Abstract
Anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) immune-mediated necrotizing myopathy is a subtype of idiopathic inflammatory myopathy which may be associated with statin exposure. It presents with severe proximal muscle weakness, high creatine kinase levels and muscle fiber necrosis. Treatment with intravenous immunoglobulins and immunosuppressants is often necessary. This entity is not commonly known among dermatologists as there are usually no extramuscular manifestations. We report a rare case of statin-associated anti-HMGCR immune-mediated necrotizing myopathy with dermatomyositis-like cutaneous features. The possibility of anti-HMGCR immune-mediated necrotizing myopathy should be considered in patients with cutaneous dermatomyositis-like features associated with severe proximal muscle weakness, highly elevated creatine kinase levels and possible statin exposure. This indicates the importance of muscle biopsy and specific autoantibody testing for accurate diagnosis, as well as significant therapeutic implications.
Keywords: Anti-HMGCR, dermatomyositis, immune-mediated necrotizing myopathy, statin, myositis
Introduction
The spectrum of idiopathic inflammatory myopathies (IIMs) includes dermatomyositis (DM), overlap myositis, inclusion body myositis (IBM) and immune-mediated necrotizing myopathy (IMNM).1 IMNMs are characterized by severe proximal muscle weakness, high creatine kinase (CK) levels, predominant muscle fiber necrosis and no extramuscular manifestations.2 Anti-signal recognition particle (SRP) and anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) autoantibodies (aAbs) are associated with IMNM, and statin exposure may trigger anti-HMGCR IMNM.
We report herein a rare case of statin-associated anti-HMGCR IMNM with DM-like cutaneous features. To our knowledge, this is only the fourth case documented with substantial serologic and histologic evidence.
Case report
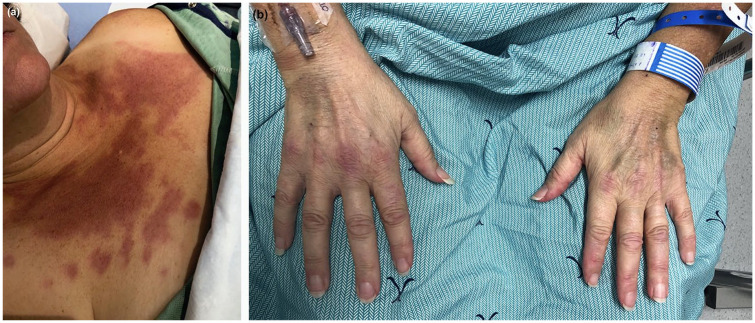
A 54-year-old Caucasian woman presented to the Myositis Clinic in a tertiary medical center for a second medical opinion for an atypical clinical presentation of DM as requested by her internist who previously hospitalized her in a regional hospital center for an acute rash accompanied by muscle weakness. Her past medical history included dyslipidemia and pre-diabetes. Atorvastatin (40 mg/day) was introduced 11 months ago. There was no family history of muscle or autoimmune disorders. Two months ago, the patient developed a rash on photoexposed areas followed by a rapidly progressive and severe proximal muscle weakness and became bedridden. She also reported dysphagia, dyspnea and fatigue. Erythemato-violaceous plaques with poikiloderma (atrophy, telangiectasias and dyspigmentation) were noted on peri-orbital regions, anterior chest, upper back, extensor surfaces of arms, lateral thighs and dorsal finger’s joints (Figure 1). Slight periungual erythema was noted with normal cuticles. Muscle strength examination revealed severe weakness. Cardiopulmonary, abdominal and neurologic examinations were otherwise normal.
Figure 1.
DM rash presenting as erythemato-violaceous papules and plaques on (a) the anterior chest area (V sign) and (b) dorsal finger’s joints with periungual erythema.
DM: dermatomyositis.
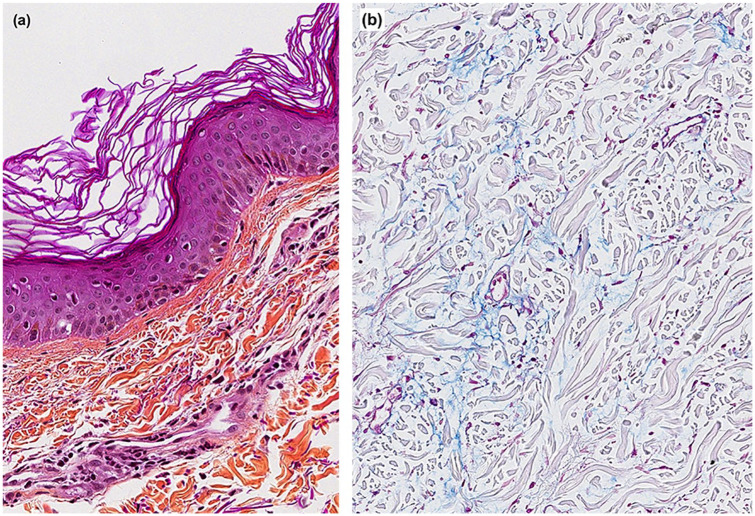
CK levels were highly elevated at 20,305 IU/L. Complete blood count, creatinine and urinalysis were normal. Antinuclear antibody (ANA), extractable nuclear antigen (ENA), anti-double stranded DNA (anti-dsDNA) and the panel for myositis-specific and myositis-associated aAbs (Euroimmun, Luebeck, Germany) were negative. Anti-HMGCR aAbs (INOVA) were positive (24.56 absorbance units (AU), normal < 20). Magnetic resonance imaging showed T2 hypersignal in the obturator, quadriceps and semi-membranous muscles. The patient had a myopathic electromyogram (EMG). Pulmonary function tests showed a moderate restrictive ventilatory defect secondary to extrapulmonary involvement, suggestive of respiratory muscle weakness. Nailfold capillaroscopy was normal. Cancer screening including thoraco-abdominopelvic computerized tomography scan, mammography, positron emission tomography scan, pelvic ultrasound, esophagogastroduodenoscopy and colonoscopy was negative. Skin biopsy revealed rare necrotic keratinocytes with discrete vacuolization of the basal cell layer at the basement membrane zone, perivascular and periadnexial lymphocytic infiltrates and increased dermal interstitial mucin (Figure 2). Quadriceps muscle biopsy showed scattered necrotic and regenerative fibers without inflammatory infiltrates or perifascicular atrophy (Figure 3). There were no sarcolemmal overexpression of major histocompatibility complex (MHC)-1, capillary dropout or capillary C5b-9 deposition. Sarcolemmal C5b-9 deposition was noted sparsely on non-necrotic fibers. Sarcoplasmic expression of myxovirus resistance protein A (MxA) was negative. Electron microscopy did not reveal tubuloreticular inclusions.
Figure 2.
Skin histology. (a) A hematoxylin phloxine saffron–stained section at 20× magnification showing rare necrotic keratinocytes with discrete vacuolization of the basal cell layer at the basement membrane zone and perivascular lymphocytic infiltrates and (b) staining with blue Alcian (pH 2.5) at 10× magnification highlighting increased dermal mucin deposition.
Figure 3.
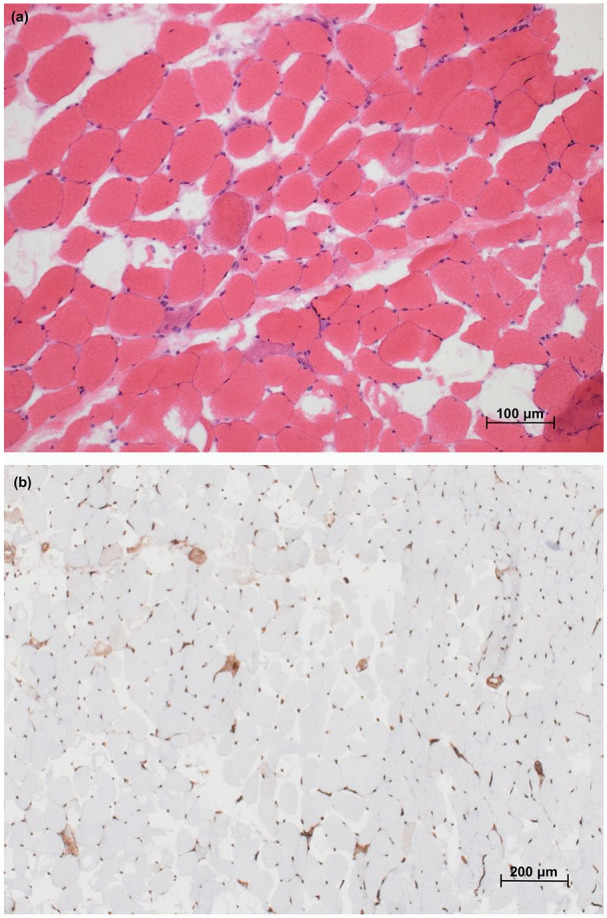
Muscle histology. (a) Hematoxylin and eosin section of semimembranosus muscle biopsy showing scattered purple staining necrotic and regenerative fibers without lymphocytic infiltration (200× magnification) and (b) immunohistochemical preparation for MHC-1 showing overexpression restricted to scattered necrotic fibers and lack of capillary dropout (100× magnification).
MHC: major histocompatibility complex.
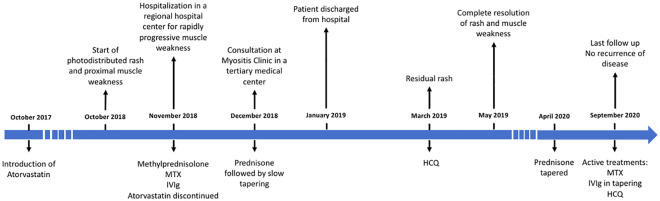
A diagnosis of statin-associated anti-HMGCR IMNM with DM-like cutaneous features was made. Statin was discontinued early, and the patient was treated with high-dose corticosteroids including methylprednisolone (pulses of 500 mg, and 40 mg BID for 2 weeks) followed by prednisone 1 mg/kg/day that was then tapered. She also received intravenous immunoglobulins (IVIgs) (1 g/kg/2 weeks), subcutaneous methotrexate (25 mg/week), hydroxychloroquine (5 mg/kg/day) and betamethasone valerate 0.1% cream (twice a day on the body until resolution of rash). The patient initially necessitated parenteral nutrition for severe dysphagia. She fully recovered after 6 months of treatment with complete resolution of the DM rash, muscle strength (Medical Research Council Scale 5/5) and swallowing difficulties. At last follow-up after almost 2 years, there was no evidence of disease recurrence. A timeline of the history and treatments is presented in Figure 4.
Figure 4.
Timeline of medical history and treatments.
IVIg: intravenous immunoglobulin; HCQ: hydroxychloroquine; MTX: methotrexate.
Discussion
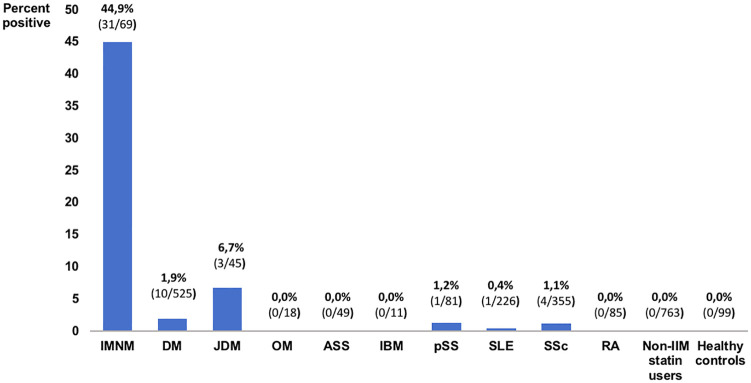
Anti-HMGCR aAb was discovered in 2010 among IMNM patients, where it recognized a 100-kDa protein corresponding to HMGCR antigen, a key enzyme in cholesterol biosynthesis targeted by statins.3,4 Anti-HMGCR aAbs may be pathogenic as their titers correlate with disease activity (muscle strength and CK levels),5 and in vitro aAbs induce muscle atrophy and impair muscle regeneration.2 These aAbs are highly specific for autoimmune myopathy as they were not found in most statin-exposed individuals, including those with self-limited statin-associated myopathy.6 The prevalence of anti-HMGCR was reported as highest in association with IMNM and only rarely in other IIM and connective tissue diseases (Figure 5).6–8 Pathophysiology of anti-HMGCR IMNM is not yet entirely understood, but genetic susceptibility has been described with HLA-DRB1*11:01.9 It is believed that this human leukocyte antigen (HLA) may present a strongly immunogenic HMGCR-derived peptide resulting from HMGCR overexpression with statin exposure.9
Figure 5.
Prevalence of anti-HMGCR aAbs among IIM, other connective tissue diseases, statin users and healthy controls according to Mammen et al.,6 Musset et al.7 and Hudson et al.8
IMNM: immune-mediated necrotizing myopathy; DM: dermatomyositis; JDM: juvenile dermatomyositis; OM: overlap myositis; ASS: anti-synthetase syndrome; IBM: inclusion body myositis; pSS: primary Sjögren’s syndrome; SLE: systemic lupus erythematosus; SSc: systemic sclerosis; RA: rheumatoid arthritis; IIM: idiopathic inflammatory myopathy.
Anti-HMGCR IMNM usually occurs between 40 and 60 years old, but pediatric cases were reported, and there is a female predominance.2,4,5 Association with statin exposure is noted in half to two-thirds of patients, and mean duration before CK elevation is 39 months (15–84 months).10 These patients present with a subacute onset of severe proximal muscle weakness, myalgias, highly elevated CK (mean around 8300 IU/L)4,5 and myopathic EMG findings. In a cohort of atorvastatin-associated anti-HMGCR IMNM, CK elevation could precede muscle weakness by months, or even years, suggesting that persistent CK elevation despite statin discontinuation and/or onset of muscle weakness should prompt for anti-HMGCR aAb testing.10 There is usually no significant extramuscular involvement.2,4,5 Muscle biopsy helps to differentiate from other myopathies and shows randomly distributed necrotic, regenerating and atrophic muscle fibers, and no or mild inflammatory infiltrates.2,5 C5b-9 deposits around fibers and/or capillaries are also observed, and MHC-I overexpression is usually negative, or slight and focal if present.11 Malignancy association with anti-HMGCR IMNM has been inconsistent.2,12
To our knowledge, our case is only the fourth reported case of statin-associated anti-HMGCR IMNM with typical cutaneous DM features with convincing serology and histology. This entity has rarely been described in few case reports and seldomly in some cohorts (including DM patients with anti-HMGCR aAbs in the latter).7,13–17 In case reports, patients were between 47 and 61 years old and were exposed to statins from 8 months to 10 years before seeking medical attention.13–15 They all presented characteristic DM rash and proximal muscle weakness for a period between 4 weeks and 4 years. CK levels were elevated (8674–37,527 IU/L) as well as anti-HMGCR aAbs. All muscle biopsies were compatible with IMNM without DM-specific findings. Skin biopsy was done in one patient and showed neutrophilic vacuolar interface dermatitis with lymphocytic and neutrophilic infiltrates in the dermis including perifollicular regions.14 Direct immunofluorescence revealed granular deposits of IgG, IgA and C3 along the dermoepidermal junction. Of note, in our patient, although the cutaneous biopsy showed periadnexial inflammatory infiltrates (more frequent in lupus erythematosus), this finding has been described in cutaneous DM18 and a diagnosis of DM-like features was retained to correlate with characteristic DM rash. All previously reported patients improved significantly within 2–12 months under high-dose corticosteroids, immunosuppressants (methotrexate or mycophenolate mofetil) and IVIgs. One patient required additional plasmapheresis to achieve near-complete remission.15
In statin-associated anti-HMGCR IMNM, statin withdrawal and corticosteroids alone are usually not sufficient.10 In a retrospective study of 55 patients, successful induction treatments were described with a combination of steroids, immunosuppressants and IVIgs in patients with muscle weakness.19 A shorter delay from presentation to treatment was significantly associated with an early remission and successful maintenance with immunosuppressant monotherapy, underlining the importance of early recognition and treatment of IMNM. The 2016 European Neuromuscular Center (ENMC) International Workshop has proposed similar treatment recommendations as the current standard of care for patients with anti-HMGCR IMNM.11 The treatments proposed in our patient are in agreement with those recommendations.
In the present case, a diagnosis of statin-associated anti-HMGCR IMNM was made instead of classic DM based on muscle biopsy consistent with IMNM, subacute and severe muscle weakness, high CK levels, statin exposure and anti-HMGCR aAb positivity.11 DM-specific myological features1 were not observed including sarcoplasmic MxA expression (77% sensitivity and 100% specificity for DM).20 This now more substantiated association has important implications on the diagnostic approach of patients who present with cutaneous features of DM since anti-HMGCR antibodies are not included in the myositis panel routinely used in most centers. In conclusion, the possibility of anti-HMGCR IMNM should be considered in patients with cutaneous DM-like features associated with severe proximal muscle weakness and highly elevated CK levels, particularly with a history of statin exposure. This indicates the importance of muscle biopsy and specific autoantibody testing for accurate diagnosis, as well as significant therapeutic implications.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: The patient provided written consent for publication of the case report.
ORCID iD: Darosa Lim  https://orcid.org/0000-0003-2707-8134
https://orcid.org/0000-0003-2707-8134
References
- 1. Benveniste O, Stenzel W, Allenbach Y. Advances in serological diagnostics of inflammatory myopathies. Curr Opin Neurol 2016; 29(5): 662–673. [DOI] [PubMed] [Google Scholar]
- 2. Allenbach Y, Benveniste O. Peculiar clinicopathological features of immune-mediated necrotizing myopathies. Curr Opin Rheumatol 2018; 30(6): 655–663. [DOI] [PubMed] [Google Scholar]
- 3. Christopher-Stine L, Casciola-Rosen LA, Hong G, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9): 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3): 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allenbach Y, Drouot L, Rigolet A, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine (Baltimore) 2014; 93(3): 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64(2): 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musset L, Allenbach Y, Benveniste O, et al. Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: a history of statins and experience from a large international multi-center study. Autoimmun Rev 2016; 15(10): 983–993. [DOI] [PubMed] [Google Scholar]
- 8. Hudson M, Luck Y, Stephenson M, et al. Anti-HMGCR antibodies in systemic sclerosis. Medicine (Baltimore) 2016; 95(44): e5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mammen AL, Gaudet D, Brisson D, et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res (Hoboken) 2012; 64(8): 1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Troyanov Y, Landon-Cardinal O, Fritzler MJ, et al. Atorvastatin-induced necrotizing autoimmune myositis: an emerging dominant entity in patients with autoimmune myositis presenting with a pure polymyositis phenotype. Medicine (Baltimore) 2017; 96(3): e5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allenbach Y, Mammen AL, Benveniste O, et al. 224th ENMC international workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016 Neuromuscul Disord 2018; 28(1): 87–99. [DOI] [PubMed] [Google Scholar]
- 12. Allenbach Y, Keraen J, Bouvier AM, et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain 2016; 139(Pt8): 2131–2135. [DOI] [PubMed] [Google Scholar]
- 13. Lavian M, Mozaffar T, Goyal N. Clinical dermatomyositis associated with anti-HMG-CoA reductase antibody positive immune mediated necrotizing myopathy: a case report (P2.125). Neurology 2017; 88(16 Supplement):P2125. [Google Scholar]
- 14. Merlant M, Fite C, Kottler D, et al. [Dermatomyositis-like syndrome revealing statin-induced necrotizing autoimmune myopathy with anti-HMGCR antibodies]. Ann Dermatol Venereol 2019; 146(8-9): 550–556. [DOI] [PubMed] [Google Scholar]
- 15. Parikh P, Tavee J, Soltanzadeh P, et al. Anti-3-hydroxy-3-methylglutaryl-coenzyme a reductase autoantibody-positive necrotizing autoimmune myopathy with dermatomyositis-like eruption. Muscle Nerve 2018; 57(6): E135–E136. [DOI] [PubMed] [Google Scholar]
- 16. Limaye V, Bundell C, Hollingsworth P, et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 2015; 52(2): 196–203. [DOI] [PubMed] [Google Scholar]
- 17. Tiniakou E, Pinal-Fernandez I, Lloyd TE, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford) 2017; 56(5): 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolstencroft PW, Rieger KE, Leatham HW, et al. Clinical factors associated with cutaneous histopathologic findings in dermatomyositis. J Cutan Pathol 2019; 46(6): 401–410. [DOI] [PubMed] [Google Scholar]
- 19. Meyer A, Troyanov Y, Drouin J, et al. Statin-induced anti-HMGCR myopathy: successful therapeutic strategies for corticosteroid-free remission in 55 patients. Arthritis Res Ther 2020; 22(1): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uruha A, Allenbach Y, Charuel JL, et al. Diagnostic potential of sarcoplasmic myxovirus resistance protein A expression in subsets of dermatomyositis. Neuropathol Appl Neurobiol 2019; 45(5): 513–522. [DOI] [PubMed] [Google Scholar]