Abstract
Background
Granulomatosis with polyangiitis (GPA), formerly known as Wegener’s granulomatosis, is a systemic inflammatory process predominantly affecting upper and lower respiratory tract and kidneys. Valvular heart disease is a rare manifestation of GPA.
Case summary
We report two cases of acute valvular heart disease mimicking acute endocarditis caused by GPA. Both patients were middle-aged females with acute aortic valve regurgitation suggestive of possible infective endocarditis. In their recent medical history, atypical otitis and sinusitis were noted. The first patient was admitted with heart failure and the second patient because of persisting fever. Echocardiogram revealed severe aortic regurgitation with an additional structure on two cusps, suggestive of infective endocarditis in both patients. Urgent surgical replacement was performed; however, intraoperative findings did not show infective endocarditis, but severe inflammatory changes of the valve and surrounding tissue. In both patients, the valve was replaced by a prosthetic valve. Microscopic examination of the valve/myocardial biopsy showed diffuse acute and chronic inflammation with necrosis and necrotizing granulomas, compatible with GPA after infectious causes were excluded. Disease remission was obtained in both patients, in one patient with Rituximab and in the other with Glucocorticoids and Cyclophosphamide. Both had an uneventful follow-up.
Discussion
Granulomatosis with polyangiitis can be a rare cause of acute aortic valve regurgitation mimicking infective endocarditis with the need for surgical valve replacement. Atypical ear, nose, and throat symptoms can be a first sign of GPA. Symptom recognition is important for early diagnosis and appropriate treatment to prevent further progression of the disease.
Keywords: Granulomatosis with polyangiitis (Wegener’s granulomatosis), Endocarditis, Aortic valve replacement, Case series
Learning points
Granulomatosis with polyangiitis (GPA) can be a rare cause of acute aortic valve regurgitation mimicking infective endocarditis and can necessitate aortic valve replacement.
Clinical presentation of GPA is often insidious and lesions of ear, nose, and throat, lung, and/or kidney are most frequently seen. Symptom recognition is important for early diagnosis and appropriate treatment to prevent further progression of the disease.
Introduction
Granulomatosis with polyangiitis (GPA), formerly known as Wegener’s granulomatosis, is a form of anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV). AAV is a necrotizing vasculitis predominantly affecting small vessels and associated with ANCAs specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA). Granulomatosis with polyangiitis is characterized by necrotizing granulomatous inflammation predominantly affecting small to medium vessels of the upper and lower respiratory tract and kidneys.1 Cardiac involvement is rather rare in GPA, however cardiac manifestations such as pericarditis, coronary arteritis, myocarditis, arrhythmias, and valvulitis have been described occasionally.2–5 Valvular disease is rarely reported and the valvular lesion is usually isolated, with aortic valve regurgitation being the most frequent finding.5–7 We report two cases of severe acute aortic valve regurgitation, both caused by aortic valve infiltration due to GPA, necessitating surgical replacement. The purpose of this case report is to remind clinicians that GPA can be a cause of acute aortic valve insufficiency and atypical ear, nose, and throat (ENT) symptoms can be the first signs of GPA.
Timeline
| Events—Case 1 | |
| 2016 | Bilateral tympanostomy tubes. |
| October 2018 | Bilateral peripheral pulmonary embolisms. |
| October 2019 | Referral to rheumatologist and treatment with hydroxychloroquine because of suspected autoimmune disease. |
| 24 October 2019 | Hospitalization due to heart failure. |
| 15 November 2019 | Readmission because of exertional dyspnoea upon minimal effort. Echocardiogram shows severe aortic regurgitation. |
| 19 November 2019 | Aortic valve replacement with mitral and tricuspid valve annuloplasty. |
| January 2020 | Start treatment with Rituximab because of granulomatosis with polyangiitis (GPA). |
| March 2020 | No more GPA disease activity. |
| Events—Case 2 | |
| June 2015 | Functional endoscopic sinus surgery and bilateral tympanostomy tubes. |
| 25 July 2015 | Hospitalization with tentative diagnosis of infective endocarditis. |
| 11 August 2015 | Persistent moderate aortic valve regurgitation with thickened aortic valve leaflets and a thickening of the aortomitral continuity. |
| 13 August 2015 | Aortic valve replacement. |
| 24 August 2015 | Dual-chamber pacemaker because of persistent third-degree atrioventricular block. |
| September 2015 | Start corticoid and cyclophosphamide therapy because of GPA. |
| 4 years of follow-up | Non-evolving, minimal paravalvular regurgitation, without GPA disease activity. |
Case presentation
Patient 1
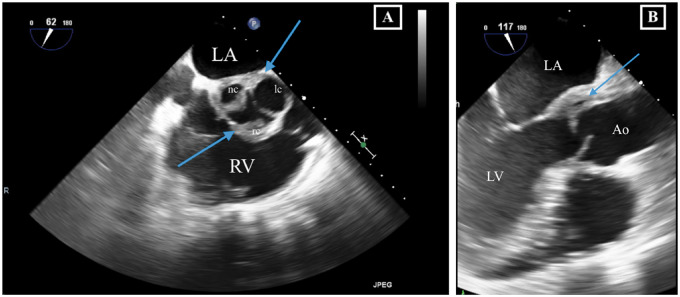
A 58-year-old woman with 3 years history of recurrent episodes of otitis media with effusion and persistent hearing loss, for which bilateral tympanostomy tubes were placed. Bilateral peripheral pulmonary embolisms were also noted in her medical history. Recently, she was referred to a rheumatologist because of slightly elevated inflammatory blood markers. Further tests showed an ANF of 1/320 (<1/80) with anti-Sjögren’s syndrome-related antigen A antibodies of more than 640 kU/L (<10), and positive ANCAs [pANCAs: 1/320 and anti-MPO: 60.4 kU/L (<10)]. The tests suggested autoimmunity for which therapy with hydroxychloroquine was started. However, due to side-effects treatment was stopped after 3 days. Later on, she was hospitalized because of exertional dyspnoea without orthopnoea. Physical examination revealed no abnormalities except for bibasal crepitus upon auscultation. There was a discretely elevated C-reactive protein (CRP) of 13 mg/L (<3.0) and D-dimers were 1437 ng/mL (0–500). Bilateral ground-glass opacities were seen in both lower lung lobes (Figure 1). Bronchoalveolar lavage isolated a methicillin-sensitive Staphylococcus aureus, for which no antibiotic treatment was deemed necessary. Transthoracic echocardiography (TTE) revealed a slight decrease of the systolic left ventricular function, a mild aortic regurgitation, a moderate mitral valve insufficiency, and a moderate tricuspid valve insufficiency. A moderate pulmonary hypertension with pulmonary artery pressure of 52 mmHg was measured. A first-degree atrioventricular (AV) block (PR interval 298 ms) and a prolonged QT (526 ms) were observed on electrocardiogram. Therapy with spironolactone and furosemide was started, and she was discharged after 8 days. After a short clinical improvement, an acute deterioration with nausea and exertional dyspnoea upon minimal effort was seen. A new diastolic murmur and bibasal crepitations were noticed. Blood pressure measured 120/50 mmHg, heart rate 88 b.p.m. and jugular venous pressure (JVP) was 6 cm above the sternal angle. Laboratory findings showed an erythrocyte sedimentation rate of 48 mm/h (0–30), a normal white blood count, minimally elevated CRP of 7.2 mg/L (<3.0), a raised serum creatinine of 1.13 mg/dL (0.55–1.02), and a N-terminal pro B-type natriuretic peptide of 7237 pg/mL (0–125). Transoesophageal echocardiography (TOE) showed a left ventricular ejection fraction of 53%, a severe aortic regurgitation with a new additional structure seen at the level of the non-coronary cusp (8 mm), and thickening of the right coronary cusp (RCC, 3 mm), and the aortomitral continuity (7 mm) (Figure 2 and Video 1). The insufficiencies of the mitral and tricuspid valve were assessed slightly worse than before and pulmonary hypertension was also increased. The tentative diagnosis of infective endocarditis was made and an empirical antibiotic therapy with ampicillin (6 × 2 g IV), flucloxacillin (6 × 2 g IV), and gentamycin (1 × 240 mg IV) was started immediately. During hospitalization, an episode of paroxysmal atrial fibrillation with spontaneous conversion to sinus rhythm was observed. She was transferred to our centre in a stable condition 3 days later and scheduled for surgery. Coronary artery imaging was not performed prior to surgery. Intraoperative findings did not show endocarditis but a pronounced thickening and oedema of the non-coronary and RCC (Figure 3). The mitral and tricuspid valves were inspected but no abnormalities were seen. An aortic valve replacement was performed with a 25 mm mechanical valve and a mitral and tricuspid valve annuloplasty with a 28 mm and 30 mm ring, respectively. The procedure was uneventful. Anatomopathological findings of the aortic valve included diffuse active and chronic inflammation with necrotizing granulomas, suggestive for GPA after exclusion of infection with micro-organisms (Figures 4 and 5). Intraoperative cultures were negative and all blood cultures also remained negative. Postoperative course was uneventful and the patient was discharged 7 days after surgery. Rituximab was administrated at 6 and 8 weeks after surgery and Methotrexate (15 mg/week) was started 3 months after surgery. During the follow-up period of 6 months, no more disease activity was seen. Inflammatory markers and kidney function remained normal and TTE showed a normal systolic function and a normal function of the valves.[AQ: Please spell out ANF (if necessary).]
Figure 1.
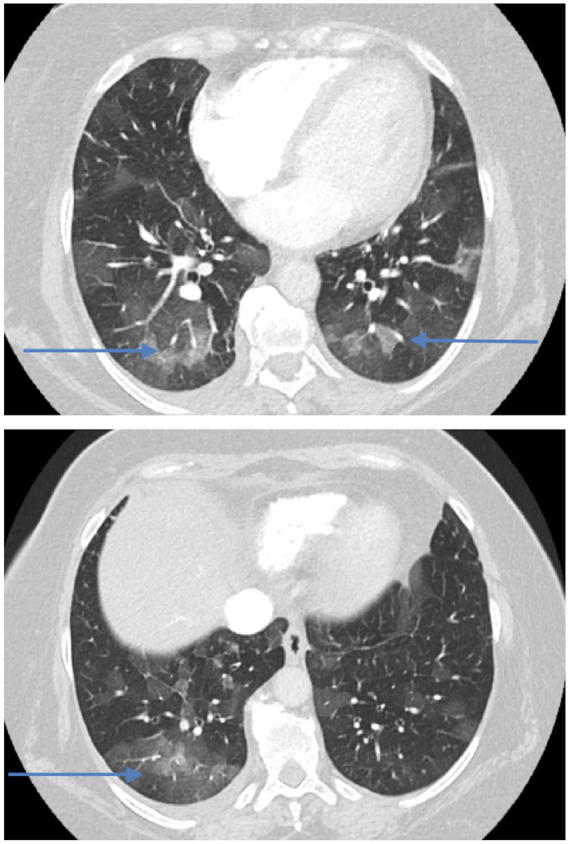
Bilateral ground-glass opacities on computed tomography thorax in Patient 1 (blue arrows).
Figure 2.
Transoesophageal echocardiography in Patient 1. (A) Mid-oesophageal right ventricular inflow–outflow view showing thickened aortic valve leaflets with an additional structure at level of the non- and right coronary cusp. (B) Mid-oesophaegeal aortic valve long-axis view showing thickening of the aortic sinus in continuity with the base of the anterior mitral leaflet. Ao, aorta; LA, left atrium; lc, left coronary sinus and cusp; LV, left ventricle; nc, non-coronary; rc, right coronary; RV, right ventricle.
Figure 3.
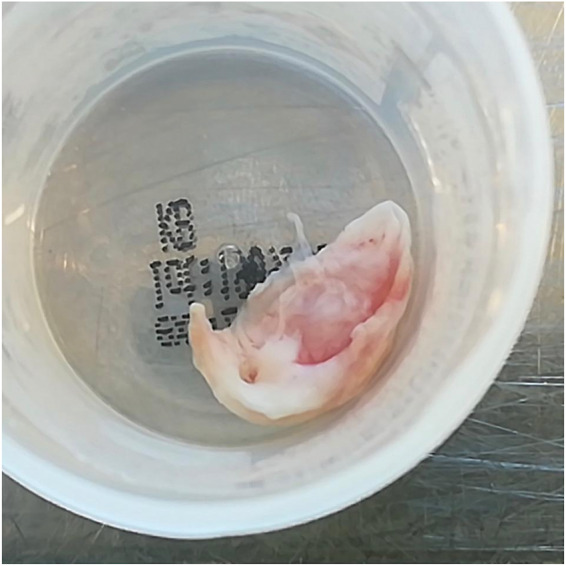
Macroscopic view of thickened and inflamed aortic valve leaflet in Patient 1.
Figure 4.
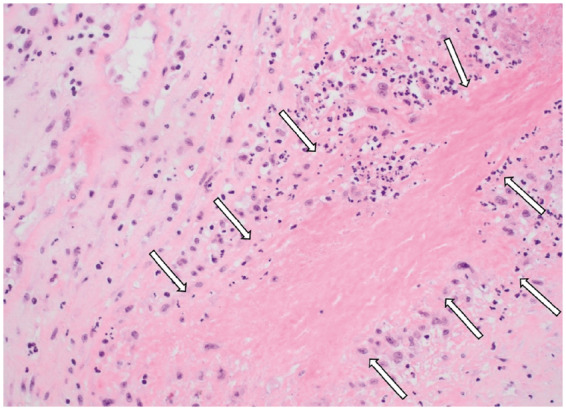
Histopathology of the aortic valve in Patient 1: geographic necrosis (arrows) (haematoxylin and eosin staining, original magnification ×200).
Figure 5.
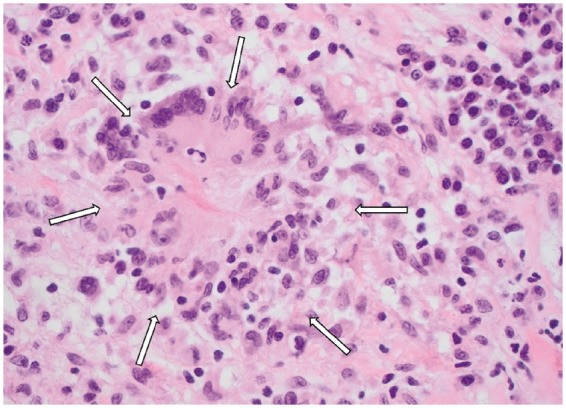
Histopathology of the aortic valve in Patient 1: small granuloma (arrows) (haematoxylin and eosin staining, original magnification ×400).
Patient 2
The second patient was a 72-year-old woman. In her medical history, a functional endoscopic sinus surgery was performed because of persisting sinusitis, and she had tympanostomy tubes placed because of recurrent otitis media with effusion. There were no cardiovascular risk factors. She was admitted to the internal medicine department with complaints of increased fatigue, dizziness, and persistent fever for 2 months. Clinical examination showed a blood pressure of 130/76 mmHg, a heart rate of 74 b.p.m., and a JVP of 6 cm above the sternal angle. A diastolic murmur was noticed and lung auscultation was normal. Initial laboratory results showed an elevated erythrocyte sedimentation rate of 114 mm/u (0–30), a slightly elevated white blood cell count of 10.99 × 10E3/µL (3.65–9.30), and a CRP level elevated up to 122 mg/L (<5.0). Serum creatinine level was 0.61 mg/dL (0.55–0.96). Computed tomography (CT) scan of the sinuses showed a bilateral maxillary sinusitis, CT scans of thorax and abdomen were negative. TOE showed normal atria and ventricles with a normal systolic function. A tricuspid aortic valve was seen with moderate aortic valve regurgitation, thickened leaflets and an additional structure at the level of the left and RCC. There was a maximal gradient of 32 mmHg and a mean gradient of 17 mmHg across the valve. The thickening of the aortic sinus continued to the base of the anterior mitral leaflet and a moderate mitral valve insufficiency was seen. Pulmonary valve and tricuspid valve were normal (Figure 6 and Video 2). Although blood cultures remained negative, a tentative diagnosis of infective endocarditis was made and treatment for endocarditis was started with ampicillin (6 × 2 g), oxacillin (6 × 2 g), and gentamycin (1 × 450 mg). On a new ultrasound an expansion of the thickening was seen (aortomitral continuity 17 mm × 9 mm; RCC 9 mm). Antibiotic therapy was empirically switched to vancomycin (1.8 mg/day continuous infusion) and gentamycin (600 mg/day) and patient was scheduled for surgery (20 days after initial antibiotic therapy). Coronary artery imaging was not performed prior to surgery. Intraoperative findings did not show endocarditis, but a massive infiltration of the ventricular side of the right and left coronary cusp was seen, expanding towards the apex in the interventricular septum, between pulmonary artery and aortic base, and towards the base of the mitral valve. An aortic valve replacement was performed with a 23 mm biological valve. Mitral valve replacement was not performed. The procedure was uneventful. Due to a persistent third-degree AV block, a dual-chamber pacemaker was placed on the 11th postoperative day. Anatomopathological findings of the valve showed a valvular and subvalvular pseudotumour caused by GPA, with necrotizing granulomas (Figure 7). Microscopic findings of the myocardial biopsy showed a mixed inflammatory process as well as necrosis and multiple, small necrotizing granulomas. The patient was discharged on postoperative Day 15; however, on postoperative Day 26, she was readmitted because of a persisting fever. Investigations showed inflammatory blood result with an elevated erythrocyte sedimentation rate of 87 mm/h (0–30), CRP of 157 mg/L (<0.5), and white blood cell count of 13.41 × 10E3/µL (3.65–9.30). There was a positive ANCA result with slightly elevated anti-MPO titer up to 5.8 IU/mL. Therapy with Methylprednisolone (32 mg daily) was started, resulting in a good clinical and biochemical response. Two weeks later, Cyclophosphamide was started (6 cycles of 500 mg, with 2 weeks interval), followed by a maintenance therapy of Azathioprine (50 mg, twice daily) and a corticoid tapering schedule (10% per month). With this therapy no GPA-activity was seen: patient had no complaints, inflammatory markers and kidney function remained normal. Cardiac evolution initially showed a thickening at the level of the left coronary cusp with a mild paravalvular regurgitation. After 4 years of follow-up, no evolution of the aortic regurgitation and no more GPA activity were noted.
Figure 6.

Transoesophageal echocardiography in Patient 2. (A) Mid-oesophageal right ventricular inflow–outflow view showing thickened aortic valve leaflets with an additional structure at level of the non- and right coronary cusp. (B) Mid-oesophaegeal aortic valve long-axis view showing thickening of two aortic valves and the aortic sinus in continuity with the base of the anterior mitral leaflet. Ao, aorta; LA, left atrium; lc, left coronary sinus and cusp; LV, left ventricle; nc, non-coronary; rc, right coronary; RV, right ventricle.
Figure 7.
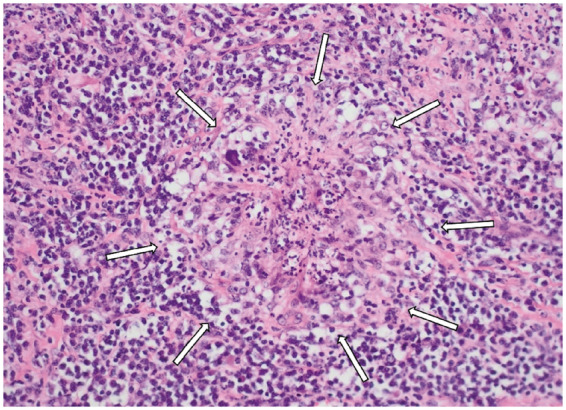
Microscopy of the aortic valve in Patient 2, demonstrating a necrotizing granuloma with surrounding acute and chronic inflammatory infiltrate (arrows) (haematoxylin and eosin staining, original magnification ×200).
Discussion
Granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, is typically characterized by inflammation of the respiratory tract and by glomerulonephritis. Cardiac lesions previously thought as rather rare in GPA, may be more frequent than expected, ranging from 6% to 44% in the literature.2,8 Granulomatosis with polyangiitis can cause pericarditis, coronary arteritis, myocarditis, valvulitis, and arrhythmias.8
We reviewed two cases transferred to our centre because of suspicion of infective endocarditis with de novo aortic regurgitation. In both patients, infective endocarditis was a possible diagnosis, but major criteria for infective endocarditis were doubtful. On the ultrasound, there was mainly a thickening of the leaflets and surrounding tissue with a new aortic regurgitation, but no typical mobile vegetation, no abscess, and no clear valve destruction.9,10 In both cases, other sources of infection were excluded and urgent surgery (within a few days) was performed, mainly because of the severity of the aortic valve regurgitation. Additionally, in the second case, ultrasound findings were worse within a couple of days, despite antibiotic treatment.
In the literature, the majority of the patients with a de novo aortic regurgitation based on GPA necessitates surgical repair. Some cases had lethal consequences by waiting. Recent overview of valvular involvement in GPA showed a total of 36 case reports. In 41.6% of cases, it concerned an isolated lesion of the aortic valve. When the aortic valve is affected, aortic valve replacement was performed in 66.7% of cases. In 8.3%, the lesions were unchanged after immunosuppressive therapy, and in another 8.3% of cases, there was an improvement or complete resolution after immunosuppressive therapy.7 Whether recurrence of the disease is possible after surgery and whether the postoperative follow-up should be different from the regular postoperative follow-up also remains unclear. Annual echocardiogram was performed in Patient 2.
Therapeutic management aims to achieve optimal overall disease control and to minimize treatment-associated toxicity. First-line therapy to rapidly control inflammation are glucocorticoids, while cyclophosphamide and rituximab are the golden standard to induce remission in severe generalized disease. Methotrexate and azathioprine are the current immunosuppressive agents used for maintenance therapy.11,12 Data concerning the effect of medical therapy on valvular disease caused by GPA seems to be lacking.
Early recognition and diagnosis of GPA is key for an effective and timely initiation of the appropriate therapy and to prevent the progression of this disease to an irreversible phase. There are no specific diagnostic tests for GPA. ENT specialists are often the first clinicians for patients with GPA, as was the case with our two patients. Therefore, in cases of atypical inflammation of the ear or sudden sensorineural hearing loss in adults without apparent reason in their medical history, GPA should be included in the differential diagnosis of the ENT specialist.13
Conclusion
We report two cases of acute aortic valve regurgitation mimicking infective endocarditis treated with an urgent aortic valve replacement. In both cases, microscopic findings of the biopsies showed acute and chronic inflammation with necrosis and necrotizing granulomas, compatible with GPA after exclusion of infectious causes. Considering their recent medical history, clinical findings, serological results, and histological results, the diagnosis of GPA was made. Granulomatosis with polyangiitis can be a rare cause of valvular heart disease, most frequently involving the aortic valve. Acute aortic valve regurgitation caused by GPA can imitate infective endocarditis and may necessitate surgical valve replacement. Clinical presentation is often insidious and lesions of ear, nose, and throat, lung, and/or kidney are most frequently seen. Symptom recognition is important for early diagnosis and appropriate treatment to prevent further progression of the disease.
Lead author biography

Dr Gilles Uijtterhaegen is a 6th year surgical resident at Ghent University Hospital. He graduated in 2014 at the medical school of the University of Ghent and has been working for several years as a surgical resident at St Jan Hospital in Bruges. He has specific interest in thoracic and vascular surgery.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Gilles Uijtterhaegen, Department of Cardiac Surgery, Ghent University Hospital, Corneel Heymanlaan 10, 9000 Ghent, Belgium.
Laura De Donder, Department of Cardiac Surgery, Ghent University Hospital, Corneel Heymanlaan 10, 9000 Ghent, Belgium.
Eline Ameloot, Department of Pathology, Ghent University Hospital, Ghent, Corneel Heymanlaan 10, 9000 Ghent, Belgium.
Kristof Lefebvre, Department of Cardiology, AZ Nikolaas, Moerlandstraat 1, 9100 Sint-Niklaas, Belgium.
Jo Van Dorpe, Department of Pathology, Ghent University Hospital, Ghent, Corneel Heymanlaan 10, 9000 Ghent, Belgium.
Michel De Pauw, Department of Cardiology, Ghent University Hospital, Corneel Heymanlaan 10, 9000 Ghent, Belgium.
Katrien François, Department of Cardiac Surgery, Ghent University Hospital, Corneel Heymanlaan 10, 9000 Ghent, Belgium.
References
- 1. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD. et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488–498. [DOI] [PubMed] [Google Scholar]
- 3. Grant SCD, Levy RD, Venning MC, Ward C, Brooks NH.. Wegener’s granulomatosis and the heart. Br Heart J 1994;71:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J 2007;28:1797–1804. [DOI] [PubMed] [Google Scholar]
- 5. Morelli S, Gurgo Di Castelmenardo AM, Conti F, Sgreccia A, Alessandri C, Bernardo ML. et al. Cardiac involvement in patients with Wegener's granulomatosis. Rheumatol Int 2000;19:209–212. [DOI] [PubMed] [Google Scholar]
- 6. Lacoste C, Mansencal N, Ben M'rad M, Goulon-Goeau C, Cohen P, Guillevin L. et al. Valvular involvement in ANCA-associated systemic vasculitis: a case report and literature review. BMC Musculoskelet Disord 2011;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Habbaa A, Rawla P, Morra ME, Abotaha AA, Sakr EE, Abdo Shehata MA. et al. Valvular involvement in granulomatosis with polyangiitis: Case report and systematic review of literature. Echocardiography 2018;35:1456–1463. [DOI] [PubMed] [Google Scholar]
- 8. Goodfield NE, Bhandari S, Plant WD, Morley-Davies A, Sutherland GR.. Cardiac involvement in Wegener's granulomatosis. Br Heart J 1995;73:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durack DT, Lukes AS, Bright DK.. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994;96:200–209. [DOI] [PubMed] [Google Scholar]
- 10. Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, Galderisi M. et al. ; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010;11:202–219. [DOI] [PubMed] [Google Scholar]
- 11. Lally L, Spiera R.. Current landscape of antineutrophil cytoplasmic antibody-associated vasculitis: classification, diagnosis, and treatment. Rheum Dis Clin North Am 2015;41:1–19, vii. [DOI] [PubMed] [Google Scholar]
- 12. Lopalco G, Rigante D, Venerito V, Emmi G, Anelli MG, Lapadula G. et al. Management of small vessel vasculitides. Curr Rheumatol Rep 2016;18:36. [DOI] [PubMed] [Google Scholar]
- 13. Greco A, Marinelli C, Fusconi M, Macri GF, Gallo A, De Virgilio A. et al. Clinic manifestations in granulomatosis with polyangiitis. Int J Immunopathol Pharmacol 2016;29:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.