Abstract
Aims:
To evaluate the association of estimated cardiovascular (CV) risk and subclinical atherosclerosis with radiographic structural damage in patients with axial spondyloarthritis (axSpA).
Methods:
Cross-sectional study including 114 patients axSpA from the SpA registry of Córdoba (CASTRO) and 132 age- and sex-matched healthy controls (HCs). Disease activity and the presence of traditional CV risk factors were recorded. The presence of atherosclerotic plaques and carotid intima media thickness (cIMT) were evaluated through carotid ultrasound and the SCORE index was calculated. Radiographic damage was measured though modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS). The association between mSASSS and SCORE was tested using generalized linear models (GLM), and an age-adjusted cluster analysis was performed to identify different phenotypes dependent on the subclinical CV risk.
Results:
Increased traditional CV risk factors, SCORE, and the presence of carotid plaques were found in axSpA patients compared with HCs. The presence of atherosclerotic plaques and SCORE were associated with radiographic structural damage. The GLM showed that the total mSASSS was associated independently with the SCORE [β coefficient 0.24; 95% confidence interval (CI) 0.10–0.38] adjusted for disease duration, age, tobacco, C-reactive protein, and non-steroidal anti-inflammatory drugs (NSAID) intake. Hard cluster analysis identified two phenotypes of patients. Patients from cluster 1, characterized by the presence of plaques and increased cIMT, had a higher prevalence of CV risk factors and SCORE, and more structural damage than cluster two patients.
Conclusion:
Radiographic structural damage is associated closely with increased estimated CV risk: higher SCORE levels in axSpA patients were found to be associated independently with mSASSS after adjusting for age, disease duration, CRP, tobacco and NSAID intake.
Keywords: axial spondyloarthritis, cardiovascular risk, carotid intima media thickness, disease activity, structural damage
Introduction
Spondyloarthritis (SpA) is a group of chronic inflammatory disorders that present different but related phenotypes including ankylosing spondylitis (AS) [currently known as radiographic axial spondyloarthritis (r-axSpA)], psoriatic arthritis (PsA), arthritis related to inflammatory bowel disease, reactive arthritis, and a subgroup of juvenile idiopathic arthritis.1 According to the predominant manifestations, spondyloarthritis can also be classified as axial SpA (axSpA) or peripheral SpA.2,3
The prevalence rate of axSpA in the population varies between 0.1% and 1.4%.4 This disease is related to the presence of some comorbidities, being most common in patients with osteoporosis or low bone mineral density, which is related directly to ankylosis, immobilization, and inflammation,5 and cardiovascular (CV) involvement, especially atherosclerosis.6 The CV burden in inflammatory rheumatic diseases has been well described,7 and recommendations have been developed advising the use of antihypertensives and statins in compliance with national guidelines. Regarding non-steroidal anti-inflammatory drugs (NSAIDs) and biologics, the recommendation is to be cautious in certain patients with CV disease.8 Recently, this CV burden has been highlighted in the context of axSpA, with most studies agreeing on the presence of a higher risk in subjects with r-axSpA than in the general population.9,10 CV risk factors and subclinical atherosclerosis are found more frequently in patients with axSpA. In addition, it has been suggested that CV risk associated with PsA and r-axSpA could be underestimated despite the use of guideline-recommended risk scores.11
The progression of structural damage in r-axSpA patients is unpredictable and has been described in early as well as in very advanced older patients.12 This spine damage is most prevalent and severe in patients with longstanding r-axSpA, although the relative contribution of radiographic damage to functional limitations decreases in patients with a longer duration of disease.13
The presence of elevated C-reactive protein (CRP) at diagnosis and during disease evolution is a traditional risk factor for radiographic progression, together with male sex, positive HLA B27, presence of radiographic damage at baseline (especially syndesmophytes), smoking, mechanical stress, obesity, Ankylosing Spondylitis Disease Activity Score (ASDAS), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and bone marrow oedema (BME) on magnetic resonance imaging (MRI).14–17
The impact of elevated CRP on traditional CV risk factors, and thus on an increased CV risk and CV mortality, has been demonstrated,18,19 and studies have shown that CRP concentration is associated with an initial absence of vascular disease and risk of coronary heart disease, ischaemic stroke, and deaths from vascular and non-vascular diseases,20 as well as with endothelial dysfunction.21 Treating inflammation in patients with r-axSpA and thus decreasing CRP levels could lead to a reduction of coronary artery disease and atherosclerosis.22
On the other hand, age is an independent and major risk factor for CV disease (CVD).23 To date, no study has evaluated the contribution of structural damage to the increased CV risk and presence of subclinical atherosclerosis in axSpA patients, regardless of age.
A deeper knowledge about the association between the characteristics related to axSpA, such as structural damage, with the increased CV risk present in these patients is needed to recognize patients at higher risk and establish earlier treatment and lifestyle measures to prevent radiographic progression and benefit the patients from a CV point of view.
The objective of this study was to evaluate the association between the estimated CV risk factors and subclinical atherosclerosis with radiographic structural damage, independent of age, in a registry of patients with spondyloarthritis (CASTRO).
Methods
Study population and data collection
A total of 114 patients with axSpA according to ASAS criteria from the Córdoba Axial Spondyloarhritis Task force, Registry and Outcomes (CASTRO),2 and 132 age- and sex-matched healthy controls (HCs) were selected for this cross-sectional study. Patients were recruited sequentially from a monographic SpA consult at the Rheumatology Department of Reina Sofia Hospital in Córdoba, and complete clinical history, physical examination and biochemical analysis were performed. CASTRO is composed of 182 patients, but those with a history of CV events (ischaemic heart disease, cerebrovascular accident, peripheral arterial disease, or heart failure) were excluded from this specific study.
The following data were collected: (1) demographic variables: sex, age and body mass index (BMI); (2) clinical data: disease duration, presence of HLA-B27, NSAIDs intake, radiographic sacroiliitis measured by simple radiography, structural damage determined by the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) index,24 disease activity measured by erythrocyte sedimentation rate (ESR), CRP, BASDAI index, ASDAScrp index, extra-articular manifestations and comorbidities; and (3) CV risk factors: CV risk was determined using the SCORE index.25,26 Carotid ultrasound was performed by an expert radiologist with a Philips Epiq-7 system and a 5–14 MHz broadband linear transducer using B-mode, duplex and color methods. The presence of atherosclerotic plaques was assessed and measured when present. Mean distal common carotid intima-media thickness (cIMT) measurement was evaluated using standardized criteria.27
For axSpA patients, radiographs of the cervical spine, lumbar spine, and sacroiliac joints were obtained at the time of CV risk assessment. Lateral views of the cervical and lumbar spine were scored according to the mSASSS index.28 Sacroiliitis was scored from right side and left side pelvic radiographs using the modified New York criteria. Sacroiliitis and the mSASSS were scored by two rheumatologist trained who were blinded to the patient characteristics. The intraclass correlation coefficient (ICC) score for agreement between readers was 0.997 [95% confidence interval (CI) 0.996–0.998] (p <0.001) for total mSASSS, 0.991 (95% CI 0.987–0.994) (p <0.001) for cervical mSASSS, and 0.992 (95% CI 0.989–0.995) (p <0.001) for lumbar mSASSS. Due to the excellent agreement between both readers, the score from the senior reader was used for the analysis.
To measure the persistence of inflammation, CRP levels (mg/l) were recorded retrospectively once, twice, or three times during the 5 years prior to study and in the moment of the study, so at least six determinations of CRP levels for each patient was available in all axSpA patients. A patient was considered to have persistent inflammation in case of increased CRP levels (>10 mg/l) in at least 50% of the determinations during the previous 5 years, i.e., if four or more of those six determinations were elevated.
The study was approved by the Ethics Committee at the Reina Sofia University Hospital (Protocol code PI-0139-2017), and each participant signed an informed consent form to be included in the study.
Statistical analysis
Data were collected and analysed using SPSS software version 25.0 (SPSS, Inc., Chicago, IL, USA). A descriptive study of the variables was carried out, calculating absolute and relative frequencies for the qualitative variables and arithmetic mean and standard deviation (SD) for the quantitative variables. The 95% CI was estimated. Independent samples t test and chi-square test were used to compare demographic and CV characteristics between the axSpA patients and HCs and in cluster groups. The linear relationship between the variables was measured by Pearson’s linear correlation coefficient in all the groups and then in groups based on the median age. Because age, disease duration, tobacco, CRP, and NSAIDs intake are biologically associated with both the structural damage and the CV risk in axSpA patients, a generalized linear model (GLM) was performed to determine the association between mSASSS and SCORE as well as with the presence of atheroma plaques by adjusting for these variables. For the association between mSASSS and SCORE, we used a Gaussian GLM since the dependent variable was not normal. For the association between mSASSS and atherosclerotic plaques, we used a logistic regression since the dependent variable was binary (yes/no).
Furthermore, to stablish different phenotypes of patients according to the carotid intima media thickness (cIMT) and the presence of atherosclerotic plaques, we performed a cluster analysis with the hard clustering method, which was adjusted for age. All comparisons were bilateral considering p ⩽ 0.05 as a significant result.
Results
CV risk in axSpA patients
Among the 114 axSpA patients and the 132 matched controls, 61.2% were male, with a mean age of 44.35 (11.55) years and 92.7% of axSpA patients were under NSAIDs. No patient was treated with biologic therapy. The demographic and clinical characteristics of the study participants are shown in Table 1.
Table 1.
Demographic and clinical details and characteristics related to cardiovascular risk of axial spondyloarthritis patients and healthy controls.
| Patients (n = 114) | HCs (n = 132) | p-value | |
|---|---|---|---|
| Age (years) | 45.73 (12.15) | 43.09 (10.86) | 0.077 |
| Sex (males) | 77 (67.50) | 71 (55.50) | 0.054 |
| HLA B27 status (positive) | 93 (82.30) | 4 (6.70) | <0.001* |
| Family history for SpA | 38 (33.60) | 2 (11.10) | |
| Radiographic sacroiliitis | 90 (81.10) | ||
| Inflammatory back pain | 96 (85.71) | 2 (10.50) | |
| Arthritis | 16 (14.10) | 0 | |
| Psoriasis | 13 (11.40) | 0 | |
| BMI (kg/m2) | 26.62 (4.25) | 20.13 (9.40) | <0.001* |
| Disease duration (years) | 19.24 (13.36) | ||
| Global VAS | 43.33 (25.19) | ||
| ASDAS-CRP | 2.44 (0.93) | ||
| ASAS HI | 4.75 (4) | ||
| BASDAI | 3.68 (2.13) | ||
| BASFI | 2.85 (2.48) | ||
| BASMI | 3.10 (1.80) | ||
| CRP (mg/l) | 5.75 (7.76) | 1.43 (1.72) | <0.001* |
| NSAIDs | 102 (92.70) | 2 (1.70) | |
| Biological treatment | 0 | 0 | |
| Total mSASSS | 15.26 (17.18) | ||
| Cervical mSASSS | 7.27 (9.09) | ||
| Lumbar mSASSS | 8.09 (9.38) | ||
| Smoking status | 37 (32.7) | 12 (14.3) | 0.003* |
| Obesity | 23 (20.7) | 4 (6.6) | 0.015* |
| Hypertension | 21 (18.6) | 2 (1.6) | <0.001* |
| Type 2 diabetes | 2 (1.8) | 0 | 0.138 |
| Glucose (mg/dl) | 83.60 (14.43) | 85.98 (13.94) | 0.244 |
| Insulin (mU/l) | 6.40 (3.99) | 8.71 (5.25) | 0.005* |
| Insulin resistance | 1.42 (1.09) | 1.89 (1.29) | 0.008* |
| Total Cholesterol (mg/dl) | 190.02 (31.37) | 197.79 (30.64) | 0.065 |
| HDL-cholesterol (mg/dl) | 55.42 (14.81) | 56.74 (14.89) | 0.514 |
| LDL-cholesterol (mg/dl) | 114.46 (29.7) | 121.50 (24.63) | 0.058 |
| Triglycerides (mg/dl) | 101.10 (60.98) | 97.79 (53.24) | 0.668 |
| Apo A (mg/dl) | 143.09 (21.13) | 150.75 (27.33) | 0.024* |
| Apo B (mg/dl) | 80.02 (17.56) | 89.43 (25.42) | 0.002* |
| Apo B/Apo A risk | 0.58 (0.18) | 0.61 (0.19) | 0.186 |
| Atherogenic risk | 12 (17.1) | 1 (7.1) | 0.685 |
| Uric acid (mg/dl) | 5.07 (1.31) | 5.06 (1.25) | 0.972 |
| Atherosclerotic carotid plaques | 16 (16.3) | 2 (3.3) | 0.011* |
| Right cIMT (mm) | 0.54 (0.12) | 0.57 (0.14) | 0.326 |
| Left cIMT (mm) | 0.56 (0.13) | 0.57 (0.09) | 0.577 |
| SCORE | 0.100 | ||
| Low risk | 73 (70.9) | 19 (86.4) | |
| Moderate, high and very high risk | 30 (29.1) | 3 (13.6) |
Data are shown as mean (standard deviation) or frequency (percentage).
Significant differences.
Apo A, apolipoprotein A; Apo B, apolipoprotein B; ASAS HI, Assessment of Spondyloarthritis International Society Health Index; ASDAS-CRP, Ankylosing Spondylitis Disease Activity Score-C Reactive Protein; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMI, body mass index; cIMT, carotid intima-media thickness; CRP, C-reactive protein; HCs, healthy controls; HDL, high density lipoprotein; LDL, low density lipoprotein; mSASSS, modified Stoke ankylosing spondylitis spinal score; NSAIDs, non steroidal antiinflammatory drugs; SpA, spondyloarthritis; VAS, visual analogic scale.
Regarding characteristics related to CV risk, axSpA patients had significantly increased smoking habits, hypertension, obesity, type 2 diabetes rates, and atherogenic risk compared with those of the age- and sex-matched control group. No differences were found in terms of glucose, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, ApoB/ApoA ratio, or uric acid levels (Table 1).
The levels of the systematic coronary risk evaluation (SCORE), which predicts the individual absolute risk for fatal CV events, were higher in the axSpA group although it did not reach statistical significance. However, when subjects were classified by low (<10% of suffering a CV event), moderate (10–20%), high (20–30%), and very high risk (>30%) depending on the SCORE levels, we observed that, among axSpA patients, 70.9% had a low CV risk and 29.1% displayed high CVD risk (moderate, high, and very high), while 86.4% of HCs were classified as low CV risk and 13.6% were classified as high CV risk (Table 1).
Association between the estimated CV risk and structural damage
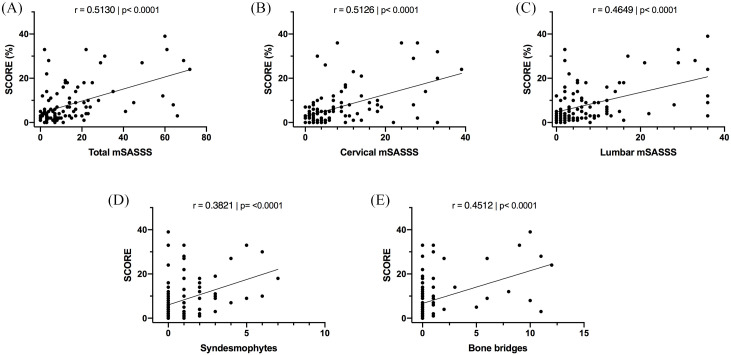
In axSpA patients, correlation studies showed that SCORE correlated significantly with structural damage: total mSASSS (Figure 1A), cervical and lumbar mSASSS (Figure 1B and C), syndesmophytes (Figure 1D), and bone bridges (Figure 1E).
Figure 1.
Association between SCORE levels and structural damage in axSpA patients. (A) Correlation between SCORE and total mSASSS. (B) Correlation between SCORE and cervical mSASSS. (C) Correlation between SCORE and lumbar mSASSS. (D) Correlation between SCORE and number of syndesmophytes. (E) Correlation between SCORE and number of bone bridges.
**p <0.01, ***p <0.001, ****p <0.0001.
axSpA, axial spondyloarthritis; mSASSS, modified Stoke ankylosing spondylitis spinal score.
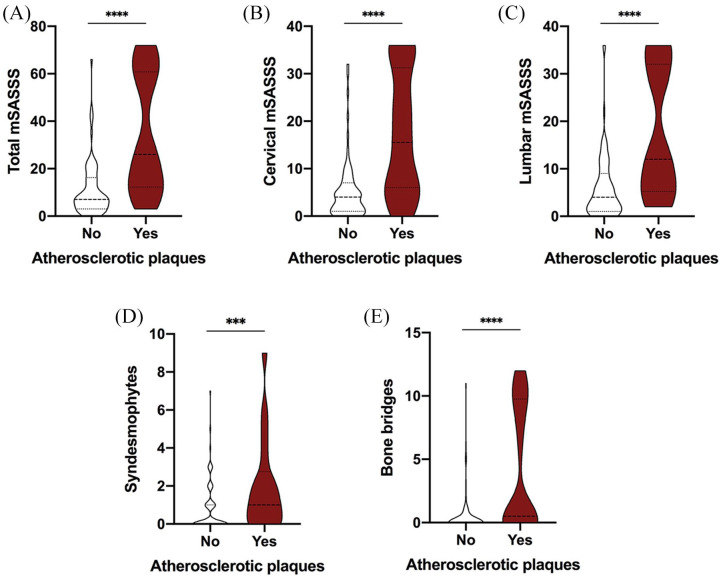
In addition, the presence of atherosclerotic carotid plaques was associated with increased total mSASSS (Figure 2A), cervical (Figure 2B) and lumbar (Figure 2C) mSASSS, syndesmophytes (Figure 2D), and bone bridges (Figure 2E).
Figure 2.
Presence of atherosclerotic carotid plaques in axSpA patients according to structural damage. (A) Total mSASSS in axSpA patients with or without atherosclerotic plaques. (B) Cervical mSASSS in axSpA patients with or without atherosclerotic plaques. (C) Lumbar mSASSS in axSpA patients with or without atherosclerotic plaques. (D) Number of syndesmophytes in axSpA patients with or without atherosclerotic plaques. (E) Number of bone bridges in axSpA patients with or without atherosclerotic plaques.
*p <0.05, ***p <0.001, ****p <0.0001
axSpA, axial spondyloarthritis; mSASSS, modified Stoke ankylosing spondylitis spinal score.
Correlation analysis was also performed in two groups according to the median age (47 years), and it was observed that the correlation between total mSASSS and SCORE was maintained in both groups: in the group with axSpA patients aged ⩽47 years old, SCORE significantly correlated with total mSASSS (r = 0.26, p = 0.049) and in patients aged >47 years old, SCORE correlated with total mSASSS (r = 0.459, p = 0.002).
Besides, after adjusting for disease duration, smoking status, increased CRP levels (at least three determinations over the last 5 years), and NSAIDs intake, the total mSASSS was associated independently with SCORE: 1 point in the total mSASSS increased the SCORE by 0.24 points, and it seems that the cervical mSASSS has the most influence on the SCORE: 1 point in the cervical mSASSS increases the SCORE by 0.42 points, these differences being statistically significant (Table 2). Total mSASSS is also associated significantly with atherosclerotic plaques presence after adjusting for disease duration, smoking status, CRP, and NSAIDs intake, and 1 point in the total mSASSS increases the risk for atherosclerotic plaques 1.05 (1.01–1.12) times (Table 3).
Table 2.
Association between mSASSS and SCORE adjusting for age, smoking status, CRP, NSAIDs intake and disease duration.
| Crude model | Adjusted for age and smoking | Adjusted for age, smoking and elevated CRP* | Adjusted for age, smoking, elevated CRP* and NSAIDs intake | Adjusted for disease duration, smoking, elevated CRP* and NSAIDs intake | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β coefficient (95% CI) | p value | β coefficient (95% CI) | p value | β coefficient (95% CI) | p value | β coefficient (95% CI) | p value | β coefficient (95% CI) | p value | |
| Total mSASSS | 0.27 (0.18–0.36) | <0.001 | 0.11 (0.03–0.20) | 0.007 | 0.17 (0.05–0.29) | 0.009 | 0.16 (0.03–0.28) | 0.018 | 0.24 (0.10–0.38) | 0.001 |
| Cervical mSASSS | 0.53 (0.35–0.70) | <0.001 | 0.24 (0.08–0.39) | 0.003 | 0.31 (0.07–0.53) | 0.012 | 0.29 (0.05–0.53) | 0.022 | 0.42 (0.14–0.71) | 0.006 |
| Lumbar mSASSS | 0.44 (0.28–0.61) | <0.001 | 0.16 (0.18–0.31) | 0.030 | 0.25 (0.06–0.45) | 0.014 | 0.24 (0.04–0.45) | 0.024 | 0.39 (0.16–0.61) | 0.002 |
Elevated CRP at more than 50% of the time-points during 5 years.
CI, confidence interval; CRP, C-reactive protein; mSASSS, modified Stoke ankylosing spondylitis spinal score; NSAIDs, non-steroidal anti-inflammatory drugs.
Table 3.
Association between mSASSS and atherosclerotic plaques adjusting for age, smoking status, CRP, NSAIDs intake and disease duration.
| Crude model | Adjusted for age and smoking | Adjusted for age, smoking and elevated CRP* | Adjusted for age, smoking, elevated CRP* and NSAIDs intake | Adjusted for disease duration, smoking, elevated CRP* and NSAIDs intake | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Total mSASSS | 1.07 (1.04–1.11) | <0.001 | 1.04 (1.01–1.08) | 0.019 | 1.05 (0.99–1.11) | 0.076 | 1.05 (0.99–1.12) | 0.096 | 1.05 (1.01–1.12) | 0.034 |
| Cervical mSASSS | 1.13 (1.07–1.20) | <0.001 | 1.08 (1.02–1.56) | 0.019 | 1.07 (0.98–1.17) | 0.128 | 1.06 (0.98–1.16) | 0.155 | 1.09 (1.00–1.19) | 0.042 |
| Lumbar mSASSS | 1.11 (1.06–1.18) | <0.001 | 1.07 (1.01–1.14) | 0.036 | 1.09 (1.00–1.21) | 0.062 | 1.09 (0.99–1.22) | 0.070 | 1.10 (1.01–1.22) | 0.037 |
Elevated CRP at more than 50% of the time-points during 5 years.
CI, confidence interval; CRP, C-reactive protein; mSASSS, modified Stoke ankylosing spondylitis spinal score; NSAIDs, non-steroidal anti-inflammatory drugs.
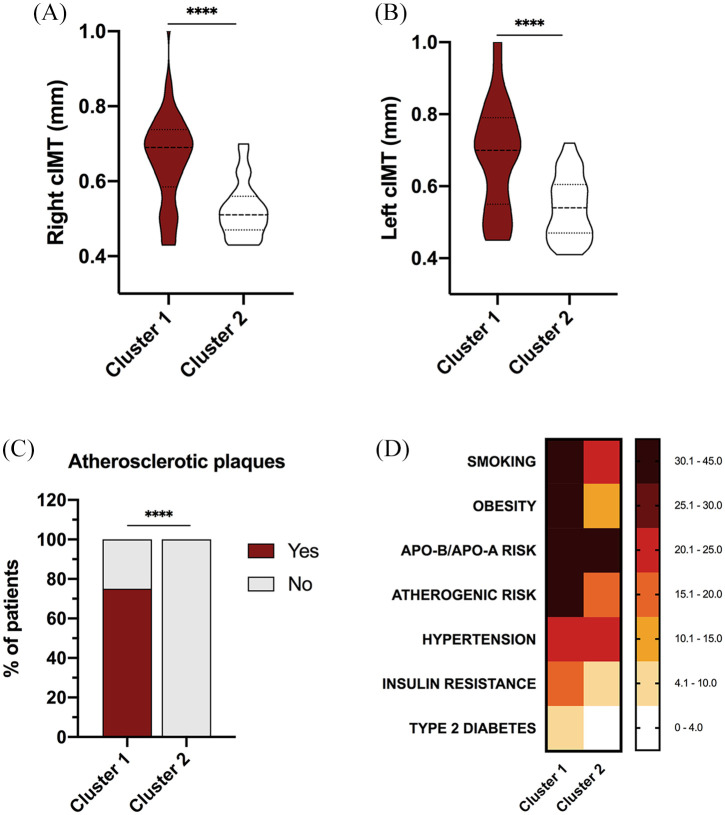
Cluster analysis
Cluster analysis including the presence of carotid plaques and right and left cIMT levels as variables (Figure 3A–C) distinguished two different phenotypes of patients according to their CV risk factor prevalence. These groups were adjusted for age. Carotid ultrasound data were used to stratify patients, since according to our previous results, SCORE does not totally reflect the presence of subclinical atherosclerosis.
Figure 3.
Cluster analysis recognizes two different phenotypes of axSpA patients according to their cardiovascular risk burden in terms of presence of carotid plaques and right and left cIMT levels. (A) Levels of right cIMT in cluster 1 and cluster 2. (B) Levels of left cIMT in cluster 1 and cluster 2. (C) Presence/absence of atherosclerotic plaques in cluster 1 and cluster 2. (D) Cluster analysis including presence of carotid plaques and right and left cIMT levels as variables distinguished two different phenotypes of patients with different cardiometabolic risk factors prevalence.
****p <0.0001.
Apo A, apolipoprotein A; Apo B, apolipoprotein B; axSpA, axial spondyloarthritis; cIMT, carotid intima media thickness.
Thus, cluster 1 was characterized by a significant increase in CV risk compared with that of cluster 2, showing higher rates of smoking habit, obesity, atherogenic index, insulin resistance, and type 2 diabetes (Figure 3D).
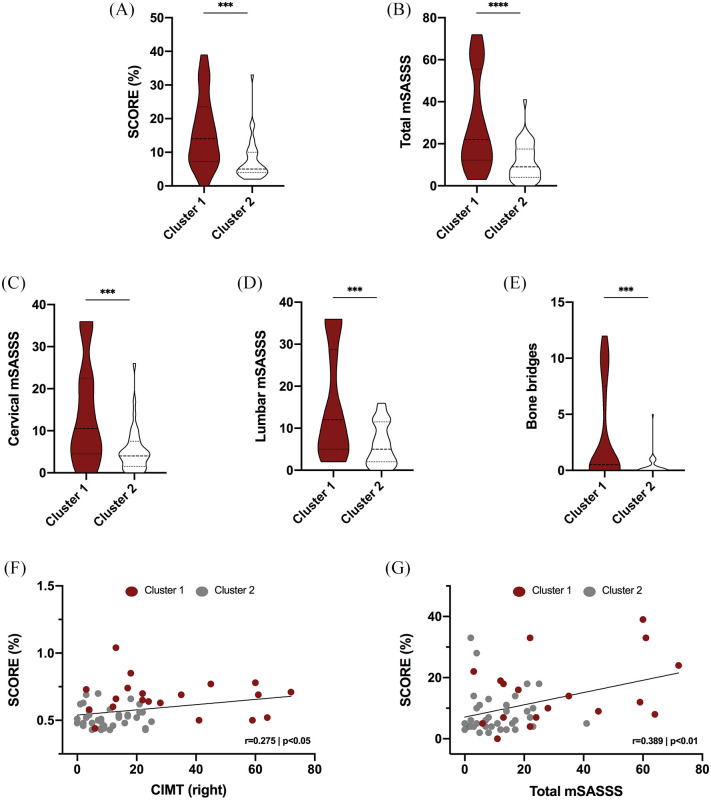
Likewise, cluster 1 had significantly increased levels of SCORE compared with those of cluster 2 (Figure 4A). Of note, cluster 1, having no difference in mean age from that of cluster 2, showed significantly augmented levels of total mSASSS (Figure 4B), cervical mSASSS (Figure 4C), lumbar mSASSS (Figure 4D), and the presence of bone bridges (Figure 4E).
Figure 4.
SCORE and structural damage in clusters recognizing two different phenotypes of axSpA patients according to the subclinical cardiovascular risk: atherosclerosis. (A) SCORE in cluster 1 and cluster 2. (B) Total mSASSS in cluster 1 and cluster 2. (C) Cervical mSASSS in cluster 1 and cluster 2. (D) Lumbar mSASSS in cluster 1 and cluster 2. (E) Number of bone bridges in cluster 1 and cluster 2. (F) Correlation between SCORE and right cIMT in cluster 1 and cluster 2. (G) Correlation between SCORE and total mSASSS in cluster 1 and cluster 2.
***p < 0.001, ****p < 0.0001.
axSpA, axial spondyloarthritis; cIMT, carotid intima media thickness; mSASSS, modified Stoke ankylosing spondylitis spinal score.
Thus, patients included in cluster 1 had increased levels of SCORE, along with higher levels of right cIMT (Figure 4F) and total mSASSS (Figure 4G).
Discussion
Age and tobacco are determining factors in the development of CV disease in the general population as well as in the progression of radiographic damage in patients with axSpA.12,23 Our study shows for the first time, to our knowledge, that axSpA patients with more structural damage display increased predicted CV risk, independently of disease duration, age, and smoking habit.
First, we confirmed the presence of an increased estimated CV risk in axSpA patients, as has been demonstrated in several previous studies.29 Thus, smoking habits have been associated with poorer outcomes in terms of structural damage, treatment response, and quality of life in SpA patients.30,31 Hypertension was found to be the most prevalent CV disease risk factor in SpA patients, particularly in Northern European countries, according to data from the COMOSPA study.32 Regarding NSAID consumption, most patients included in our study were taking NSAIDs. Recent studies have highlighted the connection between increased BMI and both axial and peripheral new bone formation and entheseal inflammation,33 although it has been proposed that obesity prevalence is higher in patients with peripheral forms of SpA.34 In addition, type 2 diabetes has also been linked to peripheral forms of SpA and psoriatic arthritis.35,36 Another factor, atherogenic risk, has previously been reported to be higher in axSpA patients,37,38 and we observed that atherogenic risk was also increased significantly in our cohort of axSpA patients.
Second, the SCORE index is the tool recommended by the 2016 European Society of Cardiology (ESC) guidelines on CV disease prevention to estimate the individual’s absolute risk for fatal CV events.39 SCORE includes age, sex, lipid levels, smoking, and blood pressure, with age being the variable with the greatest influence. In our registry, we found that the SCORE levels were increased in axSpA patients when compared with those of age-matched HCs, although this difference did not reach statistical significance, which might be because SCORE could underestimate CV risk in SpA patients.40 Despite this, when patients and controls were classified by low, moderate, high, and very high CV risk according to the SCORE levels, we observed that, among axSpA patients, 70.9% had a low CV risk, 18.4% had moderate CV risk, 6.8% had high, and 3.9% had very high CV risk, which differed from the CV risk present in HCs, with 86.4% classified as having low CV risk and 13.6% classified as having moderate CV risk.
In our study, structural damage was measured in terms of total, cervical, and lumbar mSASSS, which, to date, is the most validated and broadly used tool for measuring radiographic progression in r-axSpA because of its good correlation with indexes of disease signs and symptoms, spinal mobility, and physical function.24 The presence of syndesmophytes and bone bridges, which are the most relevant structural changes in r-axSpA according to numerous studies,14 was also evaluated. Thus, characteristics closely related to CV risk, such as SCORE levels, presence of atherosclerotic plaques, and right and left cIMT levels, were found to be associated with mSASSS and the presence of syndesmophytes and bone bridges, implying an association between radiographic damage and CV risk. In this sense, Kang and colleagues found an association between radiographic progression in the spine and a high estimated 10-year CV disease risk measured by Framingham risk score.41
Importantly, Rueda Gotor et al. demonstrated that the SCORE underestimates the real CV risk of patients with axSpA, and proposed the use of additional tools, such as carotid ultrasound, to improve the identification of axSpA patients at high risk of developing CV disease.42–44 In this context, we decided to perform cluster analysis including the presence of carotid plaques and right and left cIMT levels as variables. Interestingly, our cluster analysis based on the presence of carotid plaques and right and left cIMT levels allowed us to distinguish two different phenotypes of patients according to their CV risk factor prevalence, and these two different groups were adjusted for age. In the first cluster, with a higher number of atherosclerotic plaques and thicker carotid intima media, there was an increased prevalence of smoking, obesity, atherogenic index, insulin resistance and type 2 diabetes, with significantly increased levels of SCORE, but, surprisingly, this cluster also showed significantly augmented levels of total, cervical, and lumbar mSASSS, and an increased presence of bone bridges with a higher grade of left and right sacroiliitis.
A study by Eriksson et al. compared the prevalence of CV events in rheumatoid arthritis (RA) and axSpA patients, and found that the risk was similar for stroke, but axSpA patients had a lower risk for thrombotic events and acute coronary syndromes.45 While in RA the increased CV risk and its association with the inflammatory burden are well described,46–48 few studies have evaluated this association in axSpA. In our study, persistent inflammation was defined in case of abnormal CRP levels in >50% of the determinations during the last 5 years. We found that axSpA with persistent inflammation displayed increased right and left cIMT and a higher number of syndesmophytes, which points to CRP as a link between increased CV risk and structural damage in these patients.
The role of CRP in structural damage in axSpA patients has already been defined, meaning that axial radiographic progression is a result of chronic inflammation in axSpA patients,49–51 and our study not only supports this fact but also highlights inflammation as a more important link between the radiographic damage and CV risk than age. Indeed, in the relationship between structural damage and CV risk, there are four major confounding factors: age,12 smoking habit,30 CRP levels, NSAIDs intake, and disease duration. These variables directly influence both structural damage and CV risk. Therefore, when assessing the relationship between structural damage and CV risk, these variables should be taken into account, as we have done in our study, finding an independent association between total mSASSS and SCORE, and between total mSASSS and atherosclerotic plaques. Besides, mSASSS might be considered as a marker of the previous inflammatory burden, apart from the CRP levels from the beginning of the disease (and not only in the most recent 5 years) that would also explain the association with CV risk.
Regarding the potential role of NSAIDs in atherosclerosis, the metabolism of arachidonic acid is implicated in the pathophysiology of CV ischemic diseases, and special attention has been paid to the pathway catalyzed by cyclooxygenase, which leads to the production of prostanoids. NSAIDs are first line treatment in axSpA, and use of COX-2 inhibitors (COXIBS) has revealed an increase in the incidence of CV events, especially myocardial infarction, since the balance between proinflammatory and anti-inflammatory prostaglandins isomerases seems to be an important determinant of the role of COX-2 in plaque stability.52
Even though r-axSpA patients with greater structural damage, and, as a consequence, higher mSASSS may be those who take more NSAIDs and therefore they could show a higher CV risk and experience more CV events, some studies performed in patients with inflammatory rheumatic diseases have found that the long-term use of some anti-inflammatory drugs could help to decrease the CV risk related to the persistent inflammation situation in these patients. In addition, COXIBs were not associated with an increased risk for vascular mortality. Several studies in patients with RA have showed a reduction in vascular mortality with NSAID use.53 Several anti-inflammatory treatments have been proposed as potential therapeutic strategies for atherosclerosis by reducing the overall vascular risk imposed by inflammation. Furthermore, treatment of the underlying inflammatory process could contribute to improving CV risk in patients with inflammatory rheumatic diseases.54 Actually, in this study, we present results of patients with absence of CV events even though most of them were taking NSAIDs permanently. Currently, answering how treatment with NSAIDs affects the CV risk in patients with rheumatic inflammatory diseases, in particular r-axSpA, and if these should be treated with NSAIDs continuously or intermittently from a CV point of view is a significant requirement.8 In SpA, the impact of anti-inflammatory treatments on CV events or mortality has been assessed only rarely.55
Among the limitations of this study, the cross-sectional design does not allow us to find a causal relationship between structural damage and CV risk. Actually, the association between the mSASSS and CV risk has been identified using a surrogate variable, the SCORE index. However, in this analysis, we did not evaluate CV events since we excluded patients with such occurrences. To better understand the association of structural damage with CV risk assessed in terms of CV events, and the underlying mechanisms involving the association between radiographic structural damage in the spine and sacroiliac joints, the increased CV risk and persistent inflammation, and the influence of treatments, longitudinal studies with prospective follow up are required.
Despite the limitations, the strengths of this study include that all the analyses were adjusted for age and a large number of patients were included. Moreover, the association between CV risk and structural damage has been adjusted for age, tobacco, inflammation, and disease duration, which are confounding factors biologically associated with both SCORE and mSASSS.
In summary, for the first time, our results support the association of structural damage and predicted CV risk in patients with axSpA independently of age, tobacco, inflammation, and disease duration. Further prospective studies are needed in order to better elucidate this relationship between CV risk and structural damage.
Acknowledgments
The authors would like to thank all of the patients who participated in the study. In memory of María del Carmen Castro-Villegas, co-author of this article, for her deep dedication to the spondyloarthropaties, your loss leaves an enormous sadness in our hearts.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CL-P was supported by a contract from the Spanish Junta de Andalucía (“Nicolás Monardes” program). This work was supported by a grant from the ‘Junta de Andalucía’ (PI-0139-2017).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: María Lourdes Ladehesa-Pineda  https://orcid.org/0000-0002-3890-2224
https://orcid.org/0000-0002-3890-2224
Clementina López-Medina  https://orcid.org/0000-0002-2309-5837
https://orcid.org/0000-0002-2309-5837
Rafaela Ortega-Castro  https://orcid.org/0000-0002-7552-3469
https://orcid.org/0000-0002-7552-3469
Ignacio García-Gómez  https://orcid.org/0000-0002-4607-5644
https://orcid.org/0000-0002-4607-5644
Yolanda Jiménez-Gómez  https://orcid.org/0000-0002-1607-2717
https://orcid.org/0000-0002-1607-2717
Nuria Barbarroja  https://orcid.org/0000-0002-0962-6072
https://orcid.org/0000-0002-0962-6072
Contributor Information
María Lourdes Ladehesa-Pineda, Department of Rheumatology of “Reina Sofia University Hospital”, Avda. Menéndez Pidal s/n, Córdoba, 14004, Spain; Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Iván Arias de la Rosa, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Clementina López Medina, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain; Rheumatology Department, Cochin Hospital from Paris/INSERM U:1153, Clinical Epidemiology and Biostatistics, Paris, France.
María del Carmen Castro-Villegas, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
María del Carmen Ábalos-Aguilera, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Rafaela Ortega-Castro, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Ignacio Gómez-García, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Pedro Seguí-Azpilcueta, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Yolanda Jiménez-Gómez, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Alejandro Escudero-Contreras, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Chary López Pedrera, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
Nuria Barbarroja, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain; CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III, Madrid, Spain.
Eduardo Collantes-Estévez, Reina Sofia University Hospital/Maimonides Research Institute of Biomedical Medicine from Cordoba (IMIBIC)/University of Córdoba, Cordoba, Spain.
References
- 1. Dougados M, Baeten D. Spondyloarthritis. Lancet 2011; 377: 2127–2137. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, van der Heijde D, Landewé R, et al. The assessment of spondyloarthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011; 70: 25–31. [DOI] [PubMed] [Google Scholar]
- 4. López-Medina C, Moltó A. Update on the epidemiology, risk factors, and disease outcomes of axial spondyloarthritis. Best Pract Res Clin Rheum 2018; 32: 241–253. [DOI] [PubMed] [Google Scholar]
- 5. Moltó A, Etcheto D, van der Heijde R, et al. Prevalence of comorbidities and evaluation of their screening in spondyloarthritis: results of the international cross-sectional ASAS-COMOSPA study. Ann Rheum Dis 2016; 75: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 6. Moltó A, Dougados M. Comorbidities in spondyloarthritis including psoriatic arthritis. Best Pract Res Clin Rheumatol 2018; 32: 390–400. [DOI] [PubMed] [Google Scholar]
- 7. Castañeda S, Nurmohamed MT, González-Gay MA. Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol 2016; 30: 851–869. [DOI] [PubMed] [Google Scholar]
- 8. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017; 76: 17–28. [DOI] [PubMed] [Google Scholar]
- 9. Papagoras C, Voulgari PV, Drosos AA. Cardiovascular disease in spondyloarthritides. Curr Vasc Pharmacol 2020; 18: 473–487. [DOI] [PubMed] [Google Scholar]
- 10. López-Medina C, Jiménez-Gómez Y, Moltó A, et al. Cardiovascular risk factors in patients with spondyloarthritis from Northern European and Mediterranean countries: an ancillary study of the ASASCOMOSPA project. Joint Bone Spine 2018; 85: 447–453. [DOI] [PubMed] [Google Scholar]
- 11. Liew JW, Ramiro S, Gensler LS. Cardiovascular morbidity and mortality in ankylosing spondylitis and psoriatic arthritis. Best Pract Res Clin Rheumatol 2018; 32: 369–389. [DOI] [PubMed] [Google Scholar]
- 12. Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 2015; 74: 52–59. [DOI] [PubMed] [Google Scholar]
- 13. Ward MM, Learch TJ, Gensler LS, et al. Regional radiographic damage and functional limitations in patients with ankylosing spondylitis: differences in early and late disease. Arthritis Care Res (Hoboken) 2013; 65: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baraliakos X, Listing J, Rudwaleit M, et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis 2007; 66: 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aouad K, Ziade N, Baraliakos X. Structural progression in axial spondyloarthritis. Joint Bone Spine 2020; 87: 131–136. [DOI] [PubMed] [Google Scholar]
- 16. Almodóvar R, Ríos V, Ocaña S, et al. Association of biomarkers of inflammation, cartilage and bone turnover with gender, disease activity, radiological damage, and sacroiliitis by magnetic resonance imaging in patients with early spondyloarthritis. Clin Rheumatol 2014; 33: 237–241. [DOI] [PubMed] [Google Scholar]
- 17. Braun J, Baraliakos X, Hermann K-GA, et al. Serum C-reactive protein levels demonstrate predictive value for radiographic and magnetic resonance imaging outcomes in patients with active ankylosing spondylitis treated with golimumab. J Rheumatol 2016; 43: 1704–1712. [DOI] [PubMed] [Google Scholar]
- 18. Li ZH, Zhong WF, Lv YB, et al. Associations of plasma high-sensitivity C-reactive protein concentrations with all-cause and cause-specific mortality among middle-aged and elderly individuals. Immun Ageing 2019; 16: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol 2016; 67: 712–723. [DOI] [PubMed] [Google Scholar]
- 20. The Emerging Risk Factors Collaboration, Di Angelantonio E, Kaptoge S, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010; 375: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang K, Wang Y, Chu C, et al. Joint association of serum homocysteine and high-sensitivity C-reactive protein with arterial stiffness in Chinese population: a 12-year longitudinal study. Cardiology 2019; 144: 27–35. [DOI] [PubMed] [Google Scholar]
- 22. Blaum C, Brunner FJ, Kröger F, et al. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: implications for an anti-inflammatory treatment target population. Eur J Prev Cardiol 2019; 10: 2047487319885458. [DOI] [PubMed] [Google Scholar]
- 23. Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol 2016; 594: 2061–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Heijde D, Braun J, Deodhar A, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology (Oxford) 2019; 58: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piepoli MF, Hoes AW, Agewall S, et al. 2016. European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Atherosclerosis 2016; 252: 207–274. [DOI] [PubMed] [Google Scholar]
- 26. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003; 24: 987–1003. [DOI] [PubMed] [Google Scholar]
- 27. O’Leary DH, Polak JF, Kronmal RA, et al. ; Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 1999; 340: 14–22. [DOI] [PubMed] [Google Scholar]
- 28. Van der Heijde D, Braun J, Deodhar A, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology (Oxford) 2019; 58: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liew JW, Ramiro S, Gensler LS. Cardiovascular morbidity and mortality in ankylosing spondylitis and psoriatic arthritis. Best Pract Res Clin Rheumatol 2018; 32: 369–389. [DOI] [PubMed] [Google Scholar]
- 30. Poddubnyy D, Haibel H, Listing J, et al. Cigarette smoking has a dose-dependent impact on progression of structural damage in the spine in patients with axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort (GESPIC). Ann Rheum Dis 2013; 72: 1430–1432. [DOI] [PubMed] [Google Scholar]
- 31. Glintborg B, Højgaard P, Lund Hetland M, et al. Impact of tobacco smoking on response to tumor necrosis factor-alpha inhibitor treatment in patients with ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Rheumatology 2016; 55: 659–668. [DOI] [PubMed] [Google Scholar]
- 32. Moltó A, Etcheto A, van der Heijde D, et al. Prevalence of comorbidities and evaluation of their screening in spondyloarthritis: results of the international cross-sectional ASAS-COMOSPA study. Ann Rheum Dis 2016; 75: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 33. Bakirci S, Dabague J, Eder L, et al. The role of obesity on inflammation and damage in spondyloarthritis: a systematic literature review on body mass index and imaging. Clin Exp Rheumatol 2020; 38: 144–148. [PubMed] [Google Scholar]
- 34. Atzeni F, Nucera V, Galloway J, et al. Cardiovascular risk in ankylosing spondylitis and the effect of anti-TNF drugs: a narrative review. Expert Opin Biol Ther 2020; 20: 517–524. [DOI] [PubMed] [Google Scholar]
- 35. Solomon DH, Love TJ, Canning C, et al. The risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis, and psoriasis. Ann Rheum Dis 2010; 69: 2114–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. López-Medina C, Jiménez-Gómez Y, Moltó A, et al. Cardiovascular risk factors in patients with spondyloarthritis from Northern European and Mediterranean countries: an ancillary study of the ASAS-COMOSPA project. Joint Bone Spine 2018; 85: 447–453. [DOI] [PubMed] [Google Scholar]
- 37. Papagoras C, Markatseli TE, Saougou I, et al. Cardiovascular risk profile in patients with spondyloarthritis. Joint Bone Spine 2014; 81: 57–63. [DOI] [PubMed] [Google Scholar]
- 38. Arida A, Protogerou AD, Kitas GD, et al. Systemic inflammatory response and atherosclerosis: the paradigm of chronic inflammatory rheumatic diseases. Int J Mol Sci 2018; 19: 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piepoli MF, Hoes AW, Agewall S, et al. 2016. European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Atherosclerosis 2016; 252: 207–274. [DOI] [PubMed] [Google Scholar]
- 40. Colaco K, Ocampo V, Ayala AP, et al. Predictive utility of cardiovascular risk prediction algorithms in inflammatory rheumatic diseases: a systematic review. J Rheumatol. Epub ahead of print 15 August 2019. DOI: 10.3899/jrheum.190261. [DOI] [PubMed] [Google Scholar]
- 41. Kang KY, Her YH, Ju JH, et al. Radiographic progression is associated with increased cardiovascular risk in patients with axial spondyloarthritis. Mod Rheumatol 2016; 26: 601–606. [DOI] [PubMed] [Google Scholar]
- 42. Rueda-Gotor J, Llorca J, Corrales A, et al. Carotid ultrasound in the cardiovascular risk stratification of patients with ankylosing spondylitis: results of a population-based study. Clin Exp Rheumatol 2016; 34: 885–892. [PubMed] [Google Scholar]
- 43. Rueda-Gotor J, Quevedo-Abeledo JC, Corrales A, et al. Reclassification into very-high cardiovascular risk after carotid ultrasound in patients with axial spondyloarthritis. Clin Exp Rheumatol 2020; 38: 724–731. [PubMed] [Google Scholar]
- 44. Rueda-Gotor J, Llorca J, Corrales A, et al. Cardiovascular risk stratification in axial spondyloarthritis: carotid ultrasound is more sensitive than coronary artery calcification score to detect high-cardiovascular risk axial spondyloarthritis patients. Clin Exp Rheumatol 2018; 36: 73–80. [PubMed] [Google Scholar]
- 45. Eriksson JK, Jacobsson L, Bengtsson K, et al. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann Rheum Dis 2017; 76: 364–370. [DOI] [PubMed] [Google Scholar]
- 46. Gabriel SE, Crowson CS. Risk factors for cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol 2012; 24: 171–176. [DOI] [PubMed] [Google Scholar]
- 47. Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis 2010; 69: 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis-the next step: differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology 2011; 50: 1944–1954. [DOI] [PubMed] [Google Scholar]
- 49. Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012; 64: 1388–1398. [DOI] [PubMed] [Google Scholar]
- 50. Kang KY, Kwok SK, Ju JH, et al. The predictors of development of new syndesmophytes in female patients with ankylosing spondylitis. Scand J Rheumatol 2015; 44: 125–128. [DOI] [PubMed] [Google Scholar]
- 51. Ramiro S, van der Heijde D, van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014; 73: 1455–1461. [DOI] [PubMed] [Google Scholar]
- 52. Cipollone F, Fazia ML. COX-2 and atherosclerosis. J Cardiovasc Pharmacol 2006; 47: 26–36. [DOI] [PubMed] [Google Scholar]
- 53. Haroon NN, Paterson JM, Li P, et al. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med 2015; 163: 409–416. [DOI] [PubMed] [Google Scholar]
- 54. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prati C, Demougeot C, Guillot X, et al. Vascular involvement in axial spondyloarthropathies. Joint Bone Spine 2019; 86: 159–163. [DOI] [PubMed] [Google Scholar]