p. 137). New York: Preparation and Characterization VCH. [Google Scholar]
- Segarra KEA, Comerford C, Slaughter J, & Joye SB (2013). Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochimica et Cosmochimica Acta, 115, 15–30. [Google Scholar]
- Sivan O, Adler M, Pearson A, Gelman F, Bar-Or I, John SG, & Eckert W (2011). Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnology and Oceanography, 56, 1536–1544. [Google Scholar]
- Sivan O, Antler G, Turchyn AV, Marlow JJ, & Orphan VJ (2014). Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps. Proceedings of the National Academy of Sciences of the USA, 111, 4139–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz JF, Ellis DJ, Blum JS, Ahmann D, Lovley DR, & Oremland RS (1999). Note: Sulfurospirillum barnesii sp. nov. and Sulfurospirillum arsenophilum sp. nov., new members of the Sulfurospirillum clade of the ε-Proteobacteria. International Journal of Systematic and Evolutionary Microbiology, 49, 1177–1180. [DOI] [PubMed] [Google Scholar]
- Stookey LL (1970). Ferrozine—a new spectrophotometric reagent for iron. Analytical Chemistry, 42, 779–781. [Google Scholar]
- Stüeken EE, Buick R, & Anbar AD (2015). Selenium isotopes support free O2 in the latest Archean. Geology, 43, 259–262. [Google Scholar]
- Sturm A, Fowle DA, Jones C, Leslie KL, Nomosatryo S, Henny C, … Crowe S (2015). Rates and pathways of methane oxidation in ferruginous Lake Matano, Indonesia. Biogeosciences Discussions, 12, 1–34. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Yamada K, Yoshida N, Maruyama S, & Isozaki Y (2006). Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature, 440, 516–519. [DOI] [PubMed] [Google Scholar]
- Vargas M, Kashefi K, Blunt-Harris EL, & Lovley DR (1998). Microbiological evidence for Fe(III) reduction on early Earth. Nature, 395, 65–67. [DOI] [PubMed] [Google Scholar]
- Willems A (2014) The family Comamonadaceae In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (Eds.), The Prokaryotes (pp. 777–851). Berlin, Heidelberg: Springer. [Google Scholar]
- Zavarzina DG, Sokolova TG, Tourova TP, Chernyh NA, Kostrikina NA, & Bonch-Osmolovskaya EA (2007). Thermincola ferriacetica sp. nov., a new anaerobic, thermophilic, facultatively chemolithoautotrophic bacterium capable of dissimilatory Fe(III) reduction. Extremophiles, 11, 1–7. [DOI] [PubMed] [Google Scholar]
- Zegeye A, Bonneville S, Benning LG, Sturm A, Fowle DA, Jones C, … Nomosatryo S (2012). Green rust formation controls nutrient availability in a ferruginous water column. Geology, 40, 599–602. [Google Scholar]
- Zehnder AJ, & Brock TD (1979). Methane formation and methane oxidation by methanogenic bacteria. Journal of Bacteriology, 137, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder AJ, & Brock TD (1980). Anaerobic methane oxidation: Occurrence and ecology. Applied and Environmental Microbiology, 39, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Xu J, Yang G, & Zhuang L (2014). Methanogenesis affected by the co-occurrence of iron (III) oxides and humic substances. FEMS Microbiology Ecology, 88, 107–120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


