Abstract
Background
Diastolic mitral regurgitation (DMR) is a type of functional mitral regurgitation. Its occurrence in the diastolic phase of cardiac cycle renders DMR an easily ignored entity. Confusing it with systolic mitral regurgitation occasionally happens. The reversal of left atrioventricular pressure gradient during diastole and the incomplete closure of mitral valve are the essential conditions for DMR. Diastolic mitral regurgitation develops under various situations, where the mechanisms of diastolic reversal of left atrioventricular pressure gradient differ.
Case summary
Patient 1 was a 50-year-old man diagnosed with 2:1 second-degree atrioventricular block (AVB). Patient 2 was a 70-year-old man diagnosed with first-degree AVB. Patient 3 was a 66-year-old man diagnosed with atrial fibrillation with long intermission and occasional atrial flutter with unequal conduction. Patient 4 was a 54-year-old woman diagnosed with dilated cardiomyopathy with complete left bundle branch block. Patient 5 was a 36-year-old man diagnosed with severe acute aortic regurgitation secondary to subacute bacterial endocarditis.
Discussion
Although the degree of DMR is relatively mild, its appearance generally prompts further clinical considerations. The appreciation of DMR has an incremental value for diagnosing and evaluating the underlying cardiovascular disease.
Keywords: Case series, Diastolic mitral regurgitation, Functional mitral regurgitation, Mechanisms
Learning points
Diastolic mitral regurgitation is easily ignored because of the different characteristics from systolic mitral regurgitation.
Diastolic mitral regurgitation could be caused by different mechanisms.
It is of great significance to recognize diastolic mitral regurgitation because it may offer incremental information for clinical decision-making.
Introduction
Mitral regurgitation (MR) is the most common valve insufficiency and is classified as primary or functional.1 Based on the phase in cardiac cycle, functional MR can be further divided into systolic mitral regurgitation (SMR) and diastolic mitral regurgitation (DMR).2 Diastolic mitral regurgitation is a rare phenomenon which appears with or without structural heart disease. Although DMR has been noticed for decades and plenty of case reports have been published, far less attention is paid to DMR than to SMR. Moreover, the rarity and occasional co-existence with specific heart disease of DMR, along with its differing clinical implications and haemodynamic consequences, necessitate the differentiation between DMR and SMR. This usually requires multiple echocardiography views and electrocardiogram (ECG) recordings.3 The recognition of DMR supplements an accurate diagnosis and evaluation of cardiovascular disease. In this article, we will discuss the mechanisms, echocardiographic features, and clinical significance of DMR by five cases.
Timeline
Baseline characteristics of the five patients included in this case series.
| Patient | Age | Gender | Diagnosis | Relevant mechanism |
|---|---|---|---|---|
| 1 | 50 | Male | 2:1 second-degree atrioventricular block (AVB) | ‘Overdue’ left ventricle (LV) systole |
| 2 | 70 | Male | First-degree AVB | ‘Overdue’ LV systole |
| 3 | 66 | Male | Atrial fibrillation with long intermission and atrial flutter | ‘Overdue’ LV systole |
| 4 | 54 | Female | Dilated cardiomyopathy with complete left bundle branch block | LV systolic asynchrony |
| 5 | 36 | Male | Severe acute aortic regurgitation (subacute bacterial endocarditis) | Elevation of LV diastolic pressure |
Case presentations
Patient 1
A 50-year-old man presented to our hospital complaining of exertional dyspnoea. Laboratory test showed his N-terminal pro-brain natriuretic peptide (NT-proBNP) was within normal range. Electrocardiogram showed 2:1 second-degree atrioventricular block (AVB). Transthoracic echocardiography (TTE) showed no evidence of pulmonary hypertension and an enlarged left atrium (LA) and DMR. The characteristics of DMR are shown in Figure 1 and Video 1. A pacemaker was implanted and atrioventricular rhythm was normalized thereafter. Diastolic mitral regurgitation disappeared immediately. During a 2-year follow-up, the pacemaker functioned well, and there was no DMR anymore.
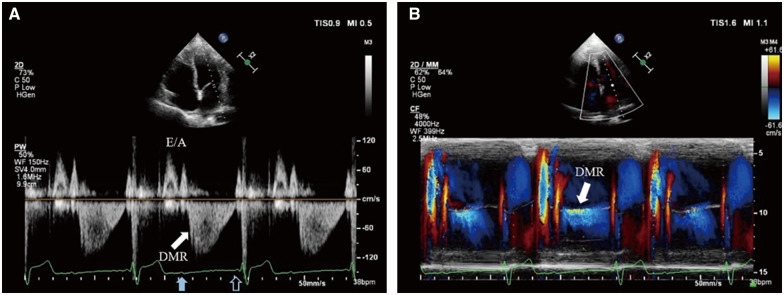
Figure 1.
P-waves with 2:1 relation with QRS complexes are displayed on the electrocardiogram. Transthoracic echocardiography: (A) PW Doppler of transmitral flow from the apical four-chamber view. After left ventricular systole, the spectrum demonstrates normal early diastolic filling E wave and left atrium contractive A wave. After the blocked sinus P-wave (solid blue arrow), diastolic mitral regurgitation (solid white arrow) appears immediately until next left atrium contraction matching the conducted sinus P-wave (hollow blue arrow). The flow velocity of diastolic mitral regurgitation is about 1.1 m/s (far lower than common systolic mitral regurgitation and slightly higher than A wave) and gradually diminishes. (B) Colour M-mode of transmitral flow from the apical four-chamber view. Diastolic mitral regurgitation presents between the two mitral valve forward flows driven by left atrium contraction (solid white arrow).
Patient 2
A 70-year-old man was diagnosed with first-degree AVB in his routine medical examination. His medical history was unremarkable for cardiovascular disease and he had no obvious clinical symptoms. Laboratory test showed his NT-proBNP was within normal range. Transthoracic echocardiography showed mild SMR and DMR. The characteristics of DMR are shown in Figure 2. There was no clinical intervention for the patient. During a 1-year follow-up, first-degree AVB and the DMR persisted.
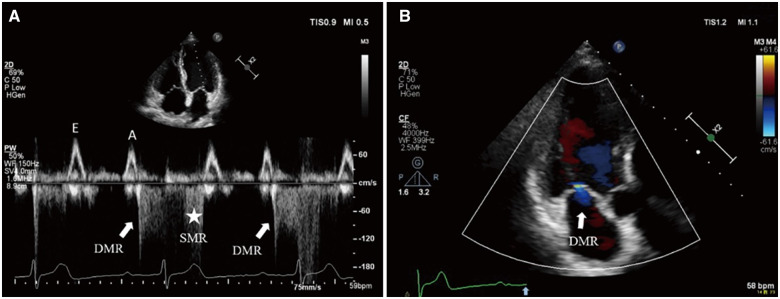
Figure 2.
P-R interval significantly prolongs over 300 ms on the electrocardiogram. Transthoracic echocardiography: (A) PW Doppler of transmitral flow from the apical four-chamber view. After left atrium systole, left ventricular contraction delays and diastolic mitral regurgitation appears with low velocity (solid white arrow) followed immediately by high velocity systolic mitral regurgitation (asterisk). (B) Colour flow Doppler still frame of transmitral flow from the apical three-chamber view. Mild diastolic mitral regurgitation jet (solid white arrow) presents after the sinus P-wave (solid blue arrow on electrocardiogram tracing).
Patient 3
A 66-year-old man presented to our hospital complaining of recurrent palpitation over 3 months. He had a history of hypertension for 8 years and diabetes mellitus for 6 years. The laboratory test revealed a slightly elevated level of NT-proBNP at 327.6 pg/mL (ref. range: 0–100 pg/mL). Electrocardiogram revealed atrial fibrillation with long intermission and occasional atrial flutter with unequal conduction. Transthoracic echocardiography showed atrial enlargement, DMR, and mild SMR. The characteristics of DMR are shown in Figure 3. This patient received medication for anticoagulation and control of ventricular rate. In the recent follow-up, the arrhythmia persisted, so did DMR.
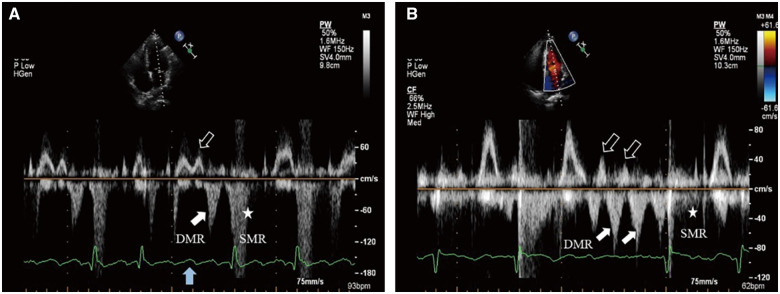
Figure 3.
(A) Atrial flutter with variable conduction on the electrocardiogram. Transthoracic echocardiography: PW Doppler of transmitral flow from the apical four-chamber view. During a 4:1 conduction, the third blocked F-wave (solid blue arrow) drives left atrium contraction accompanied by mitral valve forward flow (hollow white arrow), which is then followed by diastolic mitral regurgitation (solid white arrow) in late diastole. (B) Atrial fibrillation with a 1.5 s interval on the electrocardiogram. Transthoracic echocardiography: Multiple mitral valve forward flows (hollow white arrows) are followed closely by diastolic mitral regurgitations (solid white arrows), respectively until next left ventricular contraction. For the diastolic mitral regurgitation in both situations, the flow velocity is slightly higher than previous mitral valve forward flow.
Patient 4
A 54-year-old woman presented with 1-year history of exertional dyspnoea. The laboratory test revealed an elevated level of NT-proBNP at 1828.5 pg/mL. Electrocardiogram showed complete left bundle branch block (CLBBB). Transthoracic echocardiography demonstrated a dilated left ventricular (LV) with global hypokinesis and asynchrony. His left ventricular ejection fraction (LVEF) was 32%. Subsequent coronary computed tomography angiography revealed normal coronary arteries. A diagnosis of dilated cardiomyopathy (DCM) was made. Colour flow imaging revealed DMR and moderate SMR. The characteristics of DMR are shown in Figure 4 and Video 2. Medical therapy with betablockers, angiotensin-converting-enzyme inhibitors, and diuretics was initiated. The patient did not receive device-based therapy. His LVEF had improved to 40% after 1 and a half year of medication but DMR remained.
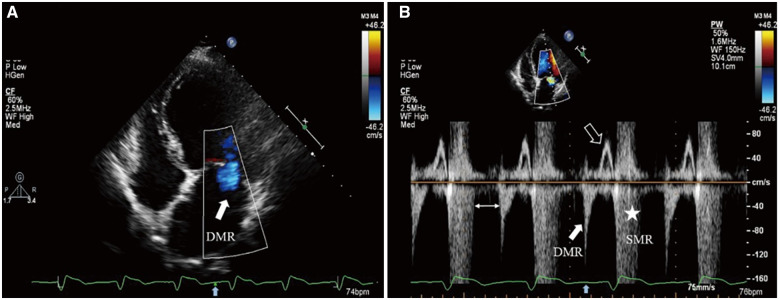
Figure 4.
Transthoracic echocardiography: (A) Colour flow Doppler still frame demonstrates diastolic mitral regurgitation jet (solid white arrow) from the apical four-chamber view after T-wave (solid blue arrow on electrocardiogram tracing). (B) PW Doppler of transmitral flow dictates two mitral regurgitation spectrums at different time of the cardiac cycle, where diastolic mitral regurgitation (solid white arrow) and systolic mitral regurgitation (asterisk) both exist. Transmitral valve forward flow signal only appears in late diastolic phase (hollow white arrow). A special ‘blank period’ existing between the two mitral regurgitation spectrums (double-headed arrow) may be due to dyssynchronous and paradoxical left ventricular movement among segments.
Patient 5
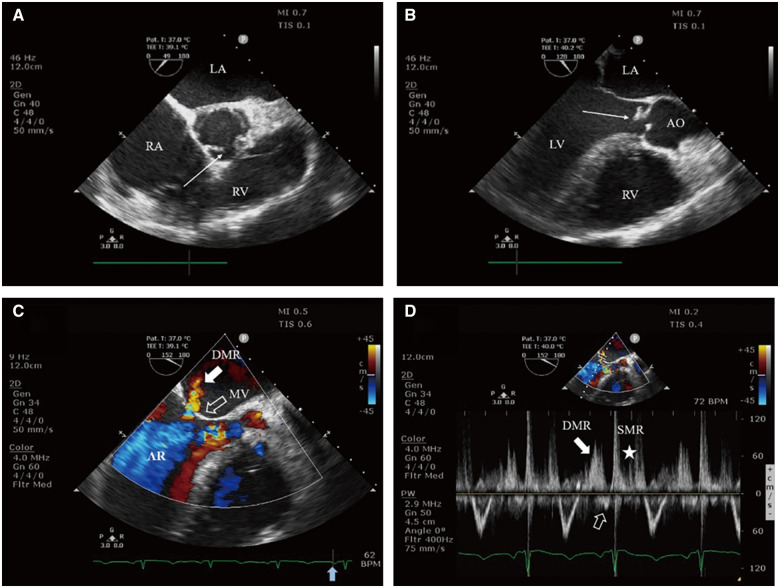
A 36-year-old man was admitted to our hospital for shortness of breath and lower extremity oedema for the previous 2 weeks. This patient reported a history of intermittent fever for the previous 2 months. On admission, his heart rate was 103 b.p.m., blood pressure was 122/47 mmHg, and temperature was 37.2°C. Cardiac examination revealed a pandiastolic murmur in the aortic area. Labs showed an elevated level of NT-proBNP at 4492.0 pg/mL. Transthoracic echocardiography demonstrated bicuspid aortic valve and severe aortic regurgitation (AR) secondary to vegetations and prolapse of aortic valve. In addition, there was DMR. Subsequent blood culture grew Streptococcus gordonii and confirmed the diagnosis of subacute bacterial endocarditis. Emergency aortic valve replacement was performed on the 4th day for aggravated heart failure. Transoesophageal echocardiography findings were similar to TTE. The characteristics of DMR are shown in Figure 5 and Video 3. After aortic valve replacement, DMR disappeared immediately. The most recent TTE showed the prosthetic valve functioned well, and there was no DMR.
Figure 5.
Transoesophageal echocardiography: (A) Midoesophageal short axis view of the aortic valve demonstrates the morphology of bicuspid aortic valve with minor thickening (arrow). (B) Midoesophageal long axis view reveals vegetations and prolapse of aortic valve (arrow). (C) Colour flow Doppler still frame demonstrates severe aortic regurgitation jet and diastolic mitral regurgitation jet (solid white arrow), and the presystolic closure of the mitral valve (hollow white arrow) before P-wave (solid blue arrow on electrocardiogram tracing). (D) PW Doppler of transmitral flow dictates two mitral regurgitation spectrums at different time of the cardiac cycle, diastolic mitral regurgitation (solid white arrow), and systolic mitral regurgitation (asterisk), and no obvious late diastolic forward flow by left atrium contraction (hollow white arrow). AO, aorta; AR, aortic regurgitation; LA, left atrium; LV, left ventricle; MV, mitral valve; RA, right atrium; RV, right ventricle.
Discussion
Essential conditions for diastolic mitral regurgitation
Blood flow is driven by pressure gradient, moving from higher pressure area to lower pressure area. Under normal conditions, a negative pressure gradient from LV to LA during diastole keeps a forward blood flow through mitral valve (MV), which then fills the LV. A reversal of this pressure gradient establishes the haemodynamic foundation for DMR, which can be achieved or maintained during various stages of diastole through different mechanisms.2,3
Full closure of the normal MV requires effective LV contraction with enough closing force, coordinated papillary muscles mechanical activation and normal mitral annular sphincteric contraction.4 Normally, after LA contraction, LV diastolic pressure will increase above LA pressure and drive MV leaflets to approximate each other.5 However, this small pressure gradient is not enough to achieve a full closure in the lack of effective LV contraction.6 Hence, the MV will keep an incomplete closure state during diastole and thus provide a potential passage for DMR. In addition, SMR patients are predisposed to DMR in the same manner.
Therefore, the reversal of left atrioventricular pressure gradient and the incomplete closure of MV are the essential conditions for DMR. Diastolic mitral regurgitation may be caused by a variety of diseases, and the mechanisms encompass ‘overdue’ LV systole, LV systolic asynchrony, and increased LV diastolic pressure.3,7 These mechanisms can induce DMR separately, or work in concert to aggravate the degree of DMR.
‘Overdue’ left ventricular systole
A delayed LV contraction mostly coincides with a certain kind of arrhythmia, such as AVB or rapid atrial arrhythmia with long intermission.8 When these arrhythmias conform to certain conditions, DMR will be induced based on the two aforementioned essential factors. It is worth mentioning that the ‘overdue’ LV systole is the most common mechanism of DMR.
Atrioventricular block
Diastolic mitral regurgitation may be observed during any degree of AVB where LA contraction is not followed by LV contraction immediately.7 Atrioventricular block can be roughly summarized as conduction failure or conduction prolongation of the sinus P-wave. In these two conditions, the mechanisms of DMR are similar.
The most classic example of sinus P-wave conduction failure-induced DMR is 2:1 second-degree AVB.9 After a conducted P-wave, LV contraction is followed by its active relaxation and an increase in LV pressure, while the next blocked P-wave causes LA contraction to further elevate the LV diastolic pressure. Subsequent LA relaxation then coincides with the elevated LV diastolic pressure. The atrioventricular pressure gradient will reverse once the LA pressure falls below that of the LV.10 Meanwhile, a properly timed LV contraction does not kick in, which leaves the MV leaflets incompletely closed. As a consequence, during the extended diastole of LV, DMR appears immediately after LA contraction.
In situation of sinus P-wave conduction prolongation, such as first-degree AVB, prolonged P-R interval delays LV contraction. Similar to 2:1 second-degree AVB, DMR may appear in the extended LV diastolic phase between LA contraction and LV contraction.
For other AVB, namely the rest second-degree and third-degree AVB, similar mechanism plays a role in the formation of DMR. Like SMR, DMR also increases the volume load of LA, while the degree is relatively slight because of the low-pressure gradient. However, inability to distinguish DMR from SMR and sometimes a confusingly large jet area of DMR can together lead to erroneous judgement of the severity of regurgitation. Diastolic mitral regurgitation caused by AVB is, in general, a benign phenomenon which can mostly be improved after treatment of AVB.11
Atrial arrhythmia
Rapid atrial arrhythmias (e.g. atrial flutter and atrial fibrillation) with long intermission are also common causes of DMR, both of which have extremely rapid atrial frequency. Once a relatively long RR interval presents, LA contractions can repeat several times between the two successive LV contractions. After every LA contraction, as in AVB, LV diastolic pressure increases while LA relaxes shortly after. Thus, one or more episodes of left atrioventricular pressure gradient reversal may happen in diastole and result in several separate occurrences of DMR until next LV contraction.
In another special situation, blocked atrial premature beat may induce DMR under certain conditions, where the electrocardiographic findings around this cardiac cycle is very similar to 2:1 second-degree AVB.12 A blocked atrial premature beat appears following the previous LV contraction and the atrioventricular pressure gradient may reverse immediately after this LA contraction, which would generate a one-time DMR.
In these atrial arrhythmias, DMR is also a benign phenomenon because of its low-pressure gradient and short duration and can be mostly relieved after the treatment of atrial arrhythmia.3
Left ventricular systolic asynchrony
Diastolic mitral regurgitation associated with LV systolic asynchrony is another interesting mechanism, and this pathological state is relatively common in DCM and CLBBB.
Left ventricular systolic dyssynchrony in DCM is due to inter- or intra-ventricular conduction delay, which can lead to asynchronous contraction of different LV wall regions.13 A few myocardial segments may perform post-systolic shortening after the closure of aortic valve, which might cause paradoxical increase of LV pressure during early diastole. The subsequent elevated pressure of LV might lead to an inversion of pressure gradient from LV to LA in early diastolic phase.3 Moreover, this pressure gradient, far below that of previous overall LV contraction process, may not be enough to drive MV towards full closure. Besides, in certain DCM, malfunctioning papillary muscles due to uncoordinated activation of corresponding segments leads to geometrical changes of MV and increased tethering.14 This process may occur in both LV overall contraction and post-systolic contraction situations. Taken together, DMR would occur in DCM patients during early diastolic phase.
This phenomenon may also be observed in CLBBB under a similar mechanism, especially in DCM combining with CLBBB.
It is worth noting that the pressure gradient reversal caused by post-systolic contraction may postpone the MV opening. Such phenomenon may be the net result of the discordant movement between segments, where partial segmental relaxation coexists with partial segmental post-systolic contraction. Therefore, although this mild DMR cannot increase the volume load of LA markedly, the early diastolic filling process of LV is hindered, and LV filling time in whole cardiac cycle is shortened to a large extent. Hence, DMR in early diastole indicates prominent LV systolic asynchrony and limited early diastolic filling. Cardiac resynchronization therapy (CRT), which helps to re-establish the synchronous contraction of different LV wall regions, may also improve the effective LV diastolic filling.6,13
Elevation of left ventricular diastolic pressure
The increase in LV diastolic pressure is another special cause of DMR. For instance, in patients with severe AR, especially acute AR, haemodynamic disturbance during diastole is the culprit of DMR. This phenomenon is relatively rare in patients with chronic AR due to LV remodelling as a result of volume load adaptation.15
In severe AR, LV accepts blood not only from LA but also from the aorta during diastole. Thus, the AR ‘overfills’ the LV and makes the filling pressure of LV rapidly increase and possibly exceed the LA pressure in late diastole, which may produce presystolic incomplete MV closure. As a consequence, in late diastole, DMR may appear and last for a period of time.
It is noteworthy that this reversal of pressure gradient impedes the transient opening of MV by atrial contraction, adds to LA volume overload and aggravates pulmonary oedema. This highlights the importance to recognize DMR in severe AR as it indicates the acuteness of AR and the need for urgent surgical intervention, while DMR in itself is a reversible process as it will disappear after successful aortic valve replacement.16
Other possible causes of the elevation of LV pressure in diastole may also lend to the formation of DMR, such as mild or moderate AR, LV diastolic dysfunction, or LV blood pooling in the setting of dynamic left ventricular outflow tract obstruction with apical hypokinesis.17–19 Although not sufficient to induce DMR separately, they may aggravate the degree of DMR in other situations (e.g. AVB) or combinatorially cause DMR.
Conclusions
Despite an uncommon finding, DMR can occur in a variety of diseases. Echocardiography with colour Doppler, spectral Doppler, and M-mode, combined with real-time ECG, represents the most useful imaging modality to identify DMR, because it enables visualization of the direction, velocity, and phase of MR. Understanding the various forming mechanisms of DMR under different disease state helps explain its relatively benign nature and the possible alternative therapeutic effect of CRT. Although the degree of DMR is relatively mild and poses insignificant regurgitant volume load to the heart, differentiation of DMR from SMR prevents overestimation of regurgitation and unnecessary clinical intervention and signifies urgent surgical correction when complicating severe AR.
Lead author biography

Quan Li is a graduate of Nanjing Medical University, Jiangsu Province, China in 2011. Currently, he worked at Department of Echocardiography, Zhongshan Hospital, Fudan University, Shanghai. His research interest is mainly focused on multimodal cardiac imaging, in particular, in valvular heart diseases and haemodynamics.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Funding
This work was supported by Natural Science Foundation of Shanghai (15ZR1438500) and Youth Backbone Foundation of Zhongshan Hospital (2017ZSGG11).
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Enriquez-Sarano M, Akins CW, Vahanian A.. Mitral regurgitation. Lancet 2009;373:1382–1394. [DOI] [PubMed] [Google Scholar]
- 2. Aydin A, Gurol T, Soylu O, Dagdeviren B.. Intermittent symptomatic functional mitral regurgitation illustrated by two cases. CVJA 2015;26:e12–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sisu RC, Vinereanu D.. Different mechanisms for diastolic mitral regurgitation illustrated by three comparative cases. Echocardiography 2011;28:476–479. [DOI] [PubMed] [Google Scholar]
- 4. Agricola E, Galderisi M, Mele D, Ansalone G, Dini FL, Di Salvo G,. et al. Echocardiographic Study Group of the Italian Society of C. Mechanical dyssynchrony and functional mitral regurgitation: pathophysiology and clinical implications. J Cardiovasc Med (Hagerstown) 2008;9:461–469. [DOI] [PubMed] [Google Scholar]
- 5. Nof E, Glikson M, Bar-Lev D, Gurevitz O, Luria D, Eldar M. et al. Mechanism of diastolic mitral regurgitation in candidates for cardiac resynchronization therapy. Am J Cardiol 2006;97:1611–1614. [DOI] [PubMed] [Google Scholar]
- 6. Agmon Y, Freeman WK, Oh JK, Seward JB.. Diastolic mitral regurgitation. Circulation 1999;99:e13. [DOI] [PubMed] [Google Scholar]
- 7. Ozeke O, Cagli K, Deveci B, Yildiz A, Ergun K, Ege MR,. et al. Imaging of diastolic mitral regurgitation in a patient with acute type A aortic dissection with diastolic prolapse of intimal flap into left ventricle. Echocardiography 2006;23:609–610. [DOI] [PubMed] [Google Scholar]
- 8. Rajesh G, Raju D, Krishnan MN. ‘ Continuously regurgitating mitral valve’: systolic and diastolic mitral regurgitation in a case of severe aortic regurgitation and complete heart block. Heart Asia 2013;5:172–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Margulescu AD, Vinereanu D, Cinteza M.. Diastolic mitral regurgitation in 2:1 atrioventricular block. Echocardiography 2009;26:228–229. [DOI] [PubMed] [Google Scholar]
- 10. Raffa S, Zito C, Oliva S, Calabro MP, La Carrubba S, Carerj S.. Diastolic mitral and tricuspid regurgitation. Echocardiography 2006;23:251–253. [DOI] [PubMed] [Google Scholar]
- 11. Aksu U, Topcu S, Gulcu O, Kalkan K, Tanboga IH.. Diastolic mitral and tricuspid regurgitation in a patient with 2:1 AV block. Int J Cardiol 2015;195:111–112. [DOI] [PubMed] [Google Scholar]
- 12. Wiggins DL, Strasburger JF, Gotteiner NL, Cuneo B, Wakai RT.. Magnetophysiologic and echocardiographic comparison of blocked atrial bigeminy and 2:1 atrioventricular block in the fetus. Heart Rhythm 2013;10:1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA,. et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 14. Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J 3rd. A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol 2004;44:1619–1625. [DOI] [PubMed] [Google Scholar]
- 15. Pulido JN, Lynch JJ, Mauermann WJ, Michelena HI, Rehfeldt KH.. Diastolic mitral regurgitation in a patient with complex native mitral and aortic valve endocarditis: a rare phenomenon with potential catastrophic consequences. Semin Cardiothorac Vasc Anesth 2016;20:100–103. [DOI] [PubMed] [Google Scholar]
- 16. Kodamag M, Koiyama S, Yamaguchi T, Hanawa H, Zhang S, Yokoyama A, et al. Diastolic mitral regurgitation in intact mitral valve detected by color Doppler echocardiography in a patient with acute aortic regurgitation. Korean J Intern Med 1992;7:64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rokutanda T, Misumi I, Usuku H, Kusuhara K, Akahoshi R, Matsumoto M, et al. Deceased left ventricular compliance contributing to diastolic mitral regurgitation in a patient with atrioventricular block. J Echocardiogr 2013;11:167–168. [DOI] [PubMed] [Google Scholar]
- 18. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 19. Agrawal A, Parikh M, Verma I, Thyagarajan B.. Diastolic mitral regurgitation in a patient with coronary artery disease and anaemia. BMJ Case Rep 2016;2016:bcr2016214473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.