Abstract
The human pathogen Bordetella pertussis targets the respiratory epithelium and causes whooping cough. Its virulence factor adenylate cyclase toxin (CyaA) plays an important role in the course of infection. Previous studies on the impact of CyaA on human epithelial cells have been carried out using cell lines derived from the airways or the intestinal tract. Here, we investigated the interaction of CyaA and its enzymatically inactive but fully pore-forming toxoid CyaA-AC– with primary human airway epithelial cells (hAEC) derived from different anatomical sites (nose and tracheo-bronchial region) in two-dimensional culture conditions. To assess possible differences between the response of primary hAEC and respiratory cell lines directly, we included HBEC3-KT in our studies. In comparative analyses, we studied the impact of both the toxin and the toxoid on cell viability, intracellular cAMP concentration and IL-6 secretion. We found that the selected hAEC, which lack CD11b, were differentially susceptible to both CyaA and CyaA-AC–. HBEC3-KT appeared not to be suitable for subsequent analyses. Since the nasal epithelium first gets in contact with airborne pathogens, we further studied the effect of CyaA and its toxoid on the innate immunity of three-dimensional tissue models of the human nasal mucosa. The present study reveals first insights in toxin–cell interaction using primary hAEC.
Keywords: Adenylate cyclase toxin, Bordetella pertussis, cyclic adenosine monophosphate, human respiratory epithelial cells, IL-6
Introduction
The innate immune response of the respiratory epithelium is essential to combat airborne viruses and bacteria. Bordetella pertussis is a human airway pathogen that targets the respiratory epithelium and causes whooping cough, which is a highly contagious and resurgent disease. This resurgence could be due to molecular changes in B. pertussis, reduced vaccine efficacy and waning immunity.1 Besides several other virulence factors, adenylate cyclase toxin (CyaA) plays an important role in B. pertussis infection. CyaA is described by Vojtova et al. as a protein fusion of a cell-invasive and highly potent adenylate cyclase (AC) enzyme with a typical repeat in toxin (RTX) cytolysin moiety.2 CyaA is able to invade eukaryotic cells by translocating its catalytic domain across the cell membrane. The AC is activated by endogenous calmodulin to catalyse unregulated conversion of adenosine triphosphate to cAMP, causing alterations in cellular physiology. CyaA is known to target cells expressing the complement receptor 3 (CR3; CD11b/CD18).3 However, it is also able to intoxicate CR3-negative cells, such as epithelial cells.4–7 Previous work on CyaA–epithelial cell interaction was carried out using human airway cell lines. The advantage of using cell lines is that they are well standardisable and show greatly enhanced life spans. However, several airway epithelial cell lines display only certain features of the corresponding primary cells.8,9 To our knowledge, the impact of CyaA on primary human airway epithelial cells (hAEC) has not yet been investigated. Challenges while using primary hAEC are that they are difficult to standardise and establish in large quantities due to complex culture techniques, shortness of donor cells, low passaging capability10 and a high potential of variability between donors.11 To obtain first insights into toxin–host cell interaction, we studied the impact of CyaA and its enzymatically inactive but fully pore-forming toxoid CyaA-AC– on primary hAEC in two-dimensional (2D) culture conditions. We analysed cell viability, intracellular cAMP production and IL-6 secretion. Since previous investigations indicate differential responses of nasal and bronchial primary hAEC upon various stimulation,12,13 we performed comparative analyses of primary hAEC obtained from different anatomical sites (nose and tracheo-bronchial region). To assess possible differences in our readouts between primary hAEC and a respiratory cell line directly, we included HBEC3-KT in our analyses. Since the nasal epithelium first gets in contact with airborne pathogens, we further studied the effect of CyaA and its toxoid on the innate immunity of three-dimensional tissue models of the human nasal mucosa.
Methods
Donors
Tracheo-bronchial tissue samples for human tracheo-bronchial epithelial cell (HTEC) isolation were obtained from six donors (three female and three male donors; 39–74 yr old) undergoing elective pulmonary resection at the University Hospital Würzburg and University Hospital Magdeburg. Specimens from nasal mucosa to isolate human nasal epithelial cells (HNEC) and fibroblasts were obtained from six donors (two female and four male donors; 26–43 yr old) at the University Hospital Würzburg. Written informed consent was obtained beforehand, and the studies were approved by the institutional ethics committees on human research of the Otto-von-Guericke University Magdeburg (vote 163/17) and Julius-Maximilians-University Würzburg (votes 182/10 and 116/17), respectively. Additionally, we used HNEC obtained from two donors, which were purchased from Epithelix Sàrl (Geneva, Switzerland; one female donor, 41 yr old, and one male donor, 63 yr old).
Primary cell isolation, cell lines and cell culture
All cells were cultured under standard conditions (37°C, 5% CO2), and cell culture media were renewed three times per wk. Primary hAEC (HNEC and HTEC) and fibroblasts were isolated and cultured according to previously published protocols.14,15 Airway epithelial cells were grown in Airway Epithelial Cell Growth Medium (AECG; #PB-C-MH-350-0099, PeloBiotech, Martinsried, Germany). Fibroblasts were grown in DMEM (#10564011; Thermo Fisher Scientific, Waltham, MA) supplemented with 10% FBS (#12103C; Merck, Darmstadt, Germany). The immortalised human bronchial epithelial cell (HBEC) line HBEC3-KT (ATCC® CRL-4051™; LGC Standards GmbH, Wesel, Germany) was cultured in K-SD medium (defined keratinocyte-SFM; #10744019; Thermo Fisher Scientific). The human monocytic cell line THP-1 (ATCC® TIB-202™, LGC Standards GmbH) was grown in Gibco™ RPMI (#61870010; Thermo Fisher Scientific) supplemented with 10% FBS and antibiotic antimycotic solution (#A5955; Sigma–Aldrich, Munich, Germany).
3D Nasal mucosa tissue model generation
As a 3D scaffold for tissue model generation, we used decellularized segments of the porcine small intestinal submucosa (SIS), as described elsewhere.9,14,15 Animal research was performed according to the German law and institutional guidelines approved by the Ethics Committee of the District of Unterfranken, Würzburg, Germany (approval number: 55.2-2532-2-256). SIS segments were fixed between two cylinders. Each scaffold was seeded with 50,000 fibroblasts from the apical side and cultured under submerged conditions. The next day, 1.5–2.5 × 105 HNEC were added from the apical side, and the whole construct was submerged for 24 h. Then, the tissue models matured under airlift conditions for 21–31 d until beating kinocilia and mucus production could be observed at the light microscopic level. For cell co-culture, a culture medium mixture made of 50% AECG and 50% fibroblast culture medium was used.
Analysis of CD11b expression
To analyse CD11b expression, the method for one-colour staining, flow cytometry and data analysis were followed, as described elsewhere.16 HNEC, HTEC, HBEC3-KT and THP-1 cells (2 × 105) were incubated with CD11b-PE Abs (1:10; #130-091-240; Miltenyi Biotec, Bergisch Gladbach, Germany). Corresponding isotype control Abs (1:10; #553989; BD Biosciences, Heidelberg, Germany) were included in all experiments. Flow cytometry was performed in triplicate from three independent experiments. Data were analysed using FlowJo™ software v10 (Tree Star, Inc., Ashland, OR).
Cell viability analysis
To quantify the effect of CyaA and CyaA-AC– on cell viability, the FITC Annexin V Apoptosis Detection Kit with 7-amino-actinomycin D (7-AAD; #640922; BioLegend, San Diego, CA) was used following the manufacturer’s instructions. In brief, 1 × 105 HNEC, HTEC and HBEC3-KT were incubated with 0.1, 0.5, 1.0 or 5.0 µg/ml CyaA or CyaA-AC– for 24 h. CyaA and CyaA-AC– were provided by Prof. P. Sebo (Czech Academy of Sciences, Czech Republic). Fifty mM Tris pH 8, 8 M urea and 2 mM CaCl2 (TUC) buffer-treated samples served as controls. After incubation, the cells were detached and stained with FITC-conjugated Annexin V followed by incubation of 7-AAD. Samples were analysed by flow cytometry using a FACSCalibur™ (Becton Dickinson, Heidelberg, Germany) and FlowJo™ software. Three independent experiments were performed in triplicate.
Immunofluorescent staining
Mucin 5B (Muc5B) staining of nasal mucosa tissue models was carried out according to a previously published protocol9 (n = 3). In brief, the 3D tissue models were fixed, paraffin-embedded and cross-sectioned. After deparaffinization, rehydration and Ag retrieval, the sections were incubated with polyclonal rabbit anti-Mucin 5B primary Abs (1:100; HPA008246; Sigma–Aldrich) overnight. Secondary Abs (polyclonal donkey anti-rabbit coupled to Alexa Fluor 488, 1:400, A-21206; Thermo Fisher Scientific) were incubated at room temperature. Sections were mounted with DAPI Fluoromount-G®.
cAMP Assay
One hundred thousand cells per well were seeded onto six-well plates. At about 90% confluency, HNEC, HTEC and HBEC3-KT cultures were incubated with 0.5 µg/ml CyaA, CyaA-AC– or TUC buffer for 1 h. Then, the cells were detached and lysed. cAMP in cell lysates was quantified by a direct competitive ELISA according to the manufacturer’s instructions (cAMP Assay Kit; #ab65355; Abcam, Cambridge, UK). The ELISA assay kit utilises a recombinant protein G–coated 96-well plate to anchor cAMP polyclonal Abs onto the plate efficiently. The amount of cAMP-HRP bound to the plate was determined by reading the colorimetric HRP activity at OD450 nm. The measured cAMP values were normalised to the total protein content using the DC™ protein assay (Bio-Rad, Feldkirchen, Germany). We performed four independent experiments, each in duplicate.
IL-6 Secretion
Cell monolayers of HNEC, HTEC and HBEC3-KT and human nasal mucosa tissue models were treated from the apical side with 1.0 µg/ml CyaA, CyaA-AC– or TUC buffer for 24 h. The supernatants were collected, and IL-6 secretion was detected by ELISA in 2D cultures (Human IL-6 ELISA Kit; catalogue #ab100572; Abcam) and by a human inflammatory cytokine kit (catalogue #551811; BD Biosciences) for 3D cultures following the manufacturer’s instructions. The assays were performed in three independent experiments in duplicate, for both 2D and 3D culture conditions. For 3D cultures, the relative IL-6 secretion was calculated.
Mucin 5B secretion
Three-dimensional nasal mucosa tissue models were treated from the apical side with 1.0 µg/ml CyaA, CyaA-AC– or TUC buffer for 24 h. Then, TUC buffer was added to the apical side of the models to dilute the secreted mucus and thus to enhance the efficiency of Muc5B collection. The apical supernatant was collected, and the secreted Muc5B was measured using a Muc5B ELISA Kit (Mucin 5 Subtype B; #ABIN414775; Abs-online GmbH, Aachen, Germany) following the manufacturer’s instructions. Three independent experiments were performed in duplicate.
Statistical analysis
One- or two-way ANOVA was performed using GraphPad Prism v7.4 (GraphPad Software, Inc., La Jolla, CA). Dunnett’s, Tukey’s or Sidak’s multiple comparison test was applied to evaluate the statistical differences between control (TUC) and treatment groups (CyaA and CyaA-AC–), differences in the control group (TUC) and cell type-specific differences. A P value of ≤ 0.05 was considered to be significant, and a P value of > 0.05 but ≤0.1 was considered as a trend.
Results
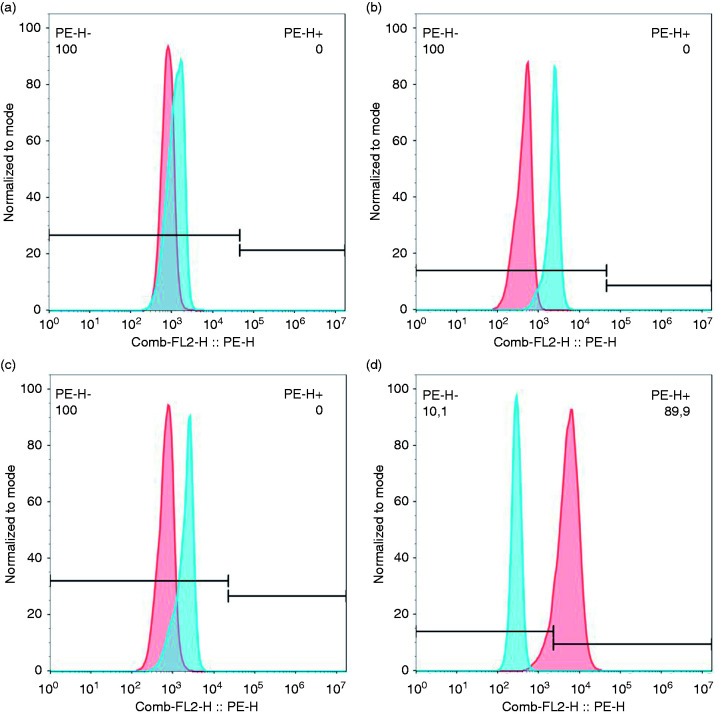
CD11b was reported to be expressed mainly in immune cells, such as macrophages or dendritic cells.3 To analyse if CD11b is present in HNEC, HTEC and HBEC3-KT, we performed flow cytometry. HNEC, HTEC and HBEC3-KT cultures were not CD11b-positive (Figure 1a–c), whereas in THP-1 populations, on average 90.5% of the cells were CD11b-positive (Figure 1d).
Figure 1.
Airway epithelial cells do not express CD11b. Expression of CD11b in airway epithelial cells and THP-1 was analysed by flow cytometry. CD11b-positive cells were not found in HNEC (a), HTEC (b) or HBEC3-KT (c) samples. THP-1 populations were CD11b-positive (d). Blue histograms show isotype control-stained cells and red histograms show cells stained with an anti-CD11b Ab. The figure shows the histograms of one representative experiment (n=3).
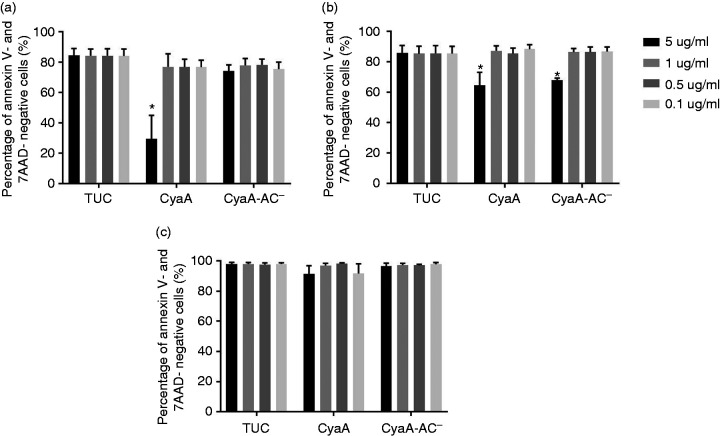
To find out if CyaA can affect hAEC viability, we incubated the three different airway epithelial cell types with 0.1, 0.5, 1.0 or 5.0 µg/ml CyaA or CyaA-AC– for 24 h and determined the percentage of Annexin V- and 7-AAD-negative cells. AEC that were treated with TUC buffer served as control. CyaA or CyaA-AC– (0.1, 0.5 µg/ml, and 1.0 µg/ml) did not change the percentage of Annexin V- and 7-AAD-negative cells in HNEC (Figure 2a) or in HTEC (Figure 2b). However, 5.0 µg/ml CyaA significantly decreased the percentage of Annexin V- and 7-AAD-negative cells to 29.5 ± 15.5% in HNEC (Figure 2a) and to 64.5 ± 8.6% in HTEC (Figure 2b). In both HNEC and HTEC, we did not detect significant changes in Annexin V-negative and 7-AAD-positive cells after treatment with CyaA (Figure 3a and b). However, the percentage of Annexin V-positive and 7-AAD-negative cells increased significantly (Figure 4a and b). Additionally, 5.0 µg/ml CyaA-AC– caused a significant decrease in the percentage of Annexin V- and 7-AAD-negative cells to 68.0 ± 1.3% in HTEC (Figure 2b). In HTEC, we also found a significant increase in Annexin V-negative and 7-AAD-positive cells after application of the toxoid (Figure 3b). Moreover, the percentage of Annexin V-positive and 7-AAD-negative cells increased significantly (Figure 4b). Both CyaA and CyaA-AC– did not affect HBEC3-KT at all (Figures 2c, 3c and 4c). Even though an application of 5.0 µg/ml CyaA is most probably not relevant in vivo, these data suggest that HNEC, HTEC and HBEC3-KT are differentially susceptible to CyaA. For subsequent experiments, we decided to apply CyaA and CyaA-AC– in concentrations < 5.0 µg/ml.
Figure 2.
Quantification of Annexin V- and 7-ADD- negative AEC after CyaA and CyaA-AC– incubation. The percentage of Annexin V- and 7-ADD-negative cells is shown for Connect up. HNEC (a), HTEC (b) and HBEC3-KT (c) after incubation with 0.1, 0.5, 1.0 µg/ml or 5.0 μg/ml of CyaA or CyaA-AC– for 24 h. Data are presented as means (bars)+SEM (error bars) of three independent experiments. Statistically significant differences compared to the control group (TUC) are indicated (*P < 0.05, ANOVA).
Figure 3.
Quantification of Annexin V-negative and 7-ADD-positive AEC after CyaA and CyaA-AC– incubation. The percentage of Annexin V-positive and 7-ADD-negative cells is shown for HNEC (a), HTEC (b) and HBEC3-KT (c) after incubation with 0.1, 0.5, 1.0 µg/ml or 5.0 μg/ml of CyaA or CyaA-AC– for 24 h. Data are presented as means (bars)+SEM (error bars) of three independent experiments. Statistically significant differences compared to the control group (TUC) are indicated (*P < 0.05, ANOVA).
Figure 4.
Quantification of Annexin V-positive and 7-ADD-negative AEC after CyaA and CyaA-AC– incubation. The percentage of Annexin V-positive and 7-ADD-negative cells is shown for HNEC (a), HTEC (b) and HBEC3-KT (c) after incubation with 0.1, 0.5, 1.0 µg/ml or 5.0 μg/ml of CyaA or CyaA-AC– for 24 h. Data are presented as means (bars) + SEM (error bars) of three independent experiments. Statistically significant differences compared to the control group (TUC) are indicated (*P < 0.05, ANOVA).
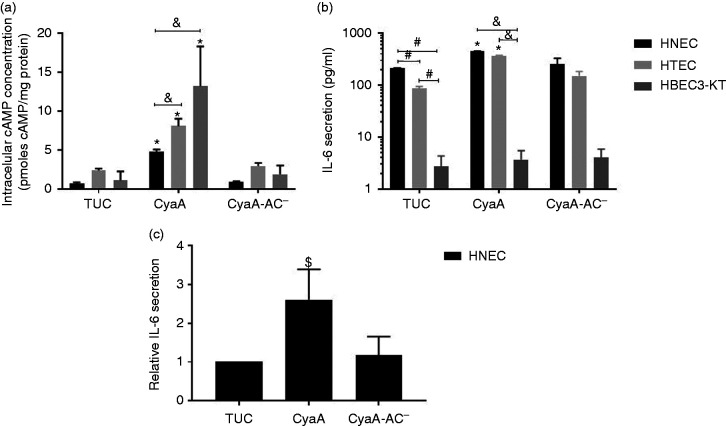
Next, we incubated monolayers of HNEC, HTEC and HBEC3-KT with CyaA or CyaA-AC– and measured the intracellular cAMP concentration to analyse the potential impact of both the toxin and toxoid on the cell cultures. AEC that were treated with TUC buffer served as control. Whereas incubation with either TUC buffer or CyaA-AC– had no effect on intracellular cAMP concentration in all three cell types, treatment with CyaA induced a significant cAMP increase (Figure 5a). After incubation with CyaA, the intracellular cAMP concentration significantly increased to 4.7 ± 0.3 pmol/mg protein in HNEC, to 8.1 ± 1.0 pmol/mg protein in HTEC and to 13.2 ± 5.1 pmol/mg protein in HBEC3-KT. Both HTEC and HBEC3-KT showed significantly higher intracellular cAMP concentrations compared to HNEC.
Figure 5.
CyaA affects intracellular cAMP concentration and IL-6 secretion in human airway epithelial cells. (a) HNEC, HTEC and HBEC3-KT were incubated with CyaA or CyaA-AC– and intracellular cAMP concentration was analysed (n = 4). (b) Quantification of human IL-6 secreted by HNEC, HTEC and HBEC3-KT populations after treatment with CyaA or CyaA-AC– was assayed by ELISA of the supernatants (n = 3). (c) Quantification of human IL-6 secreted by human nasal mucosa tissue models after treatment with CyaA or CyaA-AC– (n=3). All data are presented as means (bars) + SEM (error bars). Differences after CyaA incubation compared to the control group (TUC) are indicated (*P ≤ 0.05, $P > 0.05 ≤ 0.1, ANOVA). Statistically significant differences of IL-6 basal levels are indicated (#P < 0.05, ANOVA). Significant cell type-specific differences are indicated (&P < 0.05, ANOVA).
To study the impact on IL-6 secretion in hAEC, cell monolayers and 3D tissue models of the nasal mucosa were treated with CyaA or CyaA-AC–. TUC buffer-treated samples served as controls. In 2D monolayers, we observed cell type-specific significant differences in basal IL-6 secretion: HNEC secreted 210.4 ± 5.1 pg/ml, HTEC and HBEC3-KT secreted about 2- (86.1 ± 8.7 pg/ml) and 78-fold (2.7 ± 1.6 pg/ml) less IL-6, respectively. Whereas HBEC3-KT were affected by neither CyaA nor CyaA-AC–, CyaA treatment significantly increased IL-6 secretion of HNEC and HTEC to 451.6 ± 1.1 and 371.0 ± 4.0 pg/ml, respectively, compared to the control condition. CyaA-AC– incubation did not affect IL-6 secretion of either HNEC or HTEC (Figure 5b). Moreover, both HNEC and HTEC secreted significantly higher IL-6 concentrations than HBEC3-KT. These data indicated that the selected cell types are differentially susceptible to CyaA in 2D culture conditions. HBEC3-KT did not alter IL-6 secretion after CyaA or CyaA-AC– treatment and thus appear not to be suitable to study toxin–cell interactions further in a complex 3D environment. Since the nasal epithelium first gets in contact with airborne pathogens, we subsequently analysed the effect of CyaA and its toxoid on IL-6 and Muc5B secretion using 3D tissue models of the human nasal mucosa. Whereas CyaA-AC– did not affect relative IL-6 secretion of the 3D tissue models (1.2-fold increase, P = 0.9) compared to the control condition, CyaA treatment elicited a 2.6-fold increase, which was considered as a trend (P = 0.1; Figure 5c).
Immunofluorescent staining of 3D nasal mucosa tissue models suggested an increased Muc5B secretion after application of CyaA (Figure 6a–c). This qualitative observation was confirmed in quantitative ELISA. Whereas the toxoid did not affect Muc5B secretion compared to the control condition, CyaA treatment significantly increased Muc5B secretion to 649.85 ± 85 pg/ml (Figure 6d).
Figure 6.
CyaA modulates Muc5B secretion in 3D nasal mucosa tissue models. Immunofluorescent staining (a–c) revealed a qualitative increase in Muc5B secretion (green) upon CyaA treatment. Cell nuclei are stained with DAPI (blue). Scale bar: 100 µm. ELISA of the supernatants (c) show a quantitative increase in Muc5B secretion after stimulation with CyaA. Data are presented as means (bars) + SEM (error bars) of three independent experiments. Statistically significant differences of Muc5B secretion after treatments compared to control group (TUC) are indicated (*P < 0.05, ANOVA).
Discussion
In the present study, we focused on the interaction of CyaA and its toxoid CyaA-AC– with primary hAEC and HBEC3-KT, a HBEC line. We found that in 2D culture conditions, the selected hAEC were differentially susceptible to applied CyaA concentrations regarding cell viability, cAMP production and IL-6 secretion. In 3D tissue models of the human nasal mucosa, we demonstrated that CyaA was able to increase IL-6 and Muc5B secretion. Furthermore, we showed that the selected hAEC lack CD11b, as indicated earlier.17 It is known that CyaA binds to CR3 (CD11b/CD18) to intoxicate target cells.3 It has been reported elsewhere that CyaA is able to affect colon and airway epithelial cell lines, which do not express CD11b,5,6 however with a much lower impact compared to CR3-positive cells. The present study reveals that primary hAEC are also susceptible to CyaA in both 2D and 3D culture conditions.
In 2D culture conditions, HNEC, HTEC and HBEC3-KT were differentially susceptible to 5.0 µg/ml CyaA and its toxoid in terms of interaction with Annexin V and 7-AAD. HBEC3-KT were not affected at all. In HNEC, CyaA but not CyaA-AC– significantly increased the percentage of Annexin V-positive and 7-AAD-negative cells, which indicates that CyaA induced early apoptosis. It is known that CyaA can induce apoptosis in a macrophage cell line and that both AC and haemolytic activity are required.18 Surprisingly, we found that both CyaA and CyaA-AC– increased the percentage of Annexin V-positive and 7-AAD-negative cells in HTEC, even though the toxoid used in the present study was not expected to induce (early) apoptosis. In early apoptosis, phosphatidylserine (PS), which binds to Annexin V, is translocated from the cytoplasmic to the outer leaflet of the cell membrane. However, also pore-forming agents, such as bacterial toxins, can induce PS exposure without a loss of plasma membrane integrity. This PS exposure is considered to be part of a cell membrane repair mechanism that is mediated by the lipid scramblase TMEM16F.19 Since in HTEC the toxin and the toxoid comparably elevated the percentage of Annexin V-positive and 7-AAD-negative cells, we assume that in these cells, CyaA and CyaA-AC– induced pore formation, whereupon the cells initiated cell membrane repair. Moreover, HTEC treated with the toxoid showed a significantly increased percentage of cells with a compromised cell membrane (Annexin V-negative/7-AAD-positive cells). This increase from 5.0% in the control condition to 9.3% was statistically significant. However, an increase of 4.3% was considered minor biological relevance in this assay. The applied CyaA/CyaA-AC– concentration of 5.0 µg/ml in the present study is most probably physiologically irrelevant and does not reflect the infection situation in vivo. However, these data show the differential susceptibility of primary hAEC and HBEC3-KT to CyaA and its toxoid. The reasons for the observed cell type-specific effects remain speculative. It is known that CyaA binding to CR3 is dependent on the receptor’s glycosylation state.20 Thus, it was suggested that CyaA could interact with glycosylated structures located at the surface of CD11b-negative cells too.6 There is a lack of information on cell type-specific surface glycosylation of HNEC, HTEC and HBEC3-KT. However, it was reported that human tracheal, bronchial and submucosal gland epithelial cells differentially secrete mucin-like glycoproteins, which vary in their relative glycosylation levels.21 Conducting research into this direction could give further insights into cell type-specific susceptibility of hAEC to CyaA both in undifferentiated 2D monolayers and in differentiated respiratory tissue models.
In 2D culture conditions, all three airway epithelial cell types showed an elevated intracellular cAMP level upon stimulation with CyaA, which was cell type specific. As expected, the enzymatically inactive but fully pore-forming toxoid did not change intracellular cAMP level in either of the cell types. Previous findings on increased cAMP secretion after stimulation with CyaA in a hAEC line are comparable to what we found in primary hAEC and HBEC3-KT.6
Moreover, we found a noticeable cell type-specific baseline IL-6 secretion in 2D culture conditions. Our findings are consistent with previously published studies, where significantly higher IL-6 baseline concentrations of HNEC compared to primary HBEC were described.13,22 Further investigations of other groups revealed a differential response of HNEC and HBEC in terms of IL-6 protein level after stimulation with various cytokines, lipopolysaccharide or rhinovirus.12,13,22,23 Since these data were obtained from both paired and non-paired cultures of HNEC and HBEC and yielded consistent results, we consider our data obtained from non-paired samples as reliable in terms of cell type-specific response to CyaA and CyaA-AC–. To exclude an effect of culture conditions on our readouts, we cultured all cell types under comparable conditions. Since nasal epithelial cells are exposed to environmental factors in the first place, it makes sense from a biological point of view that these cells show a more intense innate immune response compared to tracheo-bronchial cells, which are located at lower anatomical sites.
In contrast to primary HNEC and HTEC, HBEC3-KT did not increase IL-6 secretion after CyaA treatment. It was previously found that after silica nanoparticle exposition, HBEC3-KT secreted significantly lower concentrations of IL-6 compared to the respiratory cell line BEAS-2B.24 We found in our previous study9 that another part of the innate immune system, the differentiation to the mucociliary phenotype, was impaired in HBEC3-KT. Thus, we assume that HBEC3-KT are not suitable to study the impact of CyaA further, since the innate immune response, at least in terms of IL-6 secretion and mucociliary differentiation, differs from observations made in primary respiratory epithelial cells.
Since nasal epithelial cells first get in contact with airborne pathogens, we studied the effects of CyaA and its toxoid on the innate immunity of 3D nasal mucosa tissue models to obtain first insights in toxin–host cell interaction in a more in vivo-like environment. The effect of both the toxin and the toxoid on IL-6 secretion obtained from these tissue models are consistent with our observations made in 2D cultures. However, since there was high donor variability, we could only measure a tendential increase of IL-6 secretion after CyaA treatment. Inter-individual innate immune response of respiratory epithelial cells has been reported before25 and still represents a challenge while working with differentiated hAEC. Further assays in a 3D environment showed that CyaA induced Muc5B hypersecretion, which is, besides Muc5AC, the major gel-forming respiratory mucin produced by respiratory goblet cells. It was previously shown that Muc5B secretion could be enhanced by several bacterial virulence factors but also through cytokines.26 For example, IL-6 induces Muc5B expression via the extracellular signal-regulated kinase (ERK) signalling pathway in differentiated human respiratory epithelial tissue models.27
Conclusion and outlook
Even though the hAEC used in the present study lack CD11b, CyaA is able to affect these cells. In general, the in vivo relevance of the applied CyaA concentrations is questionable. Eby et al. suggest local CyaA concentrations in human nasal samples with a maximum of around 100 ng/ml.28 However, the actual local concentrations released by B. pertussis in vivo remain to be determined. Moreover, in vivo CyaA acts with other B. pertussis virulence factors to colonise and intoxicate host cells effectively. Thus, studies focusing solely on one virulence factor can just elucidate certain aspects of host–pathogen interaction. The present study reveals first insights in toxin–host cell interaction using monolayer cultures of primary human AEC and complex tissue models of the nasal mucosa. Subsequent studies should be conducted, preferably in 3D tissue models of the human ciliated respiratory epithelium/mucosa, which show a high in vitro–in vivo correlation.
Acknowledgements
The authors thank Prof. P. Sebo for providing CyaA and CyaA-AC–, and Heike Oberwinkler and Ibukun Olufemi for excellent technical support.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG GRK 2157; 3D Tissue Models for Studying Microbial Infections by Human Pathogens to M.S.). This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
ORCID iD: Maria Steinke https://orcid.org/0000-0003-2675-1374
References
- 1.Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr 2016; 4. [DOI] [PubMed] [Google Scholar]
- 2.Vojtova J, Kamanova J, Sebo P. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr Opin Microbiol 2006; 9: 69–75. [DOI] [PubMed] [Google Scholar]
- 3.Guermonprez P, Khelef N, Blouin E, et al. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αMβ2 integrin (CD11b/CD18). J Exp Med 2001; 193: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassinet L, Fitting C, Housset B, et al. Bordetella pertussis adenylate cyclase-hemolysin induces interleukin-6 secretion by human tracheal epithelial cells. Infect Immun 2004; 72: 5530–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eby JC, Ciesla WP, Hamman W, et al. Selective translocation of the Bordetella pertussis adenylate cyclase toxin across the basolateral membranes of polarized epithelial cells. J Biol Chem 2010; 285: 10662–10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasan S, Kulkarni NN, Asbjarnarson A, et al. Bordetella pertussis adenylate cyclase toxin disrupts functional integrity of bronchial epithelial layers. Infect Immun 2018; 86: e00445-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angely C, Ladant D, Planus E, et al. Functional and structural consequences of epithelial cell invasion by Bordetella pertussis adenylate cyclase toxin. PLoS One 2020; 15: e0228606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guevara C, Zhang C, Gaddy JA, et al. Highly differentiated human airway epithelial cells: a model to study host cell–parasite interactions in pertussis. Infect Dis (Lond) 2016; 48: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodes N, Seidensticker K, Perniss A, et al. Investigation on ciliary functionality of different airway epithelial cell lines in three-dimensional cell culture. Tissue Eng Part A 2020; 26: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widdicombe JH, Sachs LA, Morrow JL, et al. Expansion of cultures of human tracheal epithelium with maintenance of differentiated structure and function. Biotechniques 2005; 39: 249–255. [DOI] [PubMed] [Google Scholar]
- 11.Stewart CE, Torr EE, Mohd Jamili NH, et al. Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy (Cairo) 2012; 2012: 943982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comer DM, Elborn JS, Ennis M. Comparison of nasal and bronchial epithelial cells obtained from patients with COPD. PLoS One 2012; 7: e32924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paplińska-Goryca M, Nejman-Gryz P, Chazan R, et al. The expression of the eotaxins IL-6 and CXCL8 in human epithelial cells from various levels of the respiratory tract. Cell Mol Biol Lett 2013; 18: 612–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweinlin M, Rossi A, Lodes N, et al. Human barrier models for the in vitro assessment of drug delivery. Drug Deliv Transl Res 2017; 7: 217–227. [DOI] [PubMed] [Google Scholar]
- 15.Steinke M, Gross R, Walles H, et al. An engineered 3D human airway mucosa model based on an SIS scaffold. Biomaterials 2014; 35: 7355–7362. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim SF, Van Den Engh G. Flow cytometry and cell sorting. Adv Biochem Eng Biotechnol 2007; 106: 19–39. [DOI] [PubMed] [Google Scholar]
- 17.Sheppard D. Airway epithelial integrins: why so many? Am J Respir Cell Mol Biol 1998; 19: 349–351. [DOI] [PubMed] [Google Scholar]
- 18.Khelef N, Guiso N. Induction of macrophage apoptosis by Bordetella pertussis adenylate cyclase-hemolysin. FEMS Microbiol Lett 1995; 134: 27–32. [DOI] [PubMed] [Google Scholar]
- 19.Wu N, Cernysiov V, Davidson D, et al. Critical role of lipid scramblase TMEM16F in phosphatidylserine exposure and repair of plasma membrane after pore formation. Cell Rep 2020; 30: 1129–1140.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morova J, Osicka R, Masin J, et al. RTX cytotoxins recognize beta2 integrin receptors through N-linked oligosaccharides. Proc Natl Acad Sci U S A 2008; 105: 5355–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor GW, Chopra DP, Mathieu PA. Differences in secretory profiles of epithelial cell cultures derived from human tracheal and bronchial mucosa and submucosal glands. Epithelial Cell Biol 1993; 2: 163–169. [PubMed] [Google Scholar]
- 22.Alves MP, Schögler A, Ebener S, et al. Comparison of innate immune responses towards rhinovirus infection of primary nasal and bronchial epithelial cells. Respirology 2016; 21: 304–312. [DOI] [PubMed] [Google Scholar]
- 23.Pringle EJ, Richardson HB, Miller D, et al. Nasal and bronchial airway epithelial cell mediator release in children. Pediatr Pulmonol 2012; 47: 1215–1225. [DOI] [PubMed] [Google Scholar]
- 24.Låg M, Skuland T, Godymchuk A, et al. Silica nanoparticle-induced cytokine responses in BEAS-2B and HBEC3-KT cells: significance of particle size and signalling pathways in different lung cell cultures. Basic Clin Pharmacol Toxicol 2018; 122: 620–632. [DOI] [PubMed] [Google Scholar]
- 25.Ilyushina NA, Dickensheets H, Donnelly RP. A comparison of interferon gene expression induced by influenza A virus infection of human airway epithelial cells from two different donors. Virus Res 2019; 264: 1–7. [DOI] [PubMed] [Google Scholar]
- 26.Hauber H-P, Foley SC, Hamid Q. Mucin overproduction in chronic inflammatory lung disease. Can Respir J 2006; 13: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Thai P, Zhao Y-H, et al. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 2003; 278: 17036–17043. [DOI] [PubMed] [Google Scholar]
- 28.Eby JC, Gray MC, Warfel JM, et al . Quantification of the adenylate cyclase toxin of Bordetella pertussis in vitro and during respiratory infection. Infect Immun 2013; 81: 1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]