Abstract
Wernicke’s encephalopathy (WE) is an acute neuropsychiatric state. Untreated, WE can lead to coma or death, or progress to Korsakoff syndrome (KS) – a dementia characterized by irreversible loss of anterograde memory. Thiamine (vitamin B1) deficiency lies at the heart of this condition. Yet, our understanding of thiamine regarding prophylaxis and treatment of WE remains limited. This may contribute to the current undertreatment of WE in clinical practice. The overall aim of this review is to identify the best strategies for prophylaxis and treatment of WE in regard to (a) dose of thiamine, (b) mode of administration, (c) timing of switch from one mode of administration to another, (d) duration of administration, and (e) use of magnesium along thiamine as an essential cofactor. Evidence from randomized controlled trials and other intervention studies is virtually absent. Therefore, we have to resort to basic science for proof of principle instead. Here, we present the first part of our clinical review, in which we explore the physiology of thiamine and the pathophysiology of thiamine deficiency. We first explore both of these in their historical context. We then review the pharmacodynamics and pharmacokinetics of thiamine, exploring the roles of the six currently known thiamine compounds, their transporters, and target enzymes. We also explore the significance of magnesium as a cofactor in thiamine-facilitated enzymatic reactions and thiamine transport. In the second (forthcoming) part of this review, we will use the findings of the current review to make evidence-based inferences about strategies for prophylaxis and treatment of WE.
Keywords: beriberi, Korsakoff syndrome, magnesium, thiamine, thiamine deficiency, vitamin B1, Wernicke encephalopathy
Introduction
Wernicke’s encephalopathy (WE) is an acute neuropsychiatric state. Untreated, WE can lead to coma or death, or progress to Korsakoff syndrome (KS). KS is a dementia characterized by irreversible loss of anterograde memory.1,2 Thiamine (vitamin B1) deficiency lies at the heart of this condition. Hence, understanding thiamine is essential for understanding the etiology of WE, its prophylaxis and treatment.
The overall aim of this review is to identify the best strategies for prophylaxis and treatment of WE in regard to (a) dose of thiamine, (b) mode of administration, (c) timing of switch from one mode of administration to another, (d) duration of administration, and (e) use of magnesium along thiamine as an essential cofactor. Evidence from randomized controlled trials and other intervention studies is virtually absent. Therefore, we have to resort to basic science for proof of principle instead. In the first part of our clinical review, we explore the physiology of thiamine and the pathophysiology of thiamine deficiency in their historical context. In the second (forthcoming) part of this review, we will use the findings of the current review to make evidence-based inferences about strategies for prophylaxis and treatment of WE.
Historical perspective on thiamine
First descriptions of states compatible with thiamine deficiency in form of beriberi appeared in Japan in the 9th century. The concept of WE emerged 1000 years later. Between 1876 and 1878, the neurologist Carl Wernicke at the Charité Hospital in Berlin identified three cases of a hemorrhagic encephalitis, which now bears his name.3 The etiology remained unknown at the time; the concept of vitamins emerged only 25 years later. It then took another 30 years to identify the link between thiamine deficiency and WE (Table 1). Whether “thiamin” or “thiamine” is the correct spelling has been debated ever since the name was proposed.4 Today, both spellings are used.
Table 1.
| Year | Milestone |
|---|---|
| As early as 640 | In China and Japan, the term “kakkè” 脚気/かっけ is used to describe what later turns out to be beriberi.11 |
| 1873–1874 | The Dutch naval surgeon Fredrik Johannes van Leent ascribes high mortality from beriberi among Indian crews to their diet, and reduces the incidence of beriberi from 60% to 7% by adding vegetables, meat, bacon, and butter.12 |
| 1875 | The French surgeon and ophthalmologist Charles Jules Alphonse Gayet describes a case of diffuse encephalitis, later thought to have been due to thiamine deficiency.13 |
| 1876–1878 | The German physician Carl Wernicke describes three cases of the hemorrhagic encephalopathy without being able to clarify the etiology.3 |
| 1884 | The Japanese naval medical officer Takaki Kanehiro prevents beriberi effectively by enriching the white rice diet with meat and vegetables, assuming protein deficiency as cause.6 |
| 1887 | The Russian physician Sergei Korsakoff describes cases of a dementia with anterograde amnesia without being able to clarify the etiology.14 |
| 1886 | The Dutch government sends professor Cornelis Pekelharing and neurologist Cornelis Winkler to Jakarta (at that time Batavia, part of the Dutch East Indies) to investigate beriberi. They tentatively conclude that beriberi is of bacterial origin.15 They return home and leave their assistant Christiaan Eijkman to isolate the causative organism. |
| 1890–1897 | Eijkman conducts experiments in chicken and demonstrates that a diet of polished rice, not bacteria, is associated with a beriberi-like illness.16 He confirms the causative association of polished rice and beriberi in an observational study with Adolphe Vorderman in 279,623 prison inmates.17 He postulates the presence of an antidote for the disease in the silver skin of rice. |
| 1901 | Gerrit Grijns, successor to Eijkman in the Indonesian laboratory, concludes that there is rather a protective substance in rice, but even meat and vegetables, essential to maintain the function of the nervous system. “Partial starvation”, for example, absence of that micronutrient, which is destroyed by cooking, leads to beriberi.18 |
| 1906 | Eijkman establishes that the protective substance is water-soluble.19 |
| 1910–1912 | The Polish scientist Casimir Funk identifies an “antineuritic substance”, which he called beriberi “vitamine” (vita: life; amine: nitrogen containing compound).20 |
| 1920 | The British biochemist Jack Drummond suggests the term vitamin (without “-e” and the distinction of vitamins by name of the alphabet.21 |
| 1926 | The Dutch chemist Barend Jansen together with his colleague Willem Donath isolates pure vitamin B1.22 |
| 1929 | Eijkman, jointly with Sir Frederick Hopkins, receives the Nobel Prize for Physiology or Medicine for the discovery of vitamins.23 |
| 1936 | The American chemist Robert Williams synthesises thiamine and later licenses the production process to Merck.24 |
| 1937 | Several European countries accept the name “aneurine” for vitamin B1. Williams suggests the name “thiamin”, derived from the Greek “theion” = sulfur and amine.25 |
| 1941 | A. C. P. Campbell and Ritchie Russell at the Scottish Mental Hospital’s Laboratory in Edinburgh suggest vitamin B1 deficiency as a cause of Wernicke’s encephalopathy.10 |
| 1963 | The Bread and Flour Regulations 1963 require the addition of vitamin B1 to non-wholegrain bread in Britain.26 |
| 1974 | In collaboration with the local baker, general practitioner Max Kamien fortified the bread with thiamine, niacin and iron in a small town in the Australian outback, eliminating vitamin B deficiency signs in Aboriginal people.27 Thiamine fortification of flour becomes mandatory in Australia 1991.28 |
| 1999 | Thiamine transporter 1 (SLC19A2) is cloned by several groups.29–32 Organic cation transporter (OCT) 3 is shown to have thiamine as substrate.30 |
| 2000 | Thiamine transporter 2 (SLC19A3) is identified and characterized.33 |
| 2001 | The reduced folate carrier (SLC19A1) is shown to transport phosphorylated thiamine compounds.34 |
| 2011 | Prion proteins are shown to bind thiamine.35 |
| 2014 | The human colonic thiamine pyrophosphate transporter (SLC44A4) is identified.36
OCT 1 is recognised as thiamine transporter in the liver.37 The role of multidrug and toxin extrusion proteins (MATE) and OCT 2 in renal excretion of thiamine is discovered.38 A putative thiamine transporter (SLC35F3) is characterized.39 |
Manifestations of thiamine deficiency
Thiamine deficiency can arise from (a) reduced intake, (b) impaired absorption, (c) inability to convert thiamine to its biologically active form, or (d) excessive elimination. Alcohol use disorder accounts for about 50% cases of WE. Bariatric surgery, consuming illnesses, malabsorption syndromes, and hyperemesis are other examples of possible causes.40,41 We will discuss such causes in more detail in the second, forthcoming, part of this review.
Thiamine deficiency is often divided in two different disease entities, Wernicke-Korsakoff-syndrome and beriberi. Beriberi has been categorized further according to organ involvement into dry, wet, and gastrointestinal beriberi. As WE and beriberi overlap, it may be more accurate to use thiamine deficiency as an umbrella term, to then be specified further to reflect the respective clinical problem (Table 2).42 In this way, six different thiamine deficiency states can be described, WE, KS, dry and wet beriberi, Shoshin beriberi, and thiamine-deficiency-associated lactic acidosis. Gastrointestinal beriberi is probably a manifestation of thiamine-deficiency-mediated lactic acidosis. Thiamine deficiency occurs not only in adults; both beriberi and WE have been described in children.43,44
Table 2.
| WE | Korsakoff’s syndrome | Dry beriberi | Wet beriberi | Shoshin beriberi | Thiamine deficiency mediated lactic acidosis/gastrointestinal beriberi | |
|---|---|---|---|---|---|---|
| Organ system predominantly affected | Central nervous system | Peripheral nervous system | Cardiovascular system | Ubiquitous | ||
| Onset | Acute | Chronic | Chronic | Subacute | Acute | Acute |
| Symptoms classically described | Nystagmus, ophthalmoplegia, ataxia, confusion | Memory loss, anterograde and retrograde, amnesia, apathy | Symmetrical peripheral neuropathy with both sensory and motor impairments, mostly of the distal extremities | Hyperdynamic heart failure, oedema, ↓ peripheral vascular resistance, hypotonia, metabolic acidosis | Anorexia, nausea, vomiting and abdominal pain, Kussmaul breathing ↑ lactate |
|
| Etiology | ↓ thiamine intake ↓ thiamine absorption ↑ thiamine elimination |
As WE or as or sequel of WE without current thiamine deficiency | ↓ thiamine intake | ↓ thiamine intake + ↓ folate? |
↓ thiamine intake + ↓ folate? |
↓ thiamine intake ↓ thiamine absorption ↑ thiamine elimination |
WE, Wernicke’s encephalopathy.
Dietary thiamine
The recommended daily thiamine intake depends on age, sex, and calorie and carbohydrate intake. As a rule of thumb, thiamine intake should be at least 0.4 mg/1000 kcal. The recommended dietary thiamine intake is 1.4 mg for adult males and 1.0 mg for adult females. In pregnancy, daily thiamine requirements rise to 1.6–1.8 mg daily. In the United Kingdom (UK), the average daily intake from food sources is about 1.5 mg.51 If the daily thiamine intake falls below 0.2 mg/1000 kcal, urinary excretion becomes low. Clinical symptoms of thiamine deficiency may then emerge within 8 weeks.52 Thiamine can be found in many foodstuffs including meat, wholegrain products, fortified grain products, pulses, and some fruits. Yeast extracts contain most thiamine, and sugar is devoid of thiamine. As a general rule, unless fortified, processed foods contain less thiamine than comparable non-processed food stuffs (Table 3). Of all meats, pork has the highest thiamine content. Food preparation involving heat can lead to a 20% thiamine loss.
Table 3.
| Item | Thiamine (mg/100 g) | Item | Thiamine (mg/100 g) |
|---|---|---|---|
| Higher thiamine contenta | Lower thiamine contenta | ||
| Ham | 0.80 | Turkey slices | 0.05 |
| Pork chop, grilled, lean | 0.78 | Chicken breast, coated, baked | 0.10 |
| Bacon, streaky, fried | 0.75 | Pork sausages, grilled | traces |
| Cornflakes fortified | 0.60 | Cornflakes unfortified | traces |
| Peas, frozen | 0.26 | Peas, canned in water, reheated, drained | 0.09 |
| Bread, whole meal, average | 0.25 | Cakes from “healthy eating” ranges | 0.06 |
| Bread, white, average | 0.24 | Sponge cake, home made | 0.08 |
| Chapati, made without fat | 0.23 | Rice cakes | 0.02 |
| Peanuts, dry, roasted | 0.18 | Potatoes crisps, fried in sunflower oil | 0.09 |
| Potatoes, old, boiled | 0.18 | Potato chips, from fast food outlet | 0.07 |
| Spaghetti, dried, whole wheat, cooked | 0.11 | Spaghetti, dried, white, cooked | 0.08 |
| Rice, brown, wholegrain, cooked | 0.11 | Rice, long grain, boiled | traces |
| Lentils, red, boiled | 0.11 | Chickpeas, canned in water, reheated, drained | 0.05 |
| Oranges | 0.11 | Apples | 0.04 |
| For comparison | |||
| Yeast extract | 4.10 | Sugar | 0.00 |
Estimates based on several food samples in each category.
Thiamine compounds
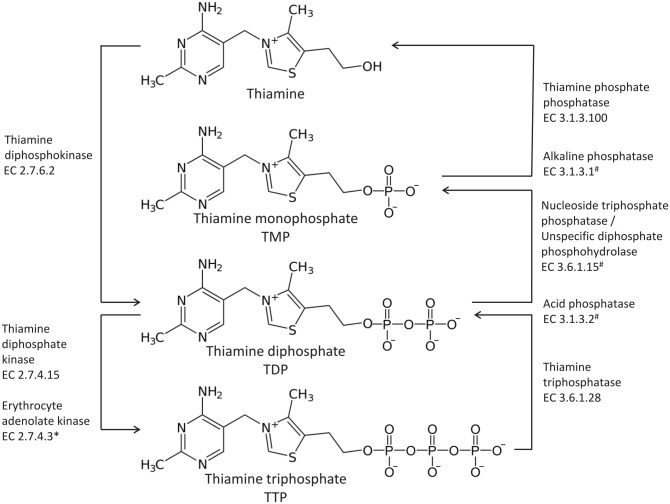
There are six known thiamine compounds, free thiamine, thiamine monophosphate (TMP), thiamine diphosphate (TDP), adenosine thiamine diphosphate (ATDP), thiamine triphosphate (TTP), and adenosine thiamine triphosphate (ATTP)5,55 (Figure 1).
Figure 1.
Thiamine compounds.
*In red blood cells.
#Thiamine phosphate esters are hydrolyzed by various phosphatases and nucleotide diphosphatases.
TDP is also referred to as thiamine pyrophosphate (TPP). In humans, free thiamine, and TMP account for 5–15% of the total thiamine. TDP is the principal biologically active form, and accounts for 80–90% of total thiamine. TDP presents in high concentrations in skeletal muscle, liver, heart, kidneys, and brain. The remaining three components, TTP, ATTP, and ATDP, account for only 1% of total thiamine in humans.55 Whole blood thiamine levels can vary significantly between populations (Table 4).
Table 4.
| n | Sex | Age range (year) | Free (nmol/l) | TMP (nmol/l) | TDP (nmol/l) | TTP (nmol/l) | Total (nmol/l) | Ratio phosphorylated thiamine (TMP + TDP + TMP)/free thiamine |
|---|---|---|---|---|---|---|---|---|
| Japana | ||||||||
| 509 | M | 21–80 | 7 (2–18) | 17 (5–47) | 124 (70–229) | 0 (0–4) | 150 (89–262) | 22 (9–58) |
| 460 | F | 18–70 | 6 (2–17) | 16 (4–60) | 114 (63–200) | 0 (0–3) | 139 (80–235) | 22 (8–58) |
| Norway 1991b | ||||||||
| 15 | M | 32–54 | 33.4 (10.4) | 10.9 (5.1) | 165.0 (40.4) | <2 | ||
| 15 | F | 23–60 | 29.6 (10.0) | 9.7 (2.3) | 121 (29.6) | <2 | ||
| The Netherlandsb | ||||||||
| 65 | 4.3 (1.9) | 4.1 (1.6) | 120 (17.5) | <4 | ||||
| Belgiumb | ||||||||
| 7 | 4 (3) | 10 (4) | 138 (33) | 13 (4) | ||||
| The Netherlandsc | ||||||||
| 98 | 115 (70–185) | |||||||
Mean (95% confidence interval).
Mean (standard deviation).
Mean (range)
F, female; M male, TDP, thiamine diphosphate; TMP, thiamine monophosphate; TTP, thiamine triphosphate.
Approximately 75% of whole blood thiamine is stored in the erythrocytes, 15% in leukocytes, and 10% in plasma.61 The compounds can be phosphorylated or dephosphorylated as required. The enzymes necessary are under genetic control (Table 5).
Table 5.
| HGNC approved gene symbol | Cytogenic location | Transporter/enzyme expressed | Reaction | Magnesium requirement | Pathologies associated with genetic change |
|---|---|---|---|---|---|
| TPK1 protein network | 7q35 | Thiamine diphosphokinase/Thiamine diphosphotransferase EC 2.7.6.2 |
Free thiamine → TDP | Divalent cations, best activation with Mg2+ | Thiamine metabolism dysfunction syndrome 5: onset of acute encephalopathic episodes in early childhood67 |
| Thiamine-diphosphate kinase EC 2.7.4.15 |
TDP → TTP | Mg2+ required by several animal organisms, but not shown for humans | |||
| AK1 | 9q34.11 | Erythrocyte adenolate kinase EC 2.7.4.3 |
TDP → TTP in red blood cells | Mg2+ required | Defect associated with hemolytic anaemia68 |
| THTPA protein network | 14q11.2 | Thiamine- triphosphatase/TTP Hydrolase EC 3.6.1.28 |
TTP → TDP | Mg2+ required | |
| ALPI | 2q27.1 1p36.12 |
Alkaline phosphatase EC 3.1.3.1 |
TDP → TMP TMP → free thiamine |
Intestine. Mg2+ used as a cofactor | |
| Multiple | Multiple | Acid phosphatase EC 3.1.3.2 |
TTP → TDP TDP → TMP |
Mg2+ used as a cofactor | |
| Not available | Not available | Nucleoside-triphosphate phosphatase/unspecific diphosphate phosphohydrolase EC 3.6.1.15 |
TDP → TMP | Mg2+ not required | |
| Not available | Not available | Thiamine phosphate (mono) phosphatase EC 3.1.3.100 |
TMP → free thiamine | Mg 2+ enhanced membrane-bound activity 1.7-fold, soluble enzyme independent of Mg 2+ (based on animal experiments) |
ALPI, alkaline phosphatase, intestinal; EC, Enzyme Commission; HGNC, HUGO Gene Nomenclature Committee; Mg2+, bivalent magnesium cation; TDP, thiamine diphosphate; THTPA, thiamine triphosphatase; TMP, thiamine monophosphate; TPK1, thiamine pyrophosphokinase; TTP, thiamine triphosphate.
Free thiamine and TMP are the thiamine transport compounds that deliver thiamine to and from the cells. TDP and TTP are the compounds that unfold the biological action. The different forms are constantly converted into each other to maintain thiamine availability.
Thiamine metabolism
Mechanism of thiamine uptake and transport through the body has crucial implications for the understanding of the etiology of WE and treatment rationale. Humans cannot synthesize thiamine but depend on two exogenous sources: dietary and bacterial thiamine.69 Transport of thiamine and conversion of various thiamine compounds into each other are under genetic control. These mechanisms require further clarification (Table 4).
Thiamine actions and implications for deficiency states
Thiamine plays a central role in energy metabolism. Thiamine is also implicated in the physiology of neurotransmission.
Energy metabolism
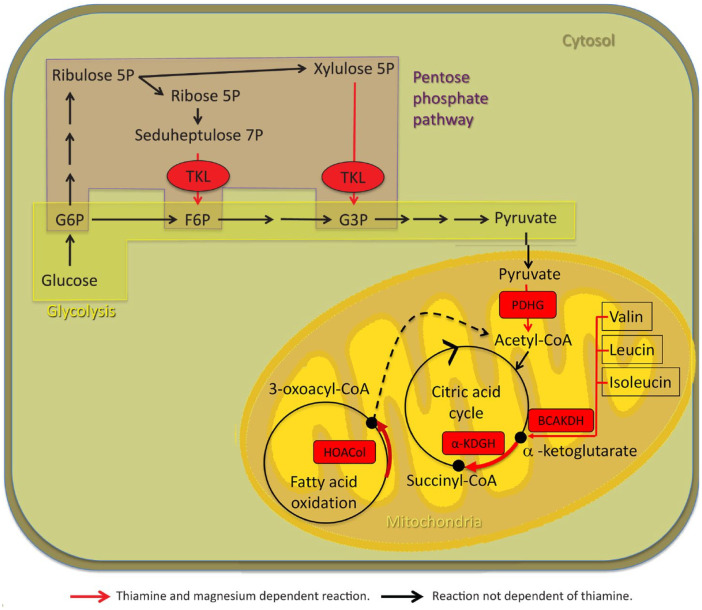
TDP acts as an important cofactor in glucose, fatty acid, and protein metabolism, as well as adenosine triphosphate (ATP) generation. Thus, TDP is a critical cofactor for transketolase (TK), pyruvate dehydrogenase (PDHG), α-ketoglutarate dehydrogenase (α-KGDH), branched chain α-keto acid dehydrogenase E1 (BCAKDH E1), and 2-hydroxyacyl CoA lyase 1 (HOACoL).5,70–76 These enzymes hold key roles in mitochondrial energy (ATP) generation in body and nerve cells, nucleic acid synthesis, carbohydrate, fatty acid metabolism, and amino acid metabolism. If these enzymes do not function properly, energy metabolism becomes impaired and oxidative stress increases. At the same time, undesirable compounds may accumulate if chemical reactions are re-routed. For instance, if α-KGDH is impaired, glutamate is produced instead of succinyl CoA. If PDHG is impaired, lactate is produced instead of acetyl CoA70 (Table 6, Figure 2).
Table 6.
| Enzyme | HGNC approved gene symbol | Cytogenic location | Subcellular location | Pathway/function | Magnesium as a further cofactor | Functions of metabolic products and resulting other pathways intermediates | Clinical implication in deficiency states |
|---|---|---|---|---|---|---|---|
| TK EC 2.2.1.1 |
TKT | 3p21.1 | Cytosol, extracellular exosome, nucleus, peroxisome, vesicles | Pentose phosphate pathway | Yes | Substrates in the glycolytic pathways Generation of d-ribose-5-P → Nucleotide synthesis → RNA/DNA synthesis NADPH as a reducing agent for fatty acid and acetylcholine synthesis, maintenance of myelin sheaths Aromatic amino acid synthesis |
↓ energy supply ↓ nucleotide synthesis ↓ fatty acid synthesis → facilitating demyelination ↑oxidative stress Amino acid imbalance |
| TK protein 1 EC 2.2.1.1 |
TKTL 1 | Xq28 | Cytosol, nucleus | As TK | Yes | May structurally alter TPP to change TPP affinity for TK78 | Unclear |
| PDHG EC 1.2.4.1 |
PDHA1 | Xp22.12 | Mitochondrion, nucleus, PDHG complex (GO:0045254)a | Glycolysis (rate-limiting co-factor) | Yes | Fatty acid, ketone bodies and acetylcholine synthesis, maintenance of myelin sheaths Generation of Acetyl CoA Generation of citrate, the first component in the TCA cycle |
↓ fatty acid synthesis → demyelination ↓ Energy (ATP) production Leigh phenotype |
| α-ketoglutarate dehydrogenase α-KGDH/OGDC EC 1.2.4.2 |
OGDH | 7p13 | Mitochondrion, nucleus, OGDC (GO: 0045252)a | TCA (citric acid) cycle | Yes | Energy production (ATP) Generation of succinyl CoA |
↑ nitric oxide and peroxidase activity →oxidative stress ↓ energy production Lactate acidosis and ↑focal extracellular glutamate → oedema → excitotoxicity → BBB permeability → neuronal death |
| BCKDH E1 subunit α EC 1.2.4.4 |
BCKDHA | 19q13.2 | Mitochondrion | Degradation of branched chain amino acids, valine, leucine and isoleucine, facilitating the oxidative decarboxylation step | Yes | Isobuturyl CoA, α-methylbuturyl CoA, isovaleryl CoA → acetyl CoA, acetoacetate, succinyl CoA → Fatty acid, ketone bodies and acetylcholine synthesis, maintenance of myelin sheaths |
↓ fatty acid synthesis → demyelination ↑ Valine, leucine and isoleucine and corresponding α-ketoacids → maple syrup urine disease |
| 2-hydroxyacyl CoA lyase 1 (HACL1) EC 4.1.-.- |
HACL1 | 3p25.1 | Cytosol, peroxisomes | Oxidation of 3-methyl branched fatty acids such as phytanic acid and 2-hydroxy fatty acids (α-oxidation) | Yes | Formate → CO2 | Peroxisome biogenesis defects → impeding the breakdown of certain nutrients including amino acids degeneration and β-oxidation of fatty acids ↑ phytanic acid → Refsum disease |
Note that this term represents a location and not a function.
ATP, adenosine triphosphate; BCKDH, branched chain α-keto acid dehydrogenase; Co A, coenzyme A; EC, Enzyme Commission numbers; GO, gene ontology; HGNC, HUGO Gene Nomenclature Committee; NADPH, nicotinamide adenine dinucleotide phosphate (hydrogenated, i.e. reduced form); ODGC, oxoglutarate dehydrogenase complex; P, phosphate; PDHG, pyruvate dehydrogenase; TCA cycle, tricarboxylic acid cycle; TK, transketolase; TPP, thiamine pyrophosphate.
Figure 2.
Thiamine- and magnesium-dependent metabolic pathways.
Red arrows, thiamine- and magnesium-dependent reactions; black arrows, reactions not dependent on thiamine.
α-KGDH, α-ketoglutarate dehydrogenase; BCAKDH, branched chain α-keto acid dehydrogenase; F6P, d-fructose 6-phosphate; G3P, d-glyceraldehyde 3-phosphate; G6P, d-glucose 6- phosphate; HOACol, 2-hydroxyacyl CoA lyase 1; PDHG, pyruvate dehydrogenase complex; TKL, transketolase.
Thiamine deficiency therefore disrupts energy metabolism and ATP production. α-KGDH and TK are two key enzymes in the pathophysiology. Decreased α-KGDH activity can develop within 4 days of thiamine deficiency.1 Decreased α-KGDH activity results in increased oxidative stress, lactate acidosis, excitotoxicity, for instance through glutamate accumulation, inflammation and disturbed blood brain barrier (BBB) permeability, cerebral edema, and, ultimately, neuronal death.71,79 Decreased TK can develop within 1 week of thiamine deficiency.1
Signal transmission
Thiamine phosphates have even non-enzymatic actions on neurotransmitters and hormones through second messengers. Whereas other B-vitamins activate the adenyl cyclase system, thiamine activates the guanyl cyclase system. cGMP is an important second messenger for peptide hormones and nitric oxide (NO), facilitating smooth muscle relaxation, mediating penile erection, regulating vascular and airway tone, peristalsis, and insulin secretion.5,75 TDP also acts as a cofactor for PDGH and facilitates acetylcholine synthesis. Thiamine even modulates choline neurotransmission non-enzymatically. This can be deducted from the observation that the metabolic thiamine antagonist oxythiamine can increase acetylcholine release.74,80 The role of TDP in the modulation of glutamate neurotransmission arises from its impact on α-KGDH. Thiamine may also regulate the activity on the astrocyte glutamate aspartate transporters.81 These transporters are under genetic control. Defects may result in insufficient clearance of glutamate and hence to an increase of interstitial glutamate. This could lead to hyperexcitability, neurotoxicity and cell death as possible consequences.81–83
Finally, thiamine may also modulate other neurotransmitters such as serotonin.1 Ultimately, much of what we know is based on in vitro and animal experiments. Our understanding of thiamine function in the brain remains limited.55,74
The journey of thiamine through the body
Thiamine transport in and out of cells: general principles
For the human organism, thiamine is an extremely valuable substance due to its central role in many metabolic processes (Table 6). As thiamine cannot be synthesised by humans, it is crucial to secure a steady supply to the cell. This involves maximizing uptake and minimizing loss. In most cells, different systems of transporters are available to facilitate uptake into the cell and into the mitochondria (Table 7). The kidneys are fine-tuned to either reabsorb or excrete thiamine depending on the actual thiamine plasma concentration. A further mechanism of facilitating thiamine uptake into the cells may involve prion proteins (PrP).84 PrP are membrane-anchored proteins, particularly abundant in neuronal cells in vertebrates. It has been shown that thiamine binds to PrP.35 The thiamine transporters are quickly saturated when extracellular thiamine concentrations exceed the physiological range. Thiamine transporter functions partly overlap. For instance, the reduced folate carrier (RFC/SLC19A1) can take over transport from Thiamine transporter 1 (ThTR-1). General cation-transporters, which are not specific to thiamine, can also transport thiamine at higher concentrations. Such include transporters belonging to the organic cation-transporter (OCT) and multidrug and toxin extrusion proteins (MATE) family (Table 7). This partial overlap of thiamine transporters may be the reason why not all thiamine transporter deficiencies are associated with pathologies.
Table 7.
| HGNC approved gene symbol | Cytogenic location | Transporter/enzyme expressed | Pathologies associated with genetic change | Comment/possible defect |
|---|---|---|---|---|
| SLC19A1 | 21q22.3 | Folate transporter (FOLT)/reduced folate carrier (RFC) | Can act as transporter for TDP (Zhao 2001) and/or TMP in some tissues when ThTR-1 fails.86 | |
| SLC19A2 | 1q24.2 | Thiamine transporter 1 (ThTR-1) | Thiamine metabolism dysfunction syndrome 1 (Thiamine-responsive megaloblastic anaemia syndrome/Rogers syndrome) | High-affinity transporter for thiamine intake.30 Association of the megaloblastic anaemia and with diabetes mellitus and hearing loss.87 Three genetic variants found in 25 patients with alcohol-associate WE, only one of these variants found in healthy controls.88 |
| SLC19A3 | 2q36.3 | Thiamine transporter 2 (ThTR-2) | Thiamine metabolism dysfunction syndrome 2 (biotin- or thiamine-responsive encephalopathy type), Leigh syndrome, Infantile lethal encephalopathy | High-affinity transporter for thiamine intake. SLC19A3 associated disorders present as neurological disorders that bear some features similar to WE.89,90 Association with seizures and brain atrophy in early infancy.67 |
| SLC22A1 | 6q25.3 | Organic cation transporter 1 (OCT 1) | Primary hepatic uptake transporter of thiamine.91 Intestinal uptake of thiamine?92,93 Expressed on luminal side of renal tubular cells.94 | |
| SLC22A2 | 6q25.3 | Organic cation transporter 2 (OCT 2) | Predominantly expressed in kidney, but even neurons95,96 and microvessels of the blood-brain barrier.97 Renal tubular secretion/reabsorption of thiamine.38 Expressed on basolateral side of renal tubular cells.94 | |
| SLC22A3 | 6q25.3 | Organic cation transporter 3 (OCT 3) | Intestinal uptake of thiamine.92,93 OCT3 in human liver cells showed a higher affinity but lower capacity compared with OCT198 but a lower affinity than ThTR. Expressed in microvessels of the blood-brain barrier97 and various brain regions.99,100 Expressed on basolateral side of renal tubular cells.101 | |
| SLC25A19 | 17q25.1 | Mitochondrial TDP carrier. | Leigh syndrome, Thiamine metabolism dysfunction syndrome 3 (microcephaly Amish type), Thiamine metabolism dysfunction syndrome 4 (bilateral striatal degeneration and progressive polyneuropathy type).102 |
Mediates thiamine uptake into mitochondria. Believed to be important for brain development and interfere with α-KGDH activity. |
| SLC35F3 | 1q42.2 | Putative thiamine transporter | Association with arterial hypertension.39 | |
| SLC44A4 | 6p21.33 | Choline transporter like protein | Autosomal dominant deafness | Acts also as a TDP transporter (human TDP transporter) in the colon and facilitate uptake of microbiota-generated thiamine. Thiamine created by colon microbiota exists in form to TDP.103,104 Autosomal dominant deafness has been attributed to defects in the choline transporter function. |
| SLC47A1 | 17p11.2 | MATE1 | Expressed on luminal side of renal tubular cells.105 Renal tubular secretion of thiamine.38 Expressed in microvessels of the blood-brain barrier.97 | |
| SLC47A2 | 17p11.2 | MATE2-K | Specifically expressed in the kidney106 but even in microvessels of the blood-brain barrier.97 Renal tubular secretion of thiamine.38 |
HGNC, HUGO Gene Nomenclature Committee; MATE1, multidrug and toxin extrusion protein; MATE2-K, kidney-specific multidrug and toxin extrusion protein; SLC, solute carrier family [number] family [letter, number] TKT; TDP, thiamine diphosphate; TMP, thiamine monophosphate; TTP, thiamine triphosphate.
From food to enterocyte
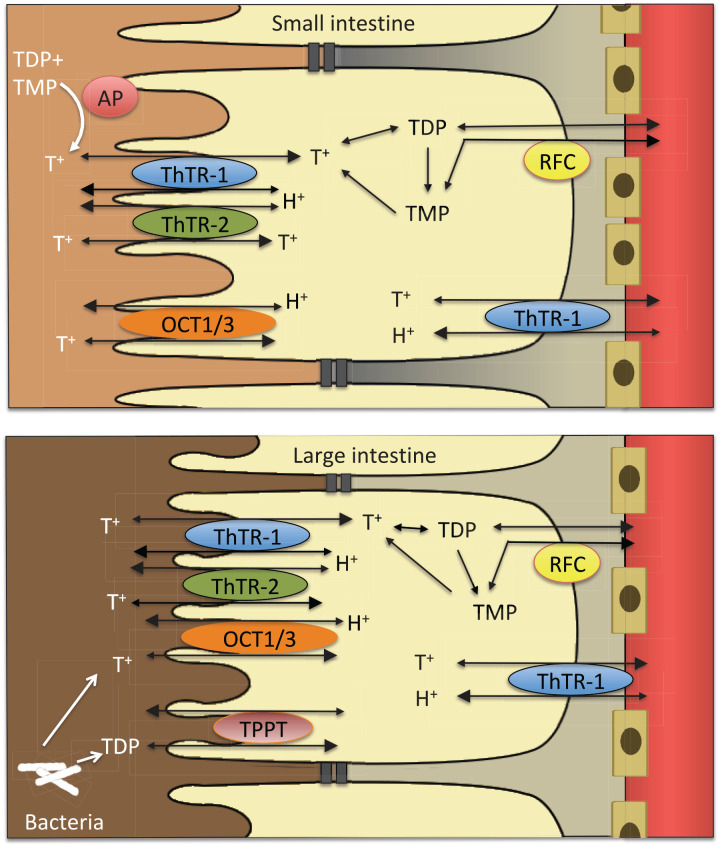
Dietary thiamine exists mainly in phosphorylated forms. Exogenously acquired thiamine is thought to be first de-phosphorylated to free thiamine by gastrointestinal phosphatases.107 Free thiamine is then absorbed in the small intestine, preferentially in the jejunum. There are two transport mechanisms, active and passive (Figure 3). The active transport system involves ThTR-1 and ThTR-2.108 The passive transport is proportional to the thiamine concentration in the intestinal lumen.109 Where epithelial and endothelial linings are made up by tight junctions, only small molecules can pass through by simple passive diffusion. As thiamine is a relatively large molecule, its passive transport most likely occurs in the form of facilitated diffusion, along an electrochemical gradient via protein channels.110 This suggests that, within limits, passive thiamine transport is a non-saturable process.109,111 Active and passive thiamine transport may operate simultaneously.112 The active transport follows a Michaelis–Menten kinetic and seems to be saturated at a concentration of 2-2.5 µmol/l.113–115 As shown in lines of heterogenous human epithelial colorectal adenocarcinoma cells (Caco-2 cells), active transport has evolved to maximize thiamine uptake in scenarios of low availability.116 Animal experiments have shown that thiamine uptake in the small intestine can be increased dramatically during thiamine deficiency. However, during chronic alcohol use, thiamine uptake is reduced. In the presence of alcohol, expression of both ThTR-1 and ThTR-2 diminished significantly.117
Figure 3.
From food to blood.
OCT, organic cation transporter; RFC, reduced folate carrier; T+, free thiamine; TDP, thiamine diphosphate; ThTR, thiamine transporter; TMP, thiamine monophosphate; TTP, thiamine triphosphate.
An alternative source of thiamine is bacterial. Bacteria flora in the large intestine may synthesize both free thiamine and TDP.36,103,104 Previously, it has been assumed that this colonic thiamine cannot be used. Now, based on animal cell culture experiments, it has been suggested that microbiota generated TDP is taken up into the colonocytes by a human TDP transporter. At present it remains unclear how much colonic thiamine contributes to physiological functions of TDP across the body (Figure 3).36,69,104
From enterocyte to blood
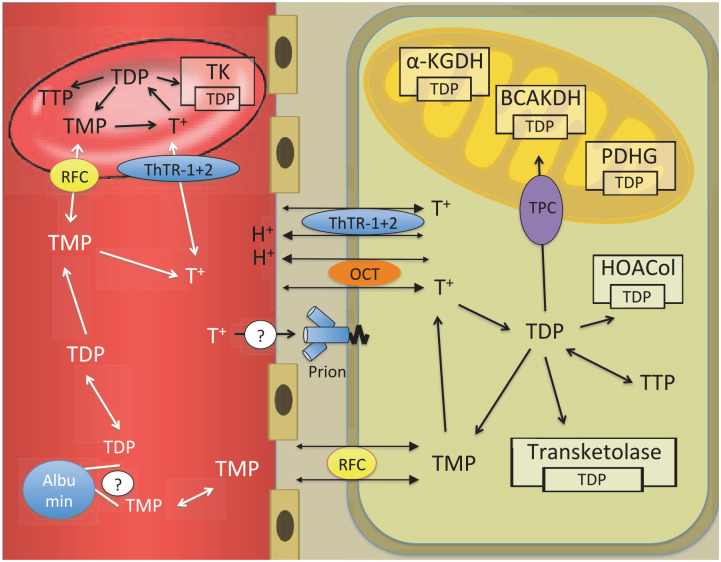
In the enterocyte, as in any other cell, free thiamine can be phosphorylated directly to TDP. The enterocyte uses a part of this TDP for its own metabolic needs. The rest is broken down to TMP and free thiamine. Free thiamine and TMP are then transported out of the cell into the plasma.64,112 The release of thiamine into plasma has been ascribed to ThTR-1,108 but other transporters may be involved. As an organic cation, thiamine can be transported by organic cation/antiport systems into the enterocyte.107 From there, organic cation transporter proteins (OCT) 1 and 3 can transport thiamine into the blood.92,112 OCT 1 and 3 can take up thiamine in concentration ranges from 10 to 500 µmol/l (Figure 4).112 This may be the mechanism behind the passive thiamine transport at high concentrations. However, it remains currently unclear how far this extracellular “flooding” of cells with free thiamine leads to higher intracellular levels of pharmacologically active phosphorylated thiamine forms. Alcohol can impair the active intestinal transport mechanism.69,117 However, alcohol does not seem to affect passive uptake of thiamine at high doses.114,118
Figure 4.
From blood to cell.
α-KGDH, α-ketoglutarate dehydrogenase; BCAKDH, branched chain α-keto acid dehydrogenase; HOACol, 2-hydroxyacyl CoA lyase 1; OCT, organic cation transporter; PDHG, pyruvate dehydrogenase; RFC, reduced folate carrier; T+, free thiamine; TDP, thiamine diphosphate; ThTR, thiamine transporter; TK, thiamine kinase; TMP, thiamine monophosphate; TPC, mitochondrial thiamine pyrophosphate carrier; TTP, thiamine triphosphate.
From blood to tissue
Once in the plasma, free thiamine and TMP are distributed throughout the body. Phosphorylated thiamine is bound partly to plasma proteins.119 Both free thiamine and TMP can enter the cell. Free thiamine in the cationic form crosses the cell membrane with help of ThTR-1.108 The mechanism by which TMP crosses the cell membrane is not well described. RFC (SLC19A1) has been implicated (Table 4).86,120 Once free thiamine enters the cell, it is phosphorylated to TDP directly without generation of TMP as an intermediary. The responsible enzyme is thiamine diphosphokinase, which catalyzes the following reaction: free thiamine + ATP → TDP + AMP. A small part can be phosphorylated further to TTP. TTP can be dephosphorylated to TDP, TDP to TMP, and TMP to free thiamine (Figure 4).55,64
In a study of thiamine kinetics in six healthy volunteers,121 large increases in thiamine blood concentration after intravenous (i.v.) injection reflected increases in extracellular plasma concentrations. Only a little free thiamine was stored in the blood cells. Further, only a small amount was phosphorylated to TDP. At the same time, thiamine was eliminated seven times faster from the plasma than blood cells. These findings hint at thiamine phosphorylation rather than thiamine transport being the rate-limiting factor for TDP availability.
From blood to brain
Data from small post-mortem studies indicate brain thiamine concentrations between 25.1 and 32.5–36.2 pmol/mg protein.58,122 These are lower concentrations than reported in rodents or other primates. TDP is by far the most prominent compound. The above concentrations translate to 283 nmol thiamine/100 g fresh brain tissue.55 Standard textbooks report “thiamine” concentrations in the brain between 0.14 mg and 0.44 mg/100 g fresh brain tissue.123,124 However, these data are not referenced. For an average brain of 1300 g, this would amount to 1.82–5.72 g or 5.6–17.5 µmol. About 2% of all available thiamine is transported into the brain.117 As any other solute, thiamine cannot freely enter the brain. Instead, all solutes need to cross either the BBB or the blood-cerebrospinal fluid barrier (BCSFB).120 The BBB is the barrier between cerebral blood vessels and brain tissues. The BCSFB is the barrier between the blood and the CSF on the one hand and the CSF and brain tissues on the other. The choroid plexus forms the interface between the blood/CSF component. The arachnoid membrane forms the interface between the CSF/brain tissue component.110,125 Within the brain, there is no barrier between the extracellular space and the CSF.110,126 Substance transport over the BCSFB is not thought to contribute relevantly to the metabolic needs of the brain.127
The anatomical structure of the BBB with tight cellular junctions limits the possibility of thiamine passing into the brain by simple passive diffusion. Indeed, has it been suggested that, “normally,” less than 10% of B vitamins is transferred into the brain by simple passive diffusion.128 How exactly thiamine enters the brain remains unclear. The BBB consists of vascular endothelium, pericytes, and astrocyte end-feet. The space between endothelial cells is rendered impermeable by tight junctions. Therefore, contrary to permeable endothelial cells in other parts of the body, substances cannot by-pass the endothelial cells in the brain. Instead, they have to pass through the endothelial cells. Like neurons and glial cells, BBB-forming cells have specific solute carriers.129 Free thiamine seems to enter the brain via active transport, most likely involving ThTR-2. The transport system is half-saturated at normal plasma concentration of 0.1–0.3 µmol/l. Lack of the other major thiamine transporter, ThTR-1, leads to thiamine-responsive megaloblastic anaemia syndrome but not to WE. Therefore, ThTR-1 may not be essential for thiamine uptake into the brain.120 Parallel to the active carrier-mediated process, a non-saturable process also exists at higher concentrations.111,130,131 Thiamine may also be taken up passively via facilitated diffusion.120,132
TMP may possibly enter the brain via active transport involving the reduced folate carrier.120 This transport system is half-saturated at normal plasma concentration of 25 µmol/l. Overall, there are about 10 µmol thiamine in the brain. The rate of thiamine turnover in the brain is about 60–100% per day. This suggests that thiamine homeostasis in the brain is managed tightly to render a steady state between thiamine entering and leaving the brain.120 Thus, administration of large amounts of thiamine for medicinal purposes may not necessarily lead to increased thiamine concentrations in the brain.56,110 An animal experiment reported in 1968 supports this assumption. Rats fed a thiamine-deficient diet developed overt encephalopathy, in which thiamine brain concentration fell to less that 20% of normal. Increasing thiamine to only 26% of normal concentration reversed symptoms to an essentially normal neurologic state.133 In humans, reversibility of symptoms is variable. Prompt treatment can reverse symptoms as long permanent damage and cell death has not occurred.2,71
Body stores
The body can store about 30 mg of thiamine.70,74 Again, most of this can be expected to be intracellular in the form of TDP. The concentrations are highest in heart, skin, kidneys, adipose tissue, lung, and colon.134 It remains unclear how long these stores last in circumstances of thiamine deficiency. One study investigated the urinary excretion of thiamine in eight young men. These consumed a 2800 kcal diet that provided 400 g of carbohydrates and 0.11–0.18 mg thiamine a day. This corresponded to 10% of the recommended nutritional intake (RNI) of thiamine. Urine thiamine decreased to <50 µg a day within 6 days and became undetectable on the 18th day of thiamine depletion.135 Another study of three volunteers found a half-time excretion for thiamine of 9.5, 13, and 18.5 days.136 These studies suggest that thiamine depletion may occur within approximately 2-3 weeks of thiamine deficiency.
Deactivation and metabolism
Thiamine can be broken down by two thiaminases: type I and II. These cleave thiamine into its pyrimidine and thiazole moieties.137 In humans, thiaminase activity is negligible under normal conditions. However, thiamine deficiency can occur when thiaminase-containing foods are ingested excessively or not processed properly. Thiaminase I is found in fish, shellfish, ferns, and some bacteria. Thiaminase II is found in some bacteria.138 Thiaminases are usually heat-labile; they can be destroyed through cooking.70 Thiaminase I in nardoo (Marsilea drummondii), an Australian fern can, however, withstand high temperatures. One historical account of beriberi due to thiaminase poisoning stems from the Burks and Wills expedition to cross Australia from coast to coast from 1860 to 1861. Of the four participating European men, only one survived. During their journey, the men began eating nardoo-based flour, preparing it the European way instead of the Aborigine way. It is speculated that they did not soak the fern sufficiently long in water to diminish the activity of nardoo thiaminase 1.139
Polyhydroxyphenols, caffeic acid, phenols, flavonoids, and tannins, can also serve as anti-thiamine factors. Such polyhydroxyphenols are found, for instance, in coffee, tea, betel nuts, blueberries, blackcurrants, Brussels sprouts, and red cabbage. They destroy thiamine by an oxidative process transforming thiamine to non-absorbable thiamine disulfide.140 Polyhydroxyphenols are heat-stable components and cannot be destroyed through cooking.70 Therefore, excessive ingestion can lead to thiamine deficiency.141
Recycling and elimination in the kidney
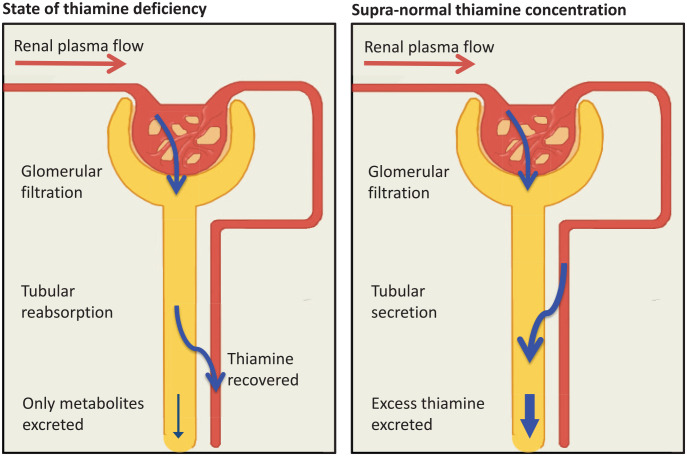
In humans, a multitude of metabolites have been identified by radioactive labelling of the pyrimidine or thiazole moiety of thiamine.136 Studies in rats demonstrated up to 22 different thiamine metabolites identifiable in the urine.142–144 The kidneys can largely adapt their handling of free thiamine to the current plasma concentration (Figure 5). Therefore, free thiamine is eliminated or reabsorbed renally, depending on thiamine status. However, thiamine metabolites cannot be reabsorbed in the kidney. Paradoxically, the amount of metabolites excreted is not decreased in a state of thiamine deficiency.135
Figure 5.
From blood to urine or urine to blood.
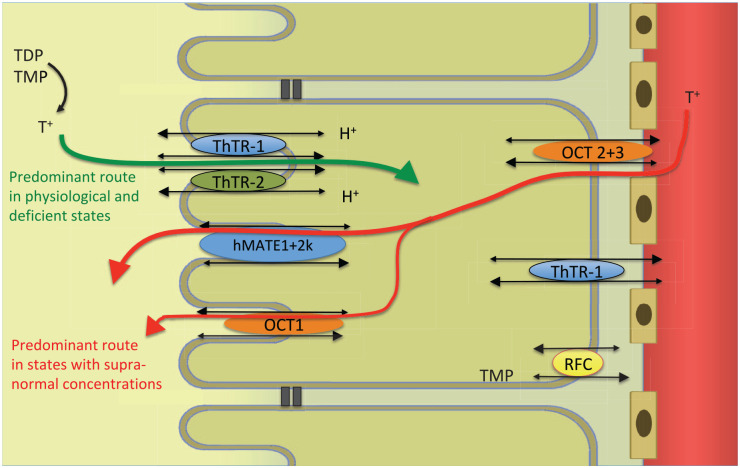
In the glomerulus, thiamine is filtrated freely, like any other small solute. Glomerularly filtrated thiamine is then processed in the proximal tubule. Thiamine stored in blood cells or bound to protein cannot be filtrated. Data on plasma protein binding is again conflicting. Thom et al. found that, under physiological conditions, up to 30% of plasma thiamine may be bound to albumin (10% TMP, 20% TDP).119 These thiamine compounds may bind to albumin via the phosphate moiety of the molecule. At thiamine concentrations over 119.5 µmol/l, albumin binding decreases to 2%. Weber et al., however, suggested that plasma thiamine was not bound to protein.145
Under physiological conditions, up to a concentration of 200 nmol/l, thiamine is reabsorbed to minimize excretion. Phosphorylated thiamine, mostly in the form of TMP, is dephosphorylated to free thiamine in the tubule.145 ThTR-1, ThTR-2, and OCT1, expressed in the brush border membrane mediate thiamine uptake from urine into the tubular cells. ThTR-1 as well as OCT2 and 3 in the basolateral membrane mediate thiamine uptake from tubular cells into the blood. ThTR transporters have a much higher affinity than OCT. They are also saturated at lower concentrations. Principally, all transporters are bidirectional.38,85,146–148
In thiamine deficient states, thiamine transporters are upregulated.149 Ziporin et al. showed that urinary thiamine excretion could decrease to undetectable levels.135
In states of thiamine excess, for example, achieved through pharmacological dosing, thiamine is eliminated completely by the kidneys.145 Elimination is increased by switching from reabsorption to active secretion. In that case, thiamine that has not been filtrated in the glomerulus is excreted via the renal tubular cells. Thiamine directly inhibits ThTR-1 mediated uptake.30 In such circumstances, the ThTR mediated pathway may be reversed from thiamine uptake to secretion.147 Additionally, the kidneys can eliminate thiamine through two types of cation transporters: OCTs and multidrug and toxin extrusion proteins (MATEs). Thiamine enters the renal tubular cells from the blood through OCT 1 and 2 located in the basolateral membrane.148 From there, thiamine is excreted across the brush border membrane into the urine through MATE1 and MATE2-k.38,150–152 This mechanism can lead to complete plasma thiamine elimination from all the blood passing through the kidneys (renal blood flow). The elimination amounts to five times the glomerular filtration rate (Figure 6).145
Figure 6.
Renal regulation of thiamine.
hMATE, human multidrug and toxin extrusion protein; OCT, organic cation transporter; RFC, reduced folate carrier; T+, free thiamine; TDP, thiamine diphosphate; ThTR, thiamine transporter; TMP, thiamine monophosphate.
Magnesium as cofactor for thiamine
Magnesium is an alkaline earth metal with an atomic weight of 24.31. In the body, magnesium occurs mostly ionized as the bivalent cation Mg2+. Mg2+ is the fourth most abundant cation in the body after sodium, potassium, and calcium, and the second most abundant intracellular cation after potassium.153–155 Mg2+ is a cofactor to more than 600 enzymatic reactions.156 Mg2+ is implicated in the energy metabolism of macronutrients, oxidative phosphorylation, protein and nucleic acid synthesis, neuro-muscular signal conduction, and regulation of cell membrane permeability.154,155 Despite its crucial contribution to physiology, magnesium is relatively under-researched compared with other nutrients, such iron or calcium. Therefore, clinicians may be much less vigilant to possible states of magnesium deficiency.156 Part of the problem is that magnesium deficiency is difficult to assess by a simple blood test; more than 99% magnesium is intracellular.154,157
Significance of magnesium for thiamine function
A significance of magnesium for alcohol-associated WE was first suggested in 1964.158 Along with thiamine, magnesium has since turned out to be an essential cofactor to several key metabolic enzymes (Table 5) that govern glucose, fatty acid, and protein metabolism as well as ATP generation. Magnesium is also required as cofactor for thiamine transport and conversion of the various thiamine compounds into each other (Table 6). Without magnesium, thiamine cannot function properly. This implies that magnesium deficiency can impair thiamine activity. But we do not know how substantial magnesium deficiency has to become to trigger clinical symptoms of thiamine deficiency (cf. magnesium deficiency below).
Dietary magnesium
Recommendations for daily intake depend on age and sex. They vary somewhat internationally. In the UK, the recommended daily magnesium intake is 300 mg for adult males and 270 mg for adult females.51 In the United States (US), the recommended daily intake is slightly higher, 420 mg for men and 320 mg for women aged between 31 and 50 years. No extra magnesium is required during pregnancy. However, an additional 50 mg per day is required for women who breastfeed to compensate for magnesium losses in breast milk.53 Magnesium is found in nearly all foods. As a central component of chlorophyll, magnesium is particularly common in leafy vegetables. Whole grain cereals, nuts, and yeast extracts are also rich sources of magnesium.51,53,157
Magnesium metabolism
Mg2+ absorption and excretion depend on nutritional uptake, intestinal absorption, and renal capacity for reabsorption. Magnesium can be taken up anywhere across the intestine. It has been suggested that the duodenum takes up 11% of all the magnesium absorbed, the jejunum 22%, the ilium 56%, and the colon 11%.157 Of all absorbed Mg2+, between 20% and 70% are excreted again in the feces.156 The remainder is distributed throughout the body. About 50–60% are stored in the bone, 20–30% in muscles and 20–25% in other organs. An average adult stores about 24 g Mg2+ in the body. Only 0.8% of magnesium is found in the blood, 0.3% in serum, and 0.5% in erythrocytes. There is a constant exchange between Mg2+ stored and Mg2+ in the blood. The kidney can filtrate about 10% of bodily Mg2+, corresponding to a renal filtration rate of 2.4 g/day. Between 5% and 70% can, however, be reabsorbed and re-entered into the redistribution cycle.153,156,157
Magnesium deficiency
Magnesium deficiency may manifest itself in neurological symptoms, such as neuromuscular hyperexcitability and weakness. Magnesium deficiency is also associated with electrocardiographic (ECG) changes and arrhythmias, hypoparathyroidism, and vitamin D deficiency. There are also biochemical changes, including hypocalcaemia, hypokalaemia, and metabolic alkalosis.159,160 However, unless severe, symptoms of magnesium deficiency can be difficult to spot. Normal magnesium serum concentration lies in a range from 0.7 to 1.0 mmol/l. Magnesium deficiency may occur when the serum magnesium concentration falls below 0.66 mmol/l. Yet, clinical symptoms may become observable only at levels below 0.5 mmol/l.161 As magnesium is over 99% intracellular, a normal magnesium serum concentration does not exclude deficiency. Such normo-magnesemic magnesium deficiency may occur in patients with chronic harmful use of alcohol.162
Magnesium deficiency can occur in three contexts: (a) decreased uptake or absorption, (b) increased gastrointestinal or renal elimination, or (c) shift from the extracellular to the intracellular space.163,164 Reduced magnesium uptake can occur in the context of total nutritional deficiency. It can also occur with one-sided nutrition with low magnesium content. Inflammatory bowel diseases and treatment with proton pump inhibitors such as omeprazole are associated with reduced magnesium absorption. Increased gastrointestinal magnesium elimination occurs for instance in the context of excessive vomiting or laxative abuse. Renal magnesium wasting can also occur in the context of treatment with loop or thiazide diuretics, cisplatin, amphotericin, aminoglycosides, foscarnet, cyclosporine, and tacrolimus.155,164 Increased renal elimination occurs when tubular reabsorption becomes impaired, for instance in the context of osmotic diuresis or some renal diseases. Such include interstitial nephritis, Gitelman, Bartter, and Fanconi syndromes. Shifts from extracellular to intracellular space occur in settings of refeeding, treatment of diabetic ketoacidosis or other metabolic acidosis, hungry bone syndrome or pancreatitis. Alcohol dependency is associated with several risk factors for magnesium deficiency, including poor nutrition, gastrointestinal problems associated with proton pump inhibitor use and reduced absorption, increased diuresis, excessive urinary excretion, and vomiting.160,165
Therapeutic implications
Thiamine is a key factor in human energy metabolism and an important contributor to neurotransmitter functions. These fundamental roles explain why thiamine deficiency can lead to devastating consequences such as WE. Thiamine stores in humans are limited, and thiamine homeostasis depends on external thiamine sources. For many thiamine-mediated reactions, even magnesium is required as a cofactor. In spite of the enormous significance of thiamine and its dependency on magnesium, surprisingly few studies have examined strategies for prophylaxis and treatment of deficiency states. In the absence of clinical evidence, a thorough understanding of the basic science behind thiamine can assist in formulating treatment strategies, with a rationale resting on pharmacodynamic and pharmacokinetic concepts. In this first part of this review, we have explored the basic science behind thiamine. In the forthcoming second part of this review, we will examine current guidelines for prophylaxis and treatment of WE in light of our understanding of the basic science behind thiamine.
Acknowledgments
We thank Leoni Mannchen for her assistance with the illustrations of the figures and Felix Filson for his assistance with the reference management.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Psychiatry, Sunderby Hospital, Region Norrbotten, Sweden.
Declaration of conflicting interests: Michael Ott has been a scientific advisory board member of Astra Zeneca Sweden, Ursula Werneke has received funding for educational activities on behalf of Norrbotten Region (Masterclass Psychiatry Programme 2014–2018 and EAPM 2016, Luleå, Sweden): Astra Zeneca, Eli Lilly, Janssen, Novartis, Otsuka/Lundbeck, Servier, Shire and Sunovion.
ORCID iDs: Michael Ott  https://orcid.org/0000-0003-2393-9750
https://orcid.org/0000-0003-2393-9750
Ursula Werneke  https://orcid.org/0000-0002-5023-3254
https://orcid.org/0000-0002-5023-3254
Contributor Information
Michael Ott, Department of Public Health and Clinical Medicine, Division of Medicine, Umeå University, Umeå, Sweden.
Ursula Werneke, Department of Clinical Sciences, Division of Psychiatry, Sunderby Research Unit, Umeå University, Umeå, Sweden.
References
- 1. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol 2007; 6: 442–455. [DOI] [PubMed] [Google Scholar]
- 2. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser 1971; 7: 1–206. [PubMed] [Google Scholar]
- 3. Wernicke C. Lehrbuch der Gehirnkrankheiten für Aertzte und Studirende. Vol. 2, Kassel & Berlin: Theodor Fisher, 1881, pp 229–246. [Google Scholar]
- 4. Williams RR. Thiamin or Thiamine? Science 1949; 109: 525. [DOI] [PubMed] [Google Scholar]
- 5. Lonsdale D. Thiamin. Adv Food Nutr Res 2018; 83: 1–56. [DOI] [PubMed] [Google Scholar]
- 6. Sugiyama Y, Seita A. Kanehiro Takaki and the control of beriberi in the Japanese Navy. J R Soc Med 2013; 106: 332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carpenter KJ. The discovery of thiamin. Ann Nutr Metab 2012; 61: 219–223. [DOI] [PubMed] [Google Scholar]
- 8. Piro A, Tagarelli G, Lagonia P, et al. Casimir Funk: his discovery of the vitamins and their deficiency disorders. Ann Nutr Metab 2010; 57: 85–88. [DOI] [PubMed] [Google Scholar]
- 9. Sneader W. Drug discovery - a history. Chichester, UK: John Wiley & Sons Ltd, 2005. [Google Scholar]
- 10. Campbell ACP, Russel WR. Wernicke’s Encephalopathy: the clinical features and their probable relationship to vitamin B deficiency. QJM 1941; 10: 41–64. [Google Scholar]
- 11. Scheube B. Die Beriberi-Krankheit: eine geographisch-medicinische Studie. Jena: Gustav Fischer, 1894. [Google Scholar]
- 12. van Leent FJ. Mededeelingen over Beri-Beri. In: Geneeskundig Tijdschrift voor Nederlandsch-Indië 1880; Vol. 20: 271–310. [Google Scholar]
- 13. Gayet C. Affection encéphalique (encéphalite diffuse probable) localisée aux étages supérieurs des pédoncles cérébraux et aux couches optiques, ainsi qu’ au plancher du quatrième ventricule et aux parois latérales du troisième. Archives de physiologie normale et pathologique 1875; 2: 341–351. [Google Scholar]
- 14. Korsakow S. Ueber eine besondere Form psychischer Störung, combinirt mit multipler Neuritis. Archiv für Psychiatrie und Nervenkrankheiten 1890; 21: 669–704. [Google Scholar]
- 15. Pekelharing CA, Winkler C. Mittheilung über die Beri-Beri. Deutsche Medicinische Wochenschrift 1887; 13: 845–848. [Google Scholar]
- 16. Eijkman C. Eine Beri Beri-ähnliche Krankheit der Hühner. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin 1897; 148: 523–532. [Google Scholar]
- 17. Eijkman C. Ein Versuch zur Bekämpfung der Beri-Beri. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin 1897; 149: 187–194. [Google Scholar]
- 18. Grijns DG. Over polyneuritis gallinarum. Geneeskundig Tijdschrift voor Nederlandsch-Indië 1901; Vol. 41: 3–110. [Google Scholar]
- 19. Eykman C. Über Ernährungspolyneuritis. Archiv für Hygiene 1906; 58: 150–170. [Google Scholar]
- 20. Funk C. The journal of state medicine. Volume XX: 341-368, 1912. The etiology of the deficiency diseases, beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra. Nutr Rev 1975; 33: 176–177. [DOI] [PubMed] [Google Scholar]
- 21. Drummond JC. The nomenclature of the so-called accessory food factors (vitamins). Biochem J 1920; 14: 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jansen BCP, Donath WF. Over het isoleeren van antiberi-beri-vitamine (Voorloopige medeeling). Geneeskundig Tijdschrift voor Nederlandsch-Indië 1926; Vol. 66: 572–574. [Google Scholar]
- 23. NobelPrize.org. The nobel prize in physiology or medicine 1929. Nobel Media AB, 2020. [Google Scholar]
- 24. Williams RR, Cline JK. Synthesis of vitamin B1. J Am Chem Soc 1936; 58: 1504–1505. [Google Scholar]
- 25. Williams RJ, Truesdail JH, Weinstock HH, et al. Pantothenic acid. II. Its concentration and purification from liver. J Am Chem Soc 1938; 60: 2719–2723. [DOI] [PubMed] [Google Scholar]
- 26. The Minister of agriculture, fisheries and food, the Minister of health. Food and drugs composition and labelling ETC. The bread and flour regulations 1963. Statutory instruments, 1963, No 1435. Legislation.gov.uk, https://www.legislation.gov.uk/uksi/1963/1435/pdfs/uksi_19631435_en.pdf (accessed 21 October 2020).
- 27. Kamien M, Woodhill JM, Nobile S, et al. Nutrition in the Australian aborigines—effects of the fortification of white flour. Aust N Z J Med 1975; 5: 123–133. [DOI] [PubMed] [Google Scholar]
- 28. Drew LR, Truswell AS. Wernicke’s encephalopathy and thiamine fortification of food: time for a new direction? Med J Aust 1998; 168: 534–535. [DOI] [PubMed] [Google Scholar]
- 29. Diaz GA, Banikazemi M, Oishi K, et al. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anaemia syndrome. Nat Genet 1999; 22: 309–312. [DOI] [PubMed] [Google Scholar]
- 30. Dutta B, Huang W, Molero M, et al. Cloning of the human thiamine transporter, a member of the folate transporter family. J Biol Chem 1999; 274: 31925–31929. [DOI] [PubMed] [Google Scholar]
- 31. Fleming JC, Tartaglini E, Steinkamp MP, et al. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet 1999; 22: 305–308. [DOI] [PubMed] [Google Scholar]
- 32. Labay V, Raz T, Baron D, et al. Mutations in SLC19A2 cause thiamine-responsive megaloblastic anaemia associated with diabetes mellitus and deafness. Nat Genet 1999; 22: 300–304. [DOI] [PubMed] [Google Scholar]
- 33. Eudy JD, Spiegelstein O, Barber RC, et al. Identification and characterization of the human and mouse SLC19A3 gene: a novel member of the reduced folate family of micronutrient transporter genes. Mol Genet Metab 2000; 71: 581–590. [DOI] [PubMed] [Google Scholar]
- 34. Zhao R, Gao F, Wang Y, et al. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. J Biol Chem 2001; 276: 1114–1118. [DOI] [PubMed] [Google Scholar]
- 35. Perez-Pineiro R, Bjorndahl TC, Berjanskii MV, et al. The prion protein binds thiamine. Febs J 2011; 278: 4002–4014. [DOI] [PubMed] [Google Scholar]
- 36. Nabokina SM, Inoue K, Subramanian VS, et al. Molecular identification and functional characterization of the human colonic thiamine pyrophosphate transporter. J Biol Chem 2014; 289: 4405–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen L, Shu Y, Liang X, et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc Natl Acad Sci U S A 2014; 111: 9983–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kato K, Mori H, Kito T, et al. Investigation of endogenous compounds for assessing the drug interactions in the urinary excretion involving multidrug and toxin extrusion proteins. Pharm Res 2014; 31: 136–147. [DOI] [PubMed] [Google Scholar]
- 39. Zhang K, Huentelman MJ, Rao F, et al. Genetic implication of a novel thiamine transporter in human hypertension. J Am Coll Cardiol 2014; 63: 1542–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ajob L, Brännstrom I, Ott M, et al. ABC om Wernickes encefalopati. Läkartidningen 2017; 114: ELZT. [PubMed] [Google Scholar]
- 41. Ota Y, Capizzano AA, Moritani T, et al. Comprehensive review of Wernicke encephalopathy: pathophysiology, clinical symptoms and imaging findings. JPN J Radiol 2020; 38: 809–820. [DOI] [PubMed] [Google Scholar]
- 42. Johnson CR, Fischer PR, Thacher TD, et al. Thiamin deficiency in low- and middle-income countries: disorders, prevalences, previous interventions and current recommendations. Nutr Health 2019; 25: 127–151. [DOI] [PubMed] [Google Scholar]
- 43. Fattal-Valevski A, Kesler A, Sela BA, et al. Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics 2005; 115: e233–e238. [DOI] [PubMed] [Google Scholar]
- 44. Qureshi UA, Wani NA, Ahmad K, et al. Infantile Wernicke’s encephalopathy. Arch Dis Child 2015; 100: 648. [DOI] [PubMed] [Google Scholar]
- 45. So YT. Wernicke encephalopathy. In: Wilterdink JL. (ed.) UpToDate, https://www.uptodate.com/contents/wernicke-encephalopathy?search=wernicke%20encephalopathy (2020, accessed 7 August 2020).
- 46. Huertas-González N, Hernando-Requejo V, Luciano-García Z, et al. Wernicke’s encephalopathy, wet beriberi, and polyneuropathy in a patient with folate and thiamine deficiency related to gastric phytobezoar. Case Rep Neurol Med 2015; 2015: 624807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep 2018; 2018: bcr2018224841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med 2016; 31: 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Donnino M. Gastrointestinal beriberi: a previously unrecognized syndrome. Ann Intern Med 2004; 141: 898–899. [DOI] [PubMed] [Google Scholar]
- 50. Dean RK, Subedi R, Gill D, et al. Consideration of alternative causes of lactic acidosis: thiamine deficiency in malignancy. Am J Emerg Med 2017; 35: 1214. e1215–1214e1216. [DOI] [PubMed] [Google Scholar]
- 51. Expert Group on Vitamins and Minerals. Safe upper levels for vitamins and minerals. London, UK: Food Standard Agency, 2003. [Google Scholar]
- 52. World Health Organization and United Nations High Comissioner for Refugees. Thiamine deficiency and its prevention and control in major emergencies. WHO/NHD/99.13. World Health Organization, https://www.who.int/nutrition/publications/emergencies/WHO_NHD_99.13/en/ (1999, accessed 10 August 2020).
- 53. Department of Health. Manual of nutrition. 12th ed. Norwich, UK: TSO (The Stationary Office), Part of Williams Lea, 2015. [Google Scholar]
- 54. Finglas PM, Roe MA, Pinchen HM, et al. McCance and Widdowson’s the composition of foods. 7th summary ed. Cambridge, UK: Royal Society of Chemistry, 2015. [Google Scholar]
- 55. Bettendorff L, Lakaye B, Kohn G, et al. Thiamine triphosphate: a ubiquitous molecule in search of a physiological role. Metab Brain Dis 2014; 29: 1069–1082. [DOI] [PubMed] [Google Scholar]
- 56. Ihara H, Hirano A, Wang L, et al. Reference values for whole blood thiamine and thiamine phosphates esters in Japanese adults. Int J Anal Bio-Sci 2005; 28: 241–246. [Google Scholar]
- 57. Tallaksen CM, Bøhmer T, Bell H, et al. Concomitant determination of thiamin and its phosphate esters in human blood and serum by high-performance liquid chromatography. J Chromatogr 1991; 564: 127–136. [DOI] [PubMed] [Google Scholar]
- 58. Gangolf M, Czerniecki J, Radermecker M, et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One 2010; 5: e13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schrijver J, Speek AJ, Klosse JA, et al. A reliable semiautomated method for the determination of total thiamine in whole blood by the thiochrome method with high-performance liquid chromatography. Ann Clin Biochem 1982; 19: 52–56. [DOI] [PubMed] [Google Scholar]
- 60. Brunnekreeft JW, Eidhof H, Gerrits J. Optimized determination of thiochrome derivatives of thiamine and thiamine phosphates in whole blood by reversed-phase liquid chromatography with precolumn derivatization. J Chromatogr 1989; 491: 89–96. [DOI] [PubMed] [Google Scholar]
- 61. Burch HB, Bessey OA, Love RH, et al. The determination of thiamine and thiamine phosphates in small quantities of blood and blood cells. J Biol Chem 1952; 198: 477–490. [PubMed] [Google Scholar]
- 62. Department of Bioinformatics & Biochemistry. Technische Universität Braunschweig, DE. Brenda, the comprehensive enzme information system, https://www.brenda-enzymes.org/ (2020, accessed 7 August 2020).
- 63. Guerrini I, Thomson AD, Gurling HM. Molecular genetics of alcohol-related brain damage. Alcohol Alcohol 2009; 44: 166–170. [DOI] [PubMed] [Google Scholar]
- 64. Ihara H, Igarashi H, Kakinoki T, et al. Classification of hypo and hyperthiaminosis. Int J Anal Bio-Sci 2013; 1: 45–49. [Google Scholar]
- 65. OMIM. Online Mendelian Inheritance in Man, an online cataloge of human genes and genetic disorders. Baltimore, MD: McKusick-Nathans Institute of Genetic Medicine, John Hopkins University School of Medicine, https://www.omim.org/ (2020, accessed 7 August 2020).
- 66. US National Library of Medicine (NIH). Genetics home reference, https://ghr.nlm.nih.gov/ (2020, accessed 7 August 2020).
- 67. Marcé-Grau A, Martí-Sánchez L, Baide-Mairena H, et al. Genetic defects of thiamine transport and metabolism: a review of clinical phenotypes, genetics, and functional studies. J Inherit Metab Dis 2019; 42: 581–597. [DOI] [PubMed] [Google Scholar]
- 68. Qualtieri A, Pedace V, Bisconte MG, et al. Severe erythrocyte adenylate kinase deficiency due to homozygous A–>G substitution at codon 164 of human AK1 gene associated with chronic haemolytic anaemia. Br J Haematol 1997; 99: 770–776. [DOI] [PubMed] [Google Scholar]
- 69. Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 2010; 299: G23–G31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Frank LL. Thiamin in clinical practice. JPEN J Parenter Enteral Nutr 2015; 39: 503–520. [DOI] [PubMed] [Google Scholar]
- 71. Hazell AS, Faim S, Wertheimer G, et al. The impact of oxidative stress in thiamine deficiency: a multifactorial targeting issue. Neurochem Int 2013; 62: 796–802. [DOI] [PubMed] [Google Scholar]
- 72. Sniekers M, Foulon V, Mannaerts GP, et al. Thiamine pyrophosphate: an essential cofactor for the alpha-oxidation in mammals–implications for thiamine deficiencies? Cell Mol Life Sci 2006; 63: 1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Casteels M, Sniekers M, Fraccascia P, et al. The role of 2-hydroxyacyl-CoA lyase, a thiamin pyrophosphate-dependent enzyme, in the peroxisomal metabolism of 3-methyl-branched fatty acids and 2-hydroxy straight-chain fatty acids. Biochem Soc Trans 2007; 35: 876–880. [DOI] [PubMed] [Google Scholar]
- 74. Lonsdale D, Marrs C. Thiamine deficiency disease, dysautonomia, and high calorie malnutrition. London: Academic Press, 2017. [Google Scholar]
- 75. Garrett RH, Grisham CM. Biochemistry. 5th ed. Belmont, CA: Brooks/Cole Cengage Learning, 2012. [Google Scholar]
- 76. Roche Biochemical Pathways. In: Michal G. (ed.) Part 1 metabolic pathways. Roche Diagnostics GmbH, Mannheim, DE, http://biochemical-pathways.com/#/map/1 (2014, accessed 19 October 2020).
- 77. The Universal Protein Resource (Uniprot) Consortium. UniProt, CH, https://www.uniprot.org/ (2020, accessed 7 August 2020).
- 78. Coy JF, Dubel S, Kioschis P, et al. Molecular cloning of tissue-specific transcripts of a transketolase-related gene: implications for the evolution of new vertebrate genes. Genomics 1996; 32: 309–316. [DOI] [PubMed] [Google Scholar]
- 79. Hazell AS, Butterworth RF. Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol 2009; 44: 141–147. [DOI] [PubMed] [Google Scholar]
- 80. Hirsch JA, Parrott J. New considerations on the neuromodulatory role of thiamine. Pharmacology 2012; 89: 111–116. [DOI] [PubMed] [Google Scholar]
- 81. Hazell AS, Rao KV, Danbolt NC, et al. Selective down-regulation of the astrocyte glutamate transporters GLT-1 and GLAST within the medial thalamus in experimental Wernicke’s encephalopathy. J Neurochem 2001; 78: 560–568. [DOI] [PubMed] [Google Scholar]
- 82. Hazell AS, Pannunzio P, Rama Rao KV, et al. Thiamine deficiency results in downregulation of the GLAST glutamate transporter in cultured astrocytes. Glia 2003; 43: 175–184. [DOI] [PubMed] [Google Scholar]
- 83. Jen JC, Wan J, Palos TP, et al. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology 2005; 65: 529–534. [DOI] [PubMed] [Google Scholar]
- 84. Lonsdale D. Thiamin and protein folding. Med Hypotheses 2019; 129: 109252. [DOI] [PubMed] [Google Scholar]
- 85. Koepsell H. Organic cation transporters in health and disease. Pharmacol Rev 2020; 72: 253–319. [DOI] [PubMed] [Google Scholar]
- 86. Zhao R, Gao F, Goldman ID. Reduced folate carrier transports thiamine monophosphate: an alternative route for thiamine delivery into mammalian cells. Am J Physiol Cell Physiol 2002; 282: C1512–C1517. [DOI] [PubMed] [Google Scholar]
- 87. Jungtrakoon P, Shirakawa J, Buranasupkajorn P, et al. Loss-of-function mutation in thiamine transporter 1 in a family with autosomal dominant diabetes. Diabetes 2019; 68: 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guerrini I, Thomson AD, Cook CC, et al. Direct genomic PCR sequencing of the high affinity thiamine transporter (SLC19A2) gene identifies three genetic variants in Wernicke Korsakoff syndrome (WKS). Am J Med Genet B Neuropsychiatr Genet 2005; 137b: 17–19. [DOI] [PubMed] [Google Scholar]
- 89. Fassone E, Wedatilake Y, DeVile CJ, et al. Treatable Leigh-like encephalopathy presenting in adolescence. BMJ Case Rep 2013; 2013: 200838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kono S, Miyajima H, Yoshida K, et al. Mutations in a thiamine-transporter gene and Wernicke’s-like encephalopathy. N Engl J Med 2009; 360: 1792–1794. [DOI] [PubMed] [Google Scholar]
- 91. Liang X, Yee SW, Chien HC, et al. Organic Cation Transporter 1 (OCT1) modulates multiple cardiometabolic traits through effects on hepatic thiamine content. PLoS Biol 2018; 16: e2002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lemos C, Faria A, Meireles M, et al. Thiamine is a substrate of organic cation transporters in Caco-2 cells. Eur J Pharmacol 2012; 682: 37–42. [DOI] [PubMed] [Google Scholar]
- 93. Han TK, Proctor WR, Costales CL, et al. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers. J Pharmacol Exp Ther 2015; 352: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tzvetkov MV, Vormfelde SV, Balen D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther 2009; 86: 299–306. [DOI] [PubMed] [Google Scholar]
- 95. Koepsell H, Busch A, Gorboulev V, et al. Structure and function of renal organic cation transporters. News Physiol Sci 1998; 13: 11–16. [DOI] [PubMed] [Google Scholar]
- 96. Busch AE, Karbach U, Miska D, et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol 1998; 54: 342–352. [DOI] [PubMed] [Google Scholar]
- 97. Geier EG, Chen EC, Webb A, et al. Profiling solute carrier transporters in the human blood-brain barrier. Clin Pharmacol Ther 2013; 94: 636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jensen O, Matthaei J, Blome F, et al. Variability and heritability of thiamine pharmacokinetics with focus on oct1 effects on membrane transport and pharmacokinetics in humans. Clin Pharmacol Ther 2020; 107: 628–638. [DOI] [PubMed] [Google Scholar]
- 99. Inazu M, Takeda H, Matsumiya T. Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. J Neurochem 2003; 84: 43–52. [DOI] [PubMed] [Google Scholar]
- 100. Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 2010; 335: 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen L, Pawlikowski B, Schlessinger A, et al. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet Genomics 2010; 20: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brown G. Defects of thiamine transport and metabolism. J Inherit Metab Dis 2014; 37: 577–585. [DOI] [PubMed] [Google Scholar]
- 103. Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011; 473: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nabokina SM, Subramanian VS, Said HM. The human colonic thiamine pyrophosphate transporter (hTPPT) is a glycoprotein and N-linked glycosylation is important for its function. Biochim Biophys Acta 2016; 1858: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Otsuka M, Matsumoto T, Morimoto R, et al. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A 2005; 102: 17923–17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Masuda S, Terada T, Yonezawa A, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol 2006; 17: 2127–2135. [DOI] [PubMed] [Google Scholar]
- 107. Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med 2000; 224: 246–255. [DOI] [PubMed] [Google Scholar]
- 108. Said HM, Nexo E. Gastrointestinal handling of water-soluble vitamins. Compr Physiol 2018; 8: 1291–1311. [DOI] [PubMed] [Google Scholar]
- 109. Hoyumpa AM, Jr, Strickland R, Sheehan JJ, et al. Dual system of intestinal thiamine transport in humans. J Lab Clin Med 1982; 99: 701–708. [PubMed] [Google Scholar]
- 110. Spector R, Johanson C. Micronutrient and urate transport in choroid plexus and kidney: implications for drug therapy. Pharm Res 2006; 23: 2515–2524. [DOI] [PubMed] [Google Scholar]
- 111. Smithline HA, Donnino M, Greenblatt DJ. Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy subjects. BMC Clin Pharmacol 2012; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Manzetti S, Zhang J, van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry 2014; 53: 821–835. [DOI] [PubMed] [Google Scholar]
- 113. Laforenza U, Patrini C, Alvisi C, et al. Thiamine uptake in human intestinal biopsy specimens, including observations from a patient with acute thiamine deficiency. Am J Clin Nutr 1997; 66: 320–326. [DOI] [PubMed] [Google Scholar]
- 114. Hoyumpa AM, Jr, Breen KJ, Schenker S, et al. Thiamine transport across the rat intestine. II. Effect of ethanol. J Lab Clin Med 1975; 86: 803–816. [PubMed] [Google Scholar]
- 115. Hoyumpa AM., Jr. Characterization of normal intestinal thiamin transport in animals and man. Ann N Y Acad Sci 1982; 378: 337–343. [DOI] [PubMed] [Google Scholar]
- 116. Nabokina SM, Subramanian VS, Valle JE, et al. Adaptive regulation of human intestinal thiamine uptake by extracellular substrate level: a role for THTR-2 transcriptional regulation. Am J Physiol Gastrointest Liver Physiol 2013; 305: G593–G599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Abdul-Muneer PM, Alikunju S, Schuetz H, et al. Impairment of thiamine transport at the GUT-BBB-AXIS contributes to Wernicke’s encephalopathy. Mol Neurobiol 2018; 55: 5937–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Holzbach E. Thiamine absorption in alcoholic delirium patients. J Stud Alcohol 1996; 57: 581–584. [DOI] [PubMed] [Google Scholar]
- 119. Thom JY, Davis RE, Icke GC. Protein binding of thiamin in human plasma. Int J Vitam Nutr Res 1986; 56: 189. [PubMed] [Google Scholar]
- 120. Spector R, Johanson CE. Vitamin transport and homeostasis in mammalian brain: focus on vitamins B and E. J Neurochem 2007; 103: 425–438. [DOI] [PubMed] [Google Scholar]
- 121. Tallaksen CM, Sande A, Bøhmer T, et al. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. Eur J Clin Pharmacol 1993; 44: 73–78. [DOI] [PubMed] [Google Scholar]
- 122. Bettendorff L, Mastrogiacomo F, Kish SJ, et al. Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain. J Neurochem 1996; 66: 250–258. [DOI] [PubMed] [Google Scholar]
- 123. Bitsch R. Thiamin: physiology. Friedrich-Schiller University, Jena, DE: Elsevier Ltd, 2016, pp.290–296. [Google Scholar]
- 124. Combs GF. Chapter 10 - Thiamin. In: Combs GF. (ed.) The vitamins. 4th ed. San Diego: Academic Press, 2012, pp.261–276. [Google Scholar]
- 125. Laterra J, Keep R, Betz LA, et al. Blood - cerebrospinal fluid barrier. In: Siegel GJ, Agranoff BW, Albers RW, et al. (eds) Basic neurochemistry: molecular, cellular and medical aspects. 6th ed. Philadelphia: Lippincott-Raven, 1999. [Google Scholar]
- 126. Spector R. Thiamin homeostasis in the central nervous system. Ann N Y Acad Sci 1982; 378: 344–354. [DOI] [PubMed] [Google Scholar]
- 127. Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv 2016; 13: 963–975. [DOI] [PubMed] [Google Scholar]
- 128. Spector R. Micronutrient homeostasis in mammalian brain and cerebrospinal fluid. J Neurochem 1989; 53: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 129. Hu C, Tao L, Cao X, et al. The solute carrier transporters and the brain: physiological and pharmacological implications. Asian J Pharm Sci 2020; 15: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Greenwood J, Love ER, Pratt OE. Kinetics of thiamine transport across the blood-brain barrier in the rat. J Physiol 1982; 327: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Thomson AD. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl 2000; 35: 2–7. [DOI] [PubMed] [Google Scholar]
- 132. Spector R. Vitamin transport diseases of brain: focus on folates, thiamine and riboflavin. Brain Disord Ther 2014; 3: 1–6. [Google Scholar]
- 133. McCandless DW, Schenker S, Cook M. Encephalopathy of thiamine deficieny: studies of intracerebral mechanisms. J Clin Invest 1968; 47: 2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gangolf M, Wins P, Thiry M, et al. Thiamine triphosphate synthesis in rat brain occurs in mitochondria and is coupled to the respiratory chain. J Biol Chem 2010; 285: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ziporin ZZ, Nunes WT, Powell RC, et al. Excretion of thiamine and its metabolites in the urine of young adult males receiving restricted intakes of the vitamin. J Nutr 1965; 85: 287–296. [DOI] [PubMed] [Google Scholar]
- 136. Ariaey-Nejad MR, Balaghi M, Baker EM, et al. Thiamin metabolism in man. Am J Clin Nutr 1970; 23: 764–778. [DOI] [PubMed] [Google Scholar]
- 137. Kreinbring CA, Remillard SP, Hubbard P, et al. Structure of a eukaryotic thiaminase I. Proc Natl Acad Sci U S A 2014; 111: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cornell College of Agriculture and Life Sciences (Cornell CALS). Thiaminases, https://poisonousplants.ansci.cornell.edu/toxicagents/thiaminase.html#:~:text=Thiaminases%20are%20enzmyes%20found%20in,metabolism%2C%20and%20render%20it%20inactive (2019, accessed 7 August 2020).
- 139. Earl JW, McCleary BV. Mystery of the poisoned expedition. Nature 1994; 368: 683–684. [DOI] [PubMed] [Google Scholar]
- 140. Kritikos G, Parr JM, Verbrugghe A. The role of thiamine and effects of deficiency in dogs and cats. Vet Sci 2017; 4: 59. [Google Scholar]
- 141. Vimokesant S, Kunjara S, Rungruangsak K, et al. Beriberi caused by antithiamin factors in food and its prevention. Ann N Y Acad Sci 1982; 378: 123–136. [DOI] [PubMed] [Google Scholar]
- 142. Neal RA, Pearson WN. Studies of thiamine metabolism in the rat. I. Metabolic products found in urine. J Nutr 1964; 83: 343–350. [DOI] [PubMed] [Google Scholar]
- 143. Balaghi M, Pearson WN. Metabolism of physiological doses of thiazole-2-14 C-labeled thiamine by the rat. J Nutr 1966; 89: 265–270. [DOI] [PubMed] [Google Scholar]
- 144. Neal RA, Pearson WN. Studies of thiamine metabolism in the rat. II. Isolation and identification of 2-methyl-4-amino-5-pyrimidinecarboxylic acid as a metabolite of thiamine in rat urine. J Nutr 1964; 83: 351–357. [DOI] [PubMed] [Google Scholar]
- 145. Weber W, Nitz M, Looby M. Nonlinear kinetics of the thiamine cation in humans: saturation of nonrenal clearance and tubular reabsorption. J Pharmacokinet Biopharm 1990; 18: 501–523. [DOI] [PubMed] [Google Scholar]
- 146. Larkin JR, Zhang F, Godfrey L, et al. Glucose-induced down regulation of thiamine transporters in the kidney proximal tubular epithelium produces thiamine insufficiency in diabetes. PLoS One 2012; 7: e53175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Boulware MJ, Subramanian VS, Said HM, et al. Polarized expression of members of the solute carrier SLC19A gene family of water-soluble multivitamin transporters: implications for physiological function. Biochem J 2003; 376: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kato K, Moriyama C, Ito N, et al. Involvement of organic cation transporters in the clearance and milk secretion of thiamine in mice. Pharm Res 2015; 32: 2192–2204. [DOI] [PubMed] [Google Scholar]
- 149. Ashokkumar B, Vaziri ND, Said HM. Thiamin uptake by the human-derived renal epithelial (HEK-293) cells: cellular and molecular mechanisms. Am J Physiol Renal Physiol 2006; 291: F796–F805. [DOI] [PubMed] [Google Scholar]
- 150. Tanihara Y, Masuda S, Sato T, et al. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol 2007; 74: 359–371. [DOI] [PubMed] [Google Scholar]
- 151. Motohashi H, Nakao Y, Masuda S, et al. Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J Pharm Sci 2013; 102: 3302–3308. [DOI] [PubMed] [Google Scholar]
- 152. Lechner C, Ishiguro N, Fukuhara A, et al. Impact of experimental conditions on the evaluation of interactions between multidrug and toxin extrusion proteins and candidate drugs. Drug Metab Dispos 2016; 44: 1381–1389. [DOI] [PubMed] [Google Scholar]
- 153. Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 2012; 5: i3–i14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schuchardt JP, Hahn A. Intestinal absorption and factors influencing bioavailability of magnesium-an update. Curr Nutr Food Sci 2017; 13: 260–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ayuk J, Gittoes NJ. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem 2014; 51: 179–188. [DOI] [PubMed] [Google Scholar]
- 156. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev 2015; 95: 1–46. [DOI] [PubMed] [Google Scholar]
- 157. Workinger JL, Doyle RP, Bortz J. Challenges in the diagnosis of magnesium status. Nutrients 2018; 10: 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Villard HP, Beauregard JM. Encéphalopathies éthyliques carentielles et le magnésium: syndrome de Gayet-Wernicke. Union Med Can 1964; 93: 50–53. [PubMed] [Google Scholar]
- 159. Agus ZS. Hypomagnesemia. J Am Soc Nephrol 1999; 10: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 160. Yu ASL. Hypomagnesemia: evaluation and treatment. In: Lam AQ. (ed.) UpToDate, https://www.uptodate.com/contents/hypomagnesemia-evaluation-and-treatment (2019, accessed 7 August 2020).
- 161. Grochowski C, Blicharska E, Baj J, et al. Serum iron, magnesium, copper, and manganese levels in alcoholism: a systematic review. Molecules 2019; 24: 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ryzen E, Nelson TA, Rude RK. Low blood mononuclear cell magnesium content and hypocalcemia in normomagnesemic patients. West J Med 1987; 147: 549–553. [PMC free article] [PubMed] [Google Scholar]
- 163. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr 2013; 4: 378s–383s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol 2009; 41: 357–362. [DOI] [PubMed] [Google Scholar]
- 165. De Marchi S, Cecchin E, Basile A, et al. Renal tubular dysfunction in chronic alcohol abuse–effects of abstinence. N Engl J Med 1993; 329: 1927–1934. [DOI] [PubMed] [Google Scholar]