Abstract
Two efflux transporters, ATP-binding cassettes B1 (ABCB1) and G2 (ABCG2), are highly expressed in the endothelial cells of the brain, where they regulate the bioavailability and distribution of several endogenous and xenobiotic compounds. However, whether ABCB1 or ABCG2 has any link with drug dependence, drug withdrawal effects, or the incidence of adverse effects in drug abuser is not known. In this study, we determined the effects of voluntary ethanol consumption following repeated exposure to cocaine or vehicle on the relative mRNA and protein expression of Abcg2/ABCG2 and Abcb1/ABCB1 in the nucleus accumbens (NAc) and medial prefrontal cortex (mPFC) of male alcohol-preferring (P) rats. Male P rats were allowed free choice access to ethanol (15 and 30% v/v) and water for 5 weeks to establish baseline drinking behavior. The following week, rats were either injected with 20 mg/kg i.p. of cocaine or saline, once a day, for 7 days. The relative mRNA and protein expression of Abcb1/ABCB1 and Abcg2/ABCG2 in the NAc and mPFC were significantly decreased in ethanol-saline- and ethanol-cocaine-exposed rats compared to control rats that received neither ethanol nor cocaine. Thus, prolonged exposure to commonly abused drugs, ethanol and cocaine, alters the expression of Abcb1/ABCB1 and Abcg2/ABCG2 mRNA and protein levels in brain areas that play a role in drug dependence.
Keywords: Ethanol, Cocaine, ABCB1, ABCG2, NAc, mPFC
Introduction
Interaction between drugs (e.g., drug-drug interactions; DDI), including drugs of abuse, can contribute to severe adverse effects. Some DDI may involve alterations in the expression or function of ATP-binding cassette (ABC) proteins [1]. These DDI may result from the non-linear elimination characteristics of the ABC transporters, as well as the competition between the substrates of ABC transporters. Based on amino acid sequence homology, the ABC transporter family has been categorized into seven main subtypes (ABCA–ABCG) and these transporters are involved in either the efflux or influx of endogenous and exogenous compounds [2]. ABC transporters, such as ABCG2 (breast cancer resistance protein, BCRP), ABCB1 (P-glycoprotein, P-gp), and ABCC2 (multidrug resistance proteins 2, MRP-2) [3, 4], are expressed in the canalicular membrane of liver cells and the apical membranes of the epithelial barriers in the kidney and intestine. The overexpression of these efflux transporters is correlated with the reduction in the systemic concentrations of their substrates [3, 5]. In addition, the overexpression of ABCB1 and ABCG2 in the brain capillary endothelial cells of the blood brain barrier (BBB) limits the penetration of certain drugs into the brain, thereby limiting or abrogating their therapeutic efficacy [6, 7].
Drug addicts commonly receive pharmacological treatments for various other medical conditions, such as certain antiretroviral and antipsychotic drugs, that are ABCB1 and ABCG2 substrates [1, 8]. These treatments could consequently alter responses to drugs of abuse, or conversely a history of illicit drug use might alter responses to these treatment medications, resulting in deleterious drug-drug interactions or even contributing to the addictive process. There is also, of course, substantial co-abuse of multiple illicit substances, particularly the co-use of ethanol with most other drugs of abuse. Previous studies, using in vitro and in vivo models, have identified roles of specific ABC proteins in transporting various drugs of abuse [9–11]. For example, it has been shown that buprenorphine is transported across the BBB via an ABCB1-mediated efflux transport system [12]. Cocaine was also found to be a substrate for ABCB1 transporters in bovine brain endothelial cells [10]. Moreover, methadone inhibits the activity of ABCB1 in vitro in Caco-2 cells [13] and in rodents [14, 15], which might therefore affect subsequent responses to methadone and other ABCB1 substrates.
Such effects on the expression of ABC transporters in response to substrate exposure or other molecular signals would be expected to be most apparent after chronic treatments. It has been reported that short-term (4 days) exposure to ethanol decreases the cellular expression of ABCG2 in cortical progenitor cells [16]. Disulfiram, a drug approved for the treatment of alcohol abuse, interacts with ABCB1 binding sites, which may induce drug resistance [17]. Thus, concurrent exposure to ethanol and disulfiram could produce adverse effects due to interactions at the level of ABC transporters. Unlike other drugs of abuse, ethanol would not be expected to be a substrate for ABC transporters and to instead cross the BBB by passive diffusion [18], but may affect the function of these transporters in other ways. For instance, chronic ethanol exposure affects the level of transcriptional factors and neuroinflammatory biomarkers that may regulate the expression of ABC transporters [19–21].
Cocaine is a psychostimulant drug that produces its addictive effects via alteration of neuronal function/activity in brain areas hypothesized to mediate reward and reinforcement [22]. Cocaine crosses the BBB by passive diffusion to some extent, but more importantly, by the proton antiporter flux system [23, 24]. However, data suggest that cocaine may also be transported by ABCB1 [10]. In addition to the pharmacodynamic mechanisms that have been postulated to mediate the development of cocaine dependence [25, 26], it has been suggested that chronic exposure to cocaine affects brain proteins that may play a role in the pathogenesis and/or pathophysiology of other diseases [27, 28]. Indeed, cocaine affects chemokine receptors of the immune system [29], and these changes could be involved in the activation or repression of ABC transporters [30, 31]. Since long-term exposure to chemotherapeutic drugs increases the expression of ABC transporters in the brain, it might be thought that exposure to these drugs might influence subsequent responses to drugs of abuse that are substrates for these transporters. Similarly, a prior history of drug abuse might also affect subsequent responses to chemotherapeutic drugs. It is therefore important to investigate the effects of chronic exposure to drugs of abuse, including cocaine, on the expression of these transporters. To begin to address this question, the present study determined the direct effect of concurrent, repeated i.p. injections of cocaine and voluntary oral ethanol consumption on mRNA and protein expression of Abcb1/ABCB1and Abcg2/ABCG2 in the NAc and mPFC of male alcohol-preferring (P) rats. This dual regimen was chosen because of previous research demonstrating substantial changes in brain mechanisms influencing the neurotoxic effects of drugs of abuse [32, 33].
Materials and Methods
Subjects
Fifteen male alcohol-preferring (P) rats (21–30 days of age) were obtained from Indiana University, School of Medicine (Indianapolis, IN, USA) and were housed in the Department of Laboratory Animal Resources, University of Toledo, Health Science Campus. The animal protocol for this study was approved by the Institutional Animal Care and Use Committee of The University of Toledo and was in accordance with all National Institutes of Health guidelines for animal research, including the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
Animals were grouped and housed in a vivarium room kept on a 12-/12-h light/dark cycle and maintained under controlled temperature (23 ± 2 °C) and humidity (50 ± 5%). The number of animals in each cage was chosen depending on the animals’ weight in accordance with IACUC guidelines.
Behavioral Drinking Paradigm
The time line of voluntary ethanol consumption and repeated cocaine administration (20 mg/kg, i.p.) is illustrated in Fig. 1. At 75 days of age, rats were housed individually under controlled temperature (23 ± 2 °C) and humidity (50 ± 5%) conditions and were maintained on a 12-/12-h light/dark cycle. Rats had ad libitum access to food and water throughout the study. Ethanol-treated rats (groups 2 and 3 below; see Fig. 1) were exposed to a free choice ethanol drinking procedure (15 and 30% ethanol v/v and water, concurrently) for a period of 5 weeks. During the fourth week of ethanol consumption, ethanol and water intake were measured three times a week for 2 weeks. Rats consuming less than 4 g/kg/day of ethanol were excluded (three rats) from the study, as described in previous studies [32, 34]. On the following week, the ethanol-exposed P rats were randomly divided into two groups, so that there were three groups overall: (A) group 1 (water control group), exposed to water throughout the study and injected i.p. with 0.9% saline (vehicle); (B) group 2 (ethanol-saline group), exposed to ethanol and water throughout the study and injected i.p. with saline once daily for 7 days; and (C) group 3 (ethanol-cocaine group), exposed to ethanol and water throughout the study and injected with 20 mg/kg i.p. of cocaine once daily for 7 days.
Fig. 1.
The time line for voluntary ethanol drinking and repeated cocaine exposure
Brain Tissue Harvesting
Carbon dioxide (27%) was used to euthanize rats 24 h after the last cocaine or saline injection (and at the same time for control subjects). Rats were decapitated using a guillotine and the brains were removed, placed on dry ice until frozen and stored at − 80 °C until samples were collected. A cryostat apparatus, set at − 20 °C, was used for dissection of samples from the mPFC and NAc. Tissue sections were taken until the brain regions of interest could be identified using the Rat Brain Atlas [35]. The isolated brain regions (left and right sides) were stored at − 80 °C for immunoblot and quantitative polymerase chain reaction (PCR) analysis.
Western Blot
A lysis buffer (1 M Tris HCL, 3 M NaCl, 0.5 M EDTA, 10% NP-40, 10% Triton, 10% SDS) containing protease inhibitors (Thermo Scientific, Rockford, IL, USA) was used to lyse the brain tissues in preparation for Western blot analysis, as previously described [36]. A protein quantification assay was performed using a DC (detergent compatible) protein assay (Bio-Rad Laboratories, USA). Subsequently, equal amounts of extracted proteins from each sample were separated on 10% polyacrylamide gels. The proteins were transferred from the gels to PVDF membranes (Bio-Rad, Hercules, CA, USA). Subsequently, TBST (50 mM Tris HCl; 150 mM NaCl, pH 7.4; 0.1% Tween 20) containing 5% non-fat dry milk was used to block the membranes at room temperature for 30 min. The membranes were then exposed to one of the following primary antibodies: mouse anti-MDR1/ABCB1 (1:200, Novus Biological), or mouse anti-ABCG2/CD338 (1: 2000, Novus Biological) at 4 °C overnight. A loading control protein was assessed throughout the study using mouse anti-GAPDH (1: 5000; Cell Signaling Technology). On the second day, the membranes were washed five times with TBST. Subsequently, TBST in 3% non-fat dry milk was used to further block the membranes for 30 min. At room temperature, membranes were exposed to the secondary antibody, anti-mouse ABCB1, ABCG2, and GABDH (1:5000; Cell Signaling Technology) for 90 min. The membranes were washed five times with TBST, dried, and incubated with the developing kits to detect proteins (SuperSignal West Pico Chemiluminescent substrate, Rockford, IL, USA). The membranes were exposed to film (Kodak BioMax MR Film, Fisher Inc., Holiston, MI, USA) and the film was developed using an SRX-101A machine (Konica Minolta Medical and Graphic Inc.). To quantify the intensity of the detected bands, an MCID system (Imaging Research Inc., Ontario, Canada) was used and obtained values were expressed as a percentage of the relative ratio of the proteins of interest to GAPDH (100% water control value) as described previously [25, 37].
The gels in the Western blot analyses were run in triplicate and not all samples could be run on the same gel. Furthermore, there are many factors that can influence, by increasing or decreasing, the intensity of the blot in each run, including room temperature, the amount of protein loaded, the ratio of the developing kit, and the film exposure time. Hence, we standardized the expression of the protein of interest for each sample to the corresponding water control sample that was run in the same gel, under the same conditions, as described in several previous studies [32, 38–41].
Real-Time, Quantitative PCR (RT-PCR, qPCR)
Triazol Reagent (Life Technologies, Carlsbad, CA, USA) was used to isolate total RNAs from the NAc and mPFC. Subsequently, using a verso cDNA synthesis kit (Thermo Scientific, Lithuania), reverse transcription (RT) was done according to the manufacturer’s protocol. An iCycler (Bio-Rad laboratories, München, Germany) was used to perform Real-Time PCR (RT-PCR). RT-PCR was done using a reaction mixture of SYBR Green as a fluorescent dye (Bio-Rad Laboratories), a 1/20 volume of cDNA preparation as a template, and the appropriate primers for the genes of interest as shown in Table 1. A threshold cycle number (CT) for each sample was obtained from the iCycler and was used to compare the relative amount of target mRNA in experimental groups with those of controls, using the 2−ΔΔCT method [33, 44]. Each sample was run in triplicate. In order to get ΔCT, the mean CT value for the control gene, GAPDH was subtracted from the mean CT value of the gene of interest. The ΔCT values for the control group (ethanol-naive) were then averaged and were subtracted from ΔCT for the experimental groups to obtain ΔΔCT. The relative fold change from control was then expressed by calculating 2−ΔΔCT for each sample and the results were reported as the group mean fold change ± SEM.
Table 1.
Primer sequence for rat Abcb1, Abcg2, and Gapdh
| Gene | Primer | Sequencea, b |
|---|---|---|
| Abcb1 | Forward primer | 5’- GTGTTTCTAGATGGCAAAGA −3’ |
| Reverse primer | 5’- CCACTCTGGTGTTGTATTTC −3’ | |
| 2Abcg2 | Forward primer | 5’- AAGACCATGAAGCAAACAAG −3’ |
| Reverse primer | 5’- ACACTGGTTGTTAGTCAGGA −3’ | |
| Gapdh | Forward primer | 5’- CCCCCAATGTATCCGTTGTG −3’ |
| Reverse primer | 5’- TAGCCCAGGATGCCCTTTAGT-3’ |
Statistical Analyses
The relative mRNA and protein expression of Abcb1/ABCB1 to Gapdh/GAPDH and Abcg2/ABCG2 to Gapdh/GAPDH for water-control, ethanol-saline, and ethanol-cocaine groups were analyzed using a one-way ANOVA. Post hoc comparisons were made using the Newman-Keuls multiple comparison test. A priori significance level was set to p < 0.05.
Results
The Effect of Voluntary Ethanol Consumption and the Repeated Cocaine Administration on the Relative mRNA Expression of Abcb1 and Abcg2 in the NAc and the mPFC
The Relative mRNA Expression of Abcb1 and Abcg2 in the NAc
Abcb1 mRNA
Statistical analysis indicated a significant decrease in the relative Abcb1 mRNA expression [F (2, 12) = 5.454, p = 0.0207] in the NAc of rats that voluntarily consumed ethanol and in rats treated chronically with 20 mg/kg i.p. of cocaine after ethanol consumption, compared to ethanol-naïve P rats (Fig. 2 and Table 2, left). There was no significant difference between ethanol-treated groups resulting from cocaine exposure.
Fig. 2.
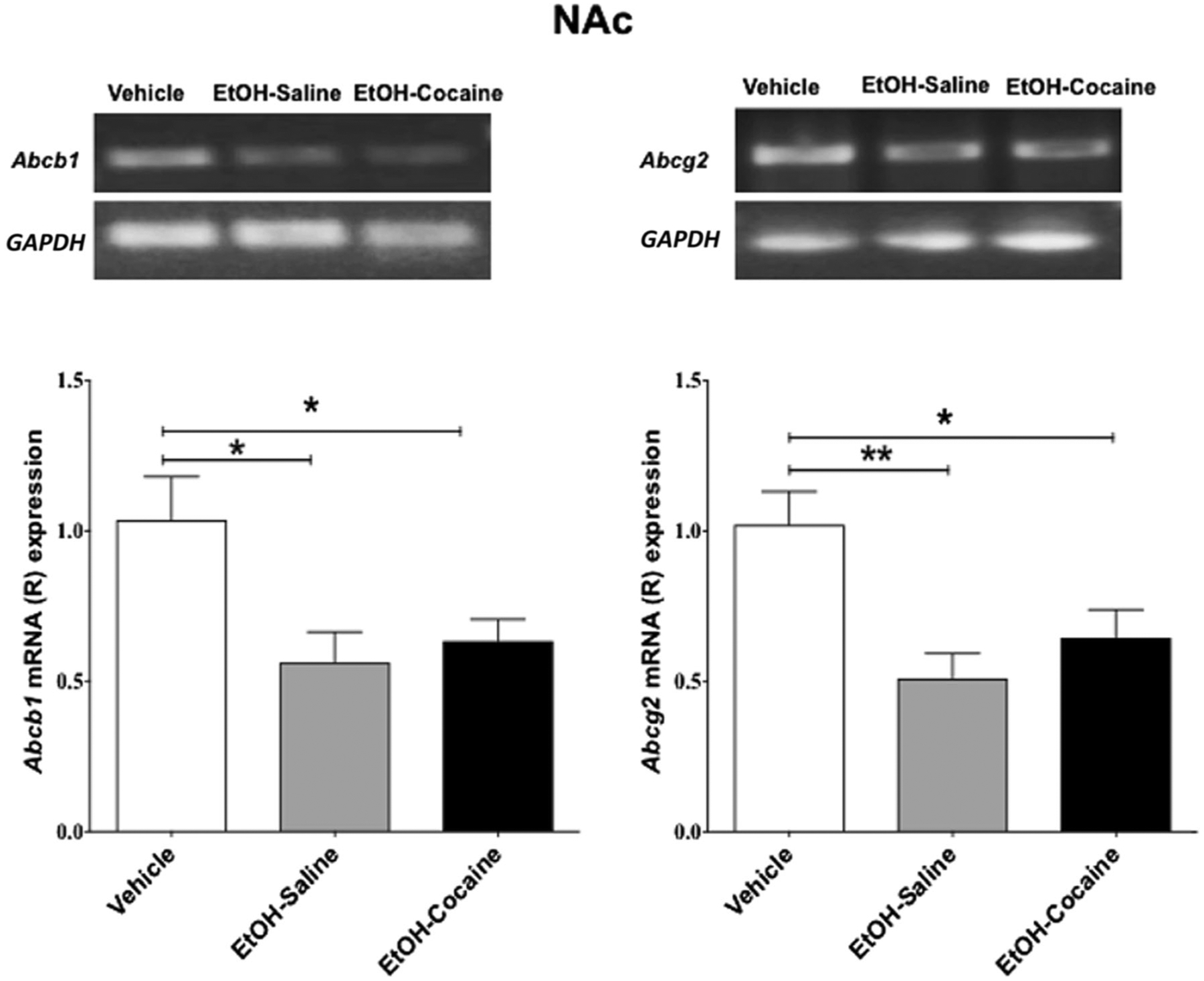
Relative mRNA expression of Abcb1 and Abcg2 in the NAc following voluntary ethanol consumption and repeated cocaine exposure (mean ± SEM). a One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in relative mRNA expression of Abcb1 in the NAc. b One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in relative mRNA expression of Abcg2 in the NAc. (*p < 0.05, **p < 0.01), (n = 5 for each group)
Table 2.
Relative mRNA expression of Abcb1 and Abcg2 in the NAc following voluntary ethanol consumption and repeated cocaine exposure expressed as mean ± SD.
| Abcb1 mRNA expression | Abcg2 mRNA expression | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | EtOH-saline | EtOH-cocaine | Vehicle | EtOH-saline | EtOH-cocaine | ||
| 0.93 | 0.47 | 0.77 | 0.85 | 0.61 | 0.44 | ||
| 1.17 | 0.51 | 0.73 | 0.95 | 0.46 | 0.82 | ||
| 0.80 | 0.45 | 0.60 | 0.84 | 0.74 | 0.72 | ||
| 1.53 | 0.96 | 0.70 | 1.02 | 0.49 | 0.84 | ||
| 0.76 | 0.42 | 0.37 | 1.44 | 0.24 | 0.40 | ||
| Mean ± SD | 1.04 ± 0.32 | 0.56 ± 0.22 | 0.63 ± 0.16 | Mean ± SD | 1.02 ± 0.25 | 0.51 ± 0.19 | 0.64 ± 0.21 |
Abcg2 mRNA
Statistical analysis revealed a significant decrease in the relative Abcg2 mRNA expression [F (2, 12) = 7.549, p = 0.0075] in the NAc of rats that voluntarily consumed ethanol and in rats treated chronically with 20 mg/kg i.p. cocaine after ethanol consumption, compared to ethanol-naïve P rats (Fig. 2 and Table 2, right). There was no significant difference between ethanol-treated groups resulting from cocaine exposure.
The Relative mRNA Expression of Abcb1 and Abcg2 in the mPFC
Abcb1 mRNA
A significant decrease in relative Abcb1 mRNA expression [F (2, 12) = 8.254, p = 0.0056] occurred in the mPFC of rats that voluntarily consumed ethanol and in rats chronically treated with 20 mg/kg i.p. cocaine after ethanol consumption, compared to ethanol-naïve P rats in mPFC (Fig. 3 and Table 3, left). There was no difference between ethanol-treated groups resulting from cocaine treatment.
Fig. 3.
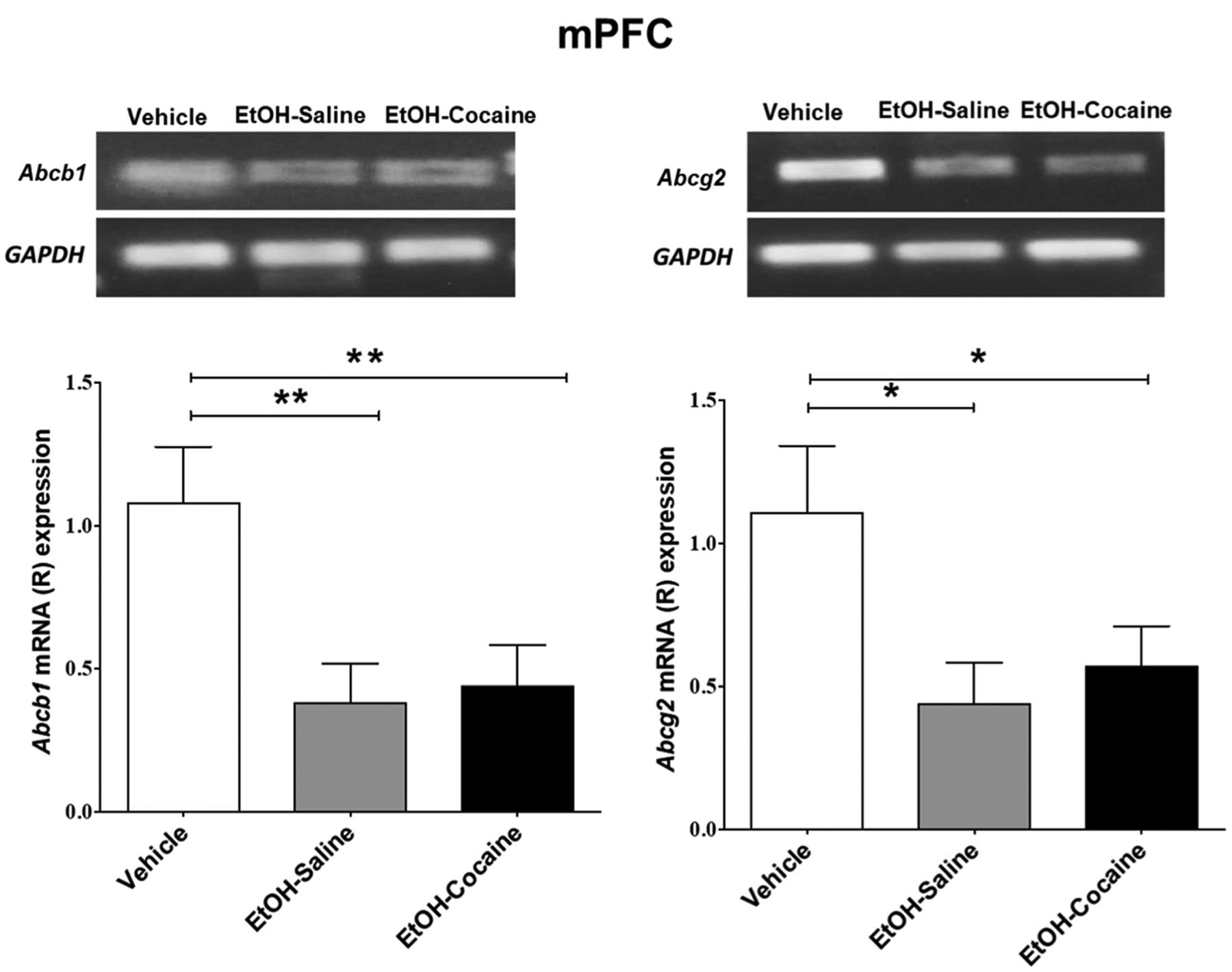
Relative mRNA expression of Abcb1 and Abcg2 in the mPFC following voluntary ethanol consumption and repeated cocaine exposure (mean ± SEM). a One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in relative mRNA expression of Abcb1 in the mPFC. b One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in relative mRNA expression of Abcg2 in the mPFC. (*p < 0.05, **p < 0.01), (n = 5 for each group)
Table 3.
Relative mRNA expression of Abcb1 and Abcg2 in the mPFC following voluntary ethanol consumption and repeated cocaine exposure expressed as as mean ± SD.
| Abcb1 mRNA expression | Abcg2 mRNA expression | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | EtOH-saline | EtOH-cocaine | Vehicle | EtOH-saline | EtOH-cocaine | ||
| 0.75 | 0.50 | 0.66 | 1.39 | 0.38 | 0.27 | ||
| 1.47 | 0.36 | 0.49 | 1.28 | 0.49 | 0.62 | ||
| 1.24 | 0.30 | 0.63 | 0.47 | 0.68 | 0.82 | ||
| 1.44 | 0.30 | 0.34 | 0.70 | 0.24 | 0.91 | ||
| 0.51 | 0.67 | 0.30 | 1.72 | 0.43 | 0.24 | ||
| Mean ± SD | 1.08 ± 0.43 | 0.43 ± 0.16 | 0.48 ± 0.16 | Mean ± SD | 1.11 ± 0.51 | 0.44 ± 0.16 | 0.57 ± 0.31 |
Abcg2 mRNA
A significant decrease in relative Abcg2 mRNA expression [F (2, 12) = 4.857, p = 0.0285] occurred in the mPFC of rats that voluntarily consumed ethanol and in rats chronically treated with 20 mg/kg i.p. of cocaine after ethanol consumption (Fig. 3 and Table 3, right). There was no difference between ethanol-treated groups resulting from cocaine treatment.
The Effect of Voluntary Ethanol Consumption and the Repeated Cocaine Administration on ABCB1 and ABCG2 Protein Expression in the NAc and the mPFC
ABCB1 and ABCG2 Expression in the NAc
ABCB1
Statistical analysis indicated that a significant decrease [F (2, 12) = 9.259, p = 0.0037] in relative ABCB1 expression occurred in the NAc of rats that voluntarily consumed ethanol and in rats treated chronically with 20 mg/kg i.p. cocaine after ethanol consumption, compared to ethanol-naïve P rats (Fig. 4 and Table 4, left). There was no difference between ethanol-treated groups resulting from cocaine treatment.
Fig. 4.
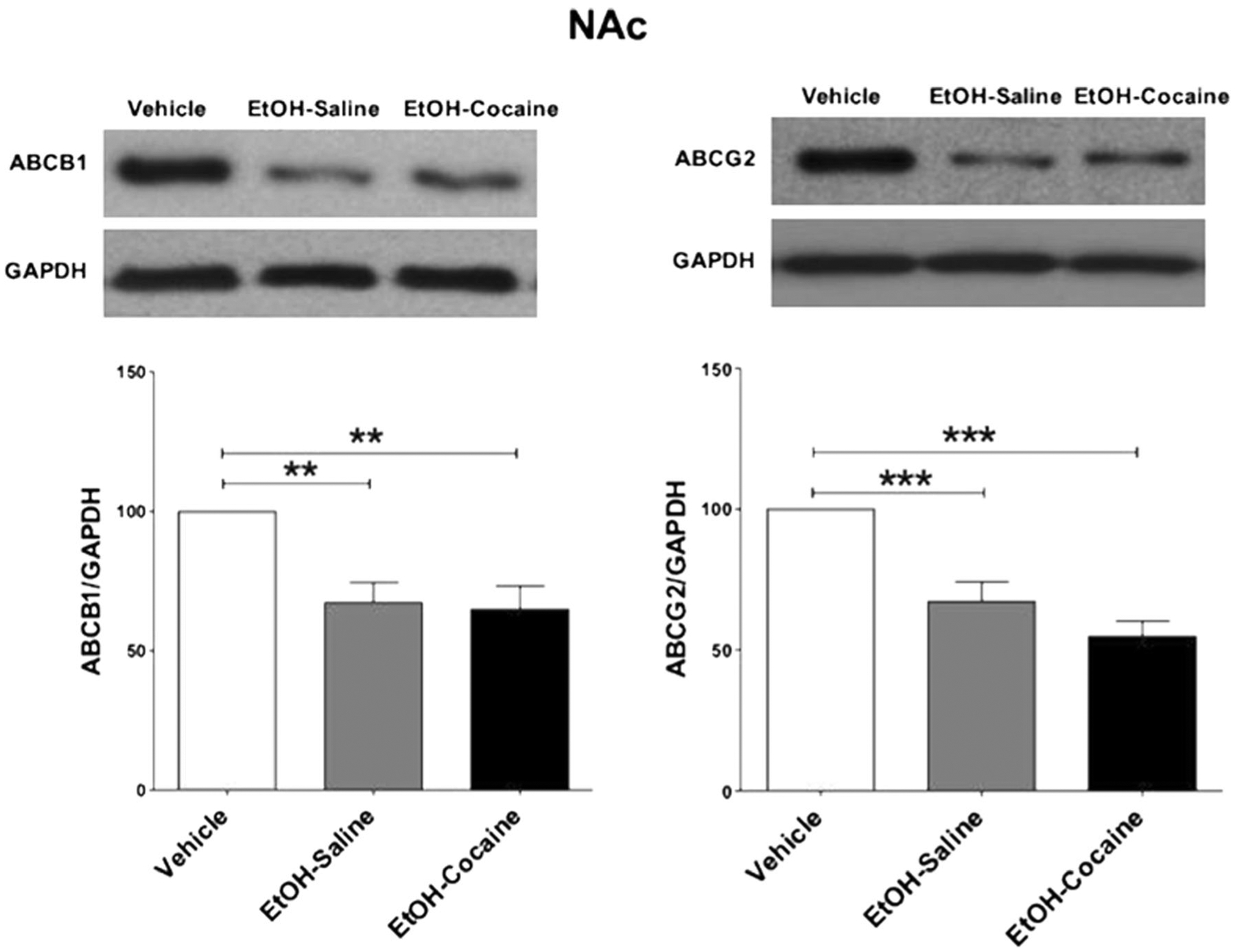
ABCB1 and ABCG2 protein expression in the NAc following voluntary ethanol consumption and repeated cocaine exposure (mean ± SEM). a One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in ABCB1 expression in the NAc. b One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in ABCG2 expression in the NAc. (**p < 0.01, ***p < 0.001), (n = 5 for each group)
Table 4.
Relative protein expression for ABCB1 and ABCG2 in the NAc following voluntary ethanol consumption and repeated cocaine exposure expressed as mean ±SD.
| ABCB1/GAPDH | ABCG2/GAPDH | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | EtOH-saline | EtOH-cocaine | Vehicle | EtOH-saline | EtOH-cocaine | ||
| 100 | 81.97 | 36.04 | 100 | 89.31 | 44.17 | ||
| 100 | 61.40 | 84.28 | 100 | 48.55 | 41.49 | ||
| 100 | 55.54 | 63.89 | 100 | 56.86 | 71.11 | ||
| 100 | 86.97 | 79.72 | 100 | 74.49 | 53.39 | ||
| 100 | 50.91 | 60.02 | 100 | 67.14 | 63.57 | ||
| Mean ± SD | 100 ± 0.00 | 67.36 ± 16.15 | 64.79 ± 19.06 | Mean ± SD | 100 ± 0.00 | 67.27 ± 15.79 | 54.75 ± 12.60 |
ABCG2
Statistical analysis indicated that a significant decrease [F (2, 12) = 20.078, p < 0.0001] in relative ABCG2 expression occurred in the NAc of rats that voluntarily consumed ethanol and in rats treated chronically with 20 mg/kg i.p. of cocaine after ethanol consumption, compared to ethanol-naïve P rats (Fig. 4 and Table 4, right). There was no difference between ethanol-treated groups resulting from cocaine treatment.
ABCB1 and ABCG2 Expression in the mPFC
ABCB1
Statistical analysis indicated that a significant decrease [F (2, 12) = 9.653, p = 0.0032] in relative ABCB1 expression occurred in the mPFC of rats that voluntarily consumed ethanol and in rats treated chronically with 20 mg/kg i.p. of cocaine after ethanol consumption, compared to ethanol-naïve P rats (Fig. 5 and Table 5, left). There was no difference between ethanol-treated groups resulting from cocaine treatment.
Fig. 5.
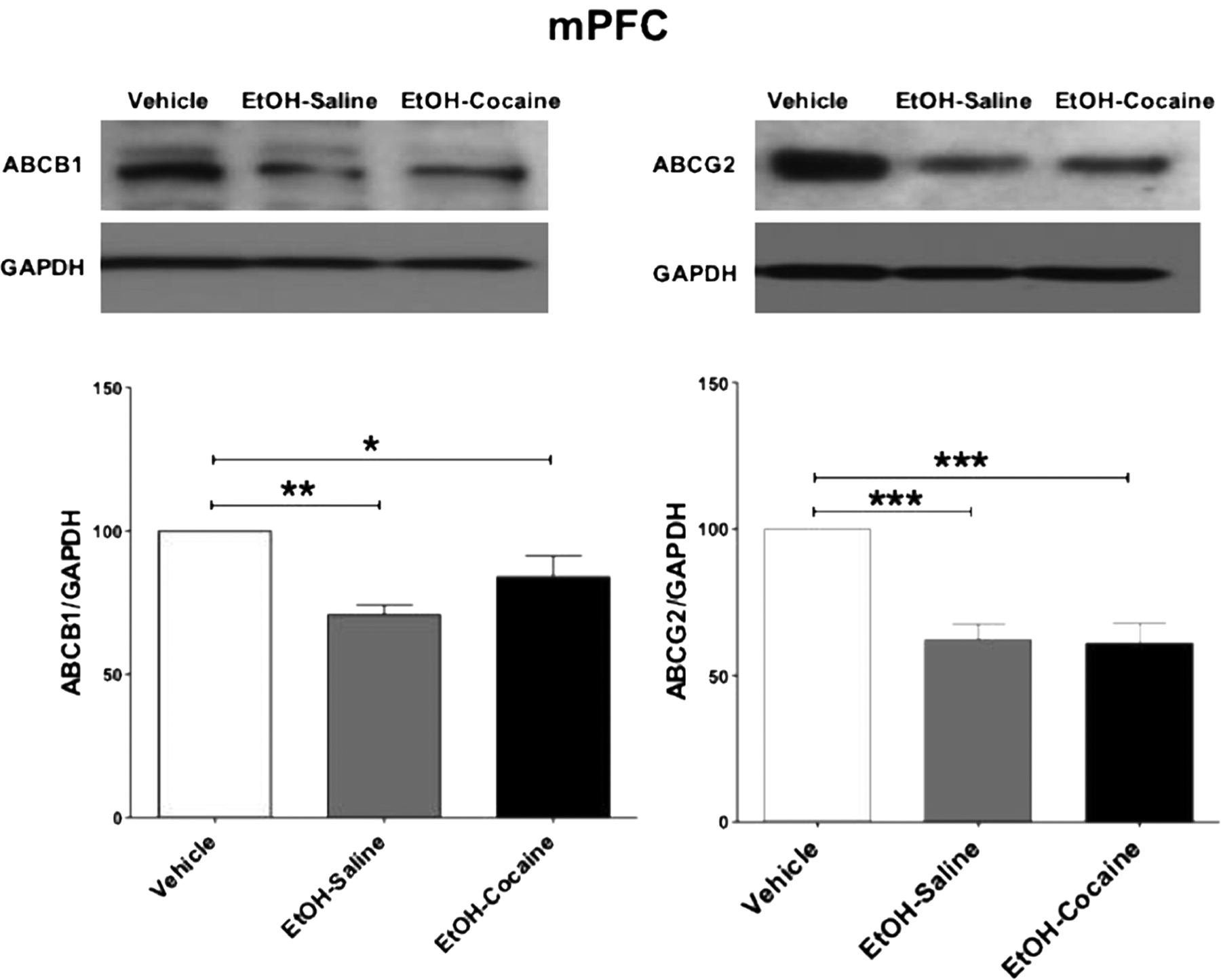
ABCB1 and ABCG2 protein expression in the mPFC following voluntary ethanol consumption and repeated cocaine exposure (mean ± SEM). a One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in ABCB1 expression in the mPFC. b One-way ANOVA followed by the Newman-Keuls multiple comparisons test revealed a significant decrease in ABCG2 expression in the mPFC. (*p < 0.05, **p < 0.01,***p < 0.001), (n = 5 for each group)
Table 5.
Relative protein expression for ABCB1 and ABCG2 in the mPFC following voluntary ethanol consumption and repeated cocaine exposure expressed as as mean ± SD.
| ABCB1/GAPDH | ABCG2/GAPDH | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | EtOH-saline | EtOH-cocaine | Vehicle | EtOH-saline | EtOH-cocaine | ||
| 100 | 56.87 | 89.02 | 100 | 55.18 | 54.11 | ||
| 100 | 72.65 | 107.85 | 100 | 57.00 | 68.38 | ||
| 100 | 75.08 | 67.97 | 100 | 76.95 | 77.68 | ||
| 100 | 73.98 | 68.99 | 100 | 48.79 | 38.76 | ||
| 100 | 75.50 | 85.92 | 100 | 73.18 | 66.77 | ||
| Mean ± SD | 100 ± 0.00 | 70.82 ± 7.87 | 83.95 ± 16.43 | Mean ± SD | 100 ± 0.00 | 62.22 ± 12.19 | 61.14 ± 15.07 |
ABCG2
Statistical analysis indicated that a significant decrease [F (2, 12) = 19.567, p = 0.0002] in relative ABCG2 expression occurred in the mPFC of rats that voluntarily consumed ethanol and in rats treated chronically with 20 mg/kg i.p. cocaine after ethanol consumption, compared to ethanol-naïve P rats (Fig. 5 and Table 5, right). There was no difference between ethanol-treated groups resulting from cocaine treatment.
Discussion
In this study, the voluntary consumption of ethanol, as well as concurrent ethanol and cocaine treatment, significantly decreased relative mRNA and protein expression of the ABCB1 and ABCG2 transporters in the NAc and mPFC of male P rats. To our knowledge, our study is the first to report that ethanol and ethanol-cocaine treatment alter the expression of mRNA and protein expression of ABCB1 and ABCG2 transporters. The relative mRNA expression was calculated according to the 2−ΔΔCT method [44], in which the relative mRNA expression of the desired target is normalized to GAPDH. This was performed to correct for variations in the amount of cDNA added for each sample and to decrease the differences caused by the cycling process and PCR set-up, parallel to a previous published report [45]. Similarly, protein expression for each target was normalized to GAPDH as a loading control widely used for whole cell lysate, and the results were presented as a percentage of the ratio of tested protein/ GAPDH, relative to ethanol-naïve (water) control groups (100% control value) similar to previously published papers [25, 34, 38].
Ethanol-cocaine treatment did not produce any further increases or decreases in Abcb1/ABCB1 or Abcg2/ABCG2 expression compared to ethanol treatment alone. Importantly, no statistical significance was shown while comparing the expression of mRNA and protein expression of ABCB1 and ABCG2 transporters in the NAc and the mPFC between ethanol-saline and ethanol-cocaine groups. However, more research is required to investigate the effects of cocaine on these transporters and the timing of changes in mRNA and protein expression relative to different treatment regimens. It remains to be seen whether cocaine treatment alone will produce similar effects to ethanol or whether combinatorial actions might occur depending on the dose and treatment regimen. Moreover, the mechanisms underlying these actions remain to be determined. In this study a three bottle consumption procedure (15 and 30% ethanol concurrently with water) was used for the voluntary home-cage exposure for a period of 5 weeks. The use of two concentrations of ethanol together with water has been proven to increase free ethanol intake [46]. This model of voluntary exposure was used in many studies from our laboratory to study factors that influence ethanol consumption and mitigate excessive ethanol intake in P rats [34, 36, 47]. The cocaine dose for co-exposure was based on studies that have established that repeated cocaine exposure (20 mg/ kg, i.p.) changes the clearance and release of different neurotransmitters in the brain, including glutamate and dopamine [48, 49]. Moreover, the repeated cocaine exposure (20 mg/kg, i.p.) has been shown to downregulate the protein expression of glial glutamate transporters [33].
Previous studies have shown that exposure to different drugs of abuse, including ethanol, cocaine, and nicotine alter neurotransmission and within the neural circuitry underlying drug reward and reinforcement. These changes include altered expression of various proteins regulating neurotransmission in both female and male rats [33, 50]. On this basis it would be potentially expected that alterations in ABCB1 and ABCG2 transporters would be observed in both sexes. More studies are warranted in order to verify this effect on female rats. The 75-day-old rats were chosen in order to examine the effect of exposure to drugs of abuse on adults rather than on adolescents. With regard to the issue of age, although similar effects of drugs of abuse were shown on the expression of different transporters, including glial glutamate transporters in adolescents and adults [25, 33, 50], adolescent animals were shown to consume more ethanol than adults [51–53]. Additional research will certainly be needed to determine if the sensitivity of ABC transporters to exposure to drugs of abuse is greater in adolescence, a period in which animals have shown altered sensitivity to drugs of abuse.
A number of studies have shown that transcriptional factors regulate the expression of ABC transporters, including ABCB1 and ABCG2, notably the aryl hydrocarbon receptor (AHR), the pregnane xenobiotic receptor (PXR), and the constitutive and rostane receptor (CAR) [54, 55]. Furthermore, AHR mRNA has been detected in the BBB of humans, whereas PXR and CAR have not been detected in the microvessels of the brain [56]. Nonetheless, CAR and PXR have been found to play a critical role in regulating the expression of ABCB1 and ABCG2 in the BBB [57, 58]. It has been reported that chronic exposure to ethanol (50 mM for 7 days + 200 mM for 6 h) significantly reduces the expression and availability of AHR in mice [19]. Furthermore, exposure to ethanol (200 mM) for 4 h significantly reduces the binding of AHR [59]. These results suggest that chronic exposure to ethanol reduces the availability and the activity of AHR, which in part may decrease the gene and protein expression of Abcb1/ABCB1 or Abcg2/ABCG2. The exposure of rats to ethanol (4 g/kg, p.o.) for 5 weeks significantly decreased the relative mRNA expression of PXR [60], suggesting that persistent daily ethanol exposure induces a reduction in the expression of this regulator of ABC transporters. More directly, it has been reported that the incubation of cortical progenitor cells with ethanol (120 and 620 mg/dL) significantly reduces the cellular expression of ABCG2 in vitro [16].
It is also possible that the ethanol-induced decrease in the expression of ABCB1 and ABCG2 transporters could result from ethanol-induced neuroinflammation [20, 21]. For example, ethanol exposure significantly increases the levels of pro-inflammatory cytokines, which are known to alter the expression of ABCB1 and ABCG2 [21]. In addition, chronic exposure (5 g/kg/day, i.g. for 10 days) may also induce neuroinflammation by stimulating the production of tumor necrosis factor-alpha (TNF-α) in the brain [61, 62]. Indeed, increased brain TNF-α has been found to be correlated with a decrease in ABCB1 and ABCG2 expression [21, 63]. Interleukin-1β (IL-1β) levels and mRNA expression in the brain were increased in animals exposed to ethanol compared to ethanol naïve animals [62, 64, 65]. IL-1β could also reduce the expression of ABCB1 and ABCG2, as well as other ABC proteins [21, 66]. In the present study, we found that the ethanol downregulated the relative mRNA and protein expression of Abcb1/ABCB1 and Abcg2/ABCG2 in both the NAc and mPFC. This effect may be mediated, in part, by an increase in the levels of neuroinflammatory cytokines in the brain, although this remains to be examined.
The expression of ABC transporters may also be regulated more broadly by the immune system. There is a significant positive correlation between chemokine receptor-4 (CXCR-4) overexpression and ABCB1 overexpression in non-small cell carcinoma [31] and peripheral blood mononuclear cells [67]. Inhibition of the CXCR-4 receptor decreases resistance to doxorubicin, which is an ABCB1 substrate [31]. Cocaine modulates the immune system, in part, by suppressing CD4+ T cell function [68]. Furthermore, in vitro, cocaine exposure at a dose of 10−9 to 10−4 M inhibits the migration of human fetal brain-derived neural precursor cells in response to the chemokine CXCL-12 and exposure at a concentration of 10−6 M for 7 days downregulates CXCR-4 [29]. Importantly, CXCR-4 induces the expression of c-Jun and consequently upregulates the expression of ABCG2 [30]. Thus, it is possible that the effects of cocaine on ABCB1 and ABCG2 transporters in our study could result from alterations in chemokines, although this remains to be determined.
Modulation of ABC transporters by drugs of abuse may have wide-ranging implications for the treatment of diverse conditions with drugs that are substrates of these transporters, as well as for addiction. A history of illicit drug use might influence responses to other medications by altering tissue penetration and other pharmacokinetic properties of drugs that are substrates of these transporters. The present data suggest that there are alterations in brain expression of these transporters, but it remains highly probably that there are alterations in the expression of these transporters in other tissues involved in the excretion of these drugs, including cancerous tissues for which the expression of these transporters is an important aspect of drug resistance. The converse may also be true that exposure to other drugs that influence the expression of these transporters might subsequently influence responses to drugs of abuse. It remains to be determined if the changes in the expression of these transporters are associated with changes in pharmacokinetic properties of drugs of abuse, and in particular brain penetration, and whether such changes might influence the subsequent behavioral and psychological impact of drugs as part of the addictive process.
The idea that the level of expression of these transporters might play a role in drug dependence is supported by other findings. The ABC transporter ABCC4 has been repeatedly found to be associated with drug dependence in genome-wide association studies (GWAS) [69]. Particular transporters may also be more specifically related to dependence to particular drugs or drug classes. ABCB1 markers have been repeatedly associated with opiate dependence [70]. There is also some suggestion that it may be associated with ethanol dependence [71]. Although the association did not reach “genome-wide” significance in that study, other approaches in animals also identified the homologous gene as being associated with responses to ethanol [72, 73]. A meta-analysis of nicotine dependence studies, seeking to identify pathways involved in the liability to nicotine dependence, identified a cluster of genes involved in xenobiotic signaling, including ABCB1, AHR, and TNF [74]. As it is the case for many genes associated with drug dependence, the genetic relationship may not be direct, but might be associated with another endophenotype or psychiatric co-morbidity, such as antisocial behavior, which has been associated with ABCB1 markers [75]. Most importantly, the deletion of Abcb1 or Abcg2 in mice has been shown to increase both blood and brain levels of Δ−9-tetrahydrocannibinal (THC) and to potentiate the THC-induced hypothermia [76], and deletion of Abcb1 potentiates the respiratory depressive effects of buprenorphine by reducing brain efflux of norbuprenorphine [77].
In summary, our work sheds the light on the direct effect of voluntary ethanol consumption (with or without repeated cocaine exposure) on the expression of ABC efflux transporters in central brain regions involved in drug reward and reinforcement. Our work provides information about some possible drug-drug interactions among the substrates of the ABC transporters, including drugs of abuse in the brain. However, this area of research is not well-studied and needs further investigation, as it has important potential implications for mechanisms that may contribute to the addictive process, as well as for the use of drugs in individuals with a history of drug abuse. Future studies are warranted to investigate the effects of chronic exposure of ethanol, cocaine, and other abused drugs on the transcriptional factors that regulate ABC transporters and other potential mediators of these effects, such as neuroinflammation and immune factors. Moreover, additional research is required to determine the relationship between ABC transporter expression and function, and behavioral and psychological responses to drugs of abuse.
Acknowledgments
The authors thank Sujan Chandra Das and Yusuf S. Althobaiti for their contribution to the experimental procedures. We thank Dr. Charles R. Ashby Jr. (St. John’s University, Queens, NY) and Ms. Charisse Montgomery (University of Toledo, Toledo, Ohio) for critically reviewing the manuscript.
Funding
This work was supported, in part, by the National Institutes of Health (R01AA019458 to Y.S.) and the University of Toledo start-up grant (F110760) to A.K.T.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Pal D, Mitra AK (2006) MDR-and CYP3A4-mediated drug–drug interactions. J NeuroImmune Pharmacol 1(3):323–339 [DOI] [PubMed] [Google Scholar]
- 2.Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2(1): 48–58 [DOI] [PubMed] [Google Scholar]
- 3.Chan LM, Lowes S, Hirst BH (2004) The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci 21(1):25–51 [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith D, Jayawardene S, Ackland P (2013) ABC of kidney disease. 2nd Edition, Vol. 76 Wiley. [Google Scholar]
- 5.Planas JM, Alfaras I, Colom H, Juan ME (2012) The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch Biochem Biophys 527(2):67–73 [DOI] [PubMed] [Google Scholar]
- 6.Löscher W, Potschka H (2005) Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2(1):86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhr M, Steckler T, Yassouridis A, Holsboer F (2000) Penetration of amitriptyline, but not of fluoxetine, into brain is enhanced in mice with blood-brain barrier deficiency due to mdr1a P-glycoprotein gene disruption. Neuropsychopharmacology 22(4):380–387 [DOI] [PubMed] [Google Scholar]
- 8.Antoniou T, Tseng AL-i (2002) Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother 36(10):1598–1613 [DOI] [PubMed] [Google Scholar]
- 9.Bouër R, Barthe L, Philibert C, Tournaire C, Woodley J, Houin G (1999) The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: in vitro studies using the rat everted intestinal sac. Fundam Clin Pharmacol 13(4):494–500 [DOI] [PubMed] [Google Scholar]
- 10.Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann JM, Declèves X (2010) Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int J Neuropsychopharmacol 13(7): 905–915 [DOI] [PubMed] [Google Scholar]
- 11.Wandel C, Kim R, Wood M, Wood A (2002) Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology 96(4):913–920 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Zaima C, Moriki Y, Fukami T, Tomono K (2007) P-glycoprotein mediates brain-to-blood efflux transport of buprenorphine across the blood–brain barrier. J Drug Target 15(1):67–74 [DOI] [PubMed] [Google Scholar]
- 13.Störmer E et al. (2001) Methadone inhibits rhodamine123 transport in Caco-2 cells. Drug Metab Dispos 29(7):954–956 [PubMed] [Google Scholar]
- 14.Dagenais C, Graff CL, Pollack GM (2004) Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol 67(2):269–276 [DOI] [PubMed] [Google Scholar]
- 15.Wang J-S, Ruan Y, Taylor RM, Donovan JL, Markowitz JS, DeVane CL (2004) Brain penetration of methadone (R)- and (S)-enantiomers is greatly increased by P-glycoprotein deficiency in the blood–brain barrier of Abcb1a gene knockout mice. Psychopharmacology 173(1–2):132–138 [DOI] [PubMed] [Google Scholar]
- 16.Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC (2005) Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci 6(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauna ZE, Peng XH, Nandigama K, Tekle S, Ambudkar SV (2004) The molecular basis of the action of disulfiram as a modulator of the multidrug resistance-linked ATP binding cassette transporters MDR1 (ABCB1) and MRP1 (ABCC1). Mol Pharmacol 65(3): 675–684 [DOI] [PubMed] [Google Scholar]
- 18.Bradbury MW (1985) The blood-brain barrier. Transport across the cerebral endothelium. Circ Res 57(2):213–222 [DOI] [PubMed] [Google Scholar]
- 19.Zhang HF, Lin XH, Yang H, Zhou LC, Guo YL, Barnett JV, Guo ZM (2012) Regulation of the activity and expression of aryl hydrocarbon receptor by ethanol in mouse hepatic stellate cells. Alcohol Clin Exp Res 36(11):1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin L, Crews FT (2012) Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation 9(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evseenko DA, Paxton JW, Keelan JA (2007) Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos 35(4):595–601 [DOI] [PubMed] [Google Scholar]
- 22.Riezzo I, Fiore C, de Carlo D, Pascale N, Neri M, Turillazzi E, Fineschi V (2012) Side effects of cocaine abuse: multiorgan toxicity and pathological consequences. Curr Med Chem 19(33):5624–5646 [DOI] [PubMed] [Google Scholar]
- 23.Chapy H et al. (2014) Carrier-mediated cocaine transport at the blood-brain barrier as a putative mechanism in addiction liability. Int J Neuropsychopharmacol 18(1):pyu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.André P, Debray M, Scherrmann JM, Cisternino S (2009) Clonidine transport at the mouse blood–brain barrier by a new H+ antiporter that interacts with addictive drugs. J Cereb Blood Flow Metab 29(7):1293–1304 [DOI] [PubMed] [Google Scholar]
- 25.Hammad AM, Alasmari F, Althobaiti YS, Sari Y (2017) Modulatory effects of ampicillin/sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behav Brain Res 332:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J et al. (1990) Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147(6):719–724 [DOI] [PubMed] [Google Scholar]
- 27.Waa Lasoñ (2001) Neurochemical and pharmacological aspects of cocaine-induced seizures. Pol J Pharmacol 53(1):57–60 [PubMed] [Google Scholar]
- 28.López-Pedrajas R, Ramírez-Lamelas DT, Muriach B, Sánchez-Villarejo MV, Almansa I, Vidal-Gil L, Romero FJ, Barcia JM, Muriach M (2015) Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front Cell Neurosci 9, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu S, Cheeran MC, Sheng WS, Ni HT, Lokensgard JR, Peterson PK (2006) Cocaine alters proliferation, migration, and differentiation of human fetal brain-derived neural precursor cells. J Pharmacol Exp Ther 318(3):1280–1286 [DOI] [PubMed] [Google Scholar]
- 30.Liu H et al. (2016) CXCR4 promotes growth and sphere formation of hypoxic breast cancer side 7 population cells via activation of c-Jun/ABCG2 pathway. Oncol Res [DOI] [PubMed] [Google Scholar]
- 31.Liptrott N, Penny M, Bray PG, Sathish J, Khoo SH, Back DJ, Owen A (2009) The impact of cytokines on the expression of drug transporters, cytochrome P450 enzymes and chemokine receptors in human PBMC. Br J Pharmacol 156(3):497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alasmari F, Abuhamdah S, Sari Y (2015) Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neurosci Lett 600:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammad AM, Althobaiti YS, Das SC, Sari Y (2017) Effects of repeated cocaine exposure and withdrawal on voluntary ethanol drinking, and the expression of glial glutamate transporters in mesocorticolimbic system of P rats. Mol Cell Neurosci 82:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL (2011) Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol 46(3):239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C (1997) The rat brain atlas in stereotaxic coordinates, compact. Academic Press, New York [Google Scholar]
- 36.Sari Y, Sreemantula S (2012) Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience 227:327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alasmari F, Crotty Alexander LE, Nelson JA, Schiefer IT, Breen E, Drummond CA, Sari Y (2017) Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α−7 nicotinic acetylcholine receptor in female CD-1 mice. Prog Neuro-Psychopharmacol Biol Psychiatry 77:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Olinger AB, Dassow MS, Abel MS (2003) Up-regulation of GABAB receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience 118(2):451–462 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Tan Y (2011) Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience 193:72–79 [DOI] [PubMed] [Google Scholar]
- 40.Simões AP, Duarte JA, Agasse F, Canas PM, Tomé AR, Agostinho P, Cunha RA (2012) Blockade of adenosine A 2A receptors prevents interleukin-1β-induced exacerbation of neuronal toxicity through a p38 mitogen-activated protein kinase pathway. J Neuroinflammation 9(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devoto VP, Bogetti ME, de Plazas SF (2013) Developmental and hypoxia-induced cell death share common ultrastructural and biochemical apoptotic features in the central nervous system. Neuroscience 252:190–200 [DOI] [PubMed] [Google Scholar]
- 42.Reichel V, Burghard S, John I, Huber O (2011) P-glycoprotein and breast cancer resistance protein expression and function at the blood–brain barrier and blood–cerebrospinal fluid barrier (choroid plexus) in streptozotocin-induced diabetes in rats. Brain Res 1370: 238–245 [DOI] [PubMed] [Google Scholar]
- 43.Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-Mcmenemy N, Harris BT, Deleo JA (2006) Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia 54(3):193–203 [DOI] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 45.Pfaffl MW (2012) Quantification strategies in real-time polymerase chain reaction Applied microbiology. Caister Academic Press; Norfolk, UK, p. 53–61 [Google Scholar]
- 46.Breslin FJ, Johnson BA, Lynch WJ (2010) Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology 207(4):529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sari Y, Sreemantula SN, Lee MR, Choi DS (2013) Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci 51(3):779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh V, Naughton SX, Shi X, Kelley LK, Yegla B, Tallarida CS, Rawls SM, Unterwald EM (2014) Cocaine-induced neuroadaptations in the dorsal striatum: glutamate dynamics and behavioral sensitization. Neurochem Int 75:54–65 [DOI] [PubMed] [Google Scholar]
- 49.Oh JH, Lee DK, Shim YB, Ryu IS, Seo SY, Kim J, Yang JH, Cho HW et al. (2015) Dopamine D4 receptors linked to protein kinase G are required for changes in dopamine release followed by locomotor activity after repeated cocaine administration. Exp Brain Res 233(5):1511–1518 [DOI] [PubMed] [Google Scholar]
- 50.Alasmari F, Bell RL, Rao PSS, Hammad AM, Sari Y (2018) Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol Biochem Behav 170: 44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bell RL, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ (2006) Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav 83(1):35–46 [DOI] [PubMed] [Google Scholar]
- 52.Doremus TL, Brunell SC, Rajendran P, Spear LP (2005) Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res 29(10):1796–1808 [DOI] [PubMed] [Google Scholar]
- 53.García-Burgos D, González F, Manrique T, Gallo M (2009) Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res 33(4):722–728 [DOI] [PubMed] [Google Scholar]
- 54.Miller DS (2015) Regulation of ABC transporters blood–brain barrier: the good, the bad, and the ugly. In Advances in cancer research. Elsevier. p 43–70 [DOI] [PubMed] [Google Scholar]
- 55.Mahringer A, Ott M, Reimold I, Reichel V, Fricker G (2011) The ABC of the blood-brain barrier-regulation of drug efflux pumps. Curr Pharm Des 17(26):2762–2770 [DOI] [PubMed] [Google Scholar]
- 56.Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, de Waziers I et al. (2008) ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood–brain barrier. J Neurochem 107(6): 1518–1528 [DOI] [PubMed] [Google Scholar]
- 57.Lemmen J, Tozakidis IEP, Bele P, Galla HJ (2013) Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood–brain barrier after CITCO activation. Brain Res 1501:68–80 [DOI] [PubMed] [Google Scholar]
- 58.Ott M, Fricker G, Bauer B (2009) Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther 329(1):141–149 [DOI] [PubMed] [Google Scholar]
- 59.Lin X, Yang H, Zhang H, Zhou LC, Guo ZM (2013) A novel transcription mechanism activated by ethanol induction of Slc7a11 gene expression via inhibition of the DNA-binding activity of transcriptional repressor octamer-binding transcription factor 1 (OCT-1). J Biol Chem 288(21):14815–14823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J-P, Xu DX, Sun MF, Chen YH, Wang H, Wei W (2005) Chronic ethanol exposure downregulates hepatic expression of pregnane X receptor and P450 3A11 in female ICR mice. Toxicology 215(3):234–244 [DOI] [PubMed] [Google Scholar]
- 61.Marshall SA, Geil CR, Nixon K (2016) Prior binge ethanol exposure potentiates the microglial response in a model of alcohol-induced neurodegeneration. Brain Sci 6(2):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation 5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walther W et al. (2015) Chemosensitization by diverging modulation by short-term and long-term TNF-α action on ABCB1 expression and NF-κB signaling in colon cancer. Int J Oncol 47(6):2276–2285 [DOI] [PubMed] [Google Scholar]
- 64.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30(24):8285–8295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marshall SA, Casachahua JD, Rinker JA, Blose AK, Lysle DT, Thiele TE (2016) IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun 51:258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hisaeda K, Inokuchi A, Nakamura T, Iwamoto Y, Kohno K, Kuwano M, Uchiumi T (2004) Interleukin-1β represses MRP2 gene expression through inactivation of interferon regulatory factor 3 in HepG2 cells. Hepatology 39(6):1574–1582 [DOI] [PubMed] [Google Scholar]
- 67.Chandler B, Detsika M, Khoo SH, Williams J, Back DJ, Owen A (2007) Factors impacting the expression of membrane-bound proteins in lymphocytes from HIV-positive subjects. J Antimicrob Chemother 60(3):685–689 [DOI] [PubMed] [Google Scholar]
- 68.Pandhare J, Addai AB, Mantri CK, Hager C, Smith RM, Barnett L, Villalta F, Kalams SA et al. (2014) Cocaine enhances HIV-1-induced CD4+ T-cell apoptosis: implications in disease progression in cocaine-abusing HIV-1 patients. Am J Pathol 184(4):927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uhl GR, Drgon T, Johnson C, Fatusin OO, Liu QR, Contoreggi C, Li CY, Buck K et al. (2008) “Higher order” addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochem Pharmacol 75(1):98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reed B, Butelman ER, Yuferov V, Randesi M, Kreek MJ (2014) Genetics of opiate addiction. Curr Psychiatry Rep 16(11):504. [DOI] [PubMed] [Google Scholar]
- 71.Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK et al. (2010) Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res 34(5):840–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morozova TV, Anholt RR, Mackay TF (2007) Phenotypic and transcriptional response to selection for alcohol sensitivity in Drosophila melanogaster. Genome Biol 8(10):R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA et al. (2006) Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A 103(16):6368–6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X, Liu M, Li X, Zhang L, Fan R, Wang J (2015) Prioritizing genes related to nicotine addiction via a multi-source-based approach. Mol Neurobiol 52(1):442–455 [DOI] [PubMed] [Google Scholar]
- 75.Salvatore JE, Edwards AC, McClintick JN, Bigdeli TB, Adkins A, Aliev F, Edenberg HJ, Foroud T et al. (2015) Genome-wide association data suggest ABCB1 and immune-related gene sets may be involved in adult antisocial behavior. Transl Psychiatry 5:e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spiro AS, Wong A, Boucher AA, Arnold JC (2012) Enhanced brain disposition and effects of Delta9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PLoS One 7(4):e35937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alhaddad H, Cisternino S, Declèves X, Tournier N, Schlatter J, Chiadmi F, Risède P, Smirnova M et al. (2012) Respiratory toxicity of buprenorphine results from the blockage of P-glycoprotein-mediated efflux of norbuprenorphine at the blood-brain barrier in mice. Crit Care Med 40(12):3215–3223 [DOI] [PubMed] [Google Scholar]


