Abstract
Treatment considerations for epilepsy patients requiring anticoagulation are changing, and actual prescribing practices have not been characterized. We used the 2010-2018 Optum Clinformatics® Data Mart Database to estimate the annual prevalence and distinguish the patterns of oral anticoagulants (OACs) co-dispensed with antiepileptic drugs (AEDs) among adults with epilepsy. Monotonic trends were assessed using the Spearman rank correlation coefficient (ρ). Multivariable logistic regression models were built to evaluate the associations of sociodemographic characteristics. Among 345,892 adults with epilepsy (56.5% female; median age 61, IQR 46-74) on studied AEDs, the prevalence per thousand of concurrent OACs increased from 58.4 in 2010 to 92.0 in 2018 (OR 1.63, CI 1.58-1.69). Direct-acting OAC (DOAC) use rapidly increased from 2010-2018 (ρ= 1.00; P<0.001), with a corresponding decrease in warfarin use (ρ= −0.97; P<0.001). Among OAC/AED dispensings in 2018, warfarin was more likely to be co-dispensed with potentially interacting, enzyme-inducing AEDs (EI-AEDs) versus presumably non-interacting, non-enzyme inducing AEDs (OR 1.48, CI 1.38-1.59). Characteristics independently associated with concurrent OAC/EI-AED use included younger age, female sex, white race, net worth <$250K, and lower education levels. Our findings demonstrate the expanding use and evolving patterns of OAC/AED co-dispensing, and ensuing critical need to further understanding regarding postulated interactions.
Keywords: drug utilization, epilepsy, enzyme-inducing antiepileptic drug, direct-acting oral anticoagulant, warfarin
1. Introduction
Epilepsy frequently occurs concurrently with or secondary to thrombotic conditions, with approximately 10% of all epilepsy and 50% of epilepsy in older adults attributable to cerebrovascular disease.[1,2] Treatment decisions for this substantial epilepsy sub-population have grown increasingly complex in light of the mounting evidence of significant pharmacokinetic interactions between many widely-used antiepileptic drugs (AEDs) and oral anticoagulants (OACs).[3–7] In particular, the impacts of enzyme-inducing AEDs (EI-AEDs) on key components of warfarin and direct-acting OAC (DOAC) metabolism, including cytochrome p450 (CYP) enzymes and permeability glycoprotein (P-gp) efflux transporters, may substantially alter these OACs’ serum levels and raise the risk of major thromboembolic or bleeding events.[3–7] Despite the potential high clinical relevance of these postulated interactions, inconsistencies in their representations among leading prescriber information sources has raised concerns about real-world practices.[8] Moreover, research and guidelines on concurrent use of these important drug classes remains stymied by the lack of data on co-prescribing in the >3 million epilepsy patients in the US.[9] Thus, we sought to investigate and elucidate combined OAC and AED use nationally in adults with epilepsy.
2. Methods
We conducted a retrospective assessment of OAC and AED dispensing using Optum’s de-identified 2010-2018 Clinformatics® Data Mart Database, a large US commercial and Medicare advantage health insurance claims database. Included enrollees were ≥18 years of age with ICD-9/10 codes for epilepsy (345 or G40/G41) or convulsions (780.3 or R56.8). Episodes of dispensing of select AEDs lasting ≥14 contiguous days in a given year were counted and classified in terms of presence of ≥1 enzyme-inducing AEDs (EI-AEDs, potentially interacting), or presence of only non-enzyme inducing AEDs (NEI-AEDs, presumably non-interacting). Selection and categorization of EI-AEDs and NEI-AEDs were based on the combination of in vitro, animal, and clinical data on their respective pharmacokinetic effects.[3–7] EI-AEDs were defined as carbamazepine, phenobarbital, phenytoin, primidone, oxcarbazepine, and topiramate. Despite being considered weaker inducers of CYP3A4 and inhibitors of CYP2C19, oxcarbazepine and topiramate were included in the EI-AED category for this analysis, as these effects are plausible mechanisms for interactions with both warfarin and DOACs.[3–7] NEI-AEDs consisted of lacosamide, lamotrigine, and levetiracetam. Other common NEI-AEDs (for example, gabapentin) were excluded due to their frequent use for indications other than epilepsy.
Annual prevalence of overlapping prescriptions for OACs with AEDs (≥14 days) was calculated over successive years, with assessment for monotonic temporal trends using the Spearman rank correlation coefficient (ρ). Based on prior evidence regarding the pharmacokinetics of EI-AEDs,[7] a minimum of 14 days of overlapping use with an OAC was required for inclusion, as prescribing considerations relative to the risk of these drug-drug interactions would become less straightforward with episodes of <14 days. Examined OACs consisted of warfarin and DOACs, including direct thrombin inhibitors (dabigatran) and factor Xa inhibitors (rivaroxaban and apixaban). Age and gender information was collected for all eligible enrollees. Additional, characteristics (race, net worth, education level, division) were collected for the sub-group co-dispensed AEDs with OACs in 2018, the most recent year for which we had data. Net worth was modeled at the household-level, and education level was census block-derived. Multivariable logistic regression models were fit to evaluate the associations of sociodemographic characteristics with concomitant prescribing patterns in 2018, adjusting for included characteristics. Missing values were imputed with multiple imputation by chained equations. Statistical analyses were conducted in Stata version 15.1 (StataCorp, College Station, TX). The University of Pennsylvania determined this research was exempt from review.
3. Results
Among 345,892 adults with epilepsy (56.5% female; median age 61, interquartile range (IQR) 46-74) on studied AEDs, the prevalence per thousand patients of concurrent OACs increased from 58.4 in 2010 to 92.0 in 2018 (odds ratio (OR) 1.63, 95% confidence interval (CI) 1.58-1.69). In 2010, the population on concurrent OACs/AEDs had a median age of 69 (IQR 58-79) and was 50.7% female; by 2018, the population was slightly older (median age 72, IQR 63-80), with a similar gender distribution (51.4% female). DOAC use in this population rapidly increased from 2010 to 2018, with yearly percentage point changes in co-dispensing with AEDs of +0.29, +0.30, +0.41, + 0.57, +0.59, +0.84, +1.06, +1.31 (ρ = 1.00; P<0.001). Simultaneously, warfarin use with AEDs decreased, with yearly percentage point changes of +0.02, −0.01, −0.08, −0.35, −0.30, −0.20, −0.34, −0.39 (ρ = −0.97; P<0.001). Figure 1A illustrates the directionality of these trends are similar when stratified by interaction potential into EI-AED versus NEI-AEDs. Figure 1B demonstrates these trends vary by specific DOAC.
Figure 1.
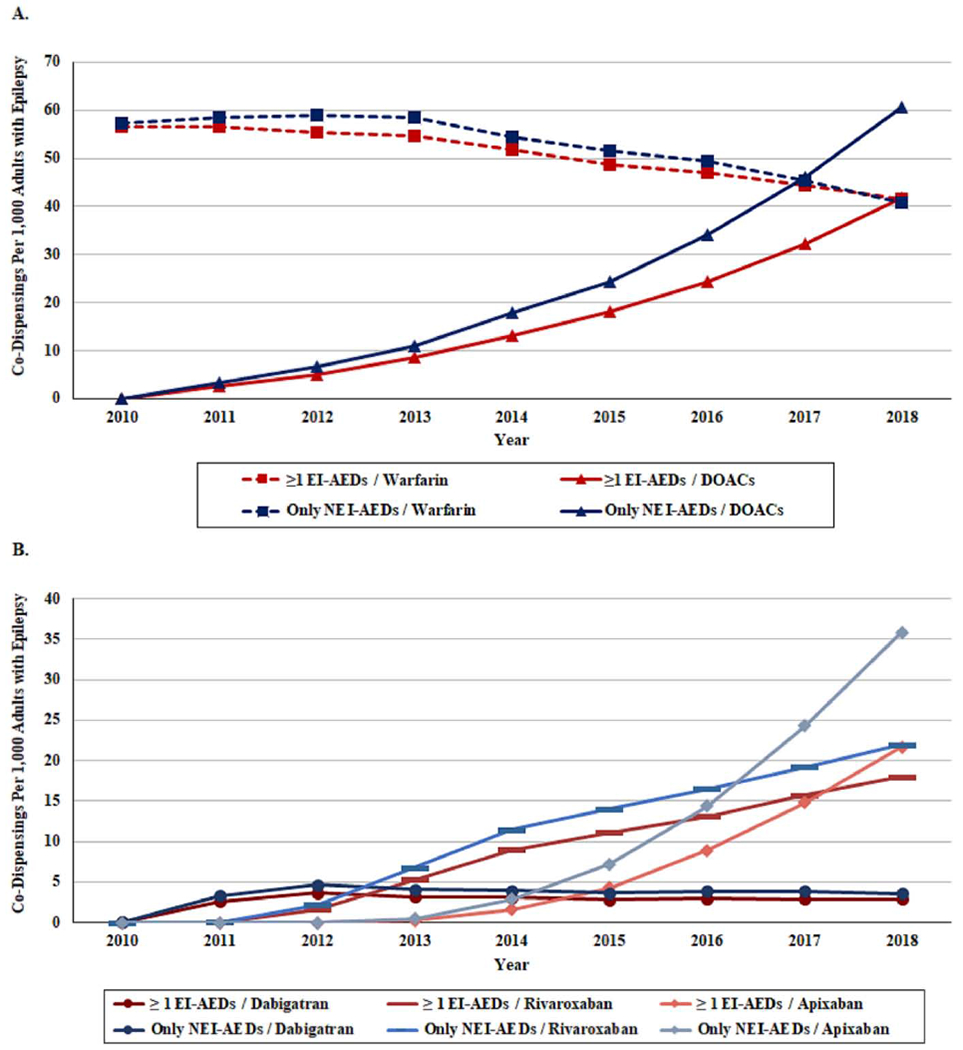
A. Yearly prevalence of adults with epilepsy co-dispensed oral anticoagulants (OACs) with ≥1 enzyme inducing antiepileptic drugs (EI-AEDs) versus only non-enzyme inducing anti epileptic drugs (NEI-AEDs), 2010-2018
B. Yearly prevalence of adults with epilepsy co-dispensed specific direct-acting oral anticoagulants (DOACs) with ≥1 enzyme inducing antiepileptic drugs (EI-AEDs) versus only non-enzyme inducing anti epileptic drugs (NEI-AEDs), 2010-2018
Abbreviations: DOAC=direct-acting oral anticoagulant; EI-AED=enzyme-inducing antiepileptic drug; NEI-AED=non-enzyme-inducing antiepileptic drug; OAC=oral anticoagulant
Among OAC/AED dispensing episodes in 2018, warfarin was more likely to be co-dispensed with potentially interacting EI-AEDs versus NEI-AEDs (50.0% EI-AED episodes versus 40.2% NEI-AED episodes, respectively; OR 1.48, CI 1.38-1.59). Table 1 displays the patient characteristics associated with exposure to these potential drug-drug interactions between EI-AEDs and OACs in 2018. Reduced odds of EI-AED/OAC co-dispensing were found with greater age (OR 0.98 per year, CI 0.98-0.99), as well as with Black (OR 0.75, CI 0.66-0.84) and Hispanic (OR 0.79, CI 0.68-0.93) race/ethnicity. Lower odds of concurrent EI-AED/OAC use were also found for those with higher net worth or education level. Increased odds of EI-AED/OAC co-dispensing were associated with female sex (OR 1.13, CI 1.05-1.22).
Table 1.
Association between patient characteristics and co-dispensing of oral anticoagulants (OACs) with ≥1 episodes of enzyme-inducing antiepileptic drugs (EI-AEDs) versus only non-enzyme-inducing antiepileptic drugs (NEI-AEDs), 2018
| Adults with Epilepsy on OACs | |||
|---|---|---|---|
| ≥1 Episodes of EI-AEDs with OACs (n= 4,837) | Only NEI-AEDs with OACs (n= 7,584) | Adjusted OR of ≥1 Episodes of EI-AEDs with OACs a,b (95% CI) | |
| Age, median (IQR), y | 70 (61-78) | 73 (64-81) | 0.98 (0.98-0.99) |
| Women, No. (%) | 2,569 (53.1%) | 3,808 (50.2%) | 1.13 (1.05-1.22) |
| Race, No. (%) | |||
| White | 2,992 (61.9%) | 4,358 (57.5%) | Reference |
| Black | 646 (13.4%) | 1,087 (14.3%) | 0.75 (0.66-0.84) |
| Hispanic | 291 (6.0%) | 497 (6.6%) | 0.79 (0.68-0.93) |
| Asian | 48 (1.0%) | 98 (1.3%) | 0.76 (0.54-1.08) |
| Unknown | 860 (17.8%) | 1,544 (20.4%) | --- |
| Net Worth, No. (%) | |||
| <$25K | 1,199 (24.8%) | 1,594 (21.0%) | Reference |
| $25K-$149K | 660 (13.6%) | 968 (12.8%) | 0.91 (0.81-1.03) |
| $150K-$249K | 350 (7.2%) | 500 (6.6%) | 0.94 (0.81-1.10) |
| $250K-$499K | 467 (9.7%) | 809 (10.7%) | 0.83 (0.72-0.97) |
| >$499K | 540 (11.2%) | 1,117 (14.7%) | 0.78 (0.67-0.91) |
| Unknown | 1,621 (33.5%) | 2,596 (34.2%) | --- |
| Education Level, No. (%) | |||
| High School Diploma or Less | 1,567 (32.4%) | 2,187 (28.8%) | Reference |
| Less than Bachelor Degree | 2,159 (44.6%) | 3,265 (43.1%) | 0.95 (0.87-1.05) |
| Bachelor Degree Plus | 373 (7.7%) | 796 (10.5%) | 0.76 (0.65-0.90) |
| Unknown | 738 (15.3%) | 1,336 (17.6%) | --- |
| Division of the Country, No. (%) | |||
| New England (CT, ME, MA, NH, RI, VT) | 225 (4.7%) | 368 (4.9%) | Reference |
| Middle Atlantic (NJ, NY, PA) | 403 (8.3%) | 737 (9.7%) | 0.89 (0.73-1.10) |
| East North Central (IL, IN, MI, OH, WI) | 706 (14.6%) | 1,005 (13.3%) | 1.08 (0.89-1.31) |
| West North Central (IA, KS, MN, MO, NE, ND, SD) | 395 (8.2%) | 534 (7.0%) | 1.09 (0.88-1.35) |
| South Atlantic (DE, DC, FL, GA, MD, NC, SC, VA, WV) | 1,315 (27.2%) | 2,140 (28.2%) | 0.95 (0.79-1.14) |
| East South Central (AL, KY, MS, TN) | 243 (5.0%) | 341 (4.5%) | 1.05 (0.83-1.33) |
| West South Central (AR, LA, OK, TX) | 630 (13.0%) | 833 (11.0%) | 1.12 (0.91-1.36) |
| Mountain (AZ, CO, ID, MT, NV, NM, UT, WY) | 460 (9.5%) | 748 (9.9%) | 0.97 (0.79-1.19) |
| Pacific (AK, CA, HI, OR, WA) | 450 (9.3%) | 863 (11.4%) | 0.91 (0.74-1.11) |
| Unknown | 10 (0.2%) | 15 (0.2%) | --- |
Adjusted for age, gender, race, net worth, education level, and division.
Missingness was imputed for gender, race, net worth, and education level using all assessed variables.
Abbreviations: EI-AED=enzyme-inducing antiepileptic drug; NEI-AED=non-enzyme-inducing antiepileptic drug; OAC=oral anticoagulant
4. Discussion
Treatment considerations for anticoagulation in those with epilepsy are complex, due to both these patients’ unique risk profiles for thrombotic/bleeding events,[1] and the interaction potential of OACs with EI-AEDs.[3–7] In this study of a large, nationally representative population of commercially insured adults and Medicare advantage enrollees, we found the proportion of people with epilepsy on AEDs that were co-prescribed OACs has risen rapidly in the past decade, reaching 9.2% by 2018. As expected,[10] DOACs have overtaken warfarin as the OACs of choice in this population. In particular, the factor Xa inhibitors, rivaroxaban and apixaban, exhibited rapid uptake following their initial Food and Drug Administration (FDA) approvals in 07/2011 and 12/2012, respectively. However, these changes in drug utilization patterns may further complicate prescribing. While there is clinical and mechanistic evidence to support the existence of interactions between warfarin and EI-AEDs, these interaction can often be detected and warfarin dosing can be suitably adjusted based on regular international normalized ratio monitoring.[3,5] In contrast, the clinical effects of potential DOAC/EI-AED interactions remain uncertain, as supporting data derives primarily from in vitro, animal, and case studies.[4,6] Moreover, as DOACs are not regularly monitored, any effects of these interactions on DOAC serum levels would likely remain undetected until the occurrence of thromboembolic events. Thus, data on any large-scale clinical impacts of DOAC/EI-AED interactions may be best derived from observational studies with rigorous pharmacoepidemiology methods, beginning with our assessment of concurrent use and characterization of the at-risk population.
Our measurements of yearly co-dispensing revealed the uptake of DOAC use with EI-AEDs has persistently lagged that with NEI-AEDs (i.e. DOAC/EI-AED episodes surpassed warfarin/EI-AED episodes in 2018, a year after DOAC/NEI-AEDs episodes surpassed warfarin/NEI-AED episodes in 2017). Consequently, among episodes of OAC/AED use in 2018, warfarin had higher odds, and DOACs had corresponding lower odds of being dispensed concurrently with potentially interacting EI-AEDs versus NEI-AEDs. The reasons for these findings are likely multifactorial. Firstly, awareness of the potential OAC/EI-AED interactions, coupled with understanding that INR monitoring for warfarin may detect clinically-relevant interactions prior to adverse event occurrence, may make prescribers more likely to use warfarin over DOACs (or make them slower to switch from using warfarin to DOACs) among epilepsy patients on EI-AEDs. Secondly, and perhaps more likely, the EI-AED/warfarin dispensing pattern may be driven by prescribers who were guided by more traditional practice styles, who were less convinced by the still developing evidence basis that broadly supports use of DOACs over warfarin for anticoagulation and NEI-AEDs over EI-AEDs for seizure control, who were more influenced in their prescribing decisions by the longer history of safety/efficacy data with the older drugs (i.e. warfarin being older than DOACs, and EI-AEDs being generally older than NEI-AEDs), or whose patient populations were generally clinically stable and well managed on chronic medication regimens that include these older drugs. Finally, uncertainty stemming from the dearth of evidence regarding the clinical relevance of DOAC/EI-AED interactions and variable drug compendia reporting for these postulated interactions may further encourage preferential use of warfarin over DOACs among patients on EI-AEDs.[4,6,8]
Specific sociodemographic characteristics were more strongly associated with being prescribed potentially interacting EI-AEDs among those with epilepsy on OACs and AEDs, including younger age, female sex, white race, net worth <$250K, and lower education levels. Interestingly, when compared to the characteristics associated with higher likelihood of EI-AED use in the general epilepsy population from large US and UK datasets, the associated characteristics in our study population were in some cases different (i.e. general EI-AED use has been associated with older age and male gender), and in others comparable (i.e. general EI-AED use has been associated with lower socioeconomic status).[11,12] These distinctions further underscore the need for more focused assessment of epilepsy populations with indications for OACs, as they represent a uniquely high-risk group for a number of adverse outcomes including major drug-drug interactions. Importantly, our findings may have been impacted by the interplay between US prescription prices for EI-AEDs (versus NEI-AEDs) and the dispensing of these drugs among different sociodemographic groups. In recent years, the increased availability of generics for lamotrigine and levetiracetam (though not lacosamide, which was still brand-only in 2018) have substantially lessened the previously well-recognized price divide between low-priced, older EI-AEDs and high-cost, newer NEI-AEDs.[13,14] In fact, when total direct healthcare costs were assessed, EI-AEDs appeared more costly than NEI-AEDs.[12,13] As such, the effects of prescription prices (or prescriber perception of these prices) on observed EI-AED dispensing patterns in those on potential interacting OACs is not clear-cut, and represents another area that merits further study.
Our study had certain limitations that are important to recognize in the interpretation and contextualization of our findings. First, this study employed claims data which would exclusively represent dispensed prescriptions. Thus, we were not able to directly assess all prescriptions written by prescribers, nor patient adherence to dispensed prescriptions. Second, our observations may not be generalizable to populations beyond commercially insured adults and Medicare advantage enrollees. Third, this investigation focused on dispensing of EI-AEDs and NEI-AEDs at the group-level, and therefore, may not be indicative of dispensing patterns for individual EI-AEDs or NEI-AEDs with OACs. Additionally, the newer DOACs (i.e. edoxaban and betrixaban) were not included in this assessment. Finally, the risk of adverse events from drug-drug interactions at the level of the individual patient depends on a multitude of interconnecting factors ranging from drug dosing to patient medical history, comorbidities, and concomitant prescriptions. These factors were not evaluated in this analysis and represent key focal points for future studies.
5. Conclusions
Taken together, our findings demonstrate the expanding use and evolving patterns of OAC/AED dispensing among adults with epilepsy, particularly the surge in co-dispensed DOACs following their regulatory approvals from 2010 onward. These results highlight the importance of better understanding prescribing and management considerations for anticoagulation therapies in epilepsy. Specifically, the growing pervasiveness of EI-AED use with OACs, and the differing trends in EI-AED use with DOACs versus warfarin, reveal the critical need for research focused on the clinical impacts of these potential interactions and guidance to advance prescribing practices.
Highlights.
We assessed the patterns of OAC co-dispensing with AEDs among adults with epilepsy
Among those on AEDs, the prevalence of concurrent OACs rose to 92.0/1,000 by 2018
DOAC use with AEDs rapidly increased, reaching 53.9 per 1,000 by 2018 (ρ= 1.00)
Warfarin use with AEDs decreased to 42.0 per 1,000 by 2018 (ρ= −0.97)
Enzyme-inducing AED use with OACs was associated with net worth and education level
Acknowledgments
Funding Information
This study was supported by a grant from the American Epilepsy Society (AES) Pre-Doctoral Research Fellowship. This work was also supported by grants from the United States Department of Health and Human Services, National Institute on Aging (R01AG060975, R01AG025152, R01AG064589) and National Institute on Drug Abuse (R01DA048001).
These funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or, in the decision to submit the article for publication.
Declaration of Competing Interest
Dr. Gelfand receives clinical trial support from Aquestive, Biogen, Cerevel, Eisai, Epilepsy Foundation, Engage Therapeutics, Livanova, Otsuka, SK Life Science, and UCB. Dr. Hennessy leads an academic training program at the University of Pennsylvania that receives support from Pfizer Inc. Dr. Hennessy has received salary support from contracts between the University of Pennsylvania and Johnson & Johnson and Pfizer Inc. Dr. Hennessy also serves on the steering committee of the Medullary Thyroid Cancer Consortium, which is supported by (Novo Nordisk Inc, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline LLC, and Eli Lilly and Company). Dr. Pollard has a small ownership in Cognizance Biomarkers, a seizure biomarker company. Dr. Kasner receives grant support from Bristol Myers Squibb and consulting fees from Bristol Myers Squibb, Janssen, Bayer. The remaining authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 2016;15:106–15. 10.1016/S1474-4422(15)00225-2 [doi]. [DOI] [PubMed] [Google Scholar]
- [2].Feyissa AM, Hasan TF, Meschia JF. Stroke-related epilepsy. Eur J Neurol 2019;26:18–e3. 10.1111/ene.13813. [DOI] [PubMed] [Google Scholar]
- [3].Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol 2003;2:347–56. 10.1016/S1474-4422(03)00409-5. [DOI] [PubMed] [Google Scholar]
- [4].Galgani A, Palleria C, Iannone LF, De Sarro G, Giorgi FS, Maschio M, et al. Pharmacokinetic Interactions of Clinical Interest Between Direct Oral Anticoagulants and Antiepileptic Drugs. Front Neurol 2018;9:1067 10.3389/fneur.2018.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perucca E Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006;61:246–55. 10.1111/j.1365-2125.2005.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stollberger C, Finsterer J. Interactions between non-vitamin K oral anticoagulants and antiepileptic drugs. Epilepsy Research 2016;126:98–101. 10.1016/j.eplepsyres.2016.06.003 [doi]. [DOI] [PubMed] [Google Scholar]
- [7].Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 2013;54:11–27. 10.1111/j.1528-1167.2012.03671.x [doi]. [DOI] [PubMed] [Google Scholar]
- [8].Acton EK, Willis AW, Gelfand MA, Kasner SE. Poor concordance among drug compendia for proposed interactions between enzyme-inducing antiepileptic drugs and direct oral anticoagulants. Pharmacoepidemiol Drug Saf 2019;28:1534–8. 10.1002/pds.4896. [DOI] [PubMed] [Google Scholar]
- [9].Zack MM. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy — United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66 10.15585/mmwr.mm6631a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and Variation in Oral Anticoagulant Choice in Patients with Atrial Fibrillation, 2010-2017. Pharmacotherapy 2018;38:907–20. 10.1002/phar.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pisu M, Richman J, Piper K, Martin R, Funkhouser E, Dai C, et al. Quality of Antiepileptic Treatment Among Older Medicare Beneficiaries With Epilepsy: A Retrospective Claims Data Analysis. Med Care 2017;55:677–83. 10.1097/MLR.0000000000000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Borghs S, Thieffry S, Noack-Rink M, Dedeken P, Hong LS, Byram L, et al. Health care cost associated with the use of enzyme-inducing and non-enzyme–active antiepileptic drugs in the UK: a long-term retrospective matched cohort study. BMC Neurol 2017;17:59 10.1186/s12883-017-0837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mintzer S, Mattson RT. Should enzyme-inducing antiepileptic drugs be considered first-line agents? Epilepsia 2009;50 Suppl 8:42–50. 10.1111/j.1528-1167.2009.02235.x [doi]. [DOI] [PubMed] [Google Scholar]
- [14].Holtkamp M, Theodore WH. Generic antiepileptic drugs-Safe or harmful in patients with epilepsy? Epilepsia 2018;59:1273–81. 10.1111/epi.14439. [DOI] [PubMed] [Google Scholar]


