Abstract
Background:
Prospectively collected responses to Patient Acceptable Symptom State (PASS) questions after shoulder instability surgery are limited. Responses to these outcome measures are imperative to understanding their clinical utility.
Purpose/Hypothesis:
The purpose of this study was to evaluate which factors predict unfavorable patient-reported outcomes after shoulder instability surgery, including “no” to the PASS question. We hypothesized that poor outcomes would be associated with male adolescents, bone loss, combined labral tears, and articular cartilage injuries.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
Patients aged ≥13 years undergoing shoulder instability surgery were included in point-of-care data collection at a single institution across 12 surgeons between 2015 and 2017. Patients with anterior-inferior labral tears were included, and those with previous ipsilateral shoulder surgery were excluded. Demographics, American Shoulder and Elbow Surgeons (ASES) and Single Assessment Numeric Evaluation (SANE) scores, and surgical findings were obtained at baseline. ASES and SANE scores, PASS responses, and early revision surgery rates were obtained at a minimum of 1 year after the surgical intervention. Regression analyses were performed.
Results:
A total of 234 patients met inclusion criteria, of which 176 completed follow-up responses (75.2%). Nonresponders had a younger age, greater frequency of glenoid bone loss, fewer combined tears, and more articular cartilage injuries (P < .05). Responders’ mean age was 25.1 years, and 22.2% were female. Early revision surgery occurred in 3.4% of these patients, and 76.1% responded yes to the PASS question. A yes response correlated with a mean 25-point improvement in the ASES score and a 40-point improvement in the SANE score. On multivariate analysis, combined labral tears (anterior-inferior plus superior or posterior tears) were associated with greater odds of responding no to the PASS question, while both combined tears and injured capsules were associated with lower ASES and SANE scores (P < .05). Sex, bone loss, and grade 3 to 4 articular cartilage injuries were not associated with variations on any patient-reported outcome measure.
Conclusion:
Patients largely approved of their symptom state at ≥1 year after shoulder instability surgery. A response of yes to the PASS question was given by 76.1% of patients and was correlated with clinically and statistically significant improvements in ASES and SANE scores. Combined labral tears and injured capsules were negative prognosticators across patient-reported outcome measures, whereas sex, bone loss, and cartilage injuries were not.
Keywords: shoulder, instability, PROMs, follow-up, patient-reported outcome measures
An increasing number of validated patient-reported outcome measures (PROMs) are emerging for adolescent and adult patients undergoing a surgical intervention for shoulder instability, with minimal prospectively collected data supporting these outcome measures. Multiple series have identified patient-related and clinical factors that are associated with postoperative recurrent instability and poor patient-reported outcomes; these include preoperative recurrent instability, male adolescents, collision athletes, and bipolar bone loss.1,11,13,15,17 It has also been demonstrated that there is a lack of standardization of validated shoulder instability outcome reporting.7,8 In a 2018 publication,5 the American Shoulder and Elbow Surgeons (ASES) Value Committee provided recommendations regarding ideal PROMs and selected the following tools as part of a shoulder score “research package”: the Veterans RAND 12-Item Health Survey (VR-12) for generic quality of life; the ASES questionnaire; the Single Assessment Numeric Evaluation (SANE); the Penn Shoulder Score (PSS); and a disease-specific score, such as the Western Ontario Rotator Cuff Index or Western Ontario Shoulder Instability Index (WOSI).5 The Multicenter Orthopaedic Outcomes Network Shoulder Group recently published baseline data for a prospective multicenter cohort of 863 patients undergoing shoulder instability surgery, for which they selected the ASES questionnaire, Shoulder Activity Scale (SAS), WOSI, 36-Item Short Form Health Survey (SF-36), and SANE.10 Our institution has created a framework for the prospective collection of surgeon- and patient-reported outcomes to guide the development of treatment models and prognostication tools.
An alternative to multiple-item assessments is the single-item Patient Acceptable Symptom State (PASS). Patients are asked to respond yes or no to the following question in which the joint of interest is specified: “Taking into account all the activity you have during your daily life, your level of pain, and also your activity limitations and participation restrictions, do you consider the current state of your [joint] satisfactory?” This question can be elicited at baseline but has more commonly been proposed to patients at a set interval after an operative procedure, and it has been shown to be correlated with clinically successful outcomes after anterior cruciate ligament reconstruction.21 To our knowledge, the PASS question has not been utilized in assessing outcomes after shoulder instability surgery.
The purpose of this study was to evaluate which factors predict unfavorable patient-reported outcomes after shoulder instability surgery, including a response of no to the PASS question designated as our primary outcome. We hypothesized that poor outcomes would be associated with male adolescents (aged 12-18 years), bone loss, larger labral tears, and articular cartilage injuries.
Methods
The study was approved by our institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Patients aged ≥13 years undergoing open or arthroscopic shoulder surgery for glenohumeral stabilization were prospectively enrolled in a longitudinal cohort, and those undergoing surgery between 2015 and 2017 were included. Surgical procedures included arthroscopic or open capsulolabral repair or reconstruction, coracoid or allograft bone block transfer, remplissage or resurfacing of Hill-Sachs lesions, and biceps tenodesis for superior labral tears. We included patients with anterior and/or inferior labral tears, some of whom had an extension of these tears to the posterior or superior labrum. Exclusion criteria for this study consisted of previous ipsilateral shoulder surgery, isolated posterior or superior labral tears, and combined tears that did not involve the anterior-inferior labrum.
Patients completed a health assessment utilizing branching logic on the day of surgery and at least 1 year postoperatively through OrthoMiDaS Episode of Care (OME), our department’s prospective outcomes collection system.4 Data were stored in a REDCap database (Vanderbilt University). For patients undergoing shoulder surgery, the baseline assessment included 3 validated PROMs: the PSS, SANE, and ASES questionnaire.22 Patients who self-identified as competitive athletes also completed the Kerlan-Jobe Orthopaedic Clinic questionnaire.2 Demographic information and baseline characteristics were also recorded; these included age, sex, body mass index, years of education, smoking status, and VR-12 Mental Component Summary score. At 1 year postoperatively, patients were notified to complete a follow-up assessment that included the existing PROMs as well as the PASS question and items regarding return to work and to sport. Patients were also queried directly regarding any repeat ipsilateral or contralateral shoulder surgery. Patients were not queried regarding subjective instability events.
Immediately after the procedure, surgeons documented surgical details in OME based on the patient’s history and intraoperative findings. Information recorded included the proportion of glenoid bone loss as directly visualized (none, <20%, or >20%), extent and location of labral tears (anterior-inferior, superior, posterior, or any combination thereof), presence of Hill-Sachs lesions (none, yes and engaging, or yes and nonengaging), status of the glenohumeral capsule (normal, stretched or torn but untreated, injured and incorporated into labral repair, or injured and separately repaired), presence of articular cartilage injuries (normal to grades 1-4), and laterality (right or left). The surgeon also recorded the number and type of suture anchors utilized in labral repair or capsulolabral reconstruction.
Continuous variables were summarized using means and standard deviations, and categorical variables were summarized using frequencies and percentages. Binomial models were used to identify the drivers of continuous outcomes (ASES and SANE scores); given that the scores were left-skewed, their scale was inverted, and negative binomial models were used. Logistic regression models were used for binary outcomes (PASS response and repeat ipsilateral surgery). The bootstrap concordance index was used to measure the quality of the logistic regression model in which 0.5 indicates that the model was no better than a prediction based on random chance, 0.7 indicates a good model, and 0.8 indicates a strong model. Goodness of fit of the negative binomial models was assessed with deviance and compared with the 5% critical value based on the model’s degrees of freedom. The logistic regression model was graphically represented using a nomogram for the PASS. Analysis was conducted using R statistical computing software. Categorical variables with a low frequency of results were grouped to maintain fitness of the model. P < .05 was considered statistically significant.
Results
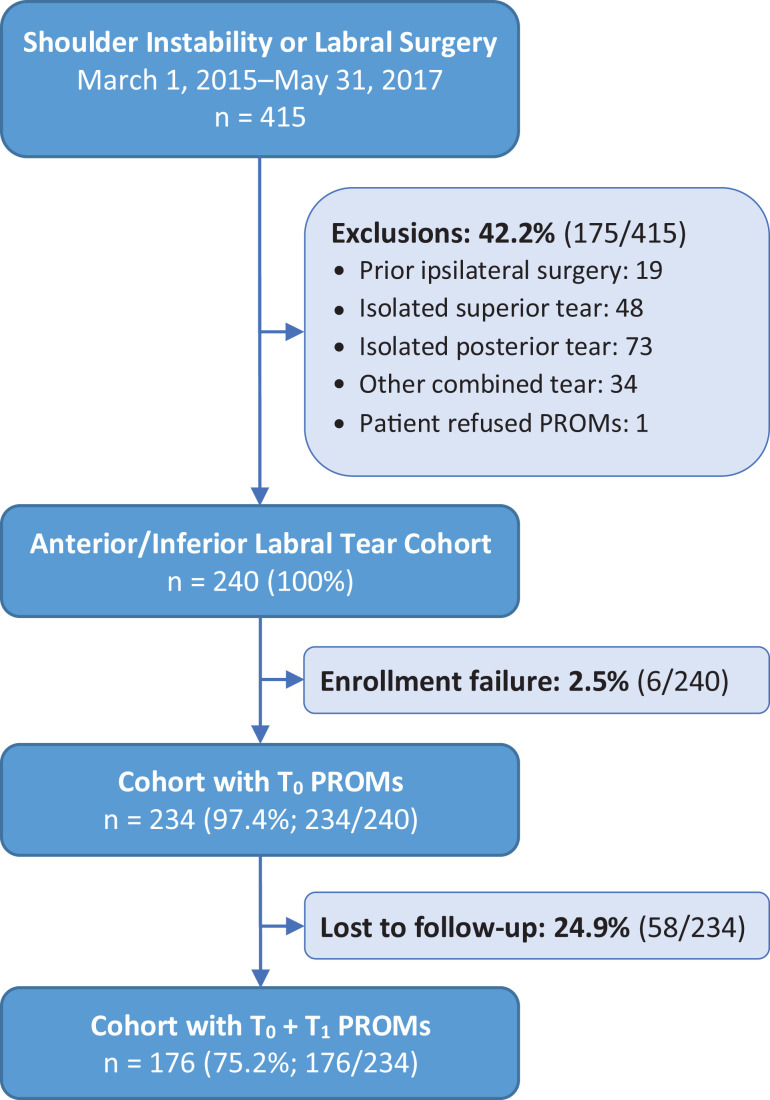
The initial cohort consisted of 415 patients aged ≥13 years with anterior, posterior, or multidirectional shoulder instability treated by 12 surgeons at 8 sites within our institution during the study period. Patients with any previous ipsilateral shoulder surgery (n = 19), isolated posterior labral tears (n = 73), isolated superior labral tears (n = 48), and combined tears not involving the anterior-inferior labrum (n = 34) were excluded (Figure 1). One patient declined the baseline assessment, and 6 patients agreed but did not complete it. The eligible cohort consisted of 234 patients across 11 surgeons. Of those, 176 completed assessments at ≥1 year postoperatively, for a 75.2% patient response rate (Figure 1). Per the OME procedures, patients were contacted by telephone, text message, email, and postal mail to facilitate the completion of follow-up.
Figure 1.
Study flowchart. PROMs, patient-reported outcome measures; T0, time zero or baseline responses; T1, one-year responses.
Nonresponders had a younger age, fewer years of education, greater frequency of glenoid bone loss, fewer combined tears, and higher grade articular cartilage injuries (P < .05) (Table 1). Responders’ mean age was 25.1 years, and 77.8% were male. Their median duration to complete the baseline assessments was 9.4 minutes and the 1-year postoperative assessments was 6.2 minutes. The median interval from surgery to the completion of postoperative assessments was 1.2 years (range, 1.0-3.5 years). There was a slight predominance of right-sided procedures at 55.1% (Table 1).
Table 1.
Descriptive Statistics for Eligible Versus Incomplete Cohort at 1-Year Follow-upa
| Variable | Eligible (n = 176) | Incomplete (n = 58) | P Value |
|---|---|---|---|
| Sex | .999 | ||
| Female | 39 (22.2) | 13 (22.4) | |
| Male | 137 (77.8) | 45 (77.6) | |
| Age at surgery, mean ± SD, y | 25.1 ± 10.3 | 21.1 ± 6.3 | .001 |
| BMI at surgery, mean ± SD | 26.6 ± 5.2 | 27.3 ± 5.5 | .355 |
| Education, mean ± SD, y | 13.0 ± 3.2 | 11.6 ± 3.6 | .011 |
| Smoking status | .381 | ||
| Never | 142 (80.7) | 43 (74.1) | |
| Current or quit | 34 (19.3) | 15 (25.9) | |
| Glenoid bone loss | .024 | ||
| None | 145 (82.4) | 39 (67.2) | |
| Some | 31 (17.6) | 19 (32.8) | |
| No. of anchors, mean ± SD | 3.5 ± 1.5 | 3.6 ± 1.2 | .853 |
| Location | .012 | ||
| Anterior-inferior | 102 (58.0) | 45 (77.6) | |
| Combined | 74 (42.0) | 13 (22.4) | |
| Laterality | .216 | ||
| Right | 97 (55.1) | 38 (65.5) | |
| Left | 79 (44.9) | 20 (34.5) | |
| Hill-Sachs lesion | .112 | ||
| None | 108 (61.4) | 30 (51.7) | |
| Engaging | 14 (8.0) | 2 (3.5) | |
| Nonengaging | 54 (30.7) | 26 (44.8) | |
| Capsule | .249 | ||
| Normal | 114 (64.8) | 32 (55.2) | |
| Injured | 62 (35.2) | 26 (44.8) | |
| Articular cartilage | .041 | ||
| Normal to grade 2 | 155 (88.1) | 44 (75.9) | |
| Grades 3-4 | 21 (11.9) | 14 (24.1) | |
| Repeat ipsilateral surgery | .341 | ||
| No | 170 (96.6) | 58 (100.0) | |
| Yes | 6 (3.4) | 0 (0.0) | |
| Baseline VR-12 MCS score,b mean ± SD | 53.4 ± 9.7 | 51.4 ± 11.5 | .238 |
| Baseline ASES total score,b mean ± SD | 66.1 ± 19.2 | 62.2 ± 20.1 | .228 |
| Baseline SANE score,b mean ± SD | 49.3 ± 22.3 | 46.9 ± 21.4 | .464 |
aData are shown as n (%) unless otherwise indicated. Bolded P values indicate statistically significant between-group differences (P < .05). ASES, American Shoulder and Elbow Surgeons; BMI, body mass index; MCS, Mental Component Summary; SANE, Single Assessment Numeric Evaluation; VR-12, Veterans RAND 12-Item Health Survey.
bThe VR-12 is norm-based at 50, whereas the ASES and SANE are on a scale of 0-100. Higher scores for all of these variables are favorable.
Surgeons identified isolated anterior-inferior labral tears in 58.0% of responders’ shoulders and combined tears extending beyond the anterior-inferior labrum in 42.0%. The capsule was reported to be injured in 35.2% of their shoulders, and 11.9% had grade 3 to 4 articular cartilage injuries. The mean number of suture anchors used was 3.5. The median duration to complete the surgical details was 1.7 minutes, and surgeons had a 100.0% response rate.
The majority of responding patients underwent arthroscopic labral repair and/or capsulorrhaphy (n = 164; 93.2%), and 3 of these patients also underwent arthroscopic remplissage. A minority of patients underwent open procedures for instability (Table 2).
Table 2.
Open Shoulder Stabilization Procedures in Eligible Cohort (n = 176)
| Procedure | n (%) |
|---|---|
| Open anterior-inferior labral repair (open Bankart repair) | 2 (1.1) |
| Open Bankart reconstruction with allograft bone augment to anterior glenoid (Glenojet) | 4 (2.3) |
| Open coracoid transfer (Latarjet) or allograft bone transfer to anterior glenoid | 3 (1.7) |
| Other combined open procedurea | 3 (1.7) |
aThis comprises procedures with a combination of humeral head resurfacing and either Glenojet or coracoid/allograft bone transfer.
Overall, 76.1% of patients responded yes to the PASS question. On univariate analysis, a response of yes was correlated with a mean 25-point improvement in the ASES score and a 40-point improvement in the SANE score (Table 3). Patients reponding no were more likely to have combined labral tears, revision surgery, and lower baseline ASES and SANE scores (P < .05). Univariate analysis also showed that a response of no was correlated with a mean 1-point improvement in ASES scores and a 16-point improvement in SANE scores. The mean age at surgery; sex; body mass index; and the findings of capsular or articular cartilage injuries, glenoid bone loss, or Hill-Sachs lesions did not differ significantly between groups. There was a 3.4% prevalence of repeat shoulder surgery across all responding patients.
Table 3.
Descriptive Statistics by PASS Response of No Versus Yes on Univariate Analysisa
| Variable | No (n = 38) | Yes (n = 134) | P Value |
|---|---|---|---|
| Sex | .351 | ||
| Female | 11 (28.9) | 27 (20.1) | |
| Male | 27 (71.1) | 107 (79.9) | |
| Age at surgery, mean ± SD, y | 27.5 ± 10.8 | 24.6 ± 10.2 | .140 |
| BMI at surgery, mean ± SD | 27.3 ± 5.7 | 26.5 ± 5.0 | .439 |
| Education, mean ± SD, y | 12.7 ± 3.4 | 13.2 ± 3.1 | .419 |
| Smoking status | .066 | ||
| Never | 26 (68.4) | 112 (83.6) | |
| Current or quit | 12 (31.6) | 22 (16.4) | |
| Glenoid bone loss | .868 | ||
| None | 32 (84.2) | 109 (81.3) | |
| Some | 6 (15.8) | 25 (18.7) | |
| No. of anchors, mean ± SD | 3.5 ± 1.7 | 3.5 ± 1.4 | .969 |
| Location | .014 | ||
| Anterior-inferior | 15 (39.5) | 85 (63.4) | |
| Combined | 23 (60.5) | 49 (36.6) | |
| Laterality | .095 | ||
| Right | 26 (68.4) | 69 (51.5) | |
| Left | 12 (31.6) | 65 (48.5) | |
| Hill-Sachs lesion | .681 | ||
| None | 24 (63.2) | 82 (61.2) | |
| Engaging | 4 (10.5) | 10 (7.5) | |
| Nonengaging | 10 (26.3) | 42 (31.3) | |
| Capsule | .759 | ||
| Normal | 23 (60.5) | 87 (64.9) | |
| Injured | 15 (39.5) | 47 (35.1) | |
| Articular cartilage | .571 | ||
| Normal to grade 2 | 35 (92.1) | 117 (87.3) | |
| Grades 3-4 | 3 (7.9) | 17 (12.7) | |
| Repeat ipsilateral surgery | <.001 | ||
| No | 32 (84.2) | 134 (100.0) | |
| Yes | 6 (15.8) | 0 (0.0) | |
| Baseline VR-12 MCS score,b mean ± SD | 51.9 ± 10.3 | 53.9 ± 9.5 | .293 |
| Baseline ASES total score,b mean ± SD | 58.7 ± 23.4 | 67.9 ± 17.5 | .039 |
| Baseline SANE score,b mean ± SD | 39.4 ± 21.5 | 52.0 ± 21.8 | .002 |
| 1-y ASES total score,b mean ± SD | 59.9 ± 22.1 | 93.2 ± 7.9 | <.001 |
| 1-y SANE score,b mean ± SD | 55.3 ± 27.1 | 91.6 ± 8.3 | <.001 |
aData are shown as n (%) unless otherwise indicated. The missing four are those who filled out everything except for the PASS. Bolded P values indicate statistically significant between-group differences (P < .05). ASES, American Shoulder and Elbow Surgeons; BMI, body mass index; MCS, Mental Component Summary; PASS, Patient Acceptable Symptom State; SANE, Single Assessment Numeric Evaluation; VR-12, Veterans RAND 12-Item Health Survey.
bThe VR-12 is norm based at 50, whereas the ASES and SANE are on a scale of 0-100. Higher scores for all of these variables are favorable.
On multivariate analysis, combined labral tears (anterior-inferior plus superior or posterior tears) were associated with 5.4 odds of responding no to the PASS question (P = .005) (Table 4), 3.1 odds of lower ASES scores at 1-year follow-up (P < .001) (Table 5), and 2.2 odds of lower SANE scores at 1-year follow-up (P = .002) (Table 6). Injured capsules were not associated with variations in PASS responses but demonstrated 2.4 odds of lower ASES scores (P = .001) (Table 5) and 2.1 odds of lower SANE scores (P = .001) (Table 6). Older age was only associated with poorer SANE scores, but the CI approached 1 (odds ratio, 1.03 [95% CI, 1.00-1.05]; P = .048) (Table 6). Each additional year corresponded to a 3-point decrease in the SANE score. Interestingly, right shoulders were also associated with poorer scores for both the ASES questionnaire (P = .01) (Table 5) and SANE (P = .046) (Table 6) compared with left shoulders. We did not evaluate hand dominance in this study. The significant variables on multivariate analysis across all of the studied PROMs are summarized in Table 7.
Table 4.
Logistic Regression Model Results for Predicting PASS Responses of Noa
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Intercept | 1.10 (0.02-49.16) | .961 |
| Sex: male (vs female) | 0.84 (0.32-2.20) | .73 |
| Age at surgery | 1.04 (0.99-1.09) | .159 |
| BMI at surgery | 1.00 (0.92-1.09) | .974 |
| Education | 0.88 (0.76-1.02) | .08 |
| Smoking status: current or quit (vs never) | 1.29 (0.45-3.66) | .639 |
| Baseline VR-12 MCS score | 0.97 (0.94-1.02) | .224 |
| Glenoid bone loss: some (vs none) | 0.61 (0.17-2.22) | .455 |
| No. of anchors | 0.76 (0.53-1.08) | .124 |
| Location: combined (vs anterior-inferior) | 5.41 (1.67-17.47) | .005 |
| Laterality: right (vs left) | 2.18 (0.91-5.23) | .082 |
| Hill-Sachs lesion | ||
| Engaging (vs none) | 2.77 (0.55-13.97) | .216 |
| Nonengaging (vs none) | 1.56 (0.52-4.72) | .427 |
| Capsule: injured (vs normal) | 2.57 (0.91-7.28) | .076 |
| Articular cartilage: grades 3-4 (vs normal to grade 2) | 0.60 (0.14-2.60) | .494 |
aBolded P value indicates statistical significance (P < .05). BMI, body mass index; MCS, Mental Component Summary; PASS, Patient Acceptable Symptom State; VR-12, Veterans RAND 12-Item Health Survey.
Table 5.
Linear Regression Model Results for Predicting Poorer ASES Scoresa
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Intercept | 4.88 (0.42-60.57) | .161 |
| Sex: male (vs female) | 1.03 (0.55-1.84) | .921 |
| Age at surgery | 1.03 (0.99-1.07) | .078 |
| BMI at surgery | 1.02 (0.97-1.07) | .471 |
| Education | 0.98 (0.89-1.07) | .638 |
| Smoking status: current or quit (vs never) | 0.60 (0.28-1.38) | .16 |
| Baseline VR-12 MCS score | 0.99 (0.96-1.01) | .382 |
| Glenoid bone loss: some (vs none) | 0.96 (0.48-2.11) | .918 |
| No. of anchors | 0.84 (0.71-1.00) | .075 |
| Location: combined (vs anterior-inferior) | 3.05 (1.66-5.70) | <.001 |
| Laterality: right (vs left) | 1.84 (1.14-2.97) | .01 |
| Hill-Sachs lesion | ||
| Engaging (vs none) | 1.40 (0.48-4.42) | .49 |
| Nonengaging (vs none) | 0.94 (0.53-1.69) | .834 |
| Capsule: injured (vs normal) | 2.44 (1.45-4.19) | .001 |
| Articular cartilage: grades 3-4 (vs normal to grade 2) | 0.98 (0.46-2.27) | .964 |
aBolded P values indicate statistical significance (P < .05). ASES, American Shoulder and Elbow Surgeons; BMI, body mass index; MCS, Mental Component Summary; VR-12, Veterans RAND 12-Item Health Survey.
Table 6.
Linear Regression Model Results for Predicting Poorer SANE Scoresa
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Intercept | 8.33 (1.31-55.08) | .016 |
| Sex: male (vs female) | 1.09 (0.67-1.73) | .701 |
| Age at surgery | 1.03 (1.00-1.05) | .048 |
| BMI at surgery | 1.01 (0.98-1.05) | .521 |
| Education | 0.96 (0.90-1.02) | .212 |
| Smoking status: current or quit (vs never) | 0.83 (0.46-1.51) | .48 |
| Baseline VR-12 MCS score | 0.99 (0.97-1.01) | .434 |
| Glenoid bone loss: some (vs none) | 0.84 (0.47-1.56) | .529 |
| No. of anchors | 0.91 (0.79-1.07) | .262 |
| Location: combined (vs anterior-inferior) | 2.20 (1.34-3.64) | .002 |
| Laterality: right (vs left) | 1.47 (0.99-2.18) | .046 |
| Hill-Sachs lesion | ||
| Engaging (vs none) | 1.01 (0.46-2.37) | .981 |
| Nonengaging (vs none) | 1.08 (0.67-1.75) | .737 |
| Capsule: injured (vs normal) | 2.14 (1.39-3.33) | .001 |
| Articular cartilage: grades 3-4 (vs normal to grade 2) | 1.08 (0.60-2.05) | .79 |
aBolded P values indicate statistical significance (P < .05). BMI, body mass index; MCS, Mental Component Summary; SANE, Single Assessment Numeric Evaluation; VR-12, Veterans RAND 12-Item Health Survey.
Table 7.
Significant Variables Predicting “No” PASS Responses and Poorer ASES/SANE Scores on Multivariate Analysisa
| Variable | PASS | ASES | SANE |
|---|---|---|---|
| Sex: male (vs female) | |||
| Age at surgery | .048 | ||
| BMI at surgery | |||
| Education | |||
| Smoking status | |||
| Quit (vs never) | |||
| Current (vs never) | |||
| Baseline VR-12 MCS score | |||
| Glenoid bone loss: some (vs none) | |||
| No. of anchors | |||
| Location: combined (vs anterior-inferior) | .005 | <.001 | .002 |
| Laterality: right (vs left) | .01 | .046 | |
| Hill-Sachs lesion | |||
| Engaging (vs none) | |||
| Nonengaging (vs none) | |||
| Capsule: injured (vs normal) | .001 | .001 | |
| Articular cartilage: grades 3-4 (vs normal to grade 2) |
aOnly P values <.05 are listed. ASES, American Shoulder and Elbow Surgeons; BMI, body mass index; MCS, Mental Component Summary; PASS, Patient Acceptable Symptom State; SANE, Single Assessment Numeric Evaluation; VR-12, Veterans RAND 12-Item Health Survey.
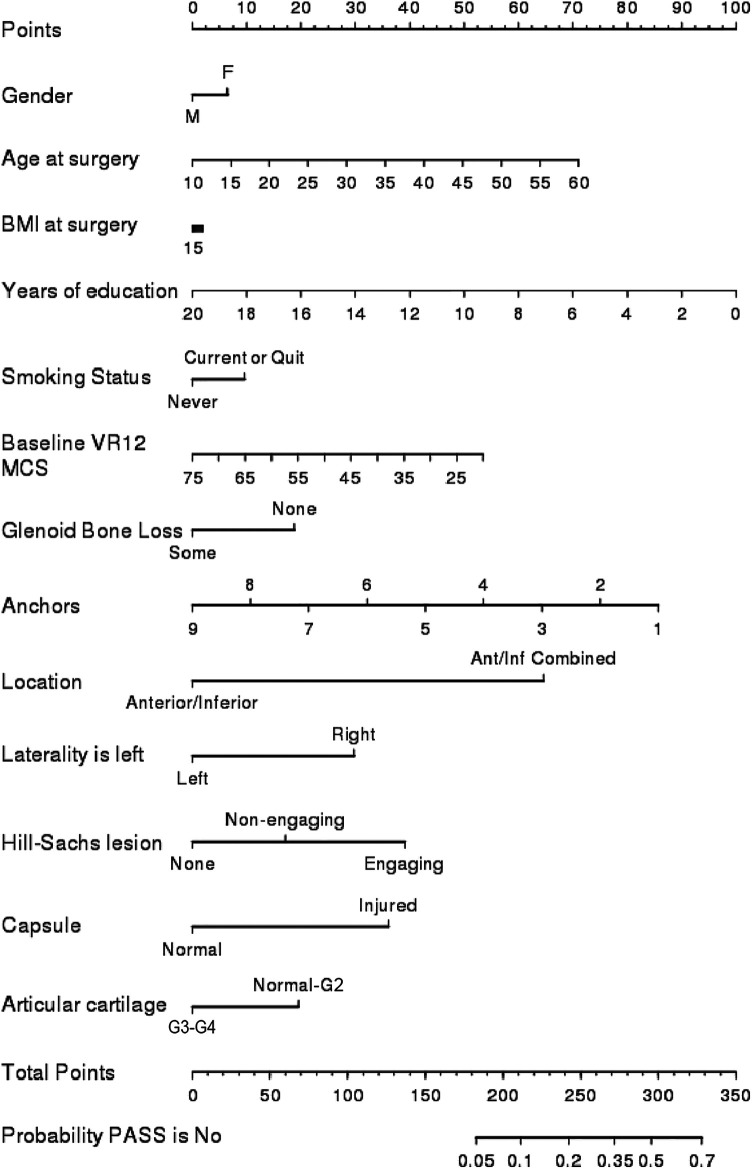
The concordance index was 0.65 for the PASS model, not meeting the level of a good (0.7) or strong (0.8) model. The nomogram for the PASS model is shown in Figure 2. The residual deviances were 179 (closest value to the 5% critical value deviance of 169) and 206 (closest value to the 5% critical value deviance of 189) for the ASES and SANE models, respectively. Sex, glenoid bone loss, Hill-Sachs lesions, and grade 3 to 4 articular cartilage injuries were not associated with variations on any PROMs on multivariate analysis (Tables 4 -6). Given the rarity of repeat surgery in this cohort (6/176; 3.4%), regression analysis to identify predictors for this variable could not be performed.
Figure 2.
Nomogram modeling probability of no PASS responses of no. Ant/Inf, anterior/inferior; BMI, body mass index; F, female; G2-G3-G4, articular cartilage grades 2-4; M, male; MCS, Mental Component Summary; PASS, Patient Acceptable Symptom State; VR-12, Veterans RAND 12-Item Health Survey.
Discussion
The purpose of the present study was to evaluate which factors predict unfavorable patient-reported outcomes after shoulder instability surgery, including our primary outcome, the PASS response. We hypothesized that poor outcomes would be associated with male adolescents, bone loss, larger labral tears, and articular cartilage injuries. This study comprised a prospective North American multisurgeon cohort of 176 patients, who had a mean age of 25.1 years, most of whom were male (77.8%), and who were undergoing surgery for anterior-inferior shoulder instability with at least 1-year follow-up. Overall, 2 patient variables related to the extent of shoulder abnormalities, combined labral tears and capsular injuries, were prognostic of poorer PROM scores (Figure 2 and Table 7), partially supporting our hypothesis. Combined labral tear was a significant variable across all of the studied PROMs (Table 7), but capsular injury was not associated with a response of no to the PASS question (P = .076) (Table 7). Older age was a negative prognosticator for the SANE only, and laterality also influenced ASES and SANE scores (Table 7). Capsular injury and labral tear extent may serve as indicators of the chronicity or magnitude of preoperative instability. Laterality may be viewed as a surrogate for hand dominance, but the cause of the poorer ASES and SANE scores for right shoulders is unclear. Contrary to our hypothesis, patient sex and the intraoperative findings of glenoid bone loss, Hill-Sachs lesions, or articular cartilage injuries did not play a role in PROM variations. The mean number of suture anchors used was >3, consistent with a previous systematic review suggesting the use of a minimum of 3 anchors for long-term labral repair durability.16 To our knowledge, this is the first report of the PASS after shoulder instability surgery, with 76.1% of our patients responding yes.
Factors Influencing Postoperative Recurrent Instability and Poor PROM Scores
We viewed early revision surgery, which occurred in 3.4% of patients, as a surrogate for recurrent dislocations. Previous data have suggested that patients with multiple shoulder dislocations preceding an index shoulder-stabilizing procedure are more likely to have poorer outcomes, but this study did not evaluate the number of instability events before the surgical intervention. Marshall et al13 reported on 173 patients undergoing arthroscopic Bankart repair with a mean age of 19.2 years, followed to a mean of 51 months postoperatively, and with 70% retention. Patients were identified retrospectively but had prospectively collected data, including Simple Shoulder Test scores, return-to-sports rates, and postoperative instability. Of the 56% of patients who underwent arthroscopic Bankart repair after a first-time anterior dislocation, 29% experienced frank postoperative instability, and 7% required revision stabilization. Of the 44% of patients with a history of recurrent anterior dislocations who then underwent arthroscopic Bankart repair, 62% had postoperative instability (odds ratio, 4.14), with 32% requiring revision (odds ratio, 6.01). These findings suggest that the outcomes of anterior soft tissue stabilization in an adolescent or young adult are considerably better after the first dislocation episode compared with after ≥2 events.
The finding of 76.1% of patients responding yes to the PASS question in the current study suggests a high satisfaction rate among patients at 1 year postoperatively and is supported by previous data. Saier et al18 provided longitudinal findings of patient satisfaction and function after arthroscopic Bankart repair in a prospective case series of 53 patients with a mean age at surgery of 29.4 years who were followed to a mean of 24 months. The authors used 2 scales to assess quality of life (the FLZ and the SF-12) and 3 others for functional outcomes (the Oxford Shoulder Instability Score, ASES questionnaire, and the shortened version of Disabilities of the Arm, Shoulder and Hand questionnaire), and they also assessed return to work. There were significant increases in quality of life within the first 12 months postoperatively, but they did not significantly vary by 24 months.
Our 3.4% rate of early revision surgery is notably lower than results from studies of similar patient cohorts that have a longer follow-up. Park et al15 reported on a retrospective series of 195 patients undergoing arthroscopic stabilization for anterior shoulder instability with 2-year follow-up, finding a 7.7% rate of revision surgery. There were 3 factors that were associated with patient dissatisfaction defined by the 15-point scale of Juniper et al.6 Greater Hill-Sachs lesion width on magnetic resonance imaging scans and the number of preoperative instability events were poor prognosticators, whereas concomitant superior labrum anterior to posterior repair was a positive prognosticator. With regard to revision surgery, greater age, glenoid bone loss, and preoperative instability events were poor prognosticators. The single factor prognostic for both patient-reported and objective outcomes was the quantity of preoperative instability events.
We did not include early revision surgery in our multivariate analysis and posit that a longer follow-up is necessary for assessing this outcome. Nonetheless, we may look to previous long-term data to see that age may be a strong prognosticator. Aboalata et al1 reported on 143 patients who underwent arthroscopic anterior shoulder stabilization with a mean age at surgery of 28.2 years and mean follow-up to 13 years. The overall redislocation rate was 18.2% and was highest in the youngest group of patients: 39.1% for age <20 years, 16.1% for age 21 to 30 years, and 13.4% for age >30 years. Furthermore, the rates were higher in patients with preoperative recurrent instability as opposed to a single dislocation.
It should also be noted that our present study evaluated for the location and extent of labral tears as opposed to the directionality of instability, with combined tears portending worse outcomes. Kraeutler et al9 reported on 151 patients with anterior, posterior, or combined instability treated using soft tissue stabilization, with the most common sports being football, rock climbing, and snowboarding. The mean age was 28.7 years, and follow-up was to 3.6 years. The authors administered the WOSI, SANE, ASES questionnaire, and SAS and found no significant difference between the instability groups on PROMs or in rates of return to sports.
Although we are unable to scrutinize the surgical indications, techniques, and athletic demands of patients in the current study, these factors have also been demonstrated to influence the rates of recurrent anterior instability. Leroux et al11 performed a systematic review of 26 studies of contact/collision athletes with anterior instability, including a single level of evidence 1 study. The mean patient age was 19.9 years, and follow-up was to 43.7 months. The pooled postoperative failure rate of anterior soft tissue stabilization was 17.8%, but the authors refined this to 7.9% in studies prescribing to “evidence-based” indications and techniques (eg, no significant bone loss identified preoperatively, lateral decubitus positioning, and a minimum of 3 suture anchors). The most common sport in athletes with recurrent instability was rugby.
PROMs and Compliance
Selecting appropriately responsive PROMs for patients with shoulder instability has been a subject of recent focus. Kasik and Saper7 also demonstrated variability in outcome reporting after arthroscopic anterior stabilization in adolescent athletes. They reviewed 8 studies comprising 282 patients’ shoulders with levels of evidence of 2 to 4. Numerous PROMs were reported, with the most common being the Rowe score, SANE, ASES questionnaire, and Constant score. Clinical outcomes included return to sports, patient satisfaction, postoperative stability, pain, and range of motion. Unger et al20 performed a systematic review of 110 shoulder studies, 29 of which investigated instability. Within that group, there were 16 different PROMs utilized, with the most common being the ASES questionnaire, Rowe score, WOSI, University of California Los Angeles shoulder rating scale, Constant score, and visual analog scale for pain. In 2018, the ASES Value Committee provided recommendations regarding PROMs for a shoulder score “research package.”5 They selected the VR-12 for generic quality of life; the ASES questionnaire; the SANE; the PSS; and a disease-specific score, such as the Western Ontario Rotator Cuff Index or WOSI.
The Multicenter Orthopaedic Outcomes Network Shoulder Group initiated a 10-center prospective cohort study with enrollment between 2012 and 2017 and reported baseline epidemiological data.10 Across 10 sites, 863 patients with a mean age of 24 years were enrolled, and approximately 75% presented with unidirectional anterior shoulder instability. The most common sports of participation were football and basketball. The preoperative assessment included range of motion, strength testing, and the following PROMs: the ASES questionnaire, WOSI, SANE, SAS, and SF-36. Findings from a series of overhead athletes within this larger study have been reported.19 As data have been reported from the larger cohort, they may be pooled with the current study in an effort to better understand and generalize the outcomes of shoulder instability management.
With regard to the PASS question, it has been previously recognized that some patients may respond yes before surgery, thereby creating a ceiling effect at a postoperative time point.21 With regard to the ASES questionnaire and SANE, a supplement to the baseline assessment may be to ask patients what they anticipate their postoperative improvement to be. Such an approach was utilized by the FAIT Group in a randomized controlled trial of patients with femoroacetabular impingement undergoing arthroscopic hip surgery versus physical therapy.14 Patients were asked at baseline to complete the Activities of Daily Living section of the Hip Outcome Score based on how they expected to fare after treatment was completed.
The PROMIS (Patient-Reported Outcomes Measurement Information System) tool has also been utilized in assessing patients with shoulder instability.3 In the study by Anthony et al,3 74 patients completed the upper extremity and physical function computer adaptive test portions of the PROMIS, which were correlated well with common shoulder and upper extremity PROMs as well as the SF-36 physical function component. However, in patients aged <21 years, there was a ceiling effect within the PROMIS upper extremity portion.
We achieved a 75.2% response rate on postoperative assessments, and increasing compliance with the electronic collection of PROM scores postoperatively has been shown to be challenging. Makhni et al12 performed arthroscopic rotator cuff–related or miscellaneous procedures, which did not include glenohumeral stabilization, on 143 patients with a mean age of 53.1 years through 2 enrolling surgeons. Compliance with assessments was 76% preoperatively, 57% at 6 months postoperatively, and 45% at 1 year. At each time point, the authors reported an approximate 20% improvement in compliance through the involvement of research staff members who approached patients at a clinic visit. This was in addition to the standard reminders sent to patients by both the research staff and automated electronic reminders. In the present study, patients lacking responses were contacted by telephone or electronic means to encourage responses. Although the cohort of Makhni et al is different in age and procedure type from our own, the findings reflect the importance of judicious follow-up with patients to solicit their PROM scores.
There are several limitations to the present study. We recognize subjectivity in surgeon reporting of intraoperatively identified soft tissue injuries and bone loss. There is heterogeneity in terms of the procedures performed, with a minority being open shoulder stabilization (6.8% of the eligible cohort). We could not longitudinally assess a patient’s return to sports, as the scope of questions did not specify the particular sports or levels of participation. We also could not determine the frequency of preoperative recurrent instability, which has been discussed to influence postoperative recurrence,13 and we did not specifically query for subjective instability events postoperatively. Postoperative instability may prompt revision surgery beyond 1-year follow-up but was outside of the scope of the current study. Hand dominance was not elicited, although right shoulders fared worse. With regard to the regression models, we did not assess for categories within the variables of glenoid bone loss (ie, subdivision of <20% and >20% glenoid bone loss) and capsular status (ie, form of capsular management). Instead, these variables were treated as binary findings to maintain adequate model fitness.
Conclusion
In this prospective cohort, patients largely approved of their symptom state at ≥1 year after anterior-inferior shoulder instability surgery. A response of yes to the PASS question was given by 76.1% of patients and was correlated with clinically and statistically significant improvements in ASES and SANE scores. Combined labral tears and injured capsules were negative prognosticators, whereas sex, cartilage injuries, and bone loss were not.
Acknowledgment
The authors thank the Cleveland Clinic’s orthopaedic patients, staff, and research personnel whose efforts related to regulatory actions, data collection, participant follow-up, data quality control, analyses, and article preparation have made this consortium successful. They also thank Brittany Stojsavljevic, editorial assistant at the Cleveland Clinic, for editorial management as well as Drs Peter Evans, Gregory Gilot, Joseph Scarcella, and Thomas Anderson for contributing cases.
Final revision submitted June 1, 2020; accepted June 16, 2020.
The content of this article is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
One or more of the authors has declared the following potential conflict of interest or source of funding: Research reported in this publication was partially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number K23AR066133, which supported a portion of M.H.J.’s professional effort. A.F.B. has received educational support from Smith & Nephew. M.S.S. has received consulting fees and nonconsulting fees from Arthrex. L.D.F. has received consulting fees from Zimmer Biomet and hospitality payments from the Musculoskeletal Transplant Foundation. M.H.J. is on the scientific advisory board for Samumed. B.W.M. has received educational support from Arthrex and hospitality payments from Rock Medical and Zimmer Biomet. A.M. has received educational support from Rock Medical; consulting fees from Amniox Medical, Arthrosurface, Linvatec, Stryker, and Trice; speaking fees from Trice; royalties from Arthrosurface, Wolters Kluwer, and Zimmer Biomet; honoraria from Arthrosurface; and hospitality payments from Arthrex, DJO, and Smith & Nephew and has stock in Arthrosurface and Trice. E.T.R. has received research support from DePuy; consulting fees from DJO, DePuy, and Encore Medical; royalties from DJO; and honoraria from DePuy and DJO. J.T.R. has received educational consulting fees from Smith & Nephew. K.P.S. has received research support from DJO and Smith & Nephew and consulting fees from Cytori Therapeutics, Flexion Therapeutics, Mitek, the National Football League, and Samumed. K.L.S. has received educational support from Arthrex and Biomet; consulting fees from Molnlycke Health Care; honoraria from Fidia Pharma; and hospitality payments from Horizon Pharma, the Musculoskeletal Transplant Foundation, Ramsay Medical, and Stryker. J.W. has received educational support from Arthrex. P.M.S. has received educational support from Rock Medical; consulting fees from Arthrex, DJO, and DePuy; nonconsulting fees from Arthrex; and hospitality payments from the Musculoskeletal Transplant Foundation. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Cleveland Clinic (No. 06-196).
Authors: Cleveland Clinic OME Sports Medicine: Ahmad F. Bayomy, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA; Department of Sports Health and Medicine, Shriners Hospitals for Children, Springfield, Massachusetts, USA); Mark S. Schickendantz, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Isaac N. Briskin, MA (Cleveland Clinic, Cleveland, Ohio, USA); Lutul D. Farrow, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Lauren E. Grobaty, AB (Cleveland Clinic, Cleveland, Ohio, USA; School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA); Morgan H. Jones, MD, MPH (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Brett W. McCoy, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Anthony Miniaci, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Eric T. Ricchetti, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); James T. Rosneck, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Elizabeth Sosic, MS (Cleveland Clinic, Cleveland, Ohio, USA); Kurt P. Spindler, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Kim L. Stearns, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); Greg J. Strnad, MS (Cleveland Clinic, Cleveland, Ohio, USA); James Williams, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA); and Paul M. Saluan, MD (Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio, USA).
References
- 1. Aboalata M, Plath JE, Seppel G, Juretzko J, Vogt S, Imhoff AB. Results of arthroscopic Bankart repair for anterior-inferior shoulder instability at 13-year follow-up. Am J Sports Med. 2016;45(4):782–787. [DOI] [PubMed] [Google Scholar]
- 2. Alberta FG, ElAttrache NS, Bissell S, et al. The development and validation of a functional assessment tool for the upper extremity in the overhead athlete. Am J Sports Med. 2010;38(5):903–911. [DOI] [PubMed] [Google Scholar]
- 3. Anthony CA, Glass NA, Hancock K, Bollier M, Wolf BR, Hettrich CM. Performance of PROMIS instruments in patients with shoulder instability. Am J Sports Med. 2016;45(2):449–453. [DOI] [PubMed] [Google Scholar]
- 4. Cleveland Clinic OME Group, Piuzzi NS, Strnad G, et al. Implementing a scientifically valid, cost-effective, and scalable data collection system at point of care: the Cleveland Clinic OME cohort. J Bone Joint Surg Am. 2019;101(5):458–464. [DOI] [PubMed] [Google Scholar]
- 5. Hawkins RJ, Thigpen CA. Selection, implementation, and interpretation of patient-centered shoulder and elbow outcomes. J Shoulder Elbow Surg. 2018;27(2):357–362. [DOI] [PubMed] [Google Scholar]
- 6. Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47(1):81–87. [DOI] [PubMed] [Google Scholar]
- 7. Kasik C, Saper MG. Variability of outcome reporting following arthroscopic Bankart repair in adolescent athletes: a systematic review. Arthroscopy. 2018;34(4):1288–1294. [DOI] [PubMed] [Google Scholar]
- 8. Kocher MS. Editorial commentary: shoulder instability outcome reporting requires standardization. Arthroscopy. 2018;34(4):1295–1296. [DOI] [PubMed] [Google Scholar]
- 9. Kraeutler MJ, Aberle NS, Brown CC, Ptasinski JJ, McCarty EC. Clinical outcomes and return to sport after arthroscopic anterior, posterior, and combined shoulder stabilization. Orthop J Sports Med. 2018;6(4):2325967118763754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraeutler MJ, McCarty EC, Belk JW, et al. Descriptive epidemiology of the MOON shoulder instability cohort. Am J Sports Med. 2018;46(5):1064–1069. [DOI] [PubMed] [Google Scholar]
- 11. Leroux TS, Saltzman BM, Meyer M, et al. The influence of evidence-based surgical indications and techniques on failure rates after arthroscopic shoulder stabilization in the contact or collision athlete with anterior shoulder instability. Am J Sports Med. 2017;45(5):1218–1225. [DOI] [PubMed] [Google Scholar]
- 12. Makhni EC, Higgins JD, Hamamoto JT, Cole BJ, Romeo AA, Verma NN. Patient compliance with electronic patient reported outcomes following shoulder arthroscopy. Arthroscopy. 2017;33(11):1940–1946. [DOI] [PubMed] [Google Scholar]
- 13. Marshall T, Vega J, Siqueira M, Cagle R, Gelber JD, Saluan P. Outcomes after arthroscopic Bankart repair: patients with first-time versus recurrent dislocations. Am J Sports Med. 2017;45(8):1776–1782. [DOI] [PubMed] [Google Scholar]
- 14. Palmer AJR, Ayyar Gupta V, Fernquest S, et al. Arthroscopic hip surgery compared with physiotherapy and activity modification for the treatment of symptomatic femoroacetabular impingement: multicentre randomised controlled trial. BMJ. 2019;364(406):1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park I, Kang J-S, Jo Y-G, Shin S-J. Factors related to patient dissatisfaction versus objective failure after arthroscopic shoulder stabilization for instability. J Bone Joint Surg Am. 2019;101(12):1070–1076. [DOI] [PubMed] [Google Scholar]
- 16. Randelli P, Ragone V, Carminati S, Cabitza P. Risk factors for recurrence after Bankart repair: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2129–2138. [DOI] [PubMed] [Google Scholar]
- 17. Sachs RA, Lin D, Stone ML, Paxton E, Kuney M. Can the need for future surgery for acute traumatic anterior shoulder dislocation be predicted? J Bone Joint Surg Am. 2007;89(8):1665–1674. [DOI] [PubMed] [Google Scholar]
- 18. Saier T, Plath JE, Waibel S, et al. How satisfied are patients with arthroscopic Bankart repair? A 2-year follow-up on quality-of-life outcome. Arthroscopy. 2017;33(10):1777–1785. [DOI] [PubMed] [Google Scholar]
- 19. Trinh TQ, Naimark MB, Bedi A, et al. Clinical outcomes after anterior shoulder stabilization in overhead athletes: an analysis of the MOON Shoulder Instability Consortium. Am J Sports Med. 2019;47(6):1404–1410. [DOI] [PubMed] [Google Scholar]
- 20. Unger RZ, Burnham JM, Gammon L, Malempati CS, Jacobs CA, Makhni EC. The responsiveness of patient-reported outcome tools in shoulder surgery is dependent on the underlying pathological condition. Am J Sports Med. 2019;47(1):241–247. [DOI] [PubMed] [Google Scholar]
- 21. Vega JF, Jacobs CA, Strnad GJ, et al. Prospective evaluation of the patient acceptable symptom state to identify clinically successful anterior cruciate ligament reconstruction. Am J Sports Med. 2019;47(5):1159–1167. [DOI] [PubMed] [Google Scholar]
- 22. Wright RW, Baumgarten KM. Shoulder outcomes measures. J Am Acad Orthop Surg. 2010;18(7):436–444. [DOI] [PubMed] [Google Scholar]